Abstract
Background:
The mammalian ovary is controlled by a number of biological rhythms, which regulate the recruitment and release of mature oocytes. The main objective of this study was to investigate the role of cellular clock proteins during follicle maturation in the mouse estrous ovary.
Methods:
Immunohistochemical (IHC) studies were performed on ovaries from 50 estrous staged mice culled at two time points of 09:00 [day] and 01:00 [mid-point of the dark cycle]. Six antibodies were used to identify the expression of core cellular clock proteins (BMAL1, CLOCK, CRY1, CRY2, PER1 and PER2) within the ovary and four follicle stages, primordial, primary, antral and corpus lutea. IHC data was scored using the Allred protocol and significance determined by Mann-Whitney tests. Differences were considered significant at p<0.05.
Results:
All four follicle stages presented greater BMAL1 and CLOCK protein scores during the day and up regulation of CRY1-2 and PER1-2 at night. In primordial follicles, BMAL1 and CLOCK increases were significant (p<0.05) and CRY-1 and PER-1 were highly significant (p<0.001), and CRY-2 did not reach significance. Primary follicles demonstrated a similar response with BMAL1 and CLOCK, and CRY-1, PER-1-2 all reaching significant expression (p<0.05; p<0.001; p<0.001 respectively). CRY-2 expression was not significant. Antral follicles did not show significant BMAL1 or CLOCK expression, CRY-1 and PER-1 were highly significant (p<0.001) and CRY-2 had a small but significant increase (p<0.05). Corpus lutea demonstrated significant BMAL1 increase but CLOCK had no significant variation. CRY-1, PER1-2 increases were highly significant (p<0.001) and CRY-2 was up regulated but failed to reach significance.
Conclusion:
The ovary demonstrated a cellular clock response to the light: dark cycle and in addition, as the ovarian follicles mature changes in the positive and negative arms of both clock responsive proteins were observed.
Keywords: Cellular clock, Ovarian follicles, Ovary
Introduction
The critical functions of the ovary are the regular release of mature oocytes and creating an optimal environment for both fertilization and early implantation. In order to provide a mature oocyte at regular intervals throughout an animal’s sexual lifetime, the development and the transition of each primordial follicle to a mature ovulatory oocyte is a finely controlled process (1, 2). In the human, the ovary needs to be able to selectively develop one primordial follicle without the neighboring follicles developing. The dormant primordial follicles (unselected) are critical to maintaining the follicular pool for future ovulation (3, 4). This process is poorly understood with numerous factors implied but still no satisfactory mechanism proposed. However, the latter stages of folliculogenesis (post-antral) are well understood and are primarily controlled by the gonadotropins; follicle stimulating hormone (FSH) and luteinizing hormone (LH) with FSH being responsible for the development of an antral follicle to a Graafian follicle and the LH surge causing follicular rupture and release of the oocyte for fertilization (5–7). Despite the hormonal control of follicle development being well understood, the pre-antral development still has many complexities which remain unresolved particularly the recruitment and selection of primordial follicles to progress to maturity (8). Two observations suggest a circadian cellular clock made be implicated in this development. Firstly in animals, the photoperiod or the light: dark cycle directly effects the timing and efficiency of ovulation. Hamsters have been shown to vary the timing of ovulation dependent on the ratio of light: dark, with the exposure to constant light disrupting regularity in ovulation (9). Furthermore, rats cease ovulation after 60 days of constant light and ovulation is lost along with a decrease in LH (10). In addition to animal studies, there is substantial evidence that disruption of the cellular clocks in humans also perturbs reproductive cycles including ovulation (11). Secondly, two proteins involved in the cellular clock (CLOCK and PER-1) have been identified in both the testis and ovaries, however, they differ in their expression between these organs. The testis, which can continually produce gametes, shows a lack of oscillation of these proteins, which is normally considered critical to the function of the cellular clock (12), whereas the ovary has an oscillating cellular clock as demonstrated by mRNA studies (13, 14).
The cellular clock has been identified in the majority of mammalian peripheral tissues after the original discovery in the suprachiasmatic nucleus (SCN). The core of the cellular clock is established by a transcriptional/translational feedback loop set up by the oscillation of clock genes and their respective proteins (15). The positive arm and the negative arm are diurnally expressed with the peaks of their expression approximately 12 hr out of synchronization (16). The mechanism is dependent on the control over the formation of the heterodimer BMAL1: CLOCK (17, 18), through a basic helix-loop-helix (bHLH) domain. The heterodimer is able to bind to the E-box regulatory element where it recruits the cellular machinery involved in the transcriptional up regulation of downstream genes (19). The E-box promoted genes can be separated into two classes; genes involved in the negative regulation of the cellular clock (PER1-3 and CRY1-2) and genes that have physiological function (clock output genes). The negative clock genes are translated and form a protein complex (CRY: PER) which is able to translocate into the nucleus where it inhibits the promotion of E-box transcription via BMAL1: CLOCK, thus ultimately reducing their Per and Cry transcription and completing the cellular clock. A critical feature of a circadian clock is that it can function autonomously, without external input. However, it can be modified or entrained to the local cellular environment (20), thereby providing a model mechanism where the cellular clock is able to control cellular activities including metabolic homeostasis, cell cycle control and hormone oscillations by altering the transcription of the clock output genes.
Although previous work has focused on cellular clock mRNA expression, it is established that mRNA expression may not predict the cellular protein level for a number of proteins and should not be used as a surrogate to demonstrate the corresponding cellular protein expression and alternative techniques such as immunohistochemistry should be used to provide definitive identification and localization of the tissue specific protein (21). In this study, immunohistochemistry was used to investigate cellular clock protein expression and it was demonstrated that the mouse estrous ovary expresses all six of the core cellular clock proteins and that they are responsive to the light:dark cycle.
Methods
Animals:
All mice used were sexually mature female C57BL/6J, 6 to 10 weeks old and were maintained in the University of Otago Animal Facility. Mice were provided food and water ab libitum and maintained on a 12:12 hour light dark cycle (Lights onset at 0700/zeitgeber time (ZT) 0). Tissues were obtained at two different chronological time points, (ZT2/0900 and ZT18/0100 [midpoint of the dark cycle]), and within each time point estrous was determined representing hormone responsive ovaries at ovulation. In addition to ovaries, kidney and liver were obtained as control tissues, which demonstrate circadian clock protein expression.
Animal procedures were under the approval of the University of Otago Animal Welfare Office [AEC: 39/08].
Estrous staging:
For estrous staging, vaginal smears were obtained and stained with 1% methylene blue for 10 min (22). Cell type and frequency were assessed where no/low epithelial cells suggested metestrous and high epithelial cell numbers, indicating mice in estrous. Estrous was determined for mice at 09:00 hr and 01:00 hr. A total of 50 mice were scored as being in estrous with 40 mice in estrous during the day and 10:00 at night.
Immunohistochemistry:
The ovaries were fixed in buffered formal saline and processed for histology. Serial sections were cut from each ovary and tissue sections were deparafinized in xylene and progressively rehydrated through graded alcohols and a final water wash. Heat antigen retrieval was performed in citrate buffer pH=6.0 for 25 min in a KOS multifunctional microwave tissue processor (Milestone, Italy). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide and non-specific binding was blocked using 3% bovine serum albumin (Invitrogen Life Sciences, USA). Tissues were incubated with each of the six clock protein primary antibodies (AbCam, UK) using optimized dilutions as shown in table 1. Following primary antibody incubation and washes, the sections were incubated with an anti-rabbit secondary antibody conjugated to biotin (AbCam, UK). Vectorstain ABC elite kits (Vector Laboratories, USA) were then used to visualize the bound primary antibody. Binding was located using commercial 3,3′-diaminobezidine hydrochloride (DAKO, Australia) and the sections were then washed and counterstained with haematoxylin, dehydrated through graded alcohols and cleared in xylene before mounting in Entellan (Merk Chemicals, Germany).
Table 1.
Primary clock related optimized antibody dilutions and incubation times
| Antibody | Dilution factor | Concentration (μg/ml) | Incubation period |
|---|---|---|---|
| CLOCK | 1.1000 | 1.0 | 1 hr at RT |
| BMAL1 | 1.200 | 5 | ON at 4°C |
| CRY1 | 1.1000 | 1 | ON at 4°C |
| CRY2 | 1.100 | 2.5 | ON at 4°C |
| PER1 | 1.500 | 2 | 1 hr at RT |
| PER2 | 1.500 | 1.1 | 1 hr at RT |
| SIRT1 | 1.2000 | 0.55 | 1 hr at RT |
RT: Room Temperature; ON: Overnight
Cell scoring:
All sections were examined microscopically by two observers and imaging data stored for analysis using an Olympus 1X71 microscope and DP control imaging system (Shinjuku, Japan). Scoring for staining location and intensity was undertaken using Allred scoring protocol (23, 24), which combines the cellular staining intensity with nuclear localization. Sections were imaged and given a score out of eight, which is derived from a staining intensity score (0–3) and a nuclear positivity score (0–5). Staining intensity included 0=negative, 1=weak, 2=moderate, and 3= intense stain. Nuclear positivity was scored by a cell count where 0%=0, 1–9%=1+, 10–32%=2+, 33–65%=3+, 66–90%=4+, 90–100%=5+. For follicles large enough, a minimum of 100 cells per follicle were counted across a minimum of 10 objective fields to ensure a representative observation and when possible 20 follicles were counted for each stage.
Statistics:
Allred scores are presented as the mean ±S.E.M. Statistical differences were determined by Mann-Whitney tests. Differences were considered significant at p<0.05 or less (SPSS Inc, Chicago, IL, USA).
Results
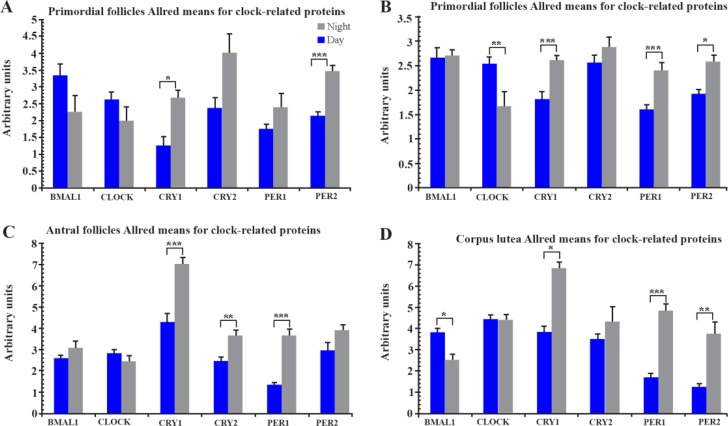
Mean Allred positivity scores (±SEM) for each respective clock protein are shown in figure 1. For each of the four follicle stages assessed, all presented with greater BMAL1 and CLOCK protein scores in the day (09:00 hr) ovarian sections. Conversely, the four negative arm clock proteins (CRY1-2 and PER 1-2) were up regulated in the night ovarian sections (01:00 hr). Of the four negative clock proteins, only CRY1 had a statistically significant difference with time for all follicle stages (primordial follicles, p<0.05; primary follicles, p<0.001; antral follicles, p<0.001; corpus lutea, p<0.05).
Figure 1.
Mean Allred positivity scores for each respective clock related proteins. The four follicle stages all display a clock where the positive clock-proteins (BMAL1 and CLOCK) presented greater scores in day sections. Conversely, the four negative clock-related proteins were upregulated in night sections. A: Positivity of clock-related proteins expressed in primordial follicles. Only CRY1 displayed a statistically significant difference across the time. B: Positivity of clock-related proteins expressed in primary follicles. C: Positivity of clock-related proteins expressed in antral follicles. D: Positivity of clock-related proteins expressed in corpus lutea. All scores are mean Allred scores±S.E.M. Mann-Whitney statistics was performed on Allred data.
*p<0.05, **p<0.01, ***p<0.0001
Primordial follicle cellular clock:
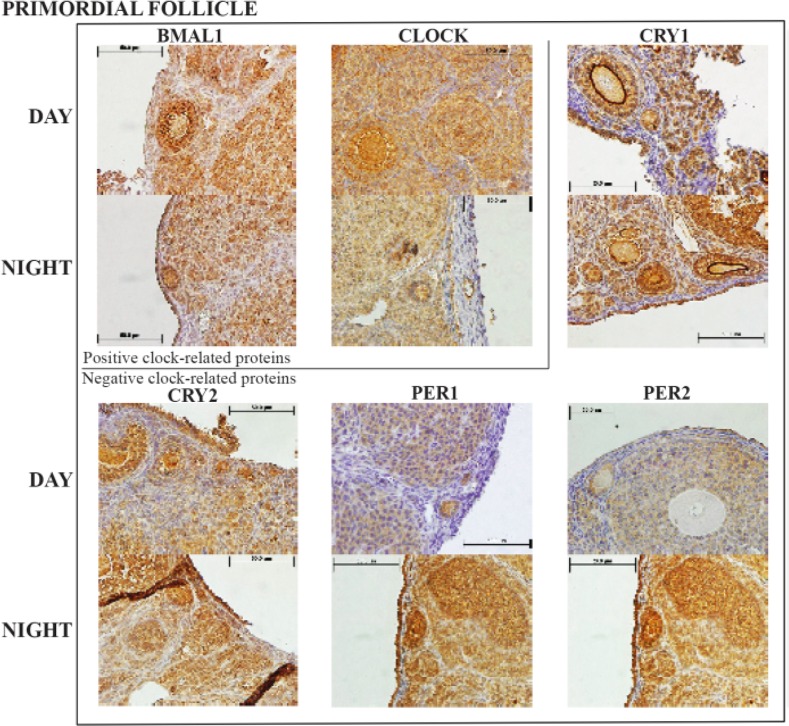
All sections for primordial follicle staining (Figure 1A and figure 2) exhibited a light:dark response with greater BMAL1 and CLOCK expression during the day. However, although BAML1 demonstrated a significant decrease at night (p<0.05), CLOCK demonstrated little variation between day and night. CRY-1 and PER-1 and 2 demonstrated highly significant increases (p<0.001) at night but CRY-2 did not increase.
Figure 2.
Representative primordial follicle histology sections with the six clock proteins expression indicated by brown staining. Box encloses BAML1 and CLOCK (positive arm) proteins
Primary follicle cellular clock:
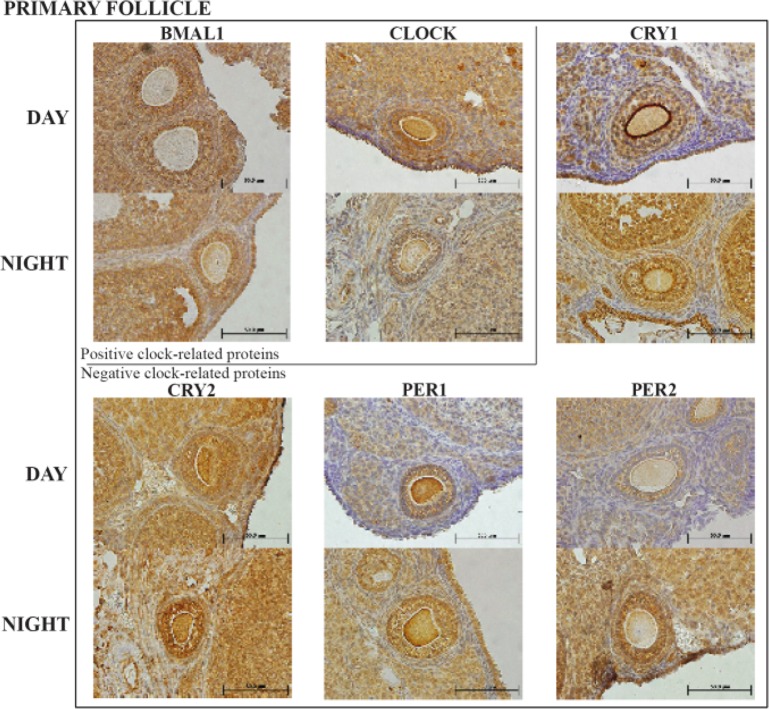
All sections for primary follicle staining (Figure 1B and figure 3) exhibited a light dark response similar to that identified in the primordial follicles for both BMAL1 and CLOCK in daytime. However, BAML1 did not decrease at night whereas CLOCK decreased at night in a similar response identified in primordial follicles. Three of the four negative arm clock proteins, CRY-1, PER-1 and 2, demonstrated highly significant increases at night (p<0.001) with only CRY-2 not increasing significantly.
Figure 3.
Representative primary follicle histology sections with the six clock proteins expression indicated by brown staining. Box encloses the BAML1 and CLOCK (positive arm) proteins
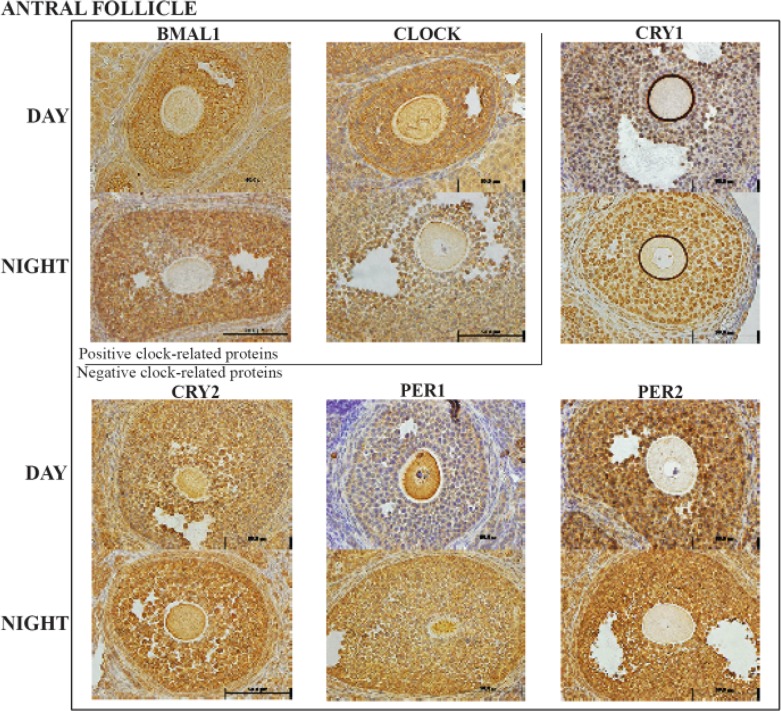
Antral follicle cellular clock:
No significant variation for either BMAL1 or CLOCK expression was demonstrated between day and night (Figure 1C and Figure 4). However, Cry-1 and PER-1 demonstrated highly significant increases at night (p< 0.001) and CRY-2 had a smaller but significant increase (p<0.05) at night. Only PER-2 failed to show a significant increase at night (p<0.05).
Figure 4.
Representative antral follicle histology sections with the six clock proteins expression indicated by brown staining. Box encloses the BAML1 and CLOCK (positive arm) proteins
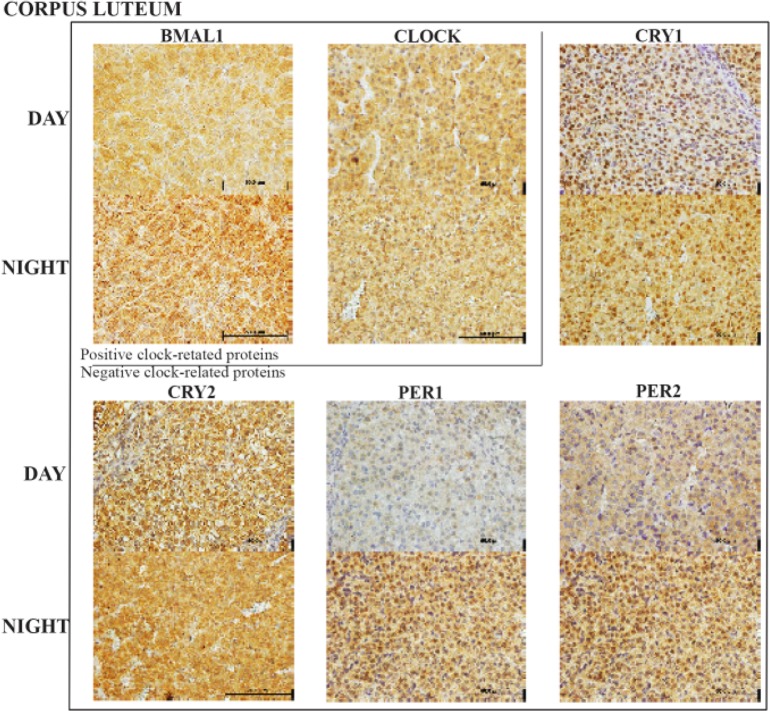
Corpus lutea cellular clock:
Whilst BMAL1 had a significant daytime increase in expression (p<0.05), CLOCK had little variation between day and night expression. All four of the negative arm clock proteins were up regulated at night (Figure 1D and figure 5) with PER-2 demonstrating the greatest increase (p<0.001). Although CRY-2 expression was also up regulated, it failed to reach statistical significance.
Figure 5.
Representative corpus luteum histology sections with the six clock proteins expression indicated by brown staining. Box encloses the BAML1 and CLOCK (positive arm) proteins
Discussion
The function of the ovary is the production and release of oocytes for fertilization at regular intervals and the provision of hormones for initiating and maintaining early pregnancy, which requires regular time keeping over a reproductive life time in a finely controlled process. The ovary maintains a steady periodic course of maturing follicles despite the diminishing pool of available follicles and it is generally considered that this fine control occurs at the pre-antral (hormone unresponsive) stage of ovarian follicle development. Despite this very well regulated organ, to date, little is known about the peripheral clock mechanism of the ovary and in particular the transition from primordial follicles to hormone responsive follicles prepared for ovulation.
The process of primordial recruitment has been vigorously studied with numerous factors implicated in this process (25–27). Anti-mullerian hormone (AMH) is a promising target as it appears to control the amount of primordial follicles able to proceed through development, acting as a regulatory to this development and impeding primordial recruitment (28). However, AMH does not provide an explanation to the complex process of selection of why does one follicle develop over another? Although this current study could not definitively answer this either, it did propose a mechanism that can be further investigated.
The central function of peripheral cellular clocks is the ability to form heterodimers between both the positive arm proteins (BAML1 and CLOCK) and the negative arm proteins (CRY 1 and 2, and PER 1 and 2) and for the heterodimers to translocate to the nucleus to control gene expression. From these data, both the primordial and primary follicles appear to have a similar positive/negative arm feedback loop. However, as follicles enter the hormone responsive antral stage cyclicity of the positive arm appears to be lost between day and night with little change in expression of BAML1 and CLOCK but the negative arm still retains the rhythm of elevated expression at night and lower expression during the day. This pattern continues in the corpus lutea. Previous research has indicated that mRNA expression of Per 2 in the SCN is increased by estrogen exposure (29), whereas Per 2 expression period is shortened in the uterus (30, 31). Furthermore, ovarian BAML1 mRNA expression has been reported to increase at pro-estrous indicating that it may well be estradiol responsive (13). Overall, there appears to be a switch in clock gene responsiveness as follicles move in to the antral stage.
Conclusion
Though the understanding of the role of peripheral cellular clocks is not fully established, there are clear indications that the switching between the positive and negative arms of the clock is strongly implicated in cellular metabolism (31), such as the control of glucose homeostasis, gluconeogenesis and intra-cellular redox (32–35). Given that each follicle stage of development may well have differing metabolic requirements, variable signals from the ovarian cellular clock may coordinate follicle responsiveness both for cell signaling within the ovary and hormone responsiveness. For the present study, this could not be determined but clock protein expression rhymicity in the ovary at estrous was established indicating that the ovary, unlike the testis, has a regulated peripheral cellular clock consistent with many other body tissues and the light: dark cycle is critical in regulating the clock. Further work is required to determine the functional relationship between other stages of the estrous cycle, time, clock protein expression and changes in ovarian metabolic activity.
Acknowledgement
We are grateful for histology advice from Ms Mandy Fisher, Manager, Histology Unit, Pathology Department, University of Otago.
Footnotes
Conflict of Interest
This work was supported by a grant from Lottery Health, New Zealand. Authors declare no conflict of interest.
References
- 1.Baker TG. A quantative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–33. [DOI] [PubMed] [Google Scholar]
- 2.Marieb E, Hoehn K. Human Anatomy and Physiology. 7th ed San Francisco: Pearson Benjamin Cummings; 2007. Chapter 27, The Reproductive System; p. 1091–101. [Google Scholar]
- 3.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–14. [DOI] [PubMed] [Google Scholar]
- 4.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298(1):149–54. [DOI] [PubMed] [Google Scholar]
- 5.Macnaughton M, Govan A. Clinics in Obstetrics and Gynaecology. Eastbourne, England: W. B Saunders Company Ltd; 1976. Chapter 2, The ovary; p. 15–22. [Google Scholar]
- 6.Ferin M. Neuroendocrine control of ovarian function in the primate. J Reprod Fertil. 1983;69(1):369–81. [DOI] [PubMed] [Google Scholar]
- 7.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4): 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche JF. Control and regulation of folliculogenesis--a symposium in perspective. Rev Reprod. 1996;1(1):19–27. [DOI] [PubMed] [Google Scholar]
- 9.Alleva JJ, Waleski MV, Alleva FR, Umberger EJ. Synchronizing effect of photoperiodicity on ovulation in hamsters. Endocrinology. 1968;82(6):1227–35. [DOI] [PubMed] [Google Scholar]
- 10.Lawton IE, Schwartz NB. Pituitary-ovarian function in rats exposed to constant light: a chronological study. Endocrinology. 1967;81(3):497–508. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17(1):141–51. [DOI] [PubMed] [Google Scholar]
- 13.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75(4):624–32. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian Clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem Biophys Res Commun. 2011;412(1):132–5. [DOI] [PubMed] [Google Scholar]
- 15.Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms. 2004;19(5):400–13. [DOI] [PubMed] [Google Scholar]
- 16.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901): 935–41. [DOI] [PubMed] [Google Scholar]
- 17.Reppert SM. A clockwork explosion! Neuron. 1998;21(1):1–4. [DOI] [PubMed] [Google Scholar]
- 18.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 Spec No 2:R271–7. [DOI] [PubMed] [Google Scholar]
- 19.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280(5369):1564–9. [DOI] [PubMed] [Google Scholar]
- 20.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293 (5529):510–4. [DOI] [PubMed] [Google Scholar]
- 21.Vilmar A, Garcia-Foncillas J, Huarriz M, Santoni-Rugiu E, Sorensen JB. RT-PCR versus immunohistochemistry for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1 in NSCLC. Lung Cancer. 2012;75(3):306–12. [DOI] [PubMed] [Google Scholar]
- 22.Van der Salm L, Legge M. A re-evaluation of methods for determining the oestrous cycle in the mouse. Animal Technol. 1994;45:43–5. [Google Scholar]
- 23.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68. [PubMed] [Google Scholar]
- 24.Makris A, Allred DC, Powles TJ, Dowsett M, Fernando IN, Trott PA, et al. Cytological evaluation of biological prognostic markers from primary breast carcinomas. Breast Cancer Res Treat. 1997;44(1): 65–74. [DOI] [PubMed] [Google Scholar]
- 25.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–64. [DOI] [PubMed] [Google Scholar]
- 26.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. 2014;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999; 140(12):5789–96. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, et al. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82(5):622–30. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab. 2008;295(5):E1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113(3):103–12. [DOI] [PubMed] [Google Scholar]
- 32.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–31. [DOI] [PubMed] [Google Scholar]
- 34.Sassone-Corsi P. Commentary: the year in circadian rhythms. Mol Endocrinol. 2010;24(11):2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]