Abstract
L-carnitine (LC) is an antioxidant with the ability to promote the growth in vitro embryo.
Objective:
The goal was to evaluate the effect of LC on some indicators of embryo development and blastocyst quality including zona pellucid (ZP) thickness, the hatching of blastocysts and their cell numbers.
Materials and Methods:
Mouse embryos were randomly divided into five groups and incubated with different concentrations of LC (I; 0, II; 0.5, III; 1, IV; 2 and V; 4 mg/ml) from 2-cell to hatched blastocyst. The percentage of blastocysts and hatched blastocysts was calculated. Blastocysts ZP thickness was measured and the number of blastocyst cells was counted using Hoechst and propidium iodide (PI) staining.
Results:
The results showed concentration of 0.5 mg/ml of LC had an antioxidant effect as in this group, the percentage of blastocysts and hatched blactocysts (p=0.01), the ZP thickness (p=0.00) and the number of blastocyst inner cell mass were significantly more favorable than the control group (p=0.03); and concentration of 4 mg/ml of LC had a toxic effect on embryo development and blastocyst quality (p=0.00).
Conclusion:
The results suggest that LC may increase the number of blastocyst cells, which probably helps to expand the blastocyst and thinning of the ZP thickness and, therefore, creating a successful hatching for implantation.
Key Words: L-carnitine, Embryo culture, Zona pellucida, Blastocyst inner cell mass
Introduction
Infertility is an important problem in developed countries. Recently using assisted reproductive technology (ART) has emerged more favorable status (1). ART was confirmed more than 30 years ago and it is estimated that about 3.9% of all births are the result of this method. One of the most common ART procedures is embryo in vitro culture (IVC) (2, 3). Embryo culture gives time to show its potential and has become a valuable tool for embryo selection. Observation of development in a group of embryos and their selection for transfer to the uterus is based on blastocyst morphology (4).
To get more blastocysts with high implantation potential, optimization of culture medium may be necessary (5). It is well known that in vitro embryo development can be damaged by several stressors, such as visible light or high oxygen concentration (6). Several studies have indicated that higher levels of reactive oxygen species (ROS) produced through IVC can reduce the rate of fertilization, embryo development and pregnancy (7, 8).
Moreover, blastocyst formation during ART is not desirable, and the use of antioxidants may improve it (9). It is important to keep embryos in culture medium from oxidative stress and for this aim, antioxidants are a valuable candidate (10, 11). L-carnitine (LC) is a water-soluble antioxidant which known to play an essential role in fatty acid metabolism (12-14). Several articles have shown that LC plays a pivotal role in β-oxidation by transporting fatty acid into mitochondria for ATP production, which can raise embryo development and improve the blastocyst formation rate (9, 15). In addition, LC has free radica-scavenging activity and inhibits lipid peroxidation, thereby protects against damage induced by hydrogen peroxide (H2O2) (9).
By observing embryo quality, the likelihood of successful implantation and pregnancy can be predicted. Despite the fact that many embryos have developed well, they have failed in implantation, and this is probably associated with hatching problems (16). Hatching is an exigent process where blastocyst escapes through the zona pellucida (ZP) before implantation. ZP Thickness is an important indicator for successful hatching and implantation of transferred embryos (17). ZP Elasticity and thinning are essential for hatching process, which can be adversely affected by increasing maternal age and embryo culture conditions. A thick ZP may be associated with low quality of embryos (18). Our hypothesis was that ZP thickness and blastocyst hatching might be affected by improving the culture media with LC.
The first discernible cell lineage during the mouse embryo development is organization of precursor cell populations of inner cell mass (ICM) and trophoectoderm (TE) (19). Du et al suggest that the number of cells in the blastocysts may be an important indicator for embryo quality and implantation (20). Abdelrazik et al reported that LC could improve the blastocyst formation rate in mice (9). Currently, there is insufficient document regarding the effect of LC on morphological parameters of blastocyst.
In this study, we evaluated the effect of different concentrations of LC on some indicators of embryo development and blastocyst quality in vitro including ZP thickness, hatching of blastocysts and their cell numbers.
Materials and methods
Mice obtained from Tehran Pasteur Institute (NMRI, female: 6-8 wks old; and male: 10 wks old), were kept in an air-conditioned room under controlled temperature (25±2oC) and controlled light (12 hr light/dark), with free access to food and water. All animal protocols were approved by the Semnan University of Medical Sciences animal Ethics Committee and enforced accordance with university guidelines.
Recovery of embryos
Female mice were superovulated by a 10 IU intra peritoneal injection of pregnant mare's serum gonadotropin (PMSG) (Sigma Aldrich, China) followed by injection of human chorionic gonadotropin (hCG) (Sigma Aldrich, China) (21). They were mated overnight with males and the mating was emphasized by the presence of vaginal plug on the morning after hCG injection. The 2-cell embryos were flushed from the oviduct at 48-50 hr after hCG injection and washed in human tubal fluid (HTF) medium containing HEPES (Sigma Aldrich, China). A total of 450 two-cell embryos were used in this study (90 embryos in each group).
Embryo culture
2-cell embryos were transferred into HTF medium (supplemented with 10% human serum albumin) (Sigma Aldrich, China) and were randomly divided into five groups with different concentrations of LC (I; 0.0, II; 0.5, III; 1, IV; 2 and V; 4 mg/ml) (Sigma Aldrich, China) (9). There was no LC in the control group. In all groups, 10 embryos were situated in a drop of 20 µl of HTF medium under mineral oil (Sigma Aldrich, USA) in a 35 mm Petri dish (Jet Biofil, Canada) and were incubated at 37oC with 95% humidity and 5% CO2 (22). In 120 hrs after incubation onset, the rate of development to hatched blastocysts was assessed as the percentage of 4-cell, 8-cell, morula, blastocyst and hatched blastocyst stages (23).
Measurement of ZP thickness
ZP thickness was randomly measured in early and full blastocyst stages. Measurement was taken from images using an inverted microscope (Nikon, Eclipse Ti-U, Japan) and motic images plus 2.0 software. The thickness of each ZP was measured at 3 points (24).
Differential staining of blastocysts
Expanded blastocysts were randomly selected for cell counting analysis. The embryos were treated with 0.1 mg/ml propidium iodide (PI) (Sigma Aldrich, China) and 1% Triton X-100 at 37oC for 10 sec and were instantly transferred into 25 µg/ml bisbenzimide (Hoechst 33258) (Sigma Aldrich, USA) and stored at 4oC overnight. The embryos were then mounted on glass slides in glycerol droplets and were observed under an inverted fluorescent microscope (Motic, AE31, Spain). ICM nuclei labeled with Hoechst (blue) and TE nuclei labeled with PI (red). The number of ICM, TE and total embryonic cells was counted (25).
Statistical analysis
Comparison of the percentage of embryonic developmental stages between the experimental groups and the control group was analyzed by 2 test. The ZP thickness and the blastocyst cell count were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey test as mean±SD. A difference with p<0.05 was considered statistically significant.
Results
Findings of developmental rate of embryos
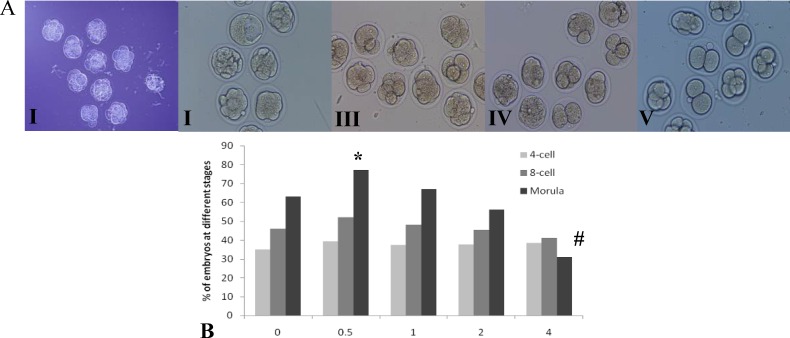
A total of 450 embryos were used to evaluate the effect of different concentrations of LC on in vitro development of 2-cell embryos to hatched blastocysts. In the early stages of embryo culture, no statistically significant difference was observed in the percentage of 4-cell and 8-cell stages between the experimental groups and the control group (p=0.67). The percentage of embryos that reached to morula stage in the 0.5 mg/ml of LC group was significantly higher than the control group (p=0.01) and there was no statistically significant difference between 1 and 2 mg/ml of LC groups than the control group (p=0.23). In addition, the percentage of morula in the 4 mg/ml of LC group was significantly lower than the control group (p=0.00) (Figure 1).
Figure 1.
A. The cleavage stages at 48 hours culture following L-carnitine exposition (×200). (I) Control group, the embryos are at 8-cell and morula stages, (II) 0.5 mg/ml, the embryos are at morula stage and in one of them blastocoele is composed, (III) 1 mg/ml, the embryos are at 4-cell and 8-cell stages, (IV) 2 mg/ml, there is a slight delay in development as the embryos are at 2-cell, 4-cell and 8-cell stages, (V) 4 mg/ml, there is a delay in development as the embryos are at 2-cell and 4-cell stages. B. The results of in vitro development of 2-cell embryos to morula stage in different concentrations of L-carnitine.
* Favorable effect of L-carnitine versus control group (p<0.05).
# Adverse effect of L-carnitine versus control group (p<0.05).
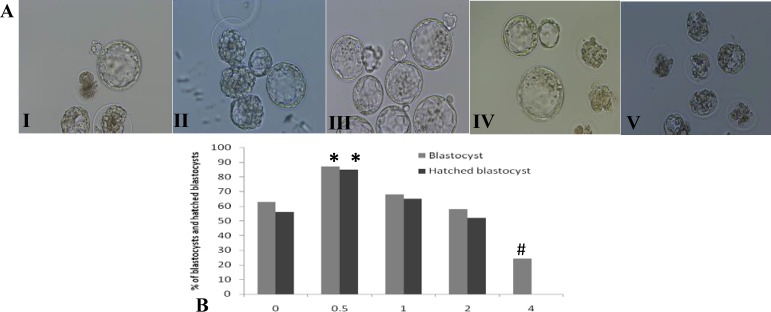
In the late stages of embryo culture, the results of percentage of embryos that reached to blastocyst and hatched blastocyst stages were almost similar to the results of the morula stage (p=0.01), as revealed an antioxidant effect of 0.5 mg/ml of LC and a toxic effect of 4 mg/ml of LC on mouse embryo development. In the 4 mg/ml of LC group, most of the blastocysts died and none were hatched (Figure 2).
Figure 2.
A. Blastocysts and hatched blastocysts in different concentrations of L-carnitine (×200). (I) Control group, some of the blastocysts have grown and some are dead, (II) 0.5 mg/ml, the blastocysts are hatching, (III) 1 mg/ml, the blastocysts have grown and have started to hatch, (IV) 2 mg/ml, some of the blastocysts have grown and some of them have developmental delay or dead, (V) 4 mg/ml, all embryos are dead. B. The results of the percentage of blastocysts and hatched blastocysts in different concentrations of L-carnitine
* Favorable effect of L-carnitine vs. control group (p<0.05).
# Adverse effect of L-carnitine vs. control group (p<0.05).
Findings of ZP thickness
Thinning of ZP occurs during the time interval between early and full blastocyst. The results showed that the ZP thickness of early and full blastocysts after incubation of embryos with 0.5 mg/ml of LC was significantly thinner than the control group (p=0.00). There was no statistically significant difference between 1 and 2 mg/ml of LC groups than the control group and the ZP thickness in the 4 mg/ml of LC group was significantly thicker than the control group (p=0.00) (Figure 3).
Figure 3.
The results of zona pellucida thickness of early and full blastocysts in different concentrations of L-carnitine.
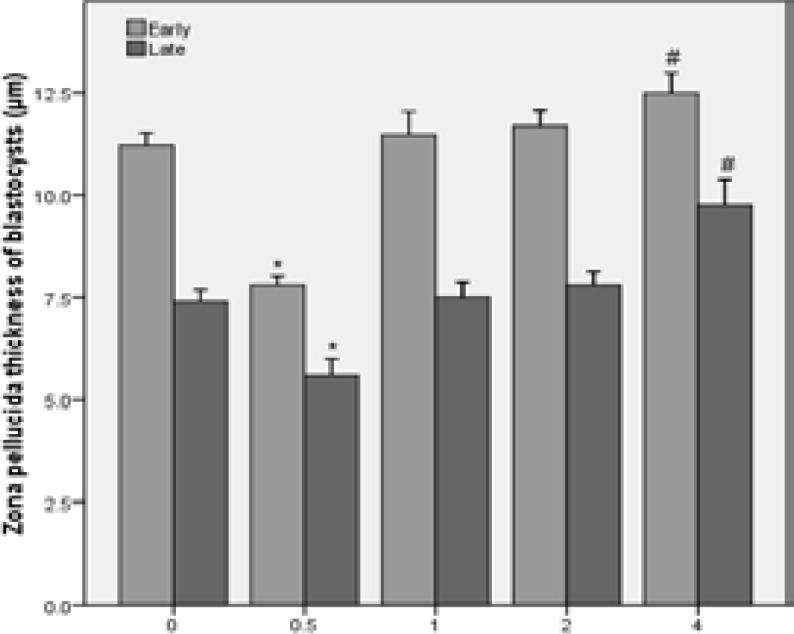
* Favorable effect of L-carnitine vs. control group (p<0.05).
# Adverse effect of L-carnitine vs. control group (p<0.05).
Findings of blastocyst cell count
Expanded blastocysts were stained with Hoechst and PI. The ICM, TE and total cell numbers were counted. The number of ICM and the total cell numbers in the 0.5 mg/ml of LC group was significantly higher than the control group (p=0.03). There was no statistically significant difference between 1 and 2 mg/ml of LC groups than the control group (p=0.87). There was no statistically significant difference in the TE cell numbers between the experimental groups and the control group (p=0.85). Since the blastocysts in the medium with 4 mg/ml of LC were not expanded, this staining was not performed in this group (Figure 4).
Figure 4.
A. Differential staining of blastocysts in different concentrations of L-carnitine (×200); (I) Control group, the average number of inner cell mass (ICM) is moderate, (II) 0.5 mg/ml, the average number of ICM is higher than the control group, (III) 1 mg/ml, the average number of ICM is almost the same as the control group, (IV) 2 mg/ml, the average number of ICM is almost the same as the control group too. B. The results of blastocyst cell numbers in different concentrations of L-carnitine.
* Favorable effect of L-carnitine versus control group (p<0.05).
Discussion
In this study, we added different concentrations of LC into embryo culture medium and evaluated some indicators of embryo development and blastocyst quality including the ZP thickness, the hatching rate and the number of blastocyst cells. Overall, the results showed the concentration of 0.5 mg/ml had an antioxidant effect and the concentration of 4 mg/ml had a toxic effect compared with the control group.
Development of a suitable culture system is necessary for successful production of embryo in vitro (26). During this process, oxidative stress is one of the detrimental agents on fertilization and embryo quality (11). Since oxidative stress during embryo culture has been known as one of the main factors responsible for poor quality of embryos, it has been suggested that environmental factors such as increasing free radical scavengers and reducing the oxygen tension are essential for improving the fertility potential in ART (26, 27).
During embryo culture, oxidative stress pressure may be regulated by adding antioxidants into culture media (10, 28). LC is an antioxidant that its antioxidant capacity has been shown in other cell types, including lymphoma cells and skeletal muscles (29, 30). Moreover, the useful effect of LC during in vitro maturation (IVM) and IVC has been reported in many mammalian species. In mice, it has been shown that adding 1mM of LC into IVM medium enhances β-oxidation, nuclear maturation and blastocyst development (31, 32). We showed that 0.5 mg/ml of LC improves development of 2-cell embryos to hatched blastocyst stage. These results are in accordance with other reports (9, 14). This positive effect is probably due to two reasons: 1. Enhancing mitochondrial lipid metabolism and 2. Act as an antioxidant to reduce oxidative stress (9, 32).
Several studies have shown that the number of blastocyst inner cell mass is indicative of embryo quality and is crucial for a successful implantation (25, 33, 34). We showed that favorable dose of LC increased the ICM. These results are in agreement with the other studies (14, 15, 31). The number of ICM is important for appropriate implantation, and several studies have reported that a reduction in the number of ICM during development can lead to reduced embryonic viability (25, 35, 36). Blastocoel fluid has H2O2 which is cytotoxic and induces apoptosis in ICM of blastocyst (37). The antioxidant effect of LC could probably increase intracellular glutathione level and since glutathione is involved in removal of H2O2, ICM would not become apoptosis (38). LC plays a key role in β-oxidation of long-chain fatty acids in mitochondria to generate cellular ATP, it can possibly increase the number of ICM by increasing ATP (14).
Embryonic cells are surrounded by ZP. Shiloh et al showed that ZP thickness of embryos isolated from cigarette smokers was thicker than non-smokers which indicated environmental factors could affect the thickness of ZP (39). Moreover, other reports have suggested ZP thickness variation is a dependable marker to select the best frozen and thawed embryos for transfer (40). ZP thickness depends on inherent properties of human embryo to produce the lytic factors required for ZP thinning. Indeed, expression of murine implantation proteinase genes (ISP1 and ISP2) has been indicated recently throughout all stages of preimplantation development from zygote to blastocyst, but synthesis of their products (ISP1-ISP2 enzyme complex) was detected only in early blastocysts (17, 41, 42).
Therefore it has been suggested that the embryonic ISP enzymes play a vital role in ZP lysis, embryo hatching, and implantation (17). We showed adding 0.5 mg/ml of LC into embryo culture medium could cause thinning of the ZP thickness and in the blastocysts with thinner ZP, hatching rate was significantly higher. These results are in agreement with other studies that the thinner ZP is essential for hatching process (43). In vitro culture of mammalian embryos changes the characteristics of their ZP; as in vitro derived embryos have thicker ZP, specially the inner layer of ZP (44). There is not enough information about the effect of antioxidants on ZP thickness of blastocyst, while the thickness of ZP is one of the most important factors for hatching and implantation (45). Hatching and implantation occur in high-quality blastocysts which have expanded enough (46).
It seems blastocyst expansion depends on the number of blastocyst cells and an increase in these cells leads to thinning of the ZP, hatching blastocyst and finally implantation (43). More research is necessary to clarify the molecular mechanisms underlying LC function on development and quality of embryos.
Conclusion
The results suggest that the concentration of 0.5 mg/ml of LC may have an antioxidant effect and the concentration of 4 mg/ml of LC may have a toxic effect on in vitro embryo development. Antioxidant effect of LC can probably increase the number of embryonic cells, which helps to expand the blastocyst and thinning of the ZP thickness and, therefore, creating a successful hatching for implantation.
Acknowledgments
This study was supported by a thesis grant from Semnan University of Medical Sciences. We would like to thank the Research Center of Nervous System Stem Cells of Semnan University of Medical Sciences for cooperation and providing facilities to this work. Finally we express our deep appreciation to Dr. Zavareh for his help.
Conflict of interest
There is no conflict of interest in this article.
References
- 1.de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, et al. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod. 2014;90:22. doi: 10.1095/biolreprod.113.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al. Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum Reprod. 2012;27:954–966. doi: 10.1093/humrep/des023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzer C, Esteves TC, Arauzo-Bravo MJ, Le Gac S, Nordhoff V, Schlatt S, et al. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum Reprod. 2012;27:2627–2640. doi: 10.1093/humrep/des223. [DOI] [PubMed] [Google Scholar]
- 4.Adler A, Lee HL, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod Biomed Online. 2014;28:485–491. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lee HJ, Yu EJ, Jee BC, Suh CS, Kim SH. Dose-dependent embryotrophic effect of recombinant granulocyte-macrophage colony-stimulating factor and brain-derived neurotrophic factor in culture medium for mouse preimplantation embryo. Obstet Gynecol Sci. 2014;57:373–378. doi: 10.5468/ogs.2014.57.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XX, Lee KB, Lee JH, Kim KJ, Kim EY, Han KW, et al. Glutathione and cysteine enhance porcine preimplantation embryo development in vitro after intracytoplasmic sperm injection. Theriogenology. 2014;81:309–314. doi: 10.1016/j.theriogenology.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Cebral E, Carrasco I, Vantman D, Smith R. Preimplantation embryotoxicity after mouse embryo exposition to reactive oxygen species. Biocell. 2007;31:51–9. [PubMed] [Google Scholar]
- 8.Mokhber Maleki E, Eimani H, Bigdeli MR, Ebrahimi B, Shahverdi AH, Golkar Narenji A, et al. A comparative study of saffron aqueous extract and its active ingredient, crocin on the in vitro maturation, in vitro fertilization, and in vitro culture of mouse oocytes. Taiwan J Obstet Gynecol. 2014;53:21–25. doi: 10.1016/j.tjog.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril. 2009;91:589–596. doi: 10.1016/j.fertnstert.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 10.Truong TT, Soh YM, Gardner DK. Antioxidants improve mouse preimplantation embryo development and viability. Hum Reprod. 2016;31:1445–1454. doi: 10.1093/humrep/dew098. [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Long H, Lyu QF, Zhang QH, Yan ZG, Liang HX, et al. Protective effect of quercetin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. PloS One. 2014;9:e89520. doi: 10.1371/journal.pone.0089520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahadori MH, Ghasemian F, Ramezani M, Asgari Z. Melatonin effect during different maturation stages of oocyte and subsequent embryo development in mice. Iran J Reprod Med. 2013;11:11–18. [PMC free article] [PubMed] [Google Scholar]
- 13.Moawad AR, Tan SL, Xu B, Chen HY, Taketo T. L-carnitine supplementation during vitrification of mouse oocytes at the germinal vesicle stage improves preimplantation development following maturation and fertilization in vitro. Biol Reprod. 2013;88:104. doi: 10.1095/biolreprod.112.107433. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Inaba Y, Somfai T, Kaneda M, Geshi M, Nagai T, et al. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod Fertil Dev. 2013;25:589–599. doi: 10.1071/RD11262. [DOI] [PubMed] [Google Scholar]
- 15.Phongnimitr T, Liang Y, Srirattana K, Panyawai K, Sripunya N, Treetampinich C, et al. Effect of L-carnitine on maturation, cryo-tolerance and embryo developmental competence of bovine oocytes. Anim Sci J. 2013;84:719–725. doi: 10.1111/asj.12067. [DOI] [PubMed] [Google Scholar]
- 16.Kilani SS, Cooke S, Kan AK, Chapman MG. Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote. 2006;14:39–44. doi: 10.1017/S0967199406003625. [DOI] [PubMed] [Google Scholar]
- 17.Balakier H, Sojecki A, Motamedi G, Bashar S, Mandel R, Librach C. Is the zona pellucida thickness of human embryos influenced by women's age and hormonal levels? Fertil Steril. 2012;98:77–83. doi: 10.1016/j.fertnstert.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Ghannadi A, Kazerooni M, Jamalzadeh F, Amiri S, Rostami P, Absalan F. The effects of laser assisted hatching on pregnancy rates. Iran J Reprod Med. 2011;9:95–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama M, Roberts RM. The effect of superovulation on the contributions of individual blastomeres from 2-cell stage CF1 mouse embryos to the blastocyst. Int J Dev Biol. 2010;54:675–681. doi: 10.1387/ijdb.092942mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du QY, Wang EY, Huang Y, Guo XY, Xiong YJ, Yu YP, et al. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil Steril. 2016;105:910–919. doi: 10.1016/j.fertnstert.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Dai SJ, Xu CL, Wang J, Sun YP, Chian RC. Effect of culture medium volume and embryo density on early mouse embryonic development: tracking the development of the individual embryo. J Assist Reprod Genet. 2012;29:617–623. doi: 10.1007/s10815-012-9744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melin J, Lee A, Foygel K, Leong DE, Quake SR, Yao MW. In vitro embryo culture in defined, sub-microliter volumes. Dev Dyn. 2009;238:950–955. doi: 10.1002/dvdy.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nematollahi-mahani SN, Nematollahi-mahani A, Moshkdanian G, Shahidzadehyazdi Z, Labibi F. The role of co-culture systems on developmental competence of preimplantation mouse embryos against pH fluctuations. J Assist Reprod Genet. 2009;26:597–604. doi: 10.1007/s10815-009-9363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filho ES, Noble JA, Wells D. A review on automatic analysis of human embryo microscope images. Open Biomed Eng J. 2010;4:170–177. doi: 10.2174/1874120701004010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3:25–29. doi: 10.1016/s1472-6483(10)61960-8. [DOI] [PubMed] [Google Scholar]
- 26.Thiyagarajan B, Valivittan K. Ameliorating effect of vitamin E on in vitro development of preimplantation buffalo embryos. J Assist Reprod Genet. 2009;26:217–225. doi: 10.1007/s10815-009-9302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoskeleton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril. 2009;91(Suppl.):2079–2086. doi: 10.1016/j.fertnstert.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 28.Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1602–1606. doi: 10.1093/humrep/den109. [DOI] [PubMed] [Google Scholar]
- 29.Qi SN, Zhang ZF, Wang ZY, Yoshida A, Ueda T. L-carnitine inhibits apoptotic DNA fragmentation induced by a new spin-labeled derivative of podophyllotoxin via caspase-3 in Raji cells. Oncol Rep. 2006;15:119–122. [PubMed] [Google Scholar]
- 30.Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, et al. L-Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283:802–810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- 31.Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol Reprod. 2011;85:548–555. doi: 10.1095/biolreprod.110.090415. [DOI] [PubMed] [Google Scholar]
- 32.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 33.Asgari Z, Ghasemian F, Ramezani M, Bahadori MH. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012;14:203–208. [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YS, Thouas GA, Gardner DK. Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Hum Reprod. 2015;30:543–552. doi: 10.1093/humrep/deu334. [DOI] [PubMed] [Google Scholar]
- 35.Houng WL, Lin CA, Shen JL, Yeh HI, Wang HH, Chang WH, et al. Dihydrolipoic acid induces cytotoxicity in mouse blastocysts through apoptosis processes. Int J Mol Sci. 2012;13:3988–4002. doi: 10.3390/ijms13033988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zullo G, Albero G, Neglia G, De Canditiis C, Bifulco G, Campanile G, et al. L-ergothioneine supplementation during culture improves quality of bovine in vitro-produced embryos. Theriogenology. 2016;85:688–697. doi: 10.1016/j.theriogenology.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011;23:561–575. doi: 10.1071/RD10148. [DOI] [PubMed] [Google Scholar]
- 38.Zare Z, Masteri Farahani R, Salehi M, Piryaei A, Ghaffari Novin M, Fadaei Fathabadi F, et al. Effect of L-carnitine supplementation on maturation and early embryo development of immature mouse oocytes selected by brilliant cresyle blue staining. J Assist Reprod Genet. 2015;32:635–643. doi: 10.1007/s10815-015-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiloh H, Lahav-Baratz S, Koifman M, Ishai D, Bidder D, Weiner-Meganzi Z, et al. The impact of cigarette smoking on zona pellucida thickness of oocytes and embryos prior to transfer into the uterine cavity. Hum Reprod. 2004;19:157–159. doi: 10.1093/humrep/deh029. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson JO, Szurek EA, Higgins AZ, Lee SR, Eroglu A. Optimization of cryoprotectant loading into murine and human oocytes. Cryobiology. 2014;68:18–28. doi: 10.1016/j.cryobiol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang L, Rancourt DE. Murine implantation serine proteinases 1 and 2: structure, function and evolution. Gene. 2005;364:30–36. doi: 10.1016/j.gene.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 42.Sharma N, Liu S, Tang L, Irwin J, Meng G, Rancourt DE. Implantation Serine Proteinases heterodimerize and are critical in hatching and implantation. BMC Dev Biol. 2006;6:61. doi: 10.1186/1471-213X-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montag M, Koll B, Holmes P, van der V. Significance of the number of embryonic cells and the state of the zona pellucida for hatching of mouse blastocysts in vitro versus in vivo. Biol Reprod. 2000;62:1738–1744. doi: 10.1095/biolreprod62.6.1738. [DOI] [PubMed] [Google Scholar]
- 44.Bassam A, Elhelw MMES, Khaled M, El Nomrosy. Assisted hatching: routine or selective application in IVF. Middle East Fertil Soc J. 2004;9:198–201. [Google Scholar]
- 45.Marco-Jimenez F, Naturil-Alfonso C, Jimenez-Trigos E, Lavara R, Vicente JS. Influence of zona pellucida thickness on fertilization, embryo implantation and birth. Anim Reprod Sci. 2012;132:96–100. doi: 10.1016/j.anireprosci.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Lu YC, Ding GL, Yang J, Zhang YL, Shi S, Zhang RJ, et al. Small-conductance calcium-activated K(+) channels 3 (SK3) regulate blastocyst hatching by control of intracellular calcium concentration. Hum Reprod. 2012;27:1421–1430. doi: 10.1093/humrep/des060. [DOI] [PubMed] [Google Scholar]