Abstract
Objective
Both Tourette's disorder (TD) and attention-deficit/hyperactivity disorder (ADHD) have been related to abnormalities in glutamatergic neurochemistry in the fronto-striatal circuitry. TD and ADHD often co-occur and the neural underpinnings of this co-occurrence have been insufficiently investigated in prior studies.
Method
We used proton magnetic resonance spectroscopy (1H-MRS) in children between 8 and 12 years of age (TD n = 15, ADHD n = 39, TD + ADHD n = 29, and healthy controls n = 53) as an in vivo method of evaluating glutamate concentrations in the fronto-striatal circuit. Spectra were collected on a 3 Tesla Siemens scanner from two voxels in each participant: the anterior cingulate cortex (ACC) and the left dorsal striatum. LC-model was used to process spectra and generate glutamate concentrations in institutional units. A one-way analysis of variance was performed to determine significant effects of diagnostic group on glutamate concentrations.
Results
We did not find any group differences in glutamate concentrations in either the ACC (F(3132) = 0.97, p = 0.41) or striatum (F(3121) = 0.59, p = 0.62). Furthermore, variation in glutamate concentration in these regions was unrelated to age, sex, medication use, IQ, tic, or ADHD severity. Obsessive–compulsive (OC) symptoms were positively correlated with ACC glutamate concentration within the participants with TD (rho = 0.35, puncorrected = 0.02).
Conclusion
We found no evidence for glutamatergic neuropathology in TD or ADHD within the fronto-striatal circuits. However, the correlation of OC-symptoms with ACC glutamate concentrations suggests that altered glutamatergic transmission is involved in OC-symptoms within TD, but this needs further investigation.
Keywords: Tourette syndrome, ADHD, Glutamate, Fronto-striatal circuit, MRS
Highlights
-
•
Large pediatric sample of ADHD and TD participants
-
•
3 Tesla proton MRS utilized to investigate fronto-striatal glutamate concentrations
-
•
No differences in glutamate concentrations in the disorder groups compared with controls
-
•
ACC glutamate concentrations associated with obsessive–compulsive symptoms in TD
1. Introduction
Tourette's disorder (TD) and attention-deficit/hyperactivity disorder (ADHD) are early onset neurodevelopmental disorders affecting approximately 1% (Robertson, 2008) and 5% (Polanczyk et al., 2007) of children and adolescents, respectively. While TD is characterized by the presence of motor and vocal tics (American Psychiatric Association, 2013) there are also psychiatric comorbidities present in up to 86% of those with TD during their lifetime (Hirschtritt et al., 2015). ADHD is the most common, occurring in approximately 40% of cases (Rickards, 2011) and even more TD patients have ADHD symptoms that do not meet the threshold for diagnosis (Robertson, 2000). Conversely, the presence of tics within patients with ADHD has been estimated at 20% (Roessner et al., 2007). ADHD itself is characterized by age inappropriate inattention and/or hyperactivity/impulsivity leading to impaired functioning (American Psychiatric Association, 2013).
Both disorders have been associated with abnormalities in fronto-striatal circuits (Leisman and Melillo, 2013, Mink, 2006), although the overlap in conditions has confounded research to date. Structural and functional neuroimaging studies have reported alterations in the caudate nuclei, putamen, and anterior cingulate cortex (ACC) in TD (Peterson et al., 2003, Ganos et al., 2013) and ADHD (Frodl and Skokauskas, 2012, Nakao et al., 2011, Hart et al., 2013) relative to controls, although not always consistently. It has been proposed that excitatory abnormalities in the striatum cause erroneous inhibition of neurons in the globus pallidus (GP) internus, which in turn leads to disinhibition of prefrontal neurons which results in tic phenomena (Albin and Mink, 2006). These striatal abnormalities may also underlie the high rate of comorbidity with other disorders, like ADHD and obsessive compulsive disorder (OCD) (Hirschtritt et al., 2015) due to the aberrant integrative interplay of different fronto-striatal circuits including connections with the ACC (Albin and Mink, 2006, Mink, 2001). Dopamine dysfunction within the fronto-striatal circuit has long been considered the primary cause of tics (Singer et al., 1982) and has been related to difficulties with attention and impulsivity (Swanson et al., 2007). However, as glutamatergic, GABAergic, serotonergic, cholinergic, and opioid as well as dopaminergic systems all operate within the fronto-striatal circuits it is plausible that multiple neurotransmitter systems may be involved in TD and ADHD (Singer et al., 2010). Glutamate is the primary excitatory neurotransmitter found in the brain (Monaghan et al., 1985, Pittenger et al., 2011), essential in fronto-striatal transmission and often co-transmitted with dopamine (Chuhma et al., 2009). Post-mortem analysis of a small number of brains from people who had TD corroborates the view that glutamate is involved in TD as reduced levels of glutamate were seen in the GP and substantia nigra of the TD brains compared to control brains (Anderson et al., 1992).
Additional insights into the underlying neurobiology of TD and ADHD can be found by investigating brain neurochemistry. This can be achieved by using proton magnetic resonance spectroscopy (1H-MRS) which allows for non-invasive in vivo quantification of specific neurometabolites. There have been just four MRS studies of TD to date, three of which focused on GABA concentrations either in the primary and secondary motor areas (Draper et al., 2014) or the sensory motor cortex (Tinaz et al., 2014, Puts et al., 2015). DeVito et al. (2005) on the other hand investigated multiple neurochemicals including Glx, the combined signal from glutamatergic compounds (glutamate + glutamine), within multiple regions; premotor cortex, caudate nucleus, putamen and thalamus with a 3 Tesla scanner in a sample of 25 (male only) children and adolescents with TD in comparison to controls. No group differences were seen in Glx in any of the regions. Within the putamen lower creatine (Cre) levels bilaterally and lower N-acetyl aspartate and choline in the left putamen were found. Reduced Cre bilaterally in the caudate nucleus was also seen but this did not reach significance.
Many more MRS studies of disorders related to TD, such as ADHD and OCD, have been conducted. For a review of these studies in ADHD, OCD and autism spectrum disorder (ASD) see Naaijen et al. (2015). However, findings were inconsistent, plagued by heterogeneous methodologies, sample selection (i.e., child or adult, inclusion or exclusion of comorbidities), voxel placement, and often inadequate field strengths to distinguish glutamate from glutamine (Naaijen et al., 2015). Despite these issues the review tentatively summarized that increased striatal Glx levels are associated with both ADHD and OCD and increased ACC Glx levels with pediatric ADHD.
In the current study we assessed a large number of children between the ages of 8 and 12 years which allowed us to focus on a group where tics are most frequently observed and not limit ourselves to the subset of patients whose tics persist into adulthood (Bloch and Leckman, 2009). Furthermore we directly addressed the confounds of comorbidity rampant in previous studies by including a TD + ADHD group in addition to ADHD, TD, and healthy control (HC) groups. Based on previous findings in childhood ADHD (Naaijen et al., 2015), we expected increased glutamate concentrations in both regions of interest. This is the first study to investigate fronto-striatal glutamate in children with TD. Given the theory that excitatory abnormalities in the striatum result in tics, we expected to observe raised glutamate in the striatum of TD patients.
2. Method
2.1. Participants
Participants with TD and/or ADHD: TD with/without ADHD n = 60, ADHD without TD n = 60 were recruited via child and adolescent psychiatry departments and patient associations throughout the Netherlands, while healthy controls (HC; n = 60) were found mainly through schools. The final numbers included for analysis (i.e. with usable data) can be found in Section 3.1 and Table 1 (n = 136 for the ACC and n = 125 for the striatum). Written informed consent was provided by the parents/guardians of all participants and written assent was also given by participants who were 12 years of age. This study was approved by the regional ethics board (CMO Regio Arnhem-Nijmegen, numbers: NL42004.091.12 & NL48377.091.14).
Table 1.
Demographic description of participants included in the ACC analysis.
| Control | TD | ADHD | TD + ADHD | Test statistic | p-Value | |
|---|---|---|---|---|---|---|
| N | 53 | 15 | 39 | 29 | ||
| Age years, mean(SD) | 10.0(1.0) | 10.4(1.2) | 10.7(1.2) | 10.7(1.6) | K-W χ2 = 2.98 | 0.40 |
| Sex, m/f | 38/15 | 13/2 | 21/18 | 25/4 | χ2 = 10.70 | 0.01⁎ |
|
aIQ, mean(SD) Range |
109(11), 86–133 | 105(12), 81–126 | 103(13), 71–137 | 106(11), 85–124 | F = 2.47 | 0.06 |
| Handed, r/l | 48/5 | 14/1 | 36/3 | 25/4 | χ2 = 0.90 | 0.82 |
| bADHD severity, mean(SD) | T = 45.5(4.9) | T = 51.7(7.0) | T = 71.9(9.7) | T = 68.7(9.1) | t = 1.37 | 0.18† |
| I = 45.5(6.0) | I = 50.9(8.2) | I = 69.3(10.0) | I = 65.2(9.8) | t = 1.69 | 0.10† | |
| H = 46.1(3.7) | H = 52.2(8.5) | H = 71.2(11.1) | H = 70.0(10.4) | t = − 0.44 | 0.66† | |
| CSBQ core autism score, mean(SD) | 1.42(1.89) | 9.53(10.58) | 12.46(7.78) | 17.66(9.40) | ||
| RBS compulsivity score, mean(SD) | 0.09(0.35) | 1.67(1.59) | 0.69(1.24) | 2.55(3.00) | ||
| cTic dx, n | – | TD = 14 | – | TD = 29 | ||
| CMT = 1 | ||||||
| cTic severity, mean(SD) | – | T = 20.4(7.9) | – | T = 20.8(9.2) | t = − 0.13 | 0.89 |
| M = 13.6(4.3) | M = 13.1(5.5) | t = 0.33 | 0.75 | |||
| V = 6.8(4.7) | V = 7.7(5.7) | K-W χ2 = 0.22 | 0.64 | |||
| cAge tic onset years, mean(SD) | – | 5.3(1.7) | – | 5.7(1.7) | t = − 0.75 | 0.46 |
| cDuration since tic onset years, mean(SD) | – | 5.0(1.8) | – | 5.0(2.0) | t = 0.06 | 0.95 |
| dOCD dx, n | – | 3 | – | 6 | ||
| dOC-symptoms, mean(SD) | – | 8.47(8.6) | – | 6.75(8.4) | K-W χ2 = 0.78 | 0.38 |
| eMedication | – | |||||
| Stimulant | 0 | 24 | 9 | |||
| Strattera | 0 | 1 | 0 | |||
| Antipsychotic | 2 | 1 | 7 | |||
| Clonidine | 0 | 0 | 2 |
ADHD, attention-deficit/hyperactivity disorder; CSBQ, Children's Social Behavioral Questionnaire; CMT, chronic motor tic; dx, diagnosis; H, hyperactive; I, inattentive; C, combined; K-W, Kruskal–Wallis; M, motor; m/f, male/female; OCD, obsessive compulsive disorder; r/l, right/left; RBS, Repetitive Behavior Scale; SD, standard deviation; t, Welch two sample t-test; T, total; TD, Tourette's disorder; V, vocal.
IQ estimated from a subtest of the Wechsler Intelligence Scale for Children-III (WISC-III Wechsler, 2002) rating.
ADHD severity ratings reflect T-scores from the Conners' Parent Rating Scale — Revised Long version (Conners et al., 1997).
Tic diagnosis and severity were determined and rated with the Yale Global Tic Severity Scale (Leckman et al., 1989). Tic severity is reported excluding impairment score.
OCD diagnosis was determined as a total-score of ≥ 16 on the Children's Yale-Brown Obsessive Compulsive Scale (Scahill et al., 1997).
Current medication status, determined from parental report of current and previous medication use.
Statistics refer to an ADHD versus TD + ADHD contrast.
p < 0.05.
Inclusion criteria for all participants included being aged 8–12 years, IQ > 70, Caucasian decent, no previous head injuries or neurological disorders, no contraindications for MRI assessment, and no major physical illness. Inclusion criteria for ADHD and TD were meeting DSM-5 criteria for these disorders. Those with sub-threshold ADHD (Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS Kaufman et al., 1997) score of 4 or 5 on either subscale) were also included. Persistent Motor or Vocal Tic Disorder (Motor type) was also allowed for the TD group. Common psychiatric comorbidities like oppositional defiant disorder were not excluded. Within the TD group, ADHD, and OCD were not excluded, while in the ADHD group those with tics and/or OCD were excluded. Within the HC group no psychiatric disorders were allowed, as determined by screening questionnaires (Child Behavior Checklist [CBCL] and Teacher Report Form [TRF] Bordin et al., 2013). Subjects were divided into four groups; HC, TD, ADHD, and TD + ADHD, see Table 1 for demographics. Participants were required to refrain from consuming caffeine on the day of testing. Medications for tics were continued as normal while stimulant medication was withheld for 48 h before testing.
2.2. Phenotypic information
TD diagnosis was confirmed, and tic severity rated, by diagnostic interview with parent(s) and child present using the Yale Global Tic Severity Scale (YGTSS Leckman et al., 1989). To determine the presence of ADHD and/or other psychiatric disorders the K-SADS (Kaufman et al., 1997) interview was administered to the parent(s). All interviews were conducted by experienced researchers who were trained and overseen by a child- and adolescent psychiatrist (JKB). The screening module was used, followed if needed by disorder-specific modules. If participants screened positive for possible OCD the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS Scahill et al., 1997) was administered with both parent(s) and child present. The CY-BOCS interview was conducted with each participant of the TD group due to the relatedness of symptoms and common co-occurance of OCD and TD.
Full-scale IQ was estimated by four subtests of the Wechsler Intelligence Scale for Children-III (WISC-III Wechsler, 2002): Vocabulary, Similarities, Block design, and Picture completion. Questionnaires were further used to assess phenotypic traits. The Conners' Parent Rating Scale — Revised Long version (CPRS-RL Conners et al., 1997) was used to rate ADHD severity. Additional questionnaires were used to assess the presence of autistic symptoms and compulsive behaviors; the Children's Social Behavioral Questionnaire (CSBQ Luteijn et al., 2000) and Repetitive Behavior Scale (RBS-R Lam and Aman, 2007). Information about medication history was gathered from parental report which has previously been shown to correlate well with pharmacy records (Kuriyan et al., 2014). Interviews on psychiatric symptoms were conducted about an unmedicated period.
2.3. T1-weighted MRI acquisition
All MRI datasets were acquired on the same 3 T Siemens Prisma (Siemens, Erlangen, Germany) scanner located in the Donders Institute for Brain, Cognition and Behaviour, Nijmegen, the Netherlands. T1-weighted anatomical images were acquired with a transversal, 3D magnetization prepared rapid gradient echo (MPRAGE) parallel imaging sequence with the following parameters: TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, FoV = 256 mm, slice thickness = 1.20 mm, flip angle 9°, in plane resolution = 1.0 × 1.0 mm, acceleration factor = 2, acquisition time = 5:30 min. Each participant also had their head stabilized with cushions during scanning and had a piece of tape across their foreheads to help awareness of possible movement while scanning. All participants were first familiarized with the MRI procedure in a mock scanner where the importance of lying still was explained.
2.4. MRS acquisition
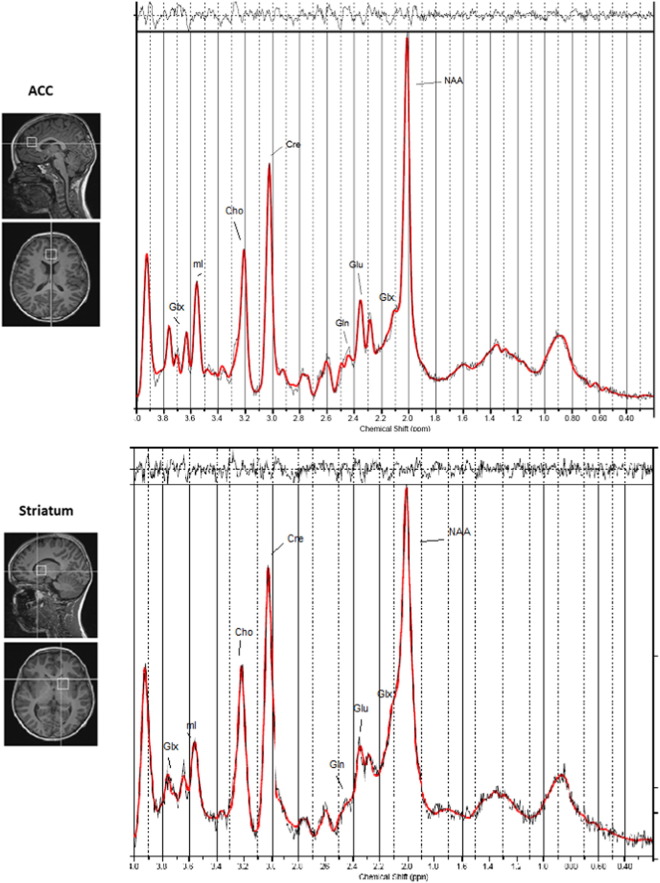
Proton spectra were acquired using a point resolved spectroscopy (PRESS) sequence in two regions of interest, with chemically selective suppression (CHESS) water suppression (Haase et al., 1985). A single 8 cm3 voxel was centered on the midline covering the pregenual ACC anterior and slightly superior to the genu of the corpus callosum (TR = 3000 ms, TE = 30 ms, number of averages = 96, bandwidth = 5 kHz, number of points = 4096). A second, similar voxel was located in the left dorsal striatum covering the caudate nucleus and putamen. Unsuppressed water reference spectra (16 averages) were also acquired as part of the standard acquisition. See Fig. 1 for location of the voxels and an example spectrum. Both voxels were placed to include a maximum amount of grey matter and a minimum amount of cerebrospinal fluid (CSF). T1-weighted images were used for voxel placement and later for tissue segmentation during processing. Acquisition time was 6 min per voxel.
Fig. 1.
Location of the two voxels are shown on a T1-weighted anatomical image for the pregenual ACC including an example spectrum (top) and the left dorsal striatum including an example spectrum (bottom). Peaks corresponding to individual metabolites are highlighted. The thin black line represents the frequency-domain data, the red line is the LCModel fit. In the top panel the residuals are plotted (the data minus the fit). Cho, choline; Cre, creatine; Gln, glutamine; Glu, glutamate; Glx, Glu + Gln; mI, myo-inositol; NAA, N-acetylaspartate.
2.5. Processing
LCModel, version V6.03-0I (Provencher, 1993, Provencher, 2001), was used to conduct spectral analysis. LCModel uses a linear combination of simulated or in vitro metabolite solution spectra as a reference to identify and quantify the major resonance of in vivo spectra.
Water referenced metabolite concentrations were automatically calculated in institutional units (i.u.). Institutional units are presented since we did not correct for the T1 and T2 relaxation times of the metabolites or the T1 relaxation time of water. The T2 of tissue water was corrected for assuming the signal had decayed by 30% at the echo time (Lu et al., 2005). No correction was made for the T2 decay of metabolites. In addition, there are other scanner-dependent factors that can affect the absolute scaling (e.g., details of coil combination), such that metabolite concentrations measured in i.u. are preferred over attempting to scale to absolute concentrations in millimolar (mM). The unified segmentation procedure within the VBM8 toolbox of SPM8 (Statistical Parametric Mapping release 8, London, UK) was used to process the T1 images and produce grey matter (GM), white matter (WM), and CSF probability maps. Spectroscopy voxels were mapped onto these maps to provide the partial volume of GM, WM, and CSF within each spectroscopy voxel (fGM, fWM, and fCSF), and to allow for group comparisons of the placement of the spectroscopy voxels. Additionally, to correct for differing amounts of water in each tissue and for partial volume effects we corrected individual metabolite concentrations for water concentrations with the following formula (Gasparovic et al., 2006):
where 43,300, 35,880, and 55,556 are the water concentrations in mM for GM, WM, and CSF (Ernst et al., 1993), respectively, as described by the LCModel manual (Provencher, 2014). These concentrations correspond to 77.9, 64.4 and 100%, respectively, assuming that CSF is pure water (Ernst et al., 1993). This includes a correction for the fraction of the MRS voxel occupied by CSF, along with corrections for the water concentration in each of the tissue types. The factor 35,880 in the denominator is included since the initial LCModel analysis was carried out assuming the voxel was pure WM.
2.6. Quality control
Statistical analyses were restricted to spectra with linewidth (full-width at half-maximum; FWHM) ≤ 0.1 ppm, Cramér-Rao lower bounds (CRLB) ≤ 20%, signal to noise ratio ≥ 5 or corrected glutamate concentrations less than two standard deviations from the mean. Furthermore, anatomical scan quality was visually checked as these were used for voxel placement and tissue segmentation. Fifteen participants were excluded from both analyses due to poor structural scan quality. A further seven spectra from the ACC (n = 136) and 20 spectra from the striatal analyses (n = 125) were excluded based on spectral quality. In addition to the exclusion of those with ≥ 20% CRLB values, we investigated group differences in CRLBs to verify that possible differences in glutamate levels were not due to differences in CRLBs (Kreis, 2015).
2.7. Statistics
Statistical analyses were conducted with the R statistical program (R Core Team, 2013). Differences between the four groups in categorical measures were tested with Pearson's chi-squared tests. Group differences in continuous measures were assessed with a one way analysis of variance (ANOVA) or Welch Two Sample t-test if assumptions of homogeneity of variance and normality of distributions were met (p > 0.05 in Bartlett test of homogeneity of variance and Shapiro–Wilk normality test). If these assumptions were violated a non-parametric Kruskal–Wallis rank sum test was used. ADHD severity was only tested between the ADHD and TD + ADHD group. Similarly measures related to tics and OC-symptoms were only tested between the TD and TD + ADHD groups.
Group differences in voxel tissue composition and spectral quality were assessed with a ANOVA. Group differences in glutamate were analyzed first with age and sex included as covariates. These covariates were removed from analysis as they did not significantly contribute to the model. This resulted in the use of an ANOVA followed if appropriate by Bonferroni corrected post-hoc pairwise t-tests. The influence of IQ, ADHD severity, ASD symptoms, repetitive and compulsive behaviors, and medication status were also examined by inclusion in an ANCOVA. Correlations between glutamate levels and phenotypic measures of those with TD (tic severity, age of onset and duration since tic onset, and OC-symptoms) were assessed with Pearson's correlation tests if normally distributed or Spearman's rank correlation rho test if not. Tests were then Bonferroni corrected for multiple comparisons. In the supplementary material we added the same analysis for Glx.
3. Results
3.1. Demographics
MR spectra were acquired for a total of 162 of the original 180 participants. Three participants from the ADHD group were found not to have ADHD (K-SADS < 4 in both subscales) and one presented with tics but did not meet criteria for TD or Chronic Motor Tic disorder (CMT). These four participants were thereafter excluded from analysis. Due to spectral or segmentation quality concerns 22 spectra were excluded from the ACC analysis (n = 136) and 35 from the striatal analysis (n = 125). The TD group was subdivided into those that also had ADHD (TD + ADHD; n = 29, 27 for the ACC and striatal analyses, respectively) and those that did not (TD; n = 15, 17 for the ACC and striatal analyses, respectively). Participants with sub-threshold ADHD were included in either the ADHD group or the TD + ADHD group if comorbid with TD. Details of the groups used for analysis of the ACC are reported in Table 1 (n = 136).
Analysis of the striatum included fewer participants (n = 125) due to exclusion based on spectral quality (n = 22). This did not significantly alter the demographic distributions between groups (n = 48, 17, 33, and 27 for the HC, TD, ADHD, and TD + ADHD groups, respectively) regarding age (K-W χ2 = 1.64, p = 0.65), sex (χ2 = 8.09, p = 0.04), IQ (F(3117) = 2.25, p = 0.09) and handedness (χ2 = 0.82, p = 0.84). ADHD severity between the ADHD and TD + ADHD groups differed slightly but not significantly with respect to total and inattentive scores (t = 1.90, p = 0.06; t = 1.84, p = 0.07; t = 0.99, p = 0.33 for total, inattentive, and hyperactive CPRS scores, respectively) while tic severity (t = 0.31, p = 0.76; t = − 0.54, p = 0.59; K-W χ2 = ~ 0, p = 0.99 for total, motor, and vocal YGTSS scores respectively) and OC-symptoms (K-W χ2 = 1.52, p = 0.22) remained similar between the TD and TD + ADHD groups.
Age of tic onset (t = − 0.51, p = 0.62) and duration since tic onset (t = − 0.06, p = 0.95) did not differ significantly between the TD and the TD + ADHD group. For both analyses sex was not balanced between groups, mainly due to a low number of girls with TD having been included. This reflects the proportionately fewer girls affected by TD compared to boys (Robertson, 2015). Sex was included in the model to account for this imbalance, however, it was found not to affect the model significantly and was therefore subsequently removed.
3.2. Spectral quality
Groups did not differ significantly in mean voxel percentage GM, WM or CSF in either ACC (F(3132) = 0.30, p = 0.83, F(3132) = 0.61, p = 0.61 and F(3132) = 0.26, p = 0.85, respectively) or striatum (F(3121) = 1.77, p = 0.16, F(3121) = 1.77, p = 0.16 and F(3121) = 1.73, p = 0.16, respectively). In the ACC voxel across all groups the tissue percentages were: GM 70 (7)%, WM 11 (2)% and CSF 18 (7)%. For the striatal voxel these were GM 58 (7)%, WM 42 (7)% and CSF 1 (1)%.
To verify that the spectral quality did not differ between the groups, we compared the CRLB estimated standard deviations in both of the voxels, using a one-way ANOVA across the four groups. CRLB's did not differ between groups in the ACC (F(3132) = 1.35, p = 0.26) or the striatum (F(3121) = 0.37, p = 0.77). Furthermore all CRLB's were in the range 3–7% SD, all SNR were > 20 and all FWHM were in the range of 0.02–0.09 reflecting overall good quality of the ACC spectrum in all four groups. For the striatum, CRLB's were in the range 5–17%, SNR were > 11 and FWHM were in the range of 0.04–0.09.
3.3. ACC
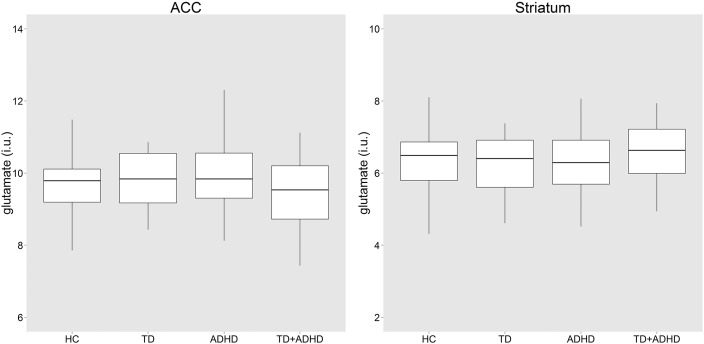
Age and sex had no significant influence on the ANCOVA model and were subsequently excluded. There was no group difference in corrected glutamate levels ANOVA (F(3, 132) = 0.97, p = 0.41, Fig. 2). There was no influence of IQ (p = 0.61), total CPRS ADHD severity T-score (p = 0.56), inattentive CPRS T-score (p = 0.70), hyperactive CPRS T-score (p = 0.48), CSBQ core autism symptom-score (p = 0.64) or RBS compulsivity score (p = 0.92). Current medication use showed no significant effect on glutamate levels when any current medication (p = 0.65), current stimulant medication (p = 0.28) or current antipsychotic medication (p = 0.56) were investigated.
Fig. 2.
Boxplots of glutamate concentrations per group in the ACC and striatum. No group differences in glutamate levels were seen. ADHD, attention-deficit/hyperactivity disorder; HC, healthy controls; i.u., institutional units; TD, Tourette's disorder; TD + ADHD, Tourette's disorder and comorbid attention-deficit/hyperactivity disorder.
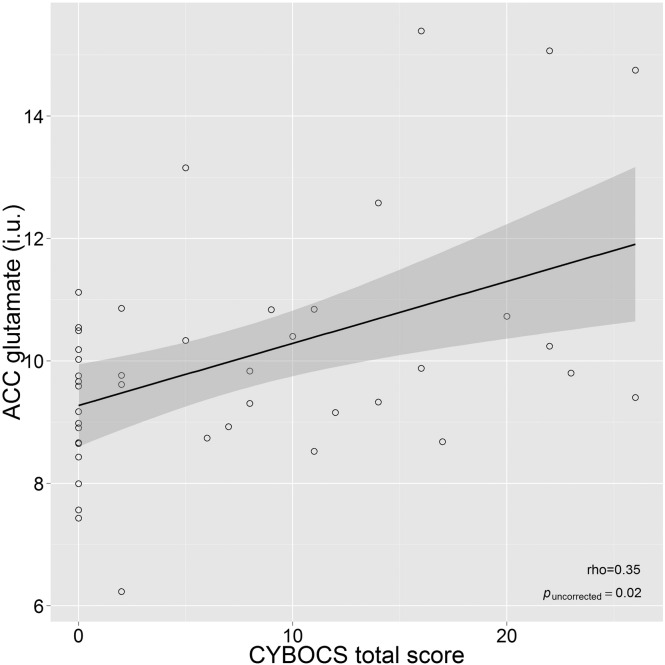
There were no correlations in those with tics between corrected glutamate concentrations and tic severity (total p = 0.77, motor p = 0.40, vocal p = 0.53), duration since (p = 0.38) or age of onset (p = 0.16). A significant positive correlation (rho = 0.35) between ACC glutamate and CY-BOCS total score (p = 0.02) was found (see Fig 3). This correlation was also present for the obsessions (rho = 0.30, p = 0.045) and compulsions (rho = 0.31, p = 0.04) severity scales separately. However, the two subscales were highly correlated (rho = 0.55, p < 0.001). However, none of the correlations with glutamate concentration survived correction for multiple comparisons (12 correlation analyses). The analysis was limited to the participants that were administered the CY-BOCS (n = 44, all with TD).
Fig. 3.
Correlation of ACC glutamate concentration with obsessive–compulsive symptoms (CY-BOCS total score) in participants with TD. ACC, anterior cingulate cortex; CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; i.u., institutional units; TD, Tourette's disorder. The solid line and its shaded area denote the linear regression fit line and its 95% confidence interval.
3.4. Striatum
Similarly for the striatal analysis there were no significant effects of age or sex on glutamate levels. Group analysis revealed no difference in striatal corrected glutamate levels (F(3, 121) = 0.59, p = 0.62, Fig. 2). Again there was no influence of IQ (p = 0.63), total CPRS ADHD severity T-score (p = 0.78), inattentive CPRS T-score (p = 0.80), hyperactive CPRS T-score (p = 0.75), CSBQ core autism symptom score (p = 0.37), RBS compulsivity score (p = 0.25) or current medication use (any p = 0.73, stimulant p = 0.80 or antipsychotic p = 0.06). There were no correlations between corrected glutamate concentrations and tic severity (total p = 0.34, motor p = 0.46 vocal p = 0.22), duration since (p = 0.19) or age of onset (p = 0.08). There was no association between glutamate and CY-BOCS total score (rho = − 0.25, p = 0.11).
3.5. Glx
There were also no group differences in Glx concentration in either the ACC or striatum (see Supplementary information for details).
4. Discussion
This is the first study to investigate glutamate concentrations in both TD and ADHD together. We found no group differences in ACC or left striatal glutamate concentrations. The findings were not confounded by any demographic differences between the groups, spectral quality, medication use or OC-symptoms. Glutamate levels in the ACC correlated with OC-symptoms within the participants with TD (n = 44) but did not relate to either tic or ADHD measures of severity.
Only one previous study examined glutamate concentrations in TD by investigating the combined Glx signal in children and adolescents and they were unable to find Glx differences in the brain regions investigated. In corroboration with results presented here (both Glx and glutamate analyses) they found no difference in the putamen but unfortunately did not also examine the ACC (DeVito et al., 2005). Previous studies did, however, suggest glutamate involvement in TD although the nature of this is yet unclear with both hyper- and hypo-glutamatergic states being hypothesized (Singer et al., 2010). For instance, a post-mortem analysis by Anderson and colleagues (Anderson et al., 1992) showed reduced glutamate levels in the GP and substantia nigra of those with TD, while a study investigating serum glutamate concentrations showed increased levels in adult TD patients compared to controls (Janik et al., 2010). Our current findings do not support the hypothesis of glutamatergic involvement in the fronto-striatal network in TD.
The current study found no difference in those with ADHD compared to healthy controls or any association between glutamate levels and ADHD severity scores in either the ACC or striatum. Previous studies have been confounded by methodological issues but do appear to show increased Glx levels in both these regions in pediatric ADHD compared to controls (Naaijen et al., 2015), an observation not replicated here, and reduced striatal Glx in adults with ADHD (Maltezos et al., 2014). Associations with symptom severity have been reported previously in adults with ADHD in which negative correlations between inattentive symptoms and glutamate-to-creatine ratios in the ACC (Dramsdahl et al., 2011) or Glx levels in the basal ganglia (Maltezos et al., 2014) were reported. However, these studies were performed in adults, who differ in both ADHD symptoms (Polanczyk et al., 2007) and glutamate levels (Horská et al., 2002) compared to children.
Possibly the most interesting finding of the current study, however, is the potential positive correlation between ACC glutamate levels and OC-symptoms within participants with TD. The correlation was present also for both the obsessions and compulsions subscales separately. This suggests the association with ACC glutamate concentration relates to the severity of OC-symptoms irrespective of these dimensions. Elevated ACC glutamate may be associated with cognitive control deficits related to obsessions and compulsions (Botvinick et al., 2004). However, these findings failed to survive correction for multiple comparisons so should be interpreted with caution. Previous literature investigating associations with symptom severity in OCD samples have also shown positive correlations with glutamatergic compounds. For instance, correlations between Glx levels in dorsal and rostral ACC (Yücel et al., 2008) and caudate nucleus (Gnanavel et al., 2014) and total symptom severity as measured with the Y-BOCS were reported before, although only in adult samples. Our findings should, however, only be interpreted in relation to OC-symptoms within TD. No studies so far have examined glutamatergic compounds in childhood TD and OCD together. Further studies are required to see if the current trend-findings extend to OC-symptoms within pediatric OCD and across other disorders that exhibit similar behaviors, such as ASD. Furthermore, this is a child sample of participants and how these findings relates to OC-symptoms in adult TD will remain unclear until further research is conducted.
The current study ranks among the first to use MRS to investigate brain neurochemical concentrations in TD and is the very first to investigate glutamate concentrations in both TD and ADHD together. However, the study was limited by the small number of TD participants who presented without ADHD/sub-threshold ADHD, these figures are in line with what is expected given the high comorbidity rates of ADHD in TD (Hirschtritt et al., 2015). It is unlikely that this significantly hindered the study as our null findings regarding tics and ADHD are supported by the lack of correlations between symptom severity and glutamate levels. These results should not be extrapolated to adults as glutamate concentrations change with age (Horská et al., 2002) and adult ADHD and TD may well constitute specific presentations of TD and ADHD that persist from childhood. Many participants were medicated, which may alter glutamate levels. However, as the effect of current medication use did not influence glutamate concentrations in either the ACC or striatum medication use was unlikely to have confounded our results. There are several additional factors that may influence glutamatergic signalling such as time of day, sleep, food intake etc. for which we unfortunately could not control (Yuen et al., 2009, Zlotnik et al., 2011). Future work is needed to confirm the influence of these factors. Most questionnaires were answered about an un-medicated period, however, a small number of parents opted to answer about a medicated period as they were more familiar with this behavior, this may have led to inconsistent measures of symptom severity in a few cases. Finally, due to low spatial resolution in MRS studies (i.e. large ROIs) it is difficult to determine regionally specific glutamatergic alterations. This is particularly relevant for the striatum ROI, which may contain functionally independent areas with regard to caudate nucleus and putamen. Further investigation should be undertaken to confirm the relation of OC-symptoms and glutamate concentrations in other disorders (OCD, ASD) and also in adult cohorts to determine if the association within TD is limited to children with TD or also present in adult TD.
In conclusion, we found no support for alterations in glutamatergic transmission in the fronto-striatal circuit of children with either ADHD, TD or a combined diagnosis. However, the current study suggests glutamatergic alterations in the ACC in relation to OC-symptoms within children with TD.
Acknowledgments
The research leading to these results received funding from the European Community's Seventh Framework Programme TACTICS (FP7/2007–2013, grant agreement no. 278948) and TS-EUROTRAIN (FP7-PEOPLE-2012-ITN grant agreement no. 316978). D.J.L. has acted as a consultant for Ixico PLC. P.J.H. has been a member of the advisory board of Shire. J.K.B. has in the past 3 years been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, Medice, Lundbeck, Roche and Servier. He is not an employee nor stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. J.N., N.J.F., S.E.A.A., T.J.C.O., A.D. and M.P.Z. do not have any conflict of interest to report.
We gratefully acknowledge and thank all the participants and their families for their enthusiastic involvement in the study. The authors also thank Nicole Driessen MSc, Saskia de Ruiter MSc and Leonie Hennissen MSc for their help with data collection and Paul Gaalman PhD for his technical support. Furthermore, we wish to acknowledge the numerous clinicians who played a vital role in recruiting participants to the study, most notably we thank Dr. Frank Visscher, Dr. Deborah Sival and Dr. Els van den Ban.
Footnotes
Presentation information: Data contributing to this study was presented as a poster at the annual meeting of the International Society for Research on Impulsivity, 2015, Amsterdam, The Netherlands and European Society for the Study of Tourette Syndrome meeting, 2016, Warsaw, Poland.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.11.013.
Appendix A. Supplementary data
Supplementary material
References
- Albin R.L., Mink J.W. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29(3):175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fifth ed. American Psychiatric Association; Washington DC: 2013. Neurodevelopmental disorders; pp. 81–82. (DSM Library). [Google Scholar]
- Anderson G.M., Pollak E.S., Chatterjee D., Leckman J.F., Riddle M.A., Cohen D.J. Brain monoamines and amino acids in Gilles de la Tourette's syndrome: a preliminary study of subcortical regions. Arch. Gen. Psychiatry. 1992;49(7):584. doi: 10.1001/archpsyc.1992.01820070078016. [DOI] [PubMed] [Google Scholar]
- Bloch M.H., Leckman J.F. Clinical course of Tourette syndrome. J. Psychosom. Res. 2009;67(6):497–501. doi: 10.1016/j.jpsychores.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordin I.A., Rocha M.M., Paula C.S. Child Behavior Checklist (CBCL),Youth Self-Report (YSR) and Teacher's Report Form(TRF): an overview of the development of the original and Brazilian versions. Cad Saude Publica. 2013;29(1):13–28. doi: 10.1590/s0102-311x2013000100004. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Chuhma N., Choi W.Y., Mingote S., Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164(3):1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Wells K.C., Parker J.D., Sitarenios G., Diamond J.M., Powell J.W. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25(6):487–497. doi: 10.1023/a:1022637815797. (Available at: http://www.ncbi.nlm.nih.gov/pubmed/9468109. Accessed February 3, 2014) [DOI] [PubMed] [Google Scholar]
- DeVito T.J., Drost D.J., Pavlosky W. Brain magnetic resonance spectroscopy in Tourette's disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(12):1301–1308. doi: 10.1097/01.chi.0000181046.52078.f4. [DOI] [PubMed] [Google Scholar]
- Dramsdahl M., Ersland L., Plessen K.J., Haavik J., Hugdahl K., Specht K. Adults with attention-deficit/hyperactivity disorder — a brain magnetic resonance spectroscopy study. Front Psychiatry. 2011;2:1–8. doi: 10.3389/fpsyt.2011.00065. (NOV) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A., Stephenson M.C., Morgan P.S. 2014. Report Increased GABA Contributes to Enhanced Control over Motor Excitability in Tourette Syndrome; pp. 2343–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T., Kreis R., Ross B. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J. Magn. Reson. Ser. B. 1993;102(1):1–8. [Google Scholar]
- Frodl T., Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012;125(2):114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Ganos C., Roessner V., Münchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci. Biobehav. Rev. 2013;37(6):1050–1062. doi: 10.1016/j.neubiorev.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Gasparovic C., Song T., Devier D. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gnanavel S., Sharan P., Khandelwal S., Sharma U., Jagannathan N.R. Neurochemicals measured by (1)H-MR spectroscopy: putative vulnerability biomarkers for obsessive compulsive disorder. MAGMA. 2014;27(5):407–417. doi: 10.1007/s10334-013-0427-y. [DOI] [PubMed] [Google Scholar]
- Haase A., Frahm J., Hänicke W., Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys. Med. Biol. 1985;30(4):341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hirschtritt M.E., Lee P.C., Pauls D.L. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72(4):325. doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horská A., Kaufmann W.E., Brant L.J., Naidu S., Harris J.C., Barker P.B. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J. Magn. Reson. Imaging. 2002;15(2):137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Janik P., Kalbarczyk A., Gutowicz M., Barańczyk-Kuźma A., Kwieciński H. The analysis of selected neurotransmitter concentrations in serum of patients with Tourette syndrome. Neurol. Neurochir. Pol. 2010;44(3):251–259. doi: 10.1016/s0028-3843(14)60039-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kreis R. The trouble with quality filtering based on relative Cram??r-Rao lower bounds. Magn. Reson. Med. 2015;18:15–18. doi: 10.1002/mrm.25568. [DOI] [PubMed] [Google Scholar]
- Kuriyan A.B., Pelham W.E., Molina B.S.G., DA W., MH S., EM G. Concordance between parent and physician medication histories for children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24(5):269–274. doi: 10.1089/cap.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.S.L., Aman M.G. The Repetitive Behavior Scale—Revised: independent validation in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Riddle M.A., Hardin M.T. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leisman G., Melillo R. The basal ganglia: motor and cognitive relationships in a clinical neurobehavioral context. Rev. Neurosci. 2013;24(1):9–25. doi: 10.1515/revneuro-2012-0067. [DOI] [PubMed] [Google Scholar]
- Lu H., Nagae-Poetscher L.M., Golay X., Lin D., Pomper M., Van Zijl P.C.M. Routine clinical brain MRI sequences for use at 3.0 Tesla. J. Magn. Reson. Imaging. 2005;22(1):13–22. doi: 10.1002/jmri.20356. [DOI] [PubMed] [Google Scholar]
- Luteijn E., Luteijn F., Jackson S., Volkmar F., Minderaa R. The Children's Social Behavior Questionnaire for milder variants of PDD problems: evaluation of the psychometric characteristics. J. Autism Dev. Disord. 2000;30(4):317–330. doi: 10.1023/a:1005527300247. [DOI] [PubMed] [Google Scholar]
- Maltezos S., Horder J., Coghlan S. Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Transl. Psychiatry. 2014;4(3) doi: 10.1038/tp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink J.W. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr. Neurol. 2001;25(3):190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Mink J.W. Advances in Neurology. Lippincott Williams & Wilkins; 2006. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs; pp. 89–98. [PubMed] [Google Scholar]
- Monaghan D.T., Yao D., Cotman C.W. L-[3H]Glutamate binds to kainate-, NMDA- and AMPA-sensitive binding sites: an autoradiographic analysis. Brain Res. 1985;340(2):378–383. doi: 10.1016/0006-8993(85)90936-9. [DOI] [PubMed] [Google Scholar]
- Naaijen J., Lythgoe D.J., Amiri H., Buitelaar J.K., Glennon J.C. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: a review of magnetic resonance spectroscopy studies. Neurosci. Biobehav. Rev. 2015;52:74–88. doi: 10.1016/j.neubiorev.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Peterson B.S., Thomas P., Kane M.J. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch. Gen. Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pittenger C., Bloch M.H., Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol. Ther. 2011;132(3):314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localizedin vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Provencher S. 2014. LCModel & LCMgui User's Manual. [Google Scholar]
- Puts N.A.J., Harris A.D., Crocetti D. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J. Neurophysiol. 2015;114(2):808–817. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. 2013. http://www.r-project.org/ Available at:
- Rickards H. Republished review: Tourette's syndrome and other tic disorders. Postgrad. Med. J. 2011;87(1024):142–149. doi: 10.1136/pgmj.2010.223685rep. [DOI] [PubMed] [Google Scholar]
- Robertson M.M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(Pt 3):425–462. doi: 10.1093/brain/123.3.425. (January 1997) [DOI] [PubMed] [Google Scholar]
- Robertson M.M. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J. Psychosom. Res. 2008;65(5):461–472. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Robertson M.M. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(1):68–87. doi: 10.1016/S2215-0366(14)00132-1. [DOI] [PubMed] [Google Scholar]
- Roessner V., Becker A., Banaschewski T., Rothenberger A. Psychopathological profile in children with chronic tic disorder and co-existing ADHD: additive effects. J. Abnorm. Child Psychol. 2007;35(1):79–85. doi: 10.1007/s10802-006-9086-z. [DOI] [PubMed] [Google Scholar]
- Scahill L., Riddle M.A., McSwiggin-Hardin M. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Singer H.S., Butler I.J., Tune L.E., Seifert W.E., Coyle J.T. Dopaminergic dysfunction in tourette syndrome. Ann. Neurol. 1982;12(4):361–366. doi: 10.1002/ana.410120408. [DOI] [PubMed] [Google Scholar]
- Singer H.S., Morris C., Grados M. Glutamatergic modulatory therapy for Tourette syndrome. Med. Hypotheses. 2010;74(5):862–867. doi: 10.1016/j.mehy.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Swanson J.M., Kinsbourne M., Nigg J. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol. Rev. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Tinaz S., Belluscio B.A., Malone P., van der Veen J.W., Hallett M., Horovitz S.G. Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum. Brain Mapp. 2014;35(12):5834–5846. doi: 10.1002/hbm.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; London: 2002. WISC-III Handleiding. [Google Scholar]
- Yücel M., Wood S.J., Wellard R.M. Anterior cingulate glutamate–glutamine levels predict symptom severity in women with obsessive–compulsive disorder. Aust N Z J Psychiatry. 2008;42(6):467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Yuen E.Y., Liu W., Karatsoreos I.N., Feng J., MCEWEN B.S., Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. 2009;106(33):14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Gruenbaum B.F., Klin Y. The effects of insulin, glucagon, glutamate, and glucose infusion on blood glutamate and plasma glucose levels in naive rats. J. Neurosurg. Anesthesiol. 2011;23(4):323–328. doi: 10.1097/ANA.0b013e3182299b15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material