Abstract
Nonalcoholic fatty liver disease (NAFLD) represents one of the most common causes of chronic liver disease, and its prevalence is rising worldwide. The occurrence of nonalcoholic steatohepatitis (NASH) is associated with a substantial increase in disease related morbidity and mortality. Accordingly, there has been a surge of innovation surrounding drug development in an effort to off-set the natural progression and long-term risks of this disease. Disease assessment within clinical trials and clinical practice for NAFLD is currently done with liver biopsies. Liver biopsy-based assessments, however, remain imprecise and are not without cost or risk. This carries significant implications for the feasibility and costs of bringing therapeutic interventions to market. A need therefore arises for reliable and highly accurate surrogate end-points that can be used in phase 2 and 3 clinical trials to reduce trial size requirements and costs, while improving feasibility and ease of implementation in clinical practice. Significant advances have now been made in magnetic resonance technology, and magnetic resonance imaging (MRI) and elastrography (MRE) have been demonstrated to be highly accurate diagnostic tools for the detection of hepatic steatosis and fibrosis. In this review article, we will summarize the currently available evidence regarding the use of MRI and MRE among NAFLD patients, and the evolving role these surrogate biomarkers will play in the rapidly advancing arena of clinical trials in NASH and hepatic fibrosis. Furthermore, we will highlight how these tools can be readily applied to routine clinical practice, where the growing burden of NAFLD will need to be met with enhanced monitoring algorithms.
Keywords: magnetic resonance imaging, magnetic resonance elastography, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
Introduction
Affecting nearly 100 million Americans, nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease in the United States, and its rates are rising internationally alongside the growing epidemics of diabetes, obesity, and metabolic syndrome.1–3 NAFLD is commonly classified into two phenotypes, nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), and the development of NASH is associated with an increased risk for morbidity and mortality through hepatic (fibrosis, cirrhosis, hepatocellular carcinoma) and non-hepatic (cardiovascular disease and cancer) complications. 4–11 Recognizing the growing burden of NAFLD worldwide, the American Association for the Study of Liver Diseases (AASLD) and other international professional societies are working with the regulatory agencies to better define therapeutic targets and treatment end-points in NASH.12 This has led to a substantial rise in the number of studies being conducted worldwide,5 and although no therapeutic interventions are currently approved for use in NASH, it is anticipated that the clinical trial landscape of NASH and hepatic fibrosis will experience a surge of innovation and exponential growth over the coming years.
Currently, therapeutic trials in NASH require liver biopsy assessment to document treatment response. The use of liver biopsies, however, is not without cost or risk (bleeding, perforation, death) and it is met with hesitation from both patients and providers alike. Additionally, its scoring is associated with a significant inter- and intra-observer variability.13–16 Furthermore, although fat accumulation within the liver tends to be diffuse, the distribution is often non-uniform which results in inaccurate assessments of disease progression or regression due to spatial variability in sampling.17–22 These limitations carry significant implications for clinical trials as the diagnostic accuracy, reliability, and responsiveness of treatment end-points impact trial size requirements, feasibility, and costs. Furthermore, translating these findings to routine practice is difficult given the inability to perform routine frequent liver biopsies in clinical practice. Thus, a need exists for reliable and highly accurate surrogate end-points that can be used in place of liver biopsies. This will be of substantial importance in early-phase clinical trials, and will help to ensure clinical trial findings are readily translatable and measurable in routine practice.
Non-invasive biomarkers have been identified for NAFLD, but their variable diagnostic accuracy, limitations in certain sub-populations, and lack of validation make it difficult to uniformly apply these as surrogate end-points in clinical trials or clinical practice.14, 23, 24 Significant advances have now been made in magnetic resonance technology, and magnetic resonance imaging (MRI) and elastrography (MRE) have been demonstrated to be highly accurate diagnostic tools for non-invasive quantitative assessment of hepatic steatosis and fibrosis.25–30 Thus, they may be suitable alternatives to liver biopsy for diagnosing NALFD, for identifying at risk populations (NASH and those with hepatic fibrosis), and for assessing response to therapeutic interventions.
In this review article, we will summarize the currently available evidence regarding the use of MRI and MRE among NAFLD patients, the advantages these imaging techniques have over other modalities, and how they may be used for the assessment of hepatic steatosis and fibrosis in clinical trials and clinical practice. The development and validation of these magnetic resonance-based technologies will have considerable implications on the evolving clinical trial environment of NALFD, and on clinical practice where the growing burden of NAFLD in the community will need to eventually be met with non-invasive screening and monitoring protocols.
Criteria for the use of biomarkers as surrogates and clinical end-points
A biomarker is a characteristic that is objectively measured and evaluated as an indication of normal biologic processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.31 A composite biomarker is a combination of 2 or more biomarkers that are combined in a stated algorithm to reach a single interpretive readout. When the biomarker is used as a reliable substitute for a clinically meaningful end-point, then it is defined as a surrogate endpoint or surrogate biomarker.32–34 This hierarchical distinction between biomarkers, and the importance of defining surrogate biomarkers, is significant as the majority of biomarkers studied fail to meet the stringent criteria required to serve as reliable surrogates for meaningful end-points.35
Biomarkers can be broadly classified into four main types: diagnostic, prognostic, predictive, and pharmacodynamic, each of which requires a separate set of operating properties to be assessed prior to their potential implementation as surrogate biomarkers.32–37 Validity – the extent to which an instrument truly measures the outcome that it is intended to assess; reliability – the consistency or repeatability of an instrument; responsiveness – the ability to detect a meaningful change in health status; and feasibility – the ease with which an instrument can be utilized in a given setting, are essential operating properties of robust evaluative instruments.37 These properties help to ensure an accurate and consistent evaluation of key outcomes across treatment interventions and patient sub-groups, while minimizing clinical trial size requirements by reducing placebo rates and enrollment bias. These operating properties can be assessed through a process of validation, which requires demonstration that the surrogate end-point (i.e. surrogate biomarker) correlates with the true clinical outcome and it captures the net effect of treatment on the clinical outcome.38–41
With these definitions in mind, we can critically examine quantitative imaging properties measured by MRI and MRE as extrinsic surrogate biomarkers of intrinsic pathologic processes (hepatic steatosis and fibrosis), as well as evaluate their potential roles as surrogate biomarkers or surrogate composite biomarkers in clinical trials and clinical practice for NAFLD. The following sections will discuss the performance characteristics of MRI- and MRE-based properties as biomarkers of hepatic steatosis and fibrosis, and the available evidence for validation of these biomarkers.
MRI-based assessment of hepatic steatosis
Technical considerations: Quantifying hepatic steatosis through proton density fat fraction (PDFF)
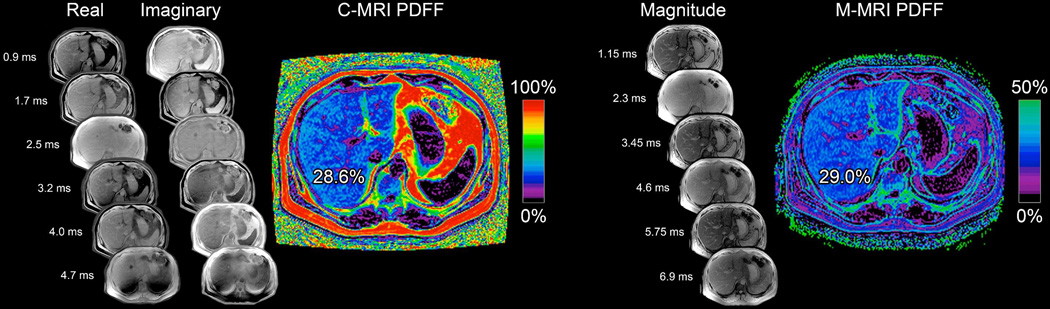
In contrast to other imaging techniques such as ultrasound and computed tomography, which use proxies to assess hepatic steatosis (i.e. attenuation and echogenicity), magnetic resonance quantifies hepatic steatosis by measuring the proton density fat fraction (PDFF) which is the fraction of MRI-visible protons bound to fat divided by all protons in the liver (bound to fat and water). Chemical shift imaging is applied to separate the liver signal into its water and fat signal components by acquiring gradient echoes at appropriately spaced echo times. A low flip angle is used to minimize T1 bias, and multiple echoes are acquired to correct for T2* effects.23, 42–48 In some variants of this approach, only the magnitude data is retained while the phase data is discarded; these variants accurately quantify the hepatic PDFF from 0 to 50%, which fortuitously captures the biological range of human hepatic steatosis, which rarely exceeds 50%.23 More sophisticated variants retain the phase data as well as the magnitude data to estimate PDFF across the full dynamic range (0–100%) of fat content in any tissue. Both variants are reproducible across MR scanners and field strengths,49–54 and can be readily interpreted in clinical practice. (Figure 1)
Figure 1. Principles of MRI-PDFF assessment and quantification of hepatic steatosis.
Moving left to right in the figure, you will first find the complex method of estimating PDFF, which acquires real and imaginary images to generate a PDFF map from 0–100%, labeled “C-MRI PDFF” here. Next are the magnitude images which are squared to generate a PDFF map from 0–50%, labeled “M-MRI PDFF” here. Both techniques acquire multiple images at echo times optimally spaced for fat water separation and T2* signal decay correction, and both apply a multi peak spectral model to correct for multi-frequency interference effects of fat proton signals. Note that for magnitude based PDFF, the calculated image only has a range of 0 to 50%. This is within the typical biological limits of liver fat content.
Validity – diagnostic accuracy and consistency across sub-populations
To be a useful diagnostic biomarker, MRI-PDFF must demonstrate a high degree of accuracy in identifying hepatic steatosis and quantifying the degree of steatosis throughout the liver in all sub-populations, as compared to the currently accepted gold standard (i.e. histology using the NASH-CRN scoring system55). Permutt et al.29 and Tang et al.53 demonstrated a strong correlation between MRI-PDFF and histology (r2=0.54, p<0.001 and p = 0.69, p<0.001, respectively) and Tang et al.53 further demonstrated that MRI based PDFF assessments had a high diagnostic accuracy (area under curve (AUC): 0.989, 95% CI 0.968 – 1.000) for differentiating between the presence (≥ 1 NASH-CRN grade) or absence (0 NASH-CRN grade) of hepatic steatosis. The diagnostic accuracy of MRI-PDFF was further validated by Idilman et al.56 and Bannas et al. 57, both of which demonstrated that MRI based PDFF assessments correlated closely with histology as assessed by liver biopsy (r = 0.82) and explant ex vivo histology assessment (r = 0.85). The consistency of application across sub-populations has also been validated by several studies demonstrating that key characteristics (age, sex, body mass index), disease components (histologic inflammation, co-existing hepatic conditions, or iron deposition) and technical factors (magnetic field strength) have no appreciable impact on the diagnostic accuracy of MRI-based PDFF assessments in NAFLD. 46, 58, 59 (Table 1) Thus, MRI-PDFF is a robust, quantitative, non-invasive, imaging-based diagnostic biomarker which can be used to improve the efficiency, increase the success rate, and decrease the required sample size of clinical trials by reducing heterogeneity in disease classification.
Table 1.
Diagnostic accuracy of MRI-PDFF for grading hepatic steatosis
| Design | Patient Characteristics | Reference Standard | MRI-PDFF cut-off | Accuracy/Correlation | |
|---|---|---|---|---|---|
| Permutt et al.29 | Cross-sectional, prospective cohort |
51 adult; biopsy proven NAFLD; AST/ALT above ULN; no alternative etiology |
Liver biopsy NASH- CRN histology; single blinded liver pathologist |
Grade 1: 8.9% Grade 2: 16.3% Grade 3: 25% |
r2 =0.56; patients with stage 4 fibrosis had lower quantified steatosis |
| Tang et al.53 | Cross-sectional, prospective cohort |
77 adult and pediatric; biopsy proven NAFLD; no alternative etiology |
Liver biopsy NASH- CRN histology; blinded liver pathologist |
Grade 1: 6.4% Grade 2: 17.4% Grade 3: 22.1% |
AUC 0.989 grade 1; AUC 0.825 ≥ grade 2; AUC 0.893 ≥ grade 3 |
| Idilman et al.56 | Retrospective, cohort |
70 adult; biopsy proven NAFLD; no alternative etiology |
Liver biopsy NASH- CRN histology; blinded liver pathologist |
Grade 2/3: 15% | AUC 0.950 ≥ grade 2; correlation decreased when fibrosis present |
| Bannas et al.57 | Cross-sectional, prospective cohort |
13 liver donors where livers were deemed unsuitable for transplant |
Five core biopsies from 9 segments (45 cores per liver); NASH-CRN histology; 2 blinded liver pathologists |
n/a | Strong correlation with histology (r2=0.850), smaller variance for MRI-PDFF than for histologic steatosis |
| Heba ER et al.58 | Retrospective, cohort |
506 adult; biopsy proven or suspected NAFLD; no alternative etiology |
Right lobe magnetic resonance spectroscopy |
n/a | 2-D echo least accurate, 3-D echo most accurate, results influenced by BMI and gender (males) |
NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; AUC: area under the curve; r2 = correlation coefficient
Reliability – Intra- and inter-examination reproducibility
The spatial variability in steatosis creates the potential for sampling error with liver biopsies, which may result in staging inaccuracies.13, 14, 22 Thus, the precision with which MRI-based PDFF assessments quantify hepatic steatosis, and the reproducibility of these estimates across observations, is significant. Negrete et al.60 demonstrated that MRI-based PDFF assessments showed excellent inter-examination precision for each hepatic segment (ICC ≥ 0.992; SD ≤ 0.66%; range ≤ 1.24%), each hepatic lobe (ICC ≥ 0.998; SD ≤ 0.34%; range ≤ 0.64%), and the whole liver (ICC = 0.999; SD ≤ 0.24%; range ≤ 0.45%). Tyagi et al.61 subsequently demonstrated that magnitude-based MRI, complex-based MRI, and MRS all had high intra- and inter-examination precision for PDFF assessments (ICC ≥ 0.990 and SD < 0.50% for all three techniques and for both intra- and inter-examination precision analyses). Bannas et al.57 further demonstrated that intra- and inter-observer agreement, along with repeatability, all showed a significantly smaller variance for MRI-PDFF than for histologic steatosis grading (p < 0.001).
These data demonstrate that MRI-PDFF is not only accurate in quantifying hepatic steatosis, but it has a high degree of precision and reproducibility, as well as greater reliability than histologic assessments. As the SDs of repeated MRI-PDFF measures have consistently been < 1%, these studies suggest that MRI-PDFF can reliably detect longitudinal changes as small as 2% points, and possibly smaller. Furthermore, meaningful differences in precision were greatest at the segmental level and lowest at the whole-liver level which may influence study power for detecting longitudinal changes depending on the anatomic level and number of averaged regions of interest (ROI) obtained.
Responsiveness – co-localizing regions of interest (ROI)
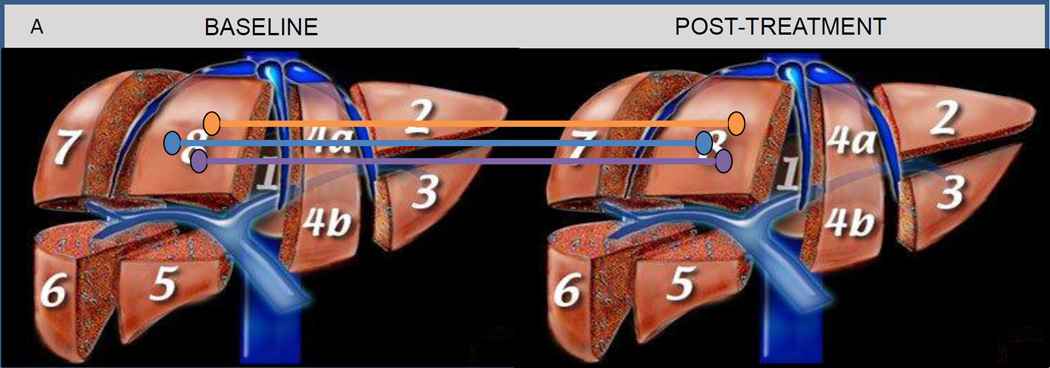
To be a useful pharmacodynamic biomarker, MRI-PDFF must demonstrate longitudinal changes that correlate with true biologic responses and/or meaningful clinical outcomes. In a prospective randomized double-blinded, placebo controlled trial Le et al.62 randomized 50 patients with biopsy-proven NASH to colesevelam or placebo and looked at changes in hepatic steatosis as measured by MRI-PDFF and MRS at 24 weeks. To account for the variability in precision when quantifying hepatic steatosis at the segmental level, the authors recorded MRI-PDFF assessments in 3 co-localized ROI (~300 – 400 mm2) in each of the 9 liver segments (27 separate ROI) at baseline and follow-up. (Figure 2A) For each segment, the three PDFF measurements were averaged and the authors validated the responsiveness and accuracy of changes over time against MRS. When using MRI-PDFF as the outcome measure, colesevelam increased hepatic steatosis in all nine-segments of the liver with a mean difference of 5.6% (p = 0.002), and MRI-PDFF correlated strongly with MRS-PDFF (r2=0.96, p<0.001). In contrast, liver biopsy-based assessment of hepatic steatosis did not detect any treatment effect. A secondary analysis by Noureddin et al.63 further demonstrated that patients who had an increase or decrease in MRI-PDFF of ≥ 1% showed a parallel increase or decrease in their body weight and serum alanine and aspartate aminotransferases at week 24 (p < 0.05), and this small increase or decrease in hepatic steatosis could not be detected with liver biopsy-based histology assessments.
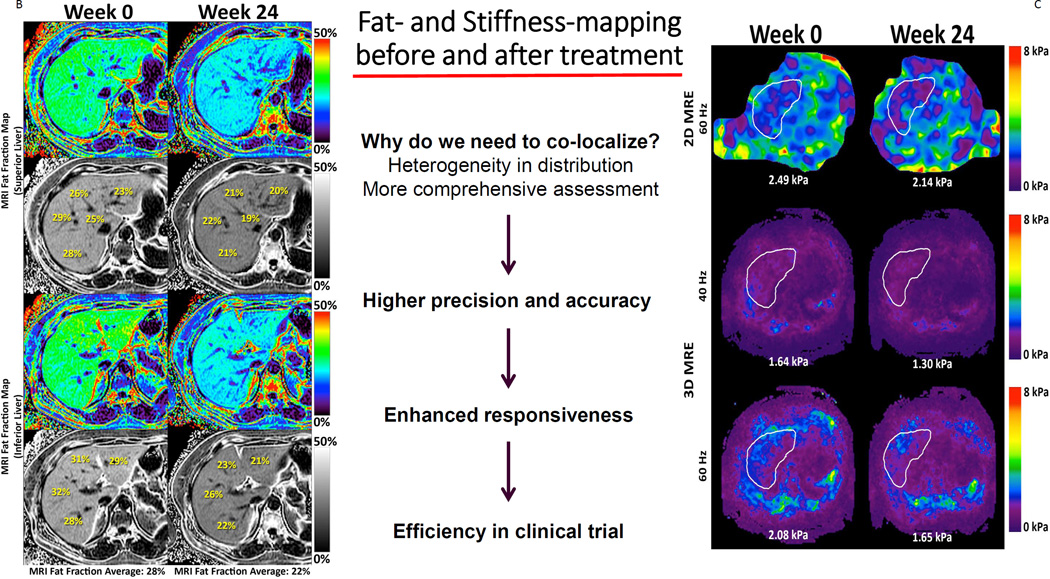
Figure 2. Co-localization of regions of interest and responsiveness of MRI-PDFF and MRE.
Figures adapted and modified from Loomba et al.119 Figure 2A: Anatomical co-localization of regions of interest. Figure 2B: Whole liver fat mapping with magnetic resonance imaging proton density fat fraction (MRI-PDFF) at weeks 0 and 24. Figure 2C: Whole liver fibrosis mapping with magnetic resonance elastography (MRE) at weeks 0 and 24.
Together these studies demonstrate that MRI-PDFF is highly responsive to changes in hepatic steatosis, this change in MRI-PDFF assessments over time correlates to a true biologic response (change in liver enzymes) and meaningful clinical outcome (change in body weight), and co-localization of ROIs is needed to ensure a high degree of accuracy for quantifying change over time. Additionally, since MRI-PDFF is more sensitive to longitudinal change in steatosis than biopsy with histology scoring, it is more likely to detect both positive and adverse therapeutic responses, providing further support for its use as a surrogate biomarker of hepatic steatosis.
Comparative effectiveness of MRI-PDFF over other imaging modalities for quantifying steatosis and integration in routine clinical practice
MRS assesses PDFF directly through a measurement of differences in water and fat peaks on a resonance frequency domain.51, 64, 65 Since MRS assesses water and fat content more directly than MRI, which estimates their content by analyzing time-dependent oscillations in the MR signal, it is considered to be potentially more accurate. There are, however, several limitations to MRS-PDFF assessments that warrant consideration. (Table 2) First, similar to liver biopsy, MRS data is typically collected from a single region or voxel positioned by the operator in the liver parenchyma using anatomic landmarks depicted by conventional imaging. Thus, similar to liver biopsies, MRS evaluates a small portion of the liver to quantify steatosis (although the ~ 8cm3 MRS voxel is orders of magnitude than a typical core biopsy) and does not depict the distribution of steatosis throughout the liver parencyhma.17 Second, the complexity of MRS requires significant expertise for its acquisition and analysis, and it is not readily available on all scanners, which limits its routine application in clinical trials and clinical practice. Finally, although MRS is considered to be potentially more accurate, the diagnostic accuracy, reliability, and responsiveness of MRI-PDFF assessments have consistently been demonstrated to be highly correlated with MRS-based PDFF assessments across multiple studies.5, 27, 58, 61–63 Thus, the ease of implementation, similarity in operating performance as compared to MRS, and ability to quantify hepatic steatosis throughout the liver, makes MRI-based PDFF assessment an attractive alternative to MRS-based assessments.
Table 2.
Comparison of imaging based assessments of hepatic steatosis in NAFLD
| MRS | MRI | Ultrasound | CAP | |
|---|---|---|---|---|
| Measurement | Directly measures differences in water and fat peaks on a resonance frequency domain |
Indirect CSI assessment of signal interface between water and fat peaks during OP and IP echoes |
Assessment through proxies (i.e. attenuation and echogenicity) |
VCTE guided assessment using a ultrasonic controlled attenuation parameter algorithm |
| Dynamic Range | Single area (8cm3 voxel) manually placed in liver parenchyma using 3-plane localizing imaging |
Quantification over a full dynamic range (0 – 100%) throughout parenchyma |
Limited when overall content of hepatic steatosis is < 20% |
Sub-optimal quantification over a broad dynamic range in ROI |
| Application | Not available on routine scanners and requires expertise |
Readily applied to routine scanners with some expertise required |
Readily available in routine practice for use |
Point of care testing |
| Accuracy | High diagnostic accuracy not significantly impacted by demographics, histologic activity, or co- xisting hepatic conditions |
High diagnostic accuracy not significantly impacted by demographics, histologic activity, or co- existing hepatic conditions |
Modest diagnostic accuracy; significantly limited by demographics (obesity), and co-existing hepatic conditions |
Higher diagnostic accuracy than standard ultrasound based assessments but lower than MRI; limited by obesity, inflammation, stage of fibrosis |
| Reliability | High precision with minimal variability |
Higher precision and lower variability than MRS and histologic assessments |
Modest reliability and agreement with training |
Improved precision and reduced variability than ultrasound through assessment of acquisition validity but lower than MRI |
| Responsiveness | Responsive to changes in steatosis in single area |
Highly responsive to changes in steatosis throughout parenchyma |
Limited responsiveness and unable to co-localize ROI for response |
Improved responsiveness over standard ultrasound |
|
Co-localization of fibrosis |
Requires alternative imaging modality for co- localizing elasticity |
Co-localization with MRE | Unable to co-localize | Co-localization with VCTE |
MRS: magnetic resonance spectroscopy; MRI-PDFF: magnetic resonance imaging proton density fat fraction; NAFLD: nonalcoholic fatty liver disease; CSI: chemical shift imaging; OP: opposed phase; IP: in-phase; MRE: magnetic resonance elastography; ROI: regions of interest; CAP: controlled attenuation parameter; VCTE: vibration controlled transient elastography
One of the major criticisms with MRI based-PDFF assessments is the equipment and cost associated with MRI scanners, along with the technical expertise required to perform and interpret readings. Thus, several investigators have begun to evaluate the potential utility of ultrasound, a non-invasive and readily available tool, for quantifying hepatic steatosis.66 Although several specific features have been identified to be unique to NAFLD when using ultrasound, and the inter-observer agreement for identifying these features has been shown to be relatively good, the sensitivity of these findings is considerably lower when the overall content of hepatic steatosis is < 20%.67–75 Furthermore, patient demographics (obesity) and co-existing conditions (hepatitis C) have been shown to further reduce the sensitivity of this diagnostic tool,75–79 which significantly impacts its ability to reliability distinguish between the presence or absence of hepatic steatosis among at-risk individuals.
Newer quantitative ultrasound-based techniques have emerged and have been shown to be highly correlated with MRI-PDFF assessments (Spearman ρ = 0.80; P < .0001),80 and the most studied of these techniques is the controlled attenuation parameter (CAP) based assessments. By concomitantly assessing hepatic steatosis and liver stiffness through transient elastography, CAP based ultrasound assessments have been shown to provide a more accurate assessment of hepatic steatosis across a wide range of patient populations.80–87 Recently, a direct comparison between MRI-PDFF and CAP for quantify hepatic steatosis was performed, and MRI-PDFF was demonstrated to be more accurate for the detection of grade 1 steatosis (MRI-PDFF: AUC 0.98, 95% CI 0.96 – 1.00 vs. CAP: AUC 0.88, 95% CI 0.80 – 0.95), for differentiating between grade 1 and 2 steatosis (MRI-PDFF: AUC 0.90, 95% CI: 0.81 – 0.98 vs. CAP: AUC 0.73, 95% CI: 0.64 – 0.81), and for differentiating between grade 2 and 3 steatosis (MRI-PDFF: AUC 0.79, 95% CI: 0.64 – 0.95 vs. CAP: AUC 0.70, 95% CI: 0.58 – 0.83).88 Thus, although ultrasound may be an inexpensive diagnostic tool for use in clinical practice with modest diagnostic accuracy, MRI-PDFF remains the most accurate diagnostic tool available for quantifying hepatic steatosis and the enhanced accuracy associated with this imaging modality will help to overcome its expense by avoiding the excess costs associated with complications from false positive or false negative results. If MRI-PDFF is not readily available for routine clinical use, then its greatest value in clinical practice will come in those individuals identified to be at risk for progression to NASH or NASH related complications, and a therapeutic intervention is planned. A baseline and follow-up assessment 6 months later, at a referral center with expertise in MRI-PDFF, will help to ensure the most accurate quantification of disease severity and response to therapy which has implications on the long-term disease related morbidity and mortality.
Importance of fibrosis when quantifying hepatic steatosis: the need for a composite biomarker
It is important to note that, although MRI-PDFF is a highly accurate, reliable, and responsive diagnostic tool for quantifying hepatic steatosis in NAFLD, its application as a biomarker in patients with more advanced liver disease is limited by the severity of fibrosis present. 29, 56 Permutt et al.29 demonstrated that average MRI-determined PDFF and histology-determined steatosis grade remained relatively stable at fibrosis stage 0–3, but dropped significantly at stage 4. Schwimmer et al.89 similarly demonstrated that the correlation between MRI-PDFF and histology was significantly (P < 0.01) weaker in children with stage 2–4 fibrosis (0.61) than children with no fibrosis (0.76) or stage 1 fibrosis (0.78). Thus, if MRI-PDFF is to be used for assessing response to therapy in patients with more aggressive and advanced disease courses, a concomitant biomarker based-assessment of fibrosis will be needed. It is likely that MRE-based stiffness measurements may address this need, as discussed below.
MRE based assessment of hepatic fibrosis
Technical considerations: Quantifying hepatic fibrosis
Fibrosis has no molecular signature that can be detected by current imaging techniques, and all imaging tests for fibrosis attempt to detect fibrosis indirectly. As collagen deposition associated with fibrosis imparts parenchymal rigidity, the leading biomarker for assessing fibrosis is through elastography. MRE uses a modified phase-contrast pulse sequence to visualize rapidly propagating mechanical shear waves (typically delivered at around 60 Hz).90 Cross-sectional elastogram images are then created depicting the stiffness generated from the wave propagation information. Technically, elastography assessments can be accomplished with most MR scanners by adding hardware to generate mechanical waves and adding specific software for acquisition and processing.90 Because the waves can be visualized and analyzed deep into the liver, MRE evaluates a large portion of the liver and can be performed in conjunction with conventional MRI.
Validity and Reliability – diagnostic accuracy and reproducibility for quantifying hepatic fibrosis
Several individual studies have investigated the diagnostic accuracy of MRE for quantifying hepatic fibrosis in NAFLD, and a recent pooled analysis of individual participant level data has demonstrated that MRE has a high diagnostic accuracy (AUC 0.90, 95% CI 0.84 – 0.94) for identifying advanced fibrosis (stage 3–4). 25, 26, 30, 91–94 (Table 3) Although the diagnostic accuracy has been demonstrated to be consistent across patient sub-groups (obesity, gender) and disease states (inflammation grade, liver stiffness), a recent prospective cohort study has demonstrated that more advanced versions of the imaging modality (3-dimensional MRE at 40Hz) are more accurate (AUC 0.981) as compared to more traditional modalities (3D-MRE at 60Hz AUC: 0.927; 2D-MRE at 40Hz AUC: 0.921).94 The inter-observer agreement in assessments for MRE has been demonstrated to be high (ICC 0.99, 95% CI 0.98 – 1.00), and the agreement for MRE assessments is higher than that with pathologist staging (ICC 0.91, 95% CI 0.86 – 0.94).95 Thus, MRE is highly accurate for detecting hepatic fibrosis, results are not influenced by patient demographics making assessments reproducible across key sub-populations, and the inter-observer agreement for staging fibrosis is nearly perfect and higher than that seen with histopathology. For these reasons, MRE is considered to be a reliable, highly accurate, and precise method for assessing hepatic fibrosis.30
Table 3.
Diagnostic accuracy of MRE for grading fibrosis
| Design | Patient Characteristics | Reference Standard | MRE cut-off | Accuracy/Correlation | |
|---|---|---|---|---|---|
| Loomba et al.30 | Cross-sectional, prospective cohort, comparative effectiveness |
117; biopsy proven NAFLD, no alternative etiology |
Liver biopsy NASH- CRN histology; blinded liver pathologist; 5-point scale (0–4); 3–4=advanced |
Stage 1: 3.02 Kpa Stage 2: 3.58 Kpa Stage 3: 3.64 Kpa Stage 4: 4.67 Kpa |
AUC 0.838 ≥ Stage 1 AUC 0.856 ≥ Stage 2 AUC 0.924 ≥ Stage 3 AUC 0.894 ≥ Stage 4 |
| Kim et al.91 | Retrospective cohort |
142; biopsy proven NAFLD within 1 year of MRE, no alternative etiology |
Livery biopsy Brunt classification; blinded liver pathologist; 5-point scale (0–4); 3–4=advanced |
Stage 0–2 vs. 3–4: 4.15 Kpa |
AUC 0.954 ≥ Stage 3; Improved accuracy as compared to FIB-4, NAFLD fibrosis score, AST/ALT ratio, APRI or BARD score |
| Cui et al.25 | Cross-sectional, prospective cohort, comparative effectiveness |
102; biopsy proven NAFLD; no alternative etiology |
Liver biopsy NASH- CRN histology; blinded liver pathologist; 5-point scale (0–4); 3–4=advanced |
3.64 Kpa had 92% sensitivity and 90% specificity for predicting advanced fibrosis |
AUC 0.957 ≥ Stage 3; Significantly (p < 0.05) better than FIB-4, Lok index, AST/ALT ratio, NAFLD fibrosis score, APRI, BARD, NASH CRN model, Bonacini score |
| Chen et al.93 | Retrospective cohort |
58; biopsy proven NAFLD within 90 days of MRE, no alternative etiology |
Livery biopsy Brunt classification; blinded liver pathologist; 5-point scale (0–4); 3–4=advanced |
Stage 0: 2.71 Kpa Stage 1: 3.43 Kpa Stage 2: 4.58 Kpa Stage 3: 5.55 Kpa Stage 4: 5.82 Kpa |
Correlation coefficient for liver stiffness with fibrosis stage of 0.651 |
| Loomba et al.94 | Cross-sectional, prospective cohort, comparative effectiveness |
100; biopsy proven NAFLD; no alternative etiology |
Liver biopsy NASH- CRN histology; blinded liver pathologist; 5-point scale (0–4); 3–4=advanced |
Stage 0–2 vs. 3–4 2D (60Hz):3.8 Kpa 3D (60Hz):3.4 Kpa 3D (40Hz):2.4 Kpa |
Stage 0–2 vs. 3–4 2D (60Hz): AUC 0.921 3D (60Hz): AUC 0.927 3D (40Hz): AUC 0.981 |
| Singh et al.92 | Systematic review |
232; biopsy proven NAFLD, individual participant level data |
Liver biopsy; 5-point scale (0–4); 3–4=advanced |
n/a | AUC 0.86 ≥ Stage 1 AUC 0.87 ≥ Stage 2 AUC 0.90 ≥ Stage 3 AUC 0.91 ≥ Stage 4 |
NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; AUC: area under the curve; MRE: magnetic resonance elastography
Validity – diagnostic accuracy for identifying NASH among NAFLD patients
Although not a requirement for the diagnosis,96 the presence of fibrosis may help to identify patients with NASH and nearly 40% of NASH patients will progress to advanced stages of fibrosis in as little as 3 years.97–101 For these reasons it has been suggested that liver biopsy be performed in all NAFLD patients to identify NASH patients earlier in the disease course so therapeutic interventions may be implored to reduce overall morbidity and mortality.101 Chen et al.93 demonstrated that MRE-based assessments of liver stiffness may have a high diagnostic accuracy (AUC 0.93) for differentiating NASH from simple steatosis, with a cut-off of 2.74 kPa yielding a sensitivity of 94% with a specificity of 73%. Furthermore, MRE-based assessments of liver stiffness increased with NASH severity independent of the presence of fibrosis. Although this would suggest that MRE may allow for the early identification of patients with NASH, even before fibrosis has begun, it needs to be interpreted with caution as this was a single center retrospective study in a small cohort of individuals. Thus further validation in prospective cohorts is needed, but these results are promising as the early identification of NASH patients prior to the onset of fibrosis would be of significant clinical importance and it would allow for prognostic and predictive enrichment of clinical trials with resultant increases in effect sizes. This would be ideal for early proof-of-concept studies where an enhanced benefit-to-risk relationship determination is needed, and in prevention trials aimed at halting progression to fibrosis where an accurate identification of patients at risk for progressing is needed.
Comparative effectiveness with other imaging modalities
Similar to MRI-based assessment of hepatic steatosis, consideration has been given to preferentially using ultrasound-based assessments of hepatic fibrosis given their ease of implementation and relative inexpensiveness. Ultrasound-based elastography methods can be classified as strain elastrography which looks at the distribution of strain but does not yet permit reproducible measurements, or shear wave elastrography which monitors the propagation of shear waves in the tissue.102, 103 Among shear wave technologies the most widely studied and compared to MRE are transient elastrography (TE), and point wave shear elastography utilizing acoustic radiation force impulse imaging (ARFI).14 Similar to their application for assessing hepatic steatosis, ultrasound-based techniques have a lower diagnostic accuracy as compared to MRE for assessing hepatic fibrosis (TE: AUC 0.82; ARFI: AUC 0.85) or cirrhosis (TE: AUC 0.92; ARFI: AUC 0.93).88, 104–107 Direct comparison studies in heterogenous study populations have also shown that MRE-based assessments have higher completion rates, and they provide significantly more reliable measurements of liver stiffness.107–110 Furthermore, ultrasound-based techniques only evaluate a portion of the liver, are technically challenging in obese patients or those with significant ascites, are operator dependent, and can be influenced by inflammatory activity or hepatic congestion.111–118 This is by far the most important limitation to its use in clinical practice, and given the importance heterogeneity of disease classification has on power calculations and study designs for therapeutic interventional trials, MRE represents a potentially important non-invasive tool for accurately identifying and quantify the severity of fibrosis in clinical trials.118
Feasibility of using MRI-PDFF and MRE as composite biomarkers in clinical trials and clinical practice
One of the final operating properties requiring validation is the feasibility of application, in both clinical trials and clinical practice. The feasibility of assessing both MRI-PDFF and MRE as composite biomarkers in clinical trials was established by the MOZART trial.119 In a randomized, double-blind, placebo controlled trial, 50 patients with biopsy-proven NASH were randomized to either ezetimibe 10 mg orally daily or placebo for 24 weeks. The authors assessed both MRI-PDFF and MRE and demonstrated that the application of co-localization of MRI-PDFF-derived fat maps and MRE-derived stiffness maps of the liver before and after treatment to noninvasively assess treatment response in NASH was feasible. (Figure 2B, Figure 2C) In clinical practice, Doycheva et al.120 assessed the feasibility of screening for NAFLD with MRI-PDFF in the primary care setting and determined that the prevalence of NAFLD among type 2 diabetics was 65%. By concomitantly assessing for hepatic fibrosis with MRE, they were further able to establish that the prevalence of advanced fibrosis among type 2 diabetics was 7%. Among those with advanced fibrosis, nearly a quarter had an MRI-PDFF assessment of < 5%. Thus, had the authors used only MRI-PDFF to screen for NAFLD they may have under-estimated the true burden of disease and severity in this population. This study helps to provide the foundation for the feasibility of using MRI-PDFF and MRE in clinical practice and highlights the importance of assessing steatosis and fibrosis simultaneously, but will need to be re-produced in subsequent studies and it will need to be compared to alternative screening modalities that are more widely available and potentially more cost-effective (i.e. CAP).121
Future Considerations
Although MRI-PDFF and MRE offer accurate non-invasive assessments of hepatic steatosis and fibrosis, the implementation of these imaging modalities in clinical practice has been limited by technical feasibility and time required to complete the test. More recently, a newer imaging modality using a multiparametric MR imaging (T1 and T2* mapping) and proton spectroscopy approach has been studied in a blinded prospective fashion and was demonstrated to be highly correlated with liver biopsies with regards to the presence of steatosis (Spearman r = 0.89, AUC 0.93) and fibrosis (Spearman r = 0.62, AUC 0.90).122 Although this imaging modality will still require further validation it offers a promising transition for using MRI for routine monitoring and assessments in clinical practice given the scan can be accomplished in approximately 23 minutes and it simultaneously assesses steatosis and fibrosis.
Summary
In conclusion, magnetic resonance imaging-based techniques are now available for non-invasive, accurate, reproducible, and precise quantification of hepatic steatosis and fibrosis through proton density fat fraction (MRI-PDFF) and elastography (MRE) assessments, respectively. These biomarkers have been demonstrated to be highly accurate, reliable, and responsive indices in NAFLD, and they carry several distinct advantages over other invasive (liver biopsy) and non-invasive (MRS and ultrasound) assessment techniques. The optimal approach to utilizing them in clinical trials will require a co-localization of regions of interests for both hepatic steatosis and fibrosis in order to accurately identify at risk populations where therapeutic interventions will be of greatest value, and to quantify longitudinal changes with therapeutic interventions. This approach can be directly translated into clinical practice, where non-invasive screening and monitoring protocols can be developed to address the growing epidemic of NAFLD in the community.
Key Point Box.
MRI-PDFF is a robust, quantitative, accurate, and reproducible non-invasive biomarker for the assessment of NAFLD
Co-localized assessment of quantitative changes in liver fat content provides more precise estimates of changes in liver fat to assess treatment response in NASH trials
MRE is emerging to be an accurate, reproducible and quantitative non-invasive biomarker for the assessment of advanced fibrosis in NAFLD
MRE is superior than ARFI and VCTE is assessment of hepatic fibrosis, especially in obese
Acknowledgments
Guarantor(s) of the article: Rohit Loomba
Conflicts and Disclosures: PSD is supported by the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32DK007202. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. Both CBS and RL are supported by R01DK106419. CBS is also supported by R01DK088925
Abbreviations
- MRI
magnetic resonance imaging
- MRE
magnetic resonance elastography
- PDFF
proton density fat fraction
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- NAFL
nonalcoholic fatty liver
- NASH
nonalcoholic steatohepatitis
- ICC
intraclass correlation coefficient
- SD
standard deviation
- CI
confidence interval
- VCTE
vibration controlled transient elastrography
- TE-CAP
Transient elastography based controlled attenuation parameter
- ARFI
acoustic radiation force impulse imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Acquisition and interpretation of data: PSD, CBS, RL
- Drafting of the manuscript: PSD
- Critical revision of the manuscript for important intellectual content: CBS, RL
- Approval of the final manuscript: PSD, CBS, RL
REFERENCES
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Spengler EK, Loomba R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin Proc. 2015;90:1233–1246. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 9.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Arulanandan A, Ang B, Bettencourt R, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:1513–1520. doi: 10.1016/j.cgh.2015.01.027. e1. [DOI] [PubMed] [Google Scholar]
- 12. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm459144.htm.
- 13.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 14.Asrani SK. Incorporation of Noninvasive Measures of Liver Fibrosis Into Clinical Practice: Diagnosis and Prognosis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 16.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonekamp S, Tang A, Mashhood A, et al. Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2014;39:1525–1532. doi: 10.1002/jmri.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.el-Hassan AY, Ibrahim EM, al-Mulhim FA, et al. Fatty infiltration of the liver: analysis of prevalence, radiological and clinical features and influence on patient management. Br J Radiol. 1992;65:774–778. doi: 10.1259/0007-1285-65-777-774. [DOI] [PubMed] [Google Scholar]
- 19.Grove A, Vyberg B, Vyberg M. Focal fatty change of the liver. A review and a case associated with continuous ambulatory peritoneal dialysis. Virchows Arch A Pathol Anat Histopathol. 1991;419:69–75. doi: 10.1007/BF01600155. [DOI] [PubMed] [Google Scholar]
- 20.Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 21.Larson SP, Bowers SP, Palekar NA, et al. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007;5:1329–1332. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392–7402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papagianni M, Sofogianni A, Tziomalos K. Non-invasive methods for the diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:638–648. doi: 10.4254/wjh.v7.i4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451. doi: 10.1016/j.cgh.2014.09.046. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idilman IS, Keskin O, Celik A, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2015 doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 28.Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downing GJ, editor. Biomarkers and Surrogate Endpoints. Elsevier: Amsterdam; 2000. NIH Definitions Working Group Biomarkers and surrogate endpoints in clinical research: definitions and conceptual model; pp. 1–9. [Google Scholar]
- 32.Aronson JK. Biomarkers and surrogate endpoints. Br J Clin Pharmacol. 2005;59:491–494. doi: 10.1111/j.1365-2125.2005.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronson JK. Research priorities in biomarkers and surrogate end-points. Br J Clin Pharmacol. 2012;73:900–907. doi: 10.1111/j.1365-2125.2012.04234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aronson JK. Surrogate end points: studying their benefits, taxonomy, and semantics. Bmj. 2012;344:e750. doi: 10.1136/bmj.e750. [DOI] [PubMed] [Google Scholar]
- 35.Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 2005;24:67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
- 36.FDA Document. Guidance for Industry Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products. 2012 Dec [Google Scholar]
- 37.Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis. 1985;38:27–36. doi: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- 38.Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers. 2002;18:41–46. doi: 10.1155/2002/929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JW, Devanarayan V, Barrett YC, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23:312–328. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- 40.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 41.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 42.Yokoo T, Bydder M, Hamilton G, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SS, Lee Y, Kim N, et al. Hepatic fat quantification using chemical shift MR imaging and MR spectroscopy in the presence of hepatic iron deposition: validation in phantoms and in patients with chronic liver disease. J Magn Reson Imaging. 2011;33:1390–1398. doi: 10.1002/jmri.22583. [DOI] [PubMed] [Google Scholar]
- 45.Guiu B, Petit JM, Loffroy R, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95–102. doi: 10.1148/radiol.2493080217. [DOI] [PubMed] [Google Scholar]
- 46.Bydder M, Shiehmorteza M, Yokoo T, et al. Assessment of liver fat quantification in the presence of iron. Magn Reson Imaging. 2010;28:767–776. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang BK, Yu ES, Lee SS, et al. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368–375. doi: 10.1097/RLI.0b013e31824baff3. [DOI] [PubMed] [Google Scholar]
- 48.Kuhn JP, Hernando D, Munoz del Rio A, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology. 2012;265:133–142. doi: 10.1148/radiol.12112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hines CD, Yu H, Shimakawa A, et al. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215–1222. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeder SB, Robson PM, Yu H, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332–1339. doi: 10.1002/jmri.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H, Shimakawa A, McKenzie CA, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang GH, Cruite I, Shiehmorteza M, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 56.Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–775. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 57.Bannas P, Kramer H, Hernando D, et al. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444–1455. doi: 10.1002/hep.28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heba ER, Desai A, Zand KA, et al. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paparo F, Cenderello G, Revelli M, et al. Diagnostic value of MRI proton density fat fraction for assessing liver steatosis in chronic viral C hepatitis. Biomed Res Int. 2015;2015:758164. doi: 10.1155/2015/758164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Negrete LM, Middleton MS, Clark L, et al. Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging. 2014;39:1265–1271. doi: 10.1002/jmri.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyagi A, Yeganeh O, Levin Y, et al. Intra- and inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging. 2015 doi: 10.1007/s00261-015-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamilton G, Middleton MS, Bydder M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145–152. doi: 10.1002/jmri.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassidy FH, Yokoo T, Aganovic L, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231–260. doi: 10.1148/rg.291075123. [DOI] [PubMed] [Google Scholar]
- 66.Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:6821–6825. doi: 10.3748/wjg.v20.i22.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 69.Riley TR, Bruno MA. Sonographic measurement of the thickness of subcutaneous tissues in nonalcoholic fatty liver disease versus other chronic liver diseases. J Clin Ultrasound. 2005;33:439–441. doi: 10.1002/jcu.20164. [DOI] [PubMed] [Google Scholar]
- 70.Riley TR, 3rd, Mendoza A, Bruno MA. Bedside ultrasound can predict nonalcoholic fatty liver disease in the hands of clinicians using a prototype image. Dig Dis Sci. 2006;51:982–985. doi: 10.1007/s10620-006-9343-6. [DOI] [PubMed] [Google Scholar]
- 71.Riley TR, 3rd, Kahn A. Risk factors and ultrasound can predict chronic hepatitis caused by nonalcoholic fatty liver disease. Dig Dis Sci. 2006;51:41–44. doi: 10.1007/s10620-006-3082-6. [DOI] [PubMed] [Google Scholar]
- 72.Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 75.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterol. 2007;102:2716–2717. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 76.Guajardo-Salinas GE, Hilmy A. Prevalence of nonalcoholic fatty liver disease (NAFLD) and utility of FIBROspect II to detect liver fibrosis in morbidly obese Hispano-American patients undergoing gastric bypass. Obes Surg. 2010;20:1647–1653. doi: 10.1007/s11695-009-0027-0. [DOI] [PubMed] [Google Scholar]
- 77.de Moura Almeida A, Cotrim HP, Barbosa DB, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14:1415–1418. doi: 10.3748/wjg.14.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perez NE, Siddiqui FA, Mutchnick MG, et al. Ultrasound diagnosis of fatty liver in patients with chronic liver disease: a retrospective observational study. J Clin Gastroenterol. 2007;41:624–629. doi: 10.1097/01.mcg.0000225680.45088.01. [DOI] [PubMed] [Google Scholar]
- 79.Chen CH, Lin ST, Yang CC, et al. The accuracy of sonography in predicting steatosis and fibrosis in chronic hepatitis C. Dig Dis Sci. 2008;53:1699–1706. doi: 10.1007/s10620-007-0048-2. [DOI] [PubMed] [Google Scholar]
- 80.Lin SC, Heba E, Wolfson T, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345. doi: 10.1016/j.cgh.2014.11.027. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasso M, Audiere S, Kemgang A, et al. Liver Steatosis Assessed by Controlled Attenuation Parameter (CAP) Measured with the XL Probe of the FibroScan: A Pilot Study Assessing Diagnostic Accuracy. Ultrasound Med Biol. 2015 doi: 10.1016/j.ultrasmedbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 83.de Ledinghen V, Vergniol J, Foucher J, et al. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 84.Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910. doi: 10.1111/j.1478-3231.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 85.Chon YE, Jung KS, Kim SU, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109. doi: 10.1111/liv.12282. [DOI] [PubMed] [Google Scholar]
- 86.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–1476. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 87.Shen F, Zheng RD, Mi YQ, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol. 2014;20:4702–4711. doi: 10.3748/wjg.v20.i16.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637. doi: 10.1053/j.gastro.2015.11.048. e7. [DOI] [PubMed] [Google Scholar]
- 89.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887–1895. doi: 10.1002/hep.27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim D, Kim WR, Talwalkar JA, et al. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2015 doi: 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Runge JH, Bohte AE, Verheij J, et al. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging. 2014;39:283–290. doi: 10.1007/s00261-013-0063-z. [DOI] [PubMed] [Google Scholar]
- 96.Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol. 2010;22:643–650. doi: 10.1097/MEG.0b013e32832ca0cb. [DOI] [PubMed] [Google Scholar]
- 97.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 98.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 99.Fassio E, Alvarez E, Dominguez N, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 100.Evans CD, Oien KA, MacSween RN, et al. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? J Clin Pathol. 2002;55:689–692. doi: 10.1136/jcp.55.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int. 2008;28:650–658. doi: 10.1111/j.1478-3231.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 102.Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161–1179. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 105.Tsochatzis EA, Manousou P, Fede G, et al. Validating non-invasive markers of fibrosis: the need for a new histological reference standard. Gut. 2011;60:1442–1443. doi: 10.1136/gut.2010.229484. author reply 1443-4. [DOI] [PubMed] [Google Scholar]
- 106.Nierhoff J, Chavez Ortiz AA, Herrmann E, et al. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040–3053. doi: 10.1007/s00330-013-2927-6. [DOI] [PubMed] [Google Scholar]
- 107.Cui J, Philo L, Nguyen P, et al. Sitagliptin versus placebo in the treatment of nonalcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon JH, Lee JM, Woo HS, et al. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol. 2013;14:202–212. doi: 10.3348/kjr.2013.14.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 110.Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772–782. doi: 10.1148/radiol.14132000. [DOI] [PubMed] [Google Scholar]
- 111.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302. doi: 10.1053/j.gastro.2012.02.017. e4. [DOI] [PubMed] [Google Scholar]
- 112.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 113.Bota S, Sporea I, Sirli R, et al. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography--analysis of a cohort of 1,031 subjects. Eur J Radiol. 2014;83:268–272. doi: 10.1016/j.ejrad.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 114.Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375:1419–1420. doi: 10.1016/S0140-6736(09)62195-4. [DOI] [PubMed] [Google Scholar]
- 115.Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology. 2008;135:299–302. doi: 10.1053/j.gastro.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 117.Gradinaru-Tascau O, Sporea I, Bota S, et al. Does experience play a role in the ability to perform liver stiffness measurements by means of supersonic shear imaging (SSI)? Med Ultrason. 2013;15:180–183. doi: 10.11152/mu.2013.2066.153.ogt1is2. [DOI] [PubMed] [Google Scholar]
- 118.EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 119.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2015 doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 122.Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]