Abstract
Background and Aims
Conventional transarterial chemoembolization (cTACE) is used to treat patients with hepatocellular carcinoma (HCC). Radioembolization is a minimally invasive procedure that involves implantation of radioactive micron-sized particles loaded with yttrium-90 (Y90) inside the blood vessels that supply a tumor. We performed a randomized, phase 2 study to compare the effects of cTACE and Y90 radioembolization in patients with HCC.
Methods
From October 2009 through October 2015, we reviewed patients with HCC of all Barcelona Clinic Liver Cancer (BCLC) stages for eligibility. Of these, 179 patients with BCLC stages A or B met our enrollment criteria and were candidates for cTACE or Y90 therapy. Patients were randomly assigned to groups that received Y90 therapy (n=24, 50% Child-Pugh A) or cTACE (n=21, 71% Child-Pugh A). The primary outcome was time to progression (TTP), evaluated by intention to treat analysis. Secondary outcomes included safety, rate of response (based on tumor size and necrosis criteria), and KM survival time. We performed inverse probability of censoring weighting and competing risk analyses.
Results
Patients in the Y90 radioembolization group had significant longer median TTP (>26 months) than patients in the cTACE group (6.8 months) (P=.0012) (hazard ratio=0.122; 95% CI, 0.027–0.557; P=.007). This was confirmed by competing risk and inverse probability of censoring weighting analyses accounting for transplantation or death. A significantly greater proportion of patients in the cTACE group developed diarrhea (21%) than in the Y90 group (0%; P=.031) or hypoalbuminemia (58% in the cTACE group vs 4% in the Y90 group) (P<.001). Similar proportions of patients in each group had a response to therapy, marked by necrosis (74% in the cTACE group vs 87% in the Y90 group) (P=.433). Median survival time, censored to liver transplantation, was 17.7 months for the cTACE group (95% CI, 8.3–NC) vs 18.6 months for the Y90 group (95% CI, 7.4–32.5) (P=.99).
Conclusions
In a phase 2 study of patients with HCC of BCLC stages A or B, we found Y90 radioembolization to provide significantly longer TTP than cTACE. Y90 radioembolization provides better tumor control and could reduce dropout from transplant waitlists. ClinicalTrials.gov no. NCT00956930
Keywords: randomized trial, chemoembolization, radioembolization, liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancers, resulting in 740,000 deaths annually. It is the 2nd most common cause of cancer mortality worldwide.1 Patients with HCC often present beyond transplant or resection eligibility. Locoregional therapies (LRTs) are now applied for the full spectrum of patients not candidates for curative options. LRTs (ablation, conventional transarterial chemoembolization (cTACE), radioembolization with yttrium-90 microspheres [Y90]), appear in guidelines as treatment options for HCC.2–4 For early disease ([Barcelona Clinic Liver Cancer] BCLC A), ablation is recommended. However, when contraindications to ablation exist, the stage-migration concept advocates the next best line of therapy (in this case cTACE) be applied. For intermediate disease (BCLC B), cTACE is the standard of care, with a demonstrated survival benefit.5–7 Compared with cTACE, Y90 has shown increased time-to-progression8, good of quality-of-life9, a neoadjuvant role prior to resection.10–12 and high antitumoral activity in patients with portal vein invasion13. Consequently, experts have strongly advocated that Y90 be studied in randomized trials using cTACE as the control arm.8, 14, 15
The purpose of this study was to compare cTACE and Y90 in a prospective, randomized phase 2 setting for the treatment of unresectable, unablatable HCC. As recommended by guidelines,16 the primary endpoint was time to progression (TTP). Secondary endpoints included safety, response rate, and overall survival. Our hypothesis was that Y90 would prolong TTP when compared to cTACE.8
METHODS
The study was an investigator initiated, open label, single-center phase 2 Prospective Randomized study of chEmoeMbolization versus radIoEmbolization for the tReatment of hEpatocellular carcinoma (PREMIERE). The study was institutional review board approved, complied with the Health Insurance Portability and Accountability Act, and registered (NCT00956930). All BCLC stage HCC patients were reviewed at multidisciplinary tumor board (transplant surgery, hepatology, medical oncology, interventional radiology) between October 2009 and October 2015. After excluding ineligible patients [portal vein invasion or infiltrative disease (n=69), hyperbilirubinemia (n=86), increased creatinine (n=28), other abnormal labs (n=20), HIV (n=15), TIPS (n=27), metastatic disease (n=36), other health problems precluding treatment (n=22), other treatment recommendations (n=44), psych/social issues (n=49), previous liver or systemic treatment (n=71), underwent resection (n=155) or ablation (n=60)], 179 BCLC A/B patients (eligible for cTACE or Y90) were offered non-interventional studies, cTACE, Y90, or a two-arm randomized clinical trial comparing cTACE to Y90 (Figure 1. Consort Diagram). Of the 179 patients, 43 declined to participate in research, 29 selected other clinical trials, 49 requested Y90, and 13 requested cTACE. 45 patients agreed to be randomized. After discussion of the protocol and signed informed consent, they were prospectively randomized 1:1 conventional chemoembolization (cTACE; control arm) or radioembolization (Y90; test arm). BCLC A patients were considered ineligible for ablation/resection due to lesion size/multifocality/location or portal hypertension/liver function. All BCLC B patients deemed appropriate for standard of care cTACE were also deemed eligible for Y90. The ultimate intent of treatment for these patients was liver transplantation with candidacy evaluated by transplant surgery.17 No donor organs were obtained from executed prisoners or other institutionalized persons. The study was halted early due to slow accrual and competing studies. The last patient was enrolled July 14, 2015, with complete imaging (TTP), transplant and survival data updated July 15, 2016. All authors had access to the study data and reviewed and approved the final manuscript.
Figure 1.
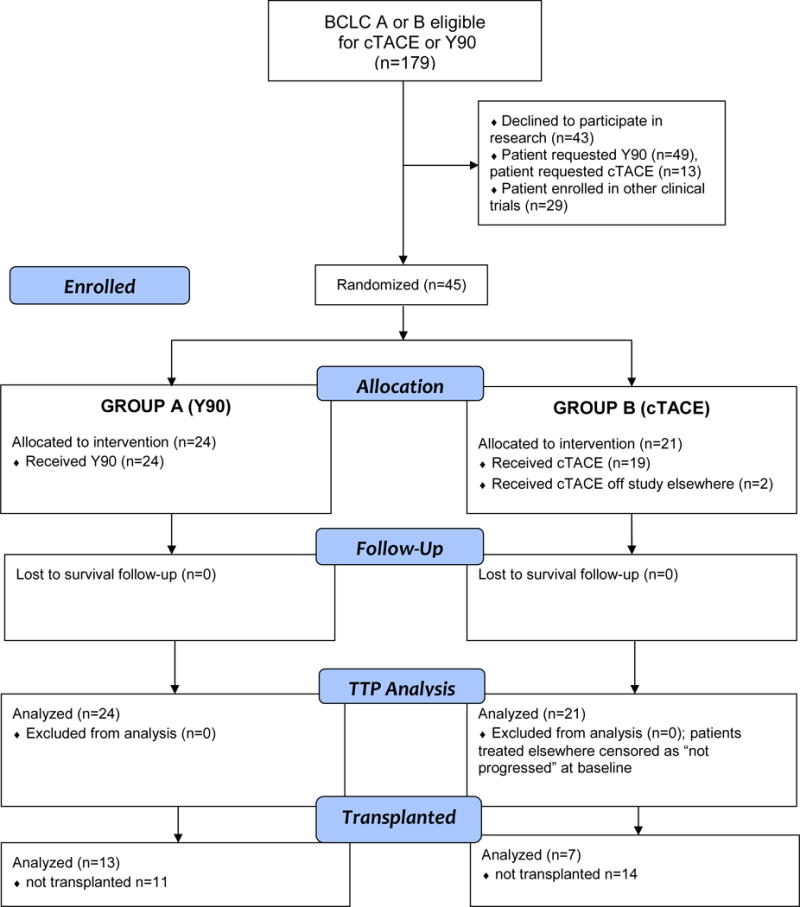
CONSORT Study Flowchart.
Study eligibility
In brief, inclusion criteria were image/biopsy-proven HCC by guidelines,4 unablatable/unresectable disease, no vascular invasion, Child-Pugh A/B, bilirubin ≤ 2.0 mg/dl and AST/ALT ≤ 5x upper limit of normal. Exclusion criteria were infiltrative/bulk disease (≥70% tumor burden), ≥50% tumor burden with albumin <3 g/dL, cardiac comorbidities, major surgery within past 4 weeks or active infection.
Evaluation and Staging
Patient demographics, risk factors, etiology, performance status, staging (BCLC), albumin-bilirubin score and Child-Pugh class were recorded.
Treatment Arms
cTACE
Chemoembolization was performed with 75 mg/m2 (max: 150 mg) dosing. Drug/lipiodol combination was followed by embolic microspheres (Embospheres, Merit Medical Systems). The percentage of drug administered was recorded, with confirmation of lipiodol deposition by non-contrast CT. Patients were admitted for 24–48 hr observation, and discharged with antibiotics/analgesics/antiemetics as needed.
Y90
Angiography and technetium-99m scintigraphy were used to estimate lung shunting, identify extrahepatic perfusion and perform coil embolization if necessary. Glass microspheres (TheraSphere®, BTG International) were used at 120 Gy dose, with treatment on an outpatient basis.18–20
Outcome Variables
All clinical and laboratory adverse events (CTCAE v4.0) were recorded, with censoring at transplantation.21
Response rates were determined by contrast-enhanced MRI (World Health Organization [WHO] bidimensional and three-dimensional European Association for the Study of the Liver [EASL]) and chest CTs.22 Since lipiodol is not visible by MRI, this was selected as the imaging modality to ensure treatment concealment. Scans were reviewed in a blinded manner by two board-certified radiologists. Third reader adjudication was performed when necessary.
TTP and overall survival (OS) analyses were calculated from day of randomization by Kaplan-Meier (KM) using intention to treat (ITT). Progression was defined by: 1) WHO: 25% increase in bidimensional cross product, 2) EASL: 25% increase in arterial enhancement, 3) portal vein tumor thrombus development, 4) index lesion: lesions requiring retreatment due to worsening enhancement, 5) new lesions or extrahepatic metastases. Small lesions difficult to characterize eventually declared as HCC were retrospectively adjudicated to the earliest date of detection. Patients were censored on the day of last imaging for all TTP analyses. OS with censoring to liver transplantation was assessed.
Statistical Plan
Sample size was based on published literature and pilot data. Our estimated effect size was 6 months, assuming exponential survival, a median TTP of 10 months for cTACE and 16 months for Y90 (HR=0.625). Using a two-tailed alpha of 0.10, a sample size of 55 per group would result in 80% power to detect this effect size. Ten percent was added to account for patient attrition, leading to an overall sample size of 124 patients (62 per group). Overall survival and TTP were compared using KM and log-rank test. The hazard ratio and 95% CI was estimated using proportional hazards regression. Inverse probability of censoring weighting (IPCW) was applied in the analysis of TTP using methods described for censoring by Hernan.23 This method assigns higher weights to the outcomes of patients who were not censored, where censoring includes transplanted patients. The covariates used in this analysis included baseline sex, tumor distribution, number of lesions, largest tumor size, alpha-fetoprotein, hepatitis C status, BCLC and Child-Pugh.24–26 Cumulative incidence of progression with transplant/death as competing risks was also compared using Gray’s test.27 Conditional power was calculated using Proschan’s method,28 as applied by Jitlal et al, a statistical methodology permitting interpretation of data from trials terminated early.29 All analyses were performed using IBM SPSS Statistics v23.0 (Armonk, NY), STATA v14.0 (StataCorp, College Station, Tx) and SAS/STAT software (SAS Institute Inc., SAS OnlineDoc® 9.4, Cary, NC: SAS Institute Inc., 2012); p<0.05 was considered significant.
RESULTS
Baseline Characteristics
The groups were well-matched. Y90 patients trended towards more portal hypertension (P=.051) and baseline bilirubin (1.3 vs 0.9, P=.058) (Table 1). Over 50% of all patients exhibited solitary lesions. No patient exhibited cancer-related symptoms or weight-loss at presentation. Supplemental Table 1 (online only) lists baseline distribution of covariates used in the IPCW analysis, variables that may affect progression and transplantation. These covariates demonstrate no significant difference between treatment arms.
Table 1.
Baseline Characteristics
| Characteristic | cTACE (n=21) | Y90 (n=24) | P value |
|---|---|---|---|
| Demographics | |||
| Age (years)* | 64 (62–70) | 62 (58–65) | 0.45 |
| Gender | |||
| Male | 16 (76) | 17 (71) | 1.00 |
| Female | 5 (24) | 7 (29) | |
| Ethnicity | |||
| Caucasian | 16 (76) | 19 (79) | 1.00 |
| African American | 3 (14) | 3 (13) | |
| Hispanic | 1 (5) | 1 (4) | |
| Asian | 1 (5) | 1 (4) | |
| Risk Factors | |||
| Etiology | |||
| Alcohol | 1 (5) | 4 (17) | 0.73 |
| HCV | 10 (48) | 12 (50) | |
| HCV + Alcohol | 2 (10) | 1 (4) | |
| HCV + Hemachromatosis | 1 (5) | 0 (0) | |
| HBV | 2 (10) | 3 (13) | |
| NASH | 1 (5) | 1 (4) | |
| Cryptogenic | 1 (5) | 0 (0) | |
| Hemachromatosis | 0 (0) | 1 (4) | |
| Unknown | 3 (14) | 1 (4) | |
| Imaging Cirrhosis | |||
| Present | 20 (95) | 24 (100) | 0.47 |
| Absent | 1 (5) | 0 (0) | |
| Tumor Burden <25% | 21 (100) | 24 (100) | – |
| Distribution | |||
| Unilobar | 14 (67) | 17 (71) | 1.00 |
| Bilobar | 7 (33) | 7 (29) | |
| No. of lesions | |||
| Solitary | 11 (52) | 13 (54) | 1.00 |
| Multifocal | 10 (48) | 11 (46) | |
| Largest Tumor Size (cm) | |||
| Median (IQR) | 2.6 (0.7) | 3.0 (1.2) | 0.18 |
| Mean (95% CI) | 3.0 (2.3–3.6) | 3.2 (2.7–3.7) | 0.55 |
| AFP (ng/mL) | |||
| <200 | 19 (90) | 21 (88) | 1.00 |
| ≥200 | 2 (10) | 3 (12) | |
| Method of Diagnosis | |||
| Biopsy | 8 (38) | 7 (29) | 0.55 |
| Imaging | 13 (62) | 17 (71) | |
| Liver Function | |||
| Bilirubin (mg/dL)* | 0.9 (0.8–1.5) | 1.3 (1.2–1.7) | 0.058 |
| Albumin (g/dL)* | 3.2 (2.9–3.4) | 3.1 (2.7–3.3) | 0.58 |
| ALBI Grade | |||
| 1 | 1 (5) | 0 (0) | 0.296 |
| 2 | 17 (81) | 17 (71) | |
| 3 | 3 (14) | 7 (29) | |
| Portal hypertension | |||
| Present | 11 (52) | 20 (83) | 0.051 |
| Absent | 10 (48) | 4 (17) | |
| Stage | |||
| BCLC | |||
| A | 17 (81) | 18 (75) | 0.73 |
| B | 4 (19) | 6 (25) | |
| Child-Pugh (at randomization) | |||
| A | 15 (71) | 12 (50) | 0.30 |
| B7 | 3 (14) | 6 (25) | |
| B8 | 2 (10) | 3 (12.5) | |
| B9 | 1 (5) | 3 (12.5) | |
| Child-Pugh (day of first treatment) | |||
| A | 16 (76) | 10 (42) | 0.085 |
| B7 | 3 (14) | 8 (33) | |
| B8 | 1 (5) | 4 (17) | |
| B9 | 1 (5) | 1 (4) | |
| C10 | 0 (0) | 1 (4) |
Note. Values expressed as median and 95% confidence intervals. BCLC, Barcelona Clinic Liver Cancer
Treatment and Dosimetry
All 24 Y90 patients were treated successfully. Two of the 21 randomized cTACE patients received treatment off study but were included in the ITT analysis.
Y90
Selective Y90 was performed in 17/24 patients; 7 were lobar treatments. Median dose was 126 Gy (95% CI, 124–176) to a median 405 cc (95% CI, 347–623) treatment volume. Median residual activity was 1.9% (95% CI, 1.6–3.5). Median lung shunt fraction was 5.1% (95% CI, 4.4–7.6) with cumulative lung dose of 4 Gy (95% CI, 2.8–6.6). All treatments were outpatient.
cTACE
Selective chemoembolization was performed in 16/19 patients; 3 were lobar infusions. On average, 52% of the total drug-lipiodol mixture was infused. Median number of days hospitalized at first procedure and cumulatively were 1 (95% CI, 0.9–2.2) and 1.5 (95% CI, 1.3–2.9), respectively.
cTACE vs Y90
Because of planning angiography, days from randomization to treatment was longer with Y90 compared to cTACE (18 days 95% CI, 15–26 vs 8 days, 95% CI, 8–11, P<.0001, respectively). cTACE patients trended towards more treatments at 1.7±1.1 (95% CI, 1.2–2.2) compared with 1.3±0.5 (95% CI, 1.0–1.5) Y90 (P=.098). One cTACE patient crossed over to Y90 after 13.8 months due to continued progression after 3 cTACE treatments.
Clinical and Laboratory Toxicities
Supplemental Table 2 (online only) summarizes toxicities. 30-day mortality was 0%. Vascular complications (n=2) included common femoral artery pseudoaneurysm, one in each group (p=1.0). There was a trend for more fatigue with Y90 (P=.08). The cTACE groups experienced more diarrhea (P=.031) and hypoalbuminemia (P<.001).
Delayed (>30 days) grade 3+ toxicities occurred in 3 cTACE patients: hyperbilirubinemia (day 49), abdominal pain from progression (day 183) and sepsis (day 309, early after 3rd cTACE cycle). Delayed grade 3+ toxicities occurred in 4 Y90 patients: ascites (day 68, 81, 179) and bacterial peritonitis (day 54).
Follow-up and Censoring
Patients were followed to last imaging date and for survival. For all 45 patients, the median length of follow-up was 17.2 months (range 1.4–62.1 months). For TACE, median (range) follow-up was 15.7 (1.4–62.1) months. For Y90, median (range) follow-up was 21.0 (2.3–59.6) months. For TACE, there were 7 transplants at a median (range) of 7.6 (3.0–17.3) months. For Y90, there were 13 transplants at a median (range) of 8.8 (4.0–15.3) months. For TTP, there were 12 progressions (TACE: 10, Y90: 2) and 33 censored (TACE: 11, Y90: 22). Of the 11 censored in TACE, 6 (54.6%) were transplanted after the last imaging for progression and were censored in the TTP analysis. Of the 22 censored in Y90, 12 (54.6%) were transplanted after the last imaging for progression and were censored in the TTP analysis. Of the 12 progressions, there was one patient in each group who had a transplant after the progression. All transplants occurred after the time used in the TTP analysis. The data for IPCW analysis may be summarized in 4 groups as: 1) censored, not subsequently transplanted: TACE: 5 (24%), Y90: 10 (42%), 2) censored, subsequently transplanted: TACE: 6 (29%), Y90: 12 (50%), 3) progressed, not subsequently transplanted: TACE: 9 (43%), Y90: 1 (4%), and 4) progressed, subsequently transplanted: TACE: 1 (5%), Y90: 1 (4%).
Time-to-progression
Figure 2 compares TTP. The median TTP (primary endpoint) was significantly longer in the Y90 group: 6.8 months for cTACE vs not reached for Y90 (>26 months), P=.0012 [HR: 0.122, 95% CI, 0.027–0.557, P=.007]. By competing risk analysis, Y90 again demonstrated significantly reduced hazard of progression compared to cTACE (subdistribution HR=0.13, 95% CI, 0.03–0.57; P=.006) with transplant/death as competing events. By IPCW analysis, risk reduction of progression in the Y90 group was more pronounced (HR: 0.071, 95% CI, 0.008–0.645, P=.019).
Figure 2.
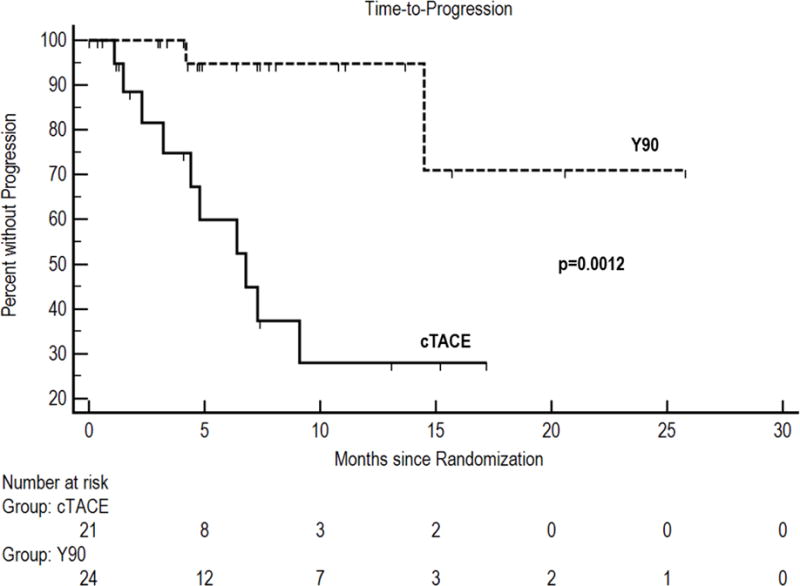
Time-to-progression.
Supplemental Table 3 (online only) summarizes pattern of progression, demonstrating that Y90 exhibited better local tumor control by enhancement criteria and new lesions. The median time-to-new hepatic lesions was 7.3 months for cTACE versus not reached for Y90 (P=.017). Two cTACE versus zero Y90 patients developed extrahepatic osseous progression (P=.16). Median time-to-extrahepatic spread was not reached.
Imaging Outcomes
Supplemental Table 4 (online only) presents imaging response. Primary index lesions (n=43) were defined in 184 reviewed studies (mean, 4.3 scans/patient), with follow-up imaging available in 42 of 43 patients (98%). Figure 3 represents a waterfall plot of maximal response. WHO response was 12/19 (63%) for cTACE versus 12/23 (52%) for Y90 (P=.542), with comparable median times to PR by group (7.3 months (95% CI, 3.9, 12.6) after cTACE vs 7.6 months (95% CI, 4.5–11.3) after Y90, P=.85, log-rank). EASL response was 14/19 (74%) for cTACE versus 20/23 (87%) for Y90 (P=.433) with comparable median times to PR/CR of 1.7 mo after Y90 (95% CI, 1.6–3.4) vs 1.4 mo after cTACE (95% CI, 1.3–4.9) (P=.62, log-rank).
Figure 3.
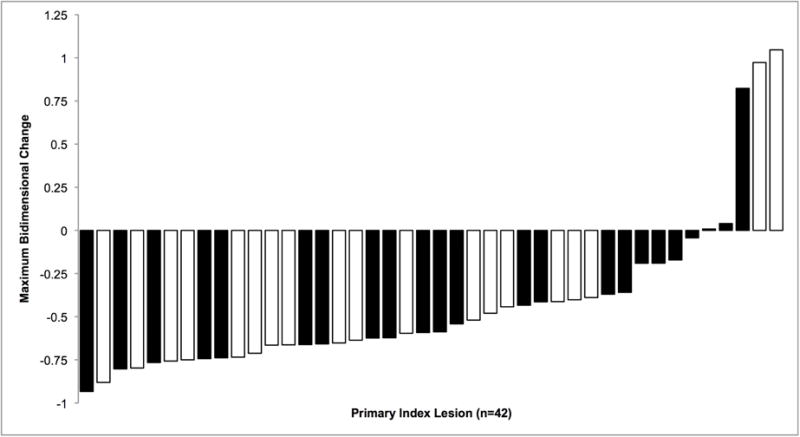
Waterfall plot of maximum size change for WHO measurements in (n=42) primary index lesions after Y90 (black bars) versus cTACE (white bars). Negative values represent reductions in tumor size with ≥50% reduction (−) defined as partial response and >25% increase (+) in size progressive disease.
Bridge to Transplant: Subgroup Analysis in Listed Patients
All transplanted patients were BCLC A at baseline; 18 Y90 versus 17 cTACE patients were within Milan criteria at baseline. The rates of transplantation in listed patients were 87% (13/15) after Y90 versus 70% (7/10) after cTACE. For cTACE, there were 7 transplants at a median (range) of 7.6 (3.0–17.3) months. For Y90, there were 13 transplants at a median (range) of 8.8 (4.0–15.3) months.
TTP Analysis in Non-transplanted Patients
Twenty-five patients did not receive liver transplant. TTP remained significantly longer with Y90 (median not reached @ 26 months, 95% CI, 14.5-NC) versus cTACE (4.8 mo, 95% CI, 1.5–7.3, P=.0002) in non-transplanted patients.
Overall Survival
Figure 4 presents KM curves (censored to liver transplantation) demonstrating median 17.7 months (95% CI, 8.3-NC) and 18.6 months (95% CI, 7.4–32.5) OS for cTACE and Y90, respectively (P=.99).
Figure 4.
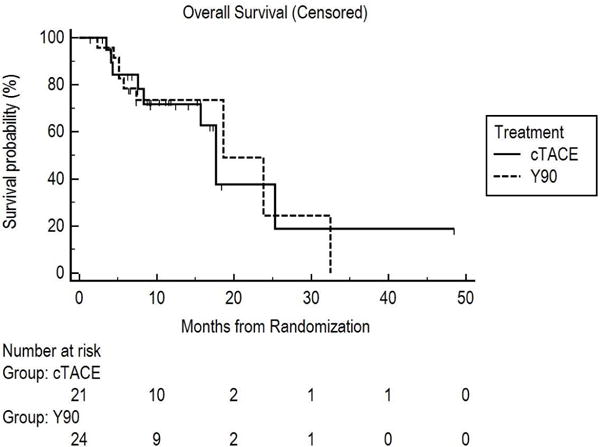
Overall survival from randomization censored to liver transplantation.
Conditional power
Overall TTP was compared using KM and log-rank test. The hazard ratio and 95% CI was estimated using proportional hazards regression. Due to slow accrual, the data safety monitoring committee recommended we close the study at 45 patients. The original power calculation determined that 55 patients per arm (110 total) were needed to have 80% power to detect a median 10 month TTP for cTACE and 16 months for Y90 (HR=0.625) (two-tailed type I error rate: 10%), with an additional 10% to account for attrition (total planned sample size of 124). There was no provision for a formal interim analysis. Since exponential survival was assumed, the power calculation expected that all 110 patients would be followed to progression events. At study halting, the actual observed median TTP after 45 patients was 6.8 months for cTACE vs not reached for Y90 (>26 months), P=.0012 [HR: 0.122, 95% CI, 0.027–0.557, P=.007]. There were 12 progression events (10 cTACE, 2 Y90). Conditional power was calculated using Proschan’s method28 as applied by Jitlal et al.29 The information fraction was calculated as the observed number of events divided by the protocol expected number of events, or 12/110=0.11. As described in the original study design, a 0.625 HR applied to both past and future data, the probability of achieving statistical significance at the end of 110 patients (conditional power) was 80%; this is the original protocol unconditional power. However, when the observed HR of 0.122 was used for accrued data but, conservatively, the original protocol HR of 0.625 for future data, the conditional power was 96.8%. Therefore, cautiously assuming that there is attenuation of the current HR=0.122 treatment effect observed in the 45 enrolled patients to HR=0.625 for an additional hypothetical 79 patients, there is 96.8% chance that statistical significance in favor of Y90 would still be declared.
DISCUSSION
Y90 prolongs TTP when compared to cTACE for early-intermediate stage HCC, suggesting more complete treatment of targeted lesions and tumor control. Longer TTP did not translate to increased OS, suggesting local control (as an isolated variable) being insufficient for survival improvement in cirrhotic patients with competing risks of death. However, improved tumor control could potentially lower dropout rate from transplant listing. With the exception of more diarrhea and hypoalbuminemia in the cTACE group, adverse events were similar between groups and compared favorably to previous retrospective reports.8, 30
Response Rate
Baseline lesion size was relatively small and hence, PRs and CRs were similar between groups. This is consistent with prior reports and does emphasize differences in assessing response with Y90 compared with cTACE when enhancement is used. Investigators have highlighted the pitfall of using early enhancement pattern in assessing response for Y90 in large lesions.31 cTACE is embolic and hence, the vascularity that permits enhancement during cross sectional imaging (arterial hypervascularity) is occluded by the drug/embolic. Y90, on the other hand, is a microembolic therapy that does not occlude the vasculature. The results of our study herein suggest that the time-to-response by enhancement criteria for small lesions are equivalent.
Time to progression, overall survival
Despite longer time from randomization to Y90 treatment, treatment failure was lower with Y90, suggesting improved cytotoxicity. Technically, cTACE and Y90 have become more similar since the adoption of cone-beam CT and microcatheter technologies, allowing improved intra-procedural visualization of tumor coverage. While coil embolization prior to radioembolization was initially common, contemporary practices require coiling infrequently.32 Therefore, we attribute TTP improvement to mechanistic differences in antitumor activity rather than technique; both groups were predominantly treated with selective techniques. Survival was lower than expected in both groups, explained by the 29%/50% Child-Pugh B advanced cirrhotics included in this clinical trial (at randomization).
Curative Transplantation
Survival is inherently linked to transplantation.17 Progression of HCC directly impacts transplant eligibility through waiting list dropout. Therefore, a locoregional therapy prolonging TTP should reduce waitlist dropout and provide higher rates of successful bridging to transplantation. This theory is supported by our findings, where patients randomized to Y90 exhibited better tumor control and listed Y90 patients received transplantation at a rate of 87%. Moreover, some centers are hesitant to perform ablation in transplant candidates due to the risk of tract seeding. In these settings, intra-arterial therapy, such as Y90, eliminates the risk of tract seeding and potentially offers a higher chance for transplantation.
Study rationale
Chemoembolization is considered the standard of care for BCLC B patients based on improved survival compared to best supportive care.5–7 Also, the applicability of cTACE is acknowledged for BCLC A patients ineligible for recommended treatments (stage migration). We followed these principles during PREMIERE, recognizing we could ethically randomize unablatable BCLC A and BCLC B patients eligible for cTACE.
Change in local practice
Our findings have motivated an institutional change in local practice. Patients bridged to transplantation now receive Y90 based on our finding of lower progression and potentially reduced dropout. Therefore, Y90 may positively influence survival by increasing the rate of curative transplant. Currently, we reserve cTACE when combination therapy with radiofrequency ablation is planned, lipiodol uptake will assist in HCC diagnosis, non-target perfusion precludes radioembolization, or when transplantation is imminent (thereby mitigating handling a radiated specimen).
Competing risk analysis
In the presence of competing risks of death and liver transplantation, Kaplan-Meier may not sufficiently estimate TTP, as either of these two events precludes recording of subsequent cancer progression. A sensitivity analysis was performed, where TTP was also analyzed using Gray’s test for comparing cumulative incidence curves, with liver transplant as the competing event, generating a cause-specific hazard ratio for TTP.27 By competing risk analysis, Y90 exhibited longer TTP compared with cTACE.
Inverse probability of censoring weighting (IPCW) analysis
To address the potential issue of dependent censoring by therapy, we applied IPCW. For this study, IPCW assigns increasing weights to patients the longer they remain on follow-up. As they proceed through follow-up, the probability of remaining on follow-up decreases. Using logistic regression, IPCW assigns the inverse of these probabilities, which are a function of baseline characteristics, to each month of follow-up, thereby increasing the weight of the months representing longer follow-up. If there is an imbalance between groups in the rate of informative or non-informative censoring, then the group with more censoring would be weighted less in this analysis, thereby equalizing the effect of censoring in the analysis. In our study, IPCW confirmed longer TTP in the Y90 group.
Strengths, limitations
Strengths include the randomized nature, comprehensive imaging review, and real-world clinically relevant patient flow of unablatable BCLC A and B patients intended for standard of care cTACE, but randomized to test arm Y90. The study also demonstrated longer TTP with Y90 despite longer time to initial treatment compared to the cTACE group. Limitations include required censoring of imaging/survival to transplant. However, given the increasing complexity of this disease, it is recognized that patients only rarely receive one HCC treatment. Patients do not move along the BCLC algorithm in a linear A→B→C→D fashion, but rather cycle from stage-to-stage in multiple directions as they receive treatment. Censoring to liver transplant is the correct statistical method to determine TTP. Survival, in such cases, is confounded by crossover to alternate therapies, an increasingly challenging endpoint when studying BCLC A and B.33 Therefore, TTP was used as a surrogate endpoint to extract meaningful data most closely linked to the intervention. Also, since transplantation and death precluded future observation of progression, competing risk and IPCW analyses were performed confirming the findings favoring Y90 over cTACE. While our enrollment rate of 25% was encouraging (45/179), the study was halted at the recommendation of our cancer center given accrual difficulties common to interventional studies. Historically, these challenges arise due to: 1) referral patterns for specific therapies or studies (43 patients declined the research, 29 selected other trials, 49 requested Y90, 13 requested cTACE), 2) the rapid improvement of technology and clinical science obviating the initial trial research questions due to updates in standard of care, 3) difficulties of patient compliance with follow-up and imaging as part of a strict protocol and, 4) compared to other cancers, the low incidence of HCC in the United States. To overcome these limitations, we advocate: 1) multidisciplinary tumor boards to review eligibility for studies, 2) shorter trial activation times and increased institutional support, 3) requisite imaging quality and frequency of scans needed for TTP analyses, 4) inclusion of neighboring hospitals referring patients for study consideration, and 5) use of composite data pooling studies. Finally, we acknowledge the slightly lower than expected survival. This is explained by the percentage of Child-Pugh B patients in both groups at randomization.
Despite accrual issues, post-hoc analysis (Proschan’s method) suggested that, with a 5.1-fold HR increase (0.122 to 0.625) associated with Y90 for the 79 remaining hypothetical patients (for complete target enrollment), there would be a 96.8% chance of a significant result at the end of the study. When controlling for dependent censoring between the two treatment arms with IPCW analysis, we find that the TTP benefit with Y90 is maintained, confirmed with competing risk analysis. While the relatively low sample size is acknowledged, the seminal studies establishing cTACE as the standard of care were also limited in sample size, single center, and enrolled mostly Child-Pugh A patients. While our TTP results favoring Y90 are in line with other uncontrolled retrospective reports in patients with compromised liver functions, our study validates such findings with prospective randomized level I evidence.34–38
CONCLUSION
Intra-arterial embolotherapy is safe and has high antitumor activity. This study represents the first, real-world, comparative effectiveness analysis of Y90 and cTACE by ITT. In light of competing risks of liver transplantation and death, Y90 significantly increased TTP compared with cTACE in a randomized phase 2 setting, translating to significantly improved local tumor control that could reduce dropout from transplant waitlists.
Supplementary Material
Acknowledgments
The authors thank Carlene del Castillo, Karen Marshall, Krystina Salzig, Melissa Williams and Jenny Karp for their commitment to patient care and dedication to clinical research.
Role of Funding: This study was supported in part by NIH grant CA126809. ACG is a Medical Scientist Training Program student (T32GM008152) with support for research provided by an Allied Scientist grant from the SIR Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: RJL, LK, RS serve as advisors to BTG. None of the other authors report a conflict of interest.
Clinical Trial Number: NCT00956930
Author Contributions:
study concept and design: Riad Salem, Andrew C Gordon, Mary F Mulcahy, Samdeep Mouli, Alfred Rademaker, Daniel Ganger, Laura Kulik, Robert J Lewandowski
acquisition of data: Riad Salem, Andrew C Gordon, Ryan Hickey, Joseph Kallini, Ahmed Gabr, Frank Miller, Vahid Yaghmai, Daniel Ganger, Laura Kulik
analysis and interpretation of data: Riad Salem, Andrew C Gordon, Joseph Kallini, Ahmed Gabr, Mary F Mulcahy, Talia Baker, Michael Abecassis, Frank Miller, Vahid Yaghmai, Kent Sato, Kush Desai, Bartley Thornburg, Al B Benson, Samdeep Mouli, Alfred Rademaker, Daniel Ganger, Laura Kulik, Robert J Lewandowski
drafting of the manuscript: Riad Salem, Andrew C Gordon, Samdeep Mouli, Alfred Rademaker, Robert J Lewandowski
critical revision of the manuscript for important intellectual content: Riad Salem, Andrew C Gordon, Ryan Hickey, Joseph Kallini, Ahmed Gabr, Mary F Mulcahy, Talia Baker, Michael Abecassis, Frank Miller, Vahid Yaghmai, Kent Sato, Kush Desai, Bartley Thornburg, Al B Benson, Samdeep Mouli, Alfred Rademaker, Daniel Ganger, Laura Kulik, Robert J Lewandowski
statistical analysis: Riad Salem, Andrew C Gordon, Samdeep Mouli, Alfred Rademaker
obtained funding: Riad Salem, Andrew C Gordon
administrative, technical, or material support: Riad Salem, Andrew C Gordon, Joseph Kallini, Ahmed Gabr, Samdeep Mouli, Alfred Rademaker
study supervision: Riad Salem, Andrew C Gordon, Joseph Kallini, Ahmed Gabr, Frank Miller, Vahid Yaghmai, Samdeep Mouli, Alfred Rademaker, Daniel Ganger, Laura Kulik, Robert J Lewandowski
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Hepatobiliary Cancers. 2016 [Google Scholar]
- 3.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salem R, Miller FH, Yaghmai V, et al. Response assessment methodologies in hepatocellular carcinoma: complexities in the era of local and systemic treatments. J Hepatol. 2013;58:1260–2. doi: 10.1016/j.jhep.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Gaba RC, Lewandowski RJ, Kulik LM, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–96. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 11.Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029–36. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabr A, Kallini JR, Gates VL, et al. Same-day 90Y radioembolization: implementing a new treatment paradigm. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3438-x. [DOI] [PubMed] [Google Scholar]
- 13.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 14.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD004787.pub2. Cd004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray CE, Jr, Haskal ZJ, Geschwind JF, et al. The use of transarterial chemoembolization in the treatment of unresectable hepatocellular carcinoma: a response to the Cochrane Collaboration review of 2011. J Vasc Interv Radiol. 2011;22:1693–6. doi: 10.1016/j.jvir.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 17.Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–82. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 18.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–39. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 19.Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S41–55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17:1571–93. doi: 10.1097/01.RVI.0000236744.34720.73. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE) 2009 [Google Scholar]
- 22.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. Jama. 2010;303:1062–9. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Alemao E, Rajagopalan S, Yang S, et al. Inverse probability weighting to control for censoring in a post hoc analysis of quality-adjusted survival data from a clinical trial of temsirolimus for renal cell carcinoma. J Med Econ. 2011;14:245–52. doi: 10.3111/13696998.2011.566296. [DOI] [PubMed] [Google Scholar]
- 26.Willems S, Schat A, van Noorden MS, et al. Correcting for dependent censoring in routine outcome monitoring data by applying the inverse probability censoring weighted estimator. Stat Methods Med Res. 2016 doi: 10.1177/0962280216628900. [DOI] [PubMed] [Google Scholar]
- 27.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988:1141–1154. [Google Scholar]
- 28.Proschan MA, Lan KG, Wittes JT. Statistical monitoring of clinical trials: a unified approach. Springer Science & Business Media; 2006. [Google Scholar]
- 29.Jitlal M, Khan I, Lee SM, et al. Stopping clinical trials early for futility: retrospective analysis of several randomised clinical studies. Br J Cancer. 2012;107:910–7. doi: 10.1038/bjc.2012.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. Journal of Vascular and Interventional Radiology. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology. 2015;62:1111–21. doi: 10.1002/hep.27915. [DOI] [PubMed] [Google Scholar]
- 32.Hamoui N, Minocha J, Memon K, et al. Prophylactic embolization of the gastroduodenal and right gastric arteries is not routinely necessary before radioembolization with glass microspheres. J Vasc Interv Radiol. 2013;24:1743–5. doi: 10.1016/j.jvir.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Buyse M, Sargent DJ, Saad ED. Survival is not a good outcome for randomized trials with effective subsequent therapies. Journal of Clinical Oncology. 2011;29:4719–4720. doi: 10.1200/JCO.2011.38.4206. [DOI] [PubMed] [Google Scholar]
- 34.Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–65. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. ASCO Annual Meeting Proceedings; 2012. [Google Scholar]
- 36.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. European Journal of Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201. doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 38.Padia SA, Kwan SW, Roudsari B, et al. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. Journal of Vascular and Interventional Radiology. 2014;25:1067–1073. doi: 10.1016/j.jvir.2014.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


