Abstract
Background/Objectives
Systemic sclerosis is a connective tissue disease, which is characterized by fibrosis of the skin and internal organs, presence of specific antibodies and vascular involvement. Capillaroscopy is a useful method for the diagnosis and follow-up of patients with systemic sclerosis.
Trichoscopy is a rapid, non-invasive technique, which has become a standard procedure in differential diagnosis of scalp and hair diseases. The aim of this study was to assess whether trichoscopy may be applied in imaging microvessels in patients with systemic sclerosis.
Methods
The study included 17 patients with systemic sclerosis, and 31 healthy patients. In every patient 10 trichoscopy images were taken with Fotofinder II.
Results
In patients with systemic sclerosis trichoscopy of the frontal scalp area revealed polymorphic microvessels in 64,7% of patients, spider vessels (76,4%), capillary loops (52,9%), arborising vessels (41,1%) and avascular areas (35,2%). In healthy individuals these features were observed in polymorphic microvessels 6,4% of patients, spider vessels 6,4%, capillary loops 100%, arborising vessels 16,1%, avascular areas 9,6%, respectively.
Conclusions
In conclusion, the presence of polymorphic vessels in frontal area in trichoscopy is characteristic for systemic sclerosis.
Keywords: capillaroscopy, connective tissue diseases, dermatomyositis, dermoscopy, hair, systemic lupus erythematosus, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a connective tissue disease, which affects mostly females (female to male ratio is 3-8:1) with an average age at onset of 30 to 50 years. SSc is characterized by fibrosing of the skin and/or internal organs, presence of specific antibodies and vascular involvement. It’s etiology is still unknown but autoimmune, genetic, inflammatory factors seems to play major role in its pathogenesis. Some authors presume that occupational or environmental exposures may implicate the occurrence of SSc.[1,7] There are two forms of the disease-limited SSc (lSSc) that affects mainly skin, with rare internal organs involvement, and diffuse SSc (dSSc) concerning skin and more commonly visceral organs. According to 2013 criteria developed by a joint committee commissioned by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) patients with a total score at least 9 are classified as having recognized SSc. In criteria, skin thickening both proximal to metacarpophalangeal joints and affecting fingers, fingertip lesions, teleangiectasia, abnormal naifold capillaries, pulmonary involvement, Raynaud’s phenomenon and specific antibodies, were taken into account. The sensitivity of criteria was 0,91, and their specifity was assessed as 0,92.[5,6]
Trichoscopy (hair and scalp dermoscopy) is a rapid, non-invasive and low-cost technique, which has become a standard procedure in differential diagnosis of hair loss.[11,12,13] The observed structures are specific blood vessels, hair shafts, hair follicle openings, the perifollicular epidermis. Blood vessels are arranged into particular pattern, avascular areas can also be observed. Other measurable parameters are hair thickness and percentage of pilosebaceous units with one, two or three hairs. Those components can help to differentiate between various hair disorders.[14,15,16] There are only limited literature data describing hair disorders in systemic sclerosis.[9,10,14] The aim of this study was to assess whether trichoscopy may be applied in imaging scalp microvessels in patients with systemic sclerosis.
Patients and methods
The study included 17 patients with systemic sclerosis, and 31 age- and sex-matched healthy individuals. In every patient 10 trichoscopy images were taken with Fotofinder II. Images were taken from the frontal and occipital area. In each area one image at a 20-fold magnification and four pictures at a 70-fold magnification were taken. The Student T-test and Pearson Chi-Square Tests were used for statistical evaluation. Data was extracted and recorded in spreadsheet (Microsoft Excell, Microsoft Corporation, Redmond, Walsh).
Results
Types of vessels
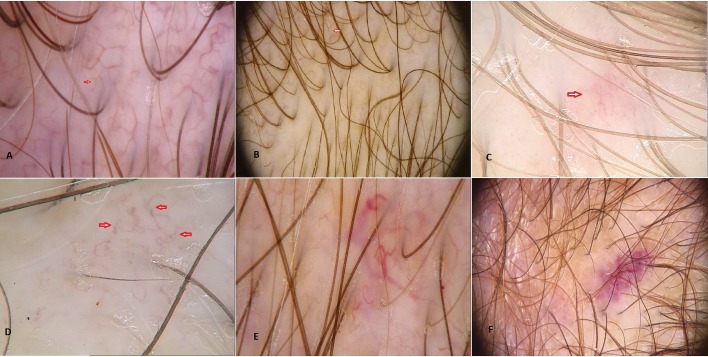
Vessels found in systemic sclerosis group were arranged into random, linear and clustered pattern. Linear pattern in SSc group was observed in 17,6% (3/17) individuals in frontal area and in 11,7% (2/17) in occipital area. Clustered pattern in SSc group was observed in 17,6% (3/17) of patients in frontal area and in 17,6% (3/17) in occipital area. Nor linear or clustered vascular pattern were seen in trichoscopy of frontal area in healthy patients. Arborizing vessels [Fig. 1A] were found both in patients with systemic sclerosis and the control group. They were more commonly observed in frontal area of patients with systemic sclerosis (47%; 7/17) than in healthy controls (16%; 5/3) (chi2(1)=33,307, p<0,001), whereas in occipital area arborising vessels accounted for 58,8% (10/17) among SSc patients and 87% (27/31) in healthy individuals. Red spider vessels [Fig. 1B] in SSc group were observed in frontal area in 76,47% (13/17) of patients, in healthy controls they were observed sporadically (2/31; chi2(1)=14,292, p<0,001). In occipital area spider vessels were found in 58,82% of patients with SSc (10/17)whereas they were not found in healthy control group (chi2(1)=29,914, p<0,001). Capillary loops [Fig. 1C] were present in frontal region in all examined healthy individuals (100%, 31/31 in control group, 52,9%, 9/17 in SSc group), and were also very common in occipital region (87%, 27/31 in control group, 64,7%, 11/17 in SSc group). Pinpoint vessels were observed alike in frontal and occipital region of healthy population and systemic sclerosis patients. In both groups pinpoint vessels were more common in frontal area (58,8%, 10/17 in SSc group, 83,8%, 26/31 in control group), than in occipital region (17,6%, 3/17 in SSc patients, 12,9%, 4/31 in healthy individuals).
Figure 1.
Types of vessels. (A) Polymorphous vessels; (B) Arborising vessels; (C) Red spider vessels; (D) Telangiectasia; (E) Telangiectasia; (F) Capillary loops.
Another common finding in frontal area of systemic sclerosis population were polymorphic vessels present among 64,7% (11/17) of patients whereas healthy individuals presented most commonly pinpoint vessels in this area (84%; 26/31) [Fig. 1D].Occipital region in both groups presented with arborizing vessels (58% SSc group vs 87% healthy control).
Telangiectasia are defined as enlarged capillary loops, deformated and budded capillaries, disturbances in vascular bed and microhaemorrhages [Fig. 1E, Fig. 1F]. They occurred in 4 patients (23,5%) with systemic sclerosis and were not observed in healthy control group.
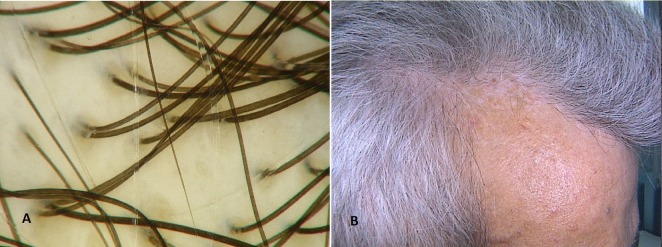
Avascular areas [Fig. 2A] in frontal region were observed in 35,29% (6/17) of patients with systemic sclerosis, and in 10% (3/31) of patients in the control group (chi2(1)=14,673, p<0,001). They were seen less commonly in occipital area in SSc group and they were not seen in healthy patients (chi(2)=6,015, p=0,014).
Figure 2.
Other trichoscopic findings. (A) Salt and pepper sign; (B) Avascular areas.
Perifollicular epidermis
Perifollicular epidermis in healthy population group was not changed whereas in SSc group ivory-whitish regions (3/17, 17,6%), salt and pepper regions (2/17, 11,7%) were observed in frontal area [Fig. 2B].
Hair shafts and hair thickness in systemic sclerosis group
Hair shafts and thinning was observed in SSc group and presented as higher percentage of thin hairs (<0,03 mm) in SSc group than in healthy controls in frontal (24,5% vs 6,6% in frontal area; t(19,558)=-3,996; p=0,001) and in occipital area (14% vs 6,6% respectively; t(20,268)=-2,517; p=0,020). The percentage of thick terminal hairs (>0,05 mm) was minor in SSc group compared to the healthy control group in frontal (12% vs 73%; t(47)=16,508; p<0,001) and in occipital area (25% vs 72%; t(47)=5,261; p<0,001). The mean hair shafts thickness did not differ statistically between both groups.
The hair loss in SSc patients was seen also as higher percentage of pilocebaceous units with single hair than in control group (frontal area- 25% vs 8,1%; t(47)=-3,258; p<0,001; occipital area 8,1% vs 3,2%; t(47)=-3,715; p=0,007). The differences in percentages of pilosebaceous units with 2 or 3 hairs was not statistically significant. Detailed results are shown in Table 1.
Table 1. Comparison of trichoscopy findings in SSc group and control group.
| Parameter | FRONTAL AREA SSc | FRONTAL AREA HEALTHY CONTROL | STATISTICAL SIGNIFICIANCE FRONTAL AREA | OCCIPITAL AREA SSc | OCCIPITAL AREA HEALTHY CONTROL | STATISTICAL SIGNIFICIANCE OCCIPITAL AREA |
|---|---|---|---|---|---|---|
| Spider vessels | 76,4% (13/17) | 6,4% (2/31) | 58,8% (10/17) | 0% (0/31) |
||
| Arborising vessels | 41,1% (7/17) |
16,1 (5/31) | 58,8% (10/17) | 87% (27/31) |
||
| Pinpiont vessels | 58,8% (10/17) | 83,8% (26/31) | 17,6% (3/17) |
12,9 (4/31) |
||
| Capillary Loops | 52,9% (9/17) |
100% (31/31) |
64,7% (11/17) | 87% (27/31) |
||
| Avascular areas | 35,2% (6/17) |
9,6% (3/31) |
17,6% (3/17) |
0% (0/31) |
||
| Polymorfic vessels | 64,7% (11/17) | 6,4% (2/31) |
5,8% (1/17) |
9,6% (3/31) |
||
| Telangiectasias | 23,5 (4/17) |
0% (0/31) |
5,8% (1/17) |
0% (0/31) |
||
| Salt and pepper sign | 11,7% (2/17) |
0% (0/31) |
0% (0/17) |
0% (0/31) |
||
| Ivory - whitish regions | 17,6% (3/17) |
0% (0/31) |
0% (0/17) |
0% (0/31) |
||
| Single hair unit (%) | 25 (SD 28,1) |
8,1 (SD 12,6) |
0,018 | 11,5 (SD 27,9) |
3,2 (SD 12,6) |
0 |
| Double hair unit (%) | 49,6 (SD 23,9) |
54,6 (SD 11,7) |
0 | 47,6 (SD 36,2) |
57,5 (SD 11,7) |
0 |
| Triple hair unit (%) | 25,4 (SD 26,0) |
37,3 (SD 11,1) |
0 | 40 (SD 31,7) |
39,2 (SD 12,3) |
0 |
| < 0,03 mm (%) | 24,5 (SD 10,8) |
6,6 (SD 4,9) |
0 | 14 (SD 10,4) |
6,3 (SD 5,1) |
0 |
| 0,03-0, 05 mm (%) | 64,5 (SD7,0) |
19,7 (SD 10,7) |
0,307 | 61 (SD 10,4) |
21.7 (SD 5,1) |
0,001 |
| > 0,05 mm (%) | 12 (SD 10,4) |
73,7 (SD 12,0) |
0,985 | 25 (SD 27,5) |
72,2 (SD 14,3) |
0,001 |
| Mean hair thickness (mm) | 0,0515 (SD 0,004) |
0,0597 (SD 0,007) |
0,068 | 0,0560 (SD 0,005) |
0,0571 (SD 0,006) |
0,401 |
Table 2. Types of vessels observed in trichoscopy.
| Spider vessels | Few linear vessels going from one, central point in different directions. |
|---|---|
| Arborising vessels | Vessels that are thickened and branched, they recall tree branches. |
| Pinpiont vessels | Small red dots densely aggregated. |
| Capillary Loops | Multiple, regularly spaced, hairpin - like vessels. |
| Telangiectasias | Enlarged capillary loops, deformed and budded capillaries, disturbances in vascular bed, microhaemorrhages. |
| Polymorphous vessels | Vessels of different kinds grouped in one scalp region. |
Discussion
Trichoscopy become a standard method in clinical dermatology. Its’ main advantages are diagnostic accuracy, repeatability, and opportunity to register outcomes. Trichoscopy is being used in different alopecias including scalp psoriasis,[26] syphilis,[27] pemphigous,[28] trichotillomania,[14] discoid lupus erythematosus.[14] There are some papers focusing on hair disorders in autoimmune diseases, however, systemic sclerosis was not yet adequate examined.[9,10,19,20]
Our study revealed that what differs systemic sclerosis patients from the control group is vessel types in forehead area. As described previously, common finding in forehead region of healthy population are pinpoint vessels and single loop vessels and they are usually uniform.[13,14,16,17] In forehead area of SSc group different types of vessels were observed. These included arborizing vessels, spider vessels and capillary loops. Spider vessels were previously described in discoid lupus erythematosus.[21] They are linear or curved vessels, with the origin in one central point and are radially arranged. Arborizing vessels are usually observed in temporal and occipital areas in healthy population and can be sporadically found in frontal area. Our findings in frontal area in patients with SSc are polymorphous pattern of vessels and a higher percentage of arborizing vessels in this region. Arborizing vessels in SSc group, unlike those seen in healthy controls, are not organized in interfollicular network but are seen between avascular areas together with spider vessels.
A typical phenomenon for systemic sclerosis were teleangiectasia observed in 23,52% of patients. Telangiectasia observed in trichoscopy most probably correlates with telangiectasia in other than scalp localizations, being one of SSc criteria. They were not observed in healthy control group so it can be assumed as pathognomic for SSc.
Ivory-whitish discoloration of the scalp which we report in patients with SSc was also previously described in cicatricial alopecias (frontal fibrosing alopecia, lichen planopilaris, etc.), but in these cases hair loss was presented clinically as patchy alopecia, whereas in SSc patients hair loss was diffused. It corresponds with fibrosis of the skin and like in fibrosing alopecias is a sign of irreversible changes affecting hair follicles.[14,21,22,23] In SSc avascular areas are located between normally arranged follicular units, they can be assumed as visible sign of fibrosis and microcirculation disorder.
Trichosocpy in SSc group shoved 'salt and pepper' areas in 2 cases of 17; 11,7%. 'Salt and pepper' sign was one of the first reported findings in systemic sclerosis.[25] 'Salt and pepper' sign was not previously described in trichoscopy in other diseases, so it can be assumed as pathognomic for SSc. Hair loss was observed as higher percentage of follicular units with single hairs, higher percentage of thin hairs (<0,03 mm) and lower of thick hairs (0,05 mm) in SSc group than in healthy controls.
In conclusion, our study revealed, that polymorphic vessels especially in forehead area and presence of teleangiectasias is characteristic for systemic sclerosis. The described vessels include arborizing vessels, spider vessels and capillary loops and usually are localized between avascular areas. Spider vessels were not previously described in the literature, besides discoid lupus erythematosus, that is why they can be assumed as typical for connective tissue diseases.[14,21]
References
- Dumoitier N, Lofek S, Mouthon L. Pathophysiology of systemic sclerosis: state of the art in 2014. Presse Med. 2014;43(10 Pt 2):e267–278. doi: 10.1016/j.lpm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- Jin J, Chou C, Lima , Zhou D, Zhou X. Systemic Sclerosis is a Complex Disease Associated Mainly with Immune Regulatory and Inflammatory Genes. Open Rheumatol J. 2014;8:29–42. doi: 10.2174/1874312901408010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretterklieber A, Painsi C, Avian A, Wutte N, Aberer E. Impaired quality of life in patients with systemic sclerosis compared to the general population and chronic dermatoses. BMC Res Notes. 2014;7:594. doi: 10.1186/1756-0500-7-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A. et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Vold AM, Gunnarsson R, Garen T, Midtvedt Ø, Molberg Ø. Performance of the 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis (SSc) in Large, Well-defined Cohorts of SSc and Mixed Connective Tissue Disease. J Rheumatol. 2015;42:60–63. doi: 10.3899/jrheum.140047. [DOI] [PubMed] [Google Scholar]
- Pekar M, Twig G, Levin A, Amital H. Systemic sclerosis: a prickly issue. Isr Med Assoc J. 2014;16:257–258. [PubMed] [Google Scholar]
- Nguyen C, Ranque B, Baubet T, Bérezné A, Mestre-Stanislas C, Rannou F. et al. Clinical, functional and health-related quality of life correlates of clinically significant symptoms of anxiety and depression in patients with systemic sclerosis: a cross-sectional survey. PLoS One. 2014;9:e90484. doi: 10.1371/journal.pone.0090484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano N, Amerio P, D'Ovidio R, Vena GA. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149:555–565. [PubMed] [Google Scholar]
- Parodi A, Cozzani E. Hair loss in autoimmune systemic diseases. G Ital Dermatol Venereol. 2014;149:79–81. [PubMed] [Google Scholar]
- Rudnicka L, Rakowska A, Kurzeja M, Olszewska M. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013;31:695–708. doi: 10.1016/j.det.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- Rakowska A. Trichoscopy (hair and scalp videodermoscopy) in the healthy female. Method standardization and norms for measurable parameters. J Dermatol Case Rep. 2009;3:14–19. doi: 10.3315/jdcr.2008.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol. 2014;71:431.e1–431.e11. doi: 10.1016/j.jaad.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: how it may help the clinician. Dermatol Clin. 2013;31:29–41. doi: 10.1016/j.det.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040–1048. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Pedrosa AF, Morais P, Lisboa C, Azevedo F. The importance of trichoscopy in clinical practice. Dermatol Res Pract. 2013;2013:986970. doi: 10.1155/2013/986970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti A, Torres F. Dermoscopy in the diagnosis of hair and scalp disorders. Actas Dermosifiliogr. 2009;100 Suppl 1:114–119. doi: 10.1016/s0001-7310(09)73176-x. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, Olszewska M, Rudnicka L. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–758. [PubMed] [Google Scholar]
- Rubegni P, Mandato F, Fimiani M. Frontal Fibrosing Alopecia: Role of Dermoscopy in Differential Diagnosis. Case Rep Dermatol. 2010;2:40–45. doi: 10.1159/000298283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71–75. doi: 10.1111/j.1346-8138.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: diagnosis simplified. Int J Trichology. 2013;5:170–178. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai VM, Balachandran C. Pseudovitiligo in Systemic Sclerosis. Dermatol Online J. 2005;11:41. [PubMed] [Google Scholar]
- Ye Y, Zhang X, Zhao Y, Gong Y, Yang J, Li H, Zhang X. The clinical and trichoscopic features of syphilitic alopecia. J Dermatol Case Rep. 2014;8:78–80. doi: 10.3315/jdcr.2014.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar M, Aktan Ş, Bilgin M. Dermoscopic findings in scalp psoriasis and seborrheic dermatitis; two new signs; signet ring vessel and hidden hair. Indian J Dermatol. 2015;60:41–45. doi: 10.4103/0019-5154.147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar-Pomian M, Kurzeja M, Rudnicka L, Olszewska M. The value of trichoscopy in the differential diagnosis of scalp lesions in pemphigus vulgaris and pemphigus foliaceus. An Bras Dermatol. 2014;89:1007–1012. doi: 10.1590/abd1806-4841.20143830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Zhao Y, Gong Y, Zhang X, Caulloo S, Zhang B, Cai Z, Yang J, McElwee KJ, Zhang X. Non-scarring patchy alopecia in patients with systemic lupus erythematosus differs from that of alopecia areata. Lupus. 2013;22:1439–1445. doi: 10.1177/0961203313508833. [DOI] [PubMed] [Google Scholar]