Abstract
Learning the temporal relationship between a warning cue (conditioned stimulus; CS) and aversive threat (unconditioned stimulus; UCS) is an important aspect of Pavlovian conditioning. Although prior functional magnetic resonance imaging (fMRI) research has identified brain regions that support Pavlovian conditioning, it remains unclear whether these regions support time-related processes important for this type of associative learning. Elucidating the neural substrates of temporal conditioning is important for a complete understanding of the Pavlovian conditioning process. Therefore, the present study used a temporal Pavlovian conditioning procedure to investigate brain activity that mediates the formation of temporal associations. During fMRI, twenty-three healthy volunteers completed a temporal conditioning procedure and a control task that does not support conditioning. Specifically, during the temporal conditioning procedure, the UCS was presented at fixed intervals (ITI: 20 s) while in the control condition the UCS was presented at random intervals (Average ITI: 20 s, ITI Range: 6-34 s). We observed greater skin conductance responses and expectancy of the UCS during fixed (i.e., temporal conditioning) relative to random (i.e., control procedure) interval trials. These findings demonstrate fixed trials support temporal conditioning, while random trials do not. During fixed interval trials, greater conditioned fMRI signal responses were observed within dorsolateral prefrontal cortex, inferior parietal lobule, inferior and middle temporal cortex, hippocampus, and amygdala. The current findings suggest these brain regions constitute a neural circuit that encodes the temporal information necessary for Pavlovian fear conditioning.
Keywords: Amygdala, Hippocampus, PFC, Timing, Fear, fMRI
Temporal information is important for successful Pavlovian conditioning. Specifically, while the formation of an association between a warning cue and threat is critical for determining a threat will occur, learning the relative timing of the threat in relation to the warning cue is necessary for determining when a threat will occur. Thus, Pavlovian conditioning is dependent on learning the warning cues that predict threat as well as the timing of threat itself (Balsam, Drew, and Gallistel, 2010; Balsam, Fairhurst, and Gallistel, 2006; Balsam and Gallistel, 2009). During traditional Pavlovian fear conditioning procedures, an initially innocuous cue (i.e., a conditioned stimulus; CS) is paired with an aversive threat (i.e., an unconditioned stimulus; UCS). Therefore, the CS serves as a warning cue that predicts the UCS (i.e., the threat). The repeated pairing of the CS and UCS results in the formation of a CS-UCS association, evidenced by the expression of a conditioned response (CR). However, one must also learn the timing of the UCS to appropriately time CR expression (Davis, Schlesinger, and Sorenson, 1989; Gallistel and Gibbon, 2000; Gibbon, Malapani, Dale, and Gallistel, 1997; Kalmbach, Ohyama, and Mauk, 2010; LaBarbera and Church, 1974). For example, timing disruptions can result in a CR that is too early or too late (Papka, Ivry, and Woodruff-Pak, 1995; Perrett, Ruiz, and Mauk, 1993). Thus, the temporal relationship between the CS and the UCS is a critical component of fear learning and memory. Although prior human neuroimaging research has identified neural circuitry that supports Pavlovian fear conditioning, the mechanism by which temporal information is encoded within this circuitry remains unclear. Thus, determining the neural substrates of temporal conditioning would provide valuable insight into processes that mediate successful associative learning.
Prior research has identified neural circuitry that supports important aspects of Pavlovian fear conditioning. In particular, both human and animal studies have shown that the amygdala is at the center of a neural circuit that mediates Pavlovian fear conditioning (Cheng, Knight, Smith, Stein, and Helmstetter, 2003; Helmstetter, 1992; Knight, Nguyen, and Bandettini, 2005; LaBar, Gatenby, Gore, LeDoux, and Phelps, 1998; LeDoux, Cicchetti, Xagoraris, and Romanski, 1990) (however, see (Fullana, Harrison, Soriano-Mas, Vervliet, Cardoner, Avila-Parcet, and Radua, 2016)). Specifically, the amygdala is essential for the formation of the CS-UCS association and mediates expression of the conditioned fear response (Cheng, Knight, Smith, and Helmstetter, 2006; Cheng et al., 2003; Helmstetter, 1992; Helmstetter and Bellgowan, 1993; Knight et al., 2005; LeDoux et al., 1990). Human neuroimaging research has also shown that other brain regions including the hippocampus, insula, prefrontal cortex (PFC), and inferior parietal lobule (IPL) also support processes that are important for various aspects of fear learning and memory (Büchel, Morris, Dolan, and Friston, 1998; Delgado, Nearing, LeDoux, and Phelps, 2008; Knight, Smith, Cheng, Stein, and Helmstetter, 2004b; Knight, Smith, Stein, and Helmstetter, 1999). However, prior brain imaging studies of Pavlovian conditioning have largely focused on brain regions that show CS-elicited CRs. These CRs represent responses to the CS and may not reflect a temporal conditioning process. Specifically, these CRs may reflect a component of the CS-UCS association that does not depend on the temporal dynamics of the CS-UCS relationship. Therefore, the neural substrates that encode temporal information during associative fear learning remain unclear.
Although the neural substrates of human temporal conditioning have yet to be determined, previous studies of Pavlovian trace conditioning may provide insight into brain regions that may mediate temporal conditioning processes. During trace conditioning, a temporal gap (called the trace interval) separates termination of the CS and onset of the UCS. Thus, successful learning requires the formation of an association between events (i.e., the CS and UCS) that are separated in time. Therefore, learning the relative timing of the CS and UCS is critical for an appropriately timed CR during trace conditioning (Davis et al., 1989; Kalmbach et al., 2010). Prior human and animal research suggests the hippocampus plays an important role in trace conditioning (Büchel, Dolan, Armony, and Friston, 1999; Cheng, Disterhoft, Power, Ellis, and Desmond, 2008; Crestani, Keist, Fritschy, Benke, Vogt, Prut, Blüthmann, Möhler, and Rudolph, 2002; Haritha, Wood, Ver Hoef, and Knight, 2013; Knight, Cheng, Smith, Stein, and Helmstetter, 2004a; Shors, Miesegaes, Beylin, Zhao, Rydel, and Gould, 2001; Solomon, Vander Schaaf, Thompson, and Weisz, 1986). Further, learning-related changes within the amygdala, PFC, IPL, and insula have been observed during trace conditioning in both animals (Belova, Paton, Morrison, and Salzman, 2007; Ferry and Di Scala, 1997; Ferry, Wirth, and Di Scala, 1999; Kalmbach et al., 2010; Takehara-Nishiuchi and McNaughton, 2008) and humans (Büchel et al., 1999; Haritha et al., 2013; Knight et al., 2004a). Thus, these brain regions may also be important for temporal conditioning.
While the previous fMRI research of trace conditioning has provided insight into human brain regions supporting time-related processes, these studies utilized discrete CS presentations which complicate interpretations related to temporal conditioning in prior work. Specifically, brain activity that supports timing-related processes overlaps with brain activity elicited by the CS and trace interval (Büchel et al., 1999; Cheng et al., 2008; Díaz-Mataix, Martinez, Schafe, LeDoux, and Doyère, 2013; Knight et al., 2004a). The overlap of these processes makes it difficult to determine whether brain activation during trace conditioning reflects a time-related process or a combination of time, CS-related, and trace interval processes. More specifically, the temporal resolution of the blood oxygen level dependent (BOLD) fMRI signal response makes it difficult to separate the fMRI signal elicited by the CS and trace interval from the response elicited by temporal processes (Büchel et al., 1999; Cheng et al., 2008; Knight et al., 2004a; Logothetis and Wandell, 2004). Thus, although timing is important for successful trace conditioning, it remains unclear whether the brain activity observed in prior conditioning studies support temporal conditioning. Therefore, fMRI research that investigates the neural substrates that encode temporal information in the absence of a discrete CS is needed for a better understanding of time-related associations during conditioning.
Even though prior research has typically employed a discrete auditory or visual CS during Pavlovian conditioning procedures, a traditional CS is not necessary for associative learning. Specifically, the UCS can be presented at regular time intervals in the absence of a discrete CS such that time itself serves as the CS (i.e., the warning cue) that predicts the UCS (Kirkpatrick and Church, 2000; Lockhart, 1966; Pendergrass and Kimmel, 1968). During this time-cued type of temporal conditioning procedure, an anticipatory CR prior to UCS presentation is taken as evidence that the association between time and the UCS has been learned (Lockhart, 1966). Thus, time-cued temporal conditioning procedures differ from traditional CS-cued conditioning procedures because an association must be formed between the passage of time and the UCS, rather than between a discrete CS and the UCS. Time-cued conditioning procedures are well suited for fMRI investigations of temporal conditioning because no discrete CS is presented. Thus, the fMRI signal response reflects brain activity that encodes temporal associations independent of CS-elicited activity.
The present study used fMRI during a time-cued Pavlovian conditioning procedure to investigate brain regions that support temporal conditioning. Participants were exposed to a temporal conditioning (i.e., fixed interval UCS presentations expected to support conditioning) and control procedure (i.e., random interval UCS presentations that should not support conditioning) (Lockhart, 1966; Pendergrass and Kimmel, 1968). We expected participants to show conditioned responses during temporal conditioning compared to the control procedure. Given prior human neuroimaging findings in trace conditioning (Büchel et al., 1999; Cheng et al., 2008; Haritha et al., 2013; Knight et al., 2004a), we hypothesized the dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), IPL, hippocampus, insula, and amygdala would show learning-related changes during temporal conditioning. The current study elucidates the neural substrates of temporal conditioning, and provides novel insight into the neural mechanisms of associative fear learning.
Materials and methods
Participants
Twenty-four healthy right-handed volunteers participated in this study. Volunteers were recruited from the Birmingham-Metropolitan area. Exclusion criteria included: left-handedness, history of blood or circulation disorders (e.g., anemia or sickle-cell), diabetes, brain or spinal abnormalities, pregnancy, previous head injury (e.g., traumatic brain injury, concussion, or loss of consciousness), or mental illness. One participant was excluded due to excessive head movement in the scanner. Data from the remaining 23 participants were included in the analysis [10 female, 13 male; age: mean = 19.92±0.59, range: 18-27 years]. All participants provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Unconditioned Stimuli
Participants were exposed to temporal conditioning and a control procedure in which the UCS was presented at fixed or random intervals respectively. A loud (100 dB) white noise UCS (0.5 s duration) was presented through MRI-compatible pneumatic headphones during these scans. The UCS was presented a total of 120 times over four 620 s scans (30 UCS presentations per scan). During the temporal conditioning scans, the UCS was presented at fixed intervals (ITI: 20 s). During the control scans, the UCS was presented at random intervals (Average ITI: 20 s, ITI range: 6-34 s). Participants completed two scans of each condition. Participants were randomly assigned to either an ABBA or BAAB scan order to control for order effects. Specifically, one half of the participants completed a fixed pattern scan followed by a random pattern scan, then completed a random pattern scan followed by a fixed pattern scan. The other half of the participants completed these scans in the opposite order. Participants were informed prior to each scan whether the UCS would be presented in a fixed or random pattern, but were not informed of the precise timing of the UCS.
UCS Expectancy
A UCS expectancy rating scale was presented on an IFIS-SA LCD (Invivo Corp.; Gainesville, FL) video screen using Presentation software (Neurobehavioral Systems, Inc.; Albany, CA). The screen was located above the participant’s head and viewed through a mirror attached to the head coil. An MRI compatible joystick (Current Designs; Philadelphia, PA) was used to monitor participants’ expectancy of UCS presentation on a moment-by-moment basis. The joystick controlled a rating bar that was presented throughout the session on the video screen. Each participant was instructed to rate their UCS expectancy on a continuous scale from 0 to 100 (0 = certain the UCS would not be presented, 50 = uncertain whether the UCS would be presented, 100 = certain the UCS would be presented). UCS expectancy was calculated as the average response during the second (40 Hz sample rate) prior to UCS onset. Additional details on this methodology have been published previously (Knight and Wood, 2011).
Skin Conductance Response
An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data. SCR was sampled (10 kHz) with a pair of disposable radio-translucent electrodes (1 cm diameter, Biopac Systems; Goleta, CA) from the thenar and hypothenar eminences of the nondominant hand. SCR data were processed using Biopac AcqKnowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 250 Hz. Conditioned SCRs were assessed from the phasic increase in conductance during the 8 s preceding UCS presentation. SCRs were calculated using procedures adapted from previous guidelines (Roth, Dawson, and Filion, 2012). Specifically, conditioned SCRs were calculated during the 8 s immediately preceding UCS presentation by subtracting the skin conductance signal at response onset from the peak skin conductance value. Trials in which an unconditioned SCR was elicited during the 8 s window prior to a subsequent UCS presentation were excluded from the analysis. Responses smaller than 0.05 μSiemens were assigned a value of zero.
MRI
Structural and functional imaging data were acquired on a 3 Tesla Siemens Allegra scanner. High resolution anatomical magnetization prepared rapid acquisition gradient echo (MPRAGE) images were obtained in the sagittal plane using a T1 weighted series (TR=2300 ms, TE=3.9 ms, flip angle=12°, FOV=25.6 cm, matrix=256×256, slice thickness=1 mm, 0.5 mm gap) to serve as an anatomical reference. Whole-brain BOLD fMRI was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2000 ms, TE=30 ms, flip angle=70°, FOV=24 cm, matrix=64×64, slice thickness=4 mm, no gap) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). The first 2 volumes of fMRI data were discarded due to transient magnetization effects. Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially smoothed using a 4 mm full-width-at-half-maximum Gaussian kernel.
Functional MRI data were analyzed at the individual subject level using multiple linear regression. The analysis modeled the hemodynamic response during the 18 s period prior to the UCS using 3dDeconvolve from the AFNI software package (Cox, 1996). Because time, instead of a discrete CS, served to signal UCS presentation in the present study, a model was selected that allowed the shape of the hemodynamic response to vary, instead of assuming a predetermined response shape (Saad, Chen, Reynolds, Christidis, Hammett, Bellgowan, and Cox, 2006). Regressors of no interest were modeled with a Gamma variate hemodynamic response function to account for brain activity not related to the anticipatory CR, and included reference waveforms for joystick movement and UCS presentation. Six motion parameters (i.e., three displacement and three rotation parameters) were included as additional regressors of no interest to account for participant head motion.
Group level analyses compared percent signal change as an index of the magnitude of the anticipatory CR. The average percent signal change data from the 8 seconds (i.e., 4 volumes) acquired prior to UCS presentation was used as an index of the CR magnitude of the fMRI signal during temporal conditioning (i.e., fixed ITI trials) and the control (i.e., random ITI trials) condition. Functional maps reflecting percent signal change were then converted to the Talairach and Tournoux stereotaxic coordinate system for group analyses (Talairach & Tournoux, 1988).
Whole-brain voxel-wise paired t-test analyses were completed using the AFNI software package (Cox, 1996). Monte Carlo simulations were used to determine the p < 0.05 (corrected) significance threshold (t > 3.58; p < 0.001; cluster volume > 400 mm3) for the whole-brain analysis. However, amygdala activity often does not meet whole-brain corrected significance thresholds given the relatively small volume of this structure. Therefore, a separate voxel-wise paired t-test analysis was completed for the amygdala using Monte Carlo simulation determined p < 0.05 (amygdala volume corrected) significance criteria (t > 2.86; p < 0.005; cluster volume > 66 mm3).
Results
UCS Expectancy
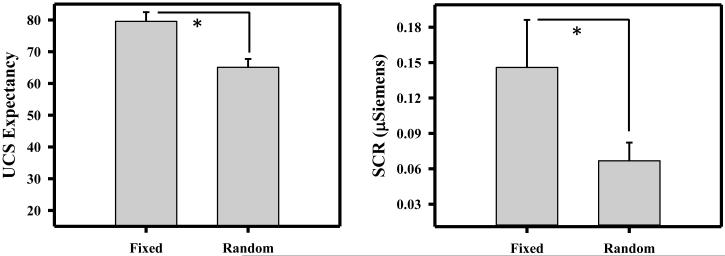
Based on prior work (Lockhart, 1966; Pendergrass and Kimmel, 1968), UCS presentations at fixed ITIs should support temporal conditioning, while UCS presentations at random ITIs should not. Given this directional hypothesis, UCS expectancy data were included in a 1-tailed paired t-test to determine whether expectation of the UCS differed between temporal conditioning (i.e., fixed ITI) and control (i.e., random ITI) conditions. UCS expectancy was higher during temporal conditioning than the control condition [t (22) = 6.82; p < 0.001; Figure 1]. These data demonstrate that participants were more certain of the timing of the UCS when separated by a fixed vs. random ITI (i.e., temporal conditioning).
Figure 1.
Unconditioned stimulus (UCS) expectancy and conditioned skin conductance response (SCR). UCS expectancy and conditioned SCR were greater during temporal conditioning (i.e., fixed interval UCS) than the control (i.e., random interval UCS) procedure. Error bars reflect the standard error of the mean. Asterisk indicates a significant difference.
Skin Conductance Response
Prior research has demonstrated temporal conditioning of SCR (Lockhart, 1966; Pendergrass and Kimmel, 1968). Therefore, we expected conditioned SCRs would be greater during temporal conditioning than the control procedure. Two participants were excluded from the SCR data analysis as nonresponders. Nonresponders were defined as participants that produced no SCRs greater than 0.05 μSiemens. Data from the remaining 21 participants were included in a 1-tailed paired t-test comparison (given our directional hypothesis) of CR magnitude for temporal conditioning and control conditions. CR magnitude was greater during temporal conditioning (i.e., fixed ITI) than control (i.e., random ITI) trials [t (20) = 2.02; p = 0.028; Figure 1]. These data demonstrate stronger temporal conditioning to fixed vs. random interval UCS presentations.
Functional MRI
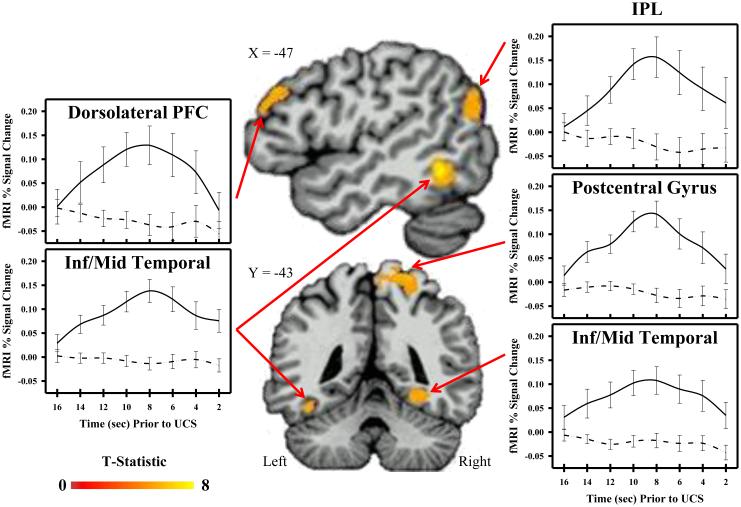
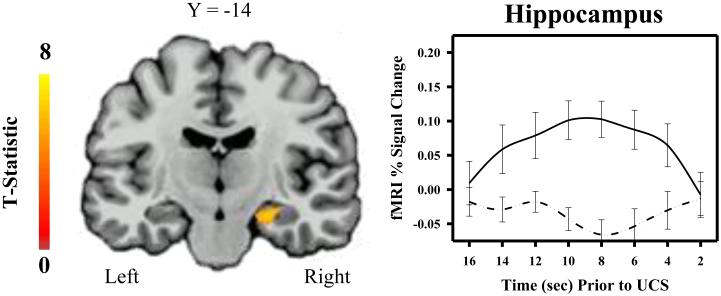
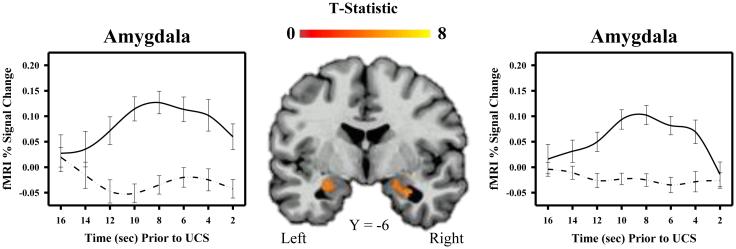
Functional MRI data from all 23 participants were included in a 2-tailed t-test to determine areas of differential activation for temporal conditioning (i.e., fixed interval UCS presentations) and control (i.e., random interval UCS presentations) conditions. Given that some brain regions (e.g., ventromedial PFC) may show task-related deactivations (i.e., greater deactivation during temporal conditioning compared to the control condition), a 2-tailed t-test was completed on the data. The whole-brain fMRI data analysis revealed learning-related differences in brain activity within a number of brain regions including the hippocampus, IPL, dlPFC, and temporal cortex (Table 1). Each of these regions showed a greater fMRI signal response during temporal conditioning than the control condition (Figures 2 and 3). Similarly, the fMRI analysis restricted to the amygdala also showed learning-related differences in bilateral amygdala activity. Specifically, amygdala activity was greater during temporal conditioning compared to the control condition (Figure 4). There were no regions that showed a greater CR to random than fixed trials.
Table 1.
Regions showing temporal conditioning.
| Structure | Hemisphere |
Peak T-
Value |
Cluster
(mm3) |
Talairach Coordinates (X,Y,Z) | ||
|---|---|---|---|---|---|---|
| Amygdala | Right | 4.86 | 378 | 27 | −4 | −19 |
| Left | 3.94 | 379 | −22 | −9 | −15 | |
| Hippocampus | Right | 6.25 | 579 | 23 | −16 | −15 |
| Right | 5.59 | 437 | 33 | −24 | −11 | |
| Dorsolateral PFC | Left | 5.92 | 453 | −49 | 32 | 25 |
| Postcentral Gyrus | Right | 6.13 | 4347 | 44 | −32 | 53 |
| Inf. Parietal Lobule | Left | 5.12 | 880 | −51 | −69 | 18 |
| Inf./Mid. Temporal Gyrus | Left | 9.18 | 2826 | −48 | −51 | −8 |
| Right | 5.14 | 649 | 30 | −40 | −9 | |
Location, volume, and coordinates from Talaraich and Tournoux (1988) for the peak voxel (t-value) for areas of activation that met significance criteria. Inf = Inferior, Mid = Middle, PFC = Prefrontal Cortex.
Figure 2.
Cortical activity and temporal conditioning. Brain activity increased during the inter-trial interval of the temporal conditioning (i.e., fixed interval UCS; solid line), but not control (i.e., random interval UCS; dotted line) procedure within dorsolateral prefrontal cortex (PFC), inferior parietal lobule (IPL), temporal cortex, and postcentral gyrus. Graphs depict mean fMRI signal (% change) prior to UCS presentation.
Figure 3.
Hippocampal activity and temporal conditioning. Hippocampal activity increased during the inter-trial interval of the temporal conditioning (i.e., fixed interval UCS; solid line), but not control (i.e., random interval UCS; dotted line) procedure. Graphs depict mean fMRI signal (% change) prior to UCS presentation.
Figure 4.
Amygdala activity and temporal conditioning. Amygdala activity increased during the inter-trial interval of the temporal conditioning (i.e., fixed interval UCS; solid line), but not control (i.e., random interval UCS; dotted line) procedure. Graphs depict mean fMRI signal (% change) prior to UCS presentation.
Discussion
Learning the relationship between the CS and UCS is an important aspect of Pavlovian conditioning (Balsam et al., 2010; Balsam et al., 2006; Balsam and Gallistel, 2009). Specifically, learning the timing of a threat during Pavlovian fear conditioning promotes defensive behaviors (e.g., escape and avoidance) that minimize the impact of the threat (Kalmbach et al., 2010; Pendergrass and Kimmel, 1968; Takehara-Nishiuchi and McNaughton, 2008). However, limited prior human fMRI research has investigated the neural substrates of temporal conditioning. Therefore, the current study was designed to investigate brain activity that supports temporal conditioning processes. Greater conditioned SCRs and higher UCS expectancies were observed during temporal conditioning (i.e., fixed interval UCS presentations) than the control (i.e., random interval UCS presentations) procedure. These findings indicate participants were able to predict the UCS on fixed, but not random trials (i.e., temporal learning). Further, learning-related differences in the fMRI signal response were observed within the amygdala, hippocampus, dlPFC, IPL, and temporal cortex. The present findings suggest these brain regions mediate temporal fear conditioning processes and provide new insight into the mechanisms of associative learning and memory.
In the current study, we observed evidence of temporal conditioning in the UCS expectancy data. Participants reported greater UCS expectancy during fixed (i.e., temporal learning) versus random interval (i.e., control condition) UCS conditions. These findings demonstrate participants learned the timing of the UCS when the UCS was presented in a fixed pattern, but not when the UCS was presented randomly (i.e., temporal conditioning). UCS expectancy is a direct measure of participant self-reported expectations of the UCS at any particular moment (Knight and Wood, 2011). While UCS expectancy has been used in prior research to infer contingency awareness (i.e., awareness of the CS-UCS association) (Knight, Nguyen, and Bandettini, 2003; 2006; Knight, Waters, and Bandettini, 2009; Schultz and Helmstetter, 2010), it can also be used to determine participant knowledge of the timing of the UCS. In the current study, UCS timing was experimentally manipulated between the fixed and random conditions. Thus, the greater expectancy during temporal conditioning (i.e., fixed interval UCS presentations) compared to the control condition (i.e., random interval UCS presentations) indicates participants learned the timing of the UCS.
To our knowledge, the current study is the first to identify human brain regions that support an association solely between the passage of time and UCS presentation (i.e., temporal conditioning). Timing information is essential for Pavlovian conditioning (Balsam et al., 2010; Balsam, Drew, and Yang, 2002; Balsam et al., 2006), however aside from the limited number of trace conditioning studies, brain activity that supports temporal conditioning processes has received little attention in the human neuroimaging literature. Although several prior trace conditioning studies have suggested brain regions like the hippocampus, dlPFC, and IPL support temporal information (Büchel et al., 1999; Cheng et al., 2008; Haritha et al., 2013; Knight et al., 2004a), these prior studies were not designed to separate time-related from CS-elicited or trace interval activation. Specifically, these prior studies used discrete CS and trace interval presentations that overlapped with the temporal information these events contained. Given the temporal dynamics of the hemodynamic response, CS and trace interval elicited activity could not be adequately distinguished from activity elicited by temporal conditioning. The present study did not use a discrete CS and thus separates brain activity that supports temporal conditioning from other confounding processes (i.e., CS-elicited and trace interval processes). Therefore, the results of the current study demonstrate temporal conditioning, identify the brain regions that support this type of learning, and extend prior findings from trace conditioning research.
Temporal learning-related changes in brain function were observed within the amygdala, hippocampus, dlPFC, IPL and temporal cortex in the present study. Specifically, greater conditioned fMRI signal responses (i.e., the CR) were observed within these brain regions during temporal conditioning compared to the control condition. No brain regions showed greater activity on control compared to temporal conditioning trials. The current findings suggest the activated brain regions form a neural circuit that encodes the temporal information that is necessary for successful Pavlovian conditioning. Prior studies of delay and trace conditioning have also reported learning-related changes in activity within these brain regions (Büchel et al., 1999; Büchel et al., 1998; Dunsmoor, Bandettini, and Knight, 2007; Haritha et al., 2013; Knight et al., 2004a; Knight et al., 1999; LaBar et al., 1998). However, these prior studies were not designed to disentangle CS-elicited from time-related activity. Given the present study did not include a discrete CS. The current findings extend prior trace conditioning research and demonstrate these brain regions process temporal information that predicts UCS presentation to support Pavlovian conditioning.
Prior research suggests the dlPFC may be particularly important for timing-related processes during associative learning. In particular, this brain region may be essential for timing the anticipatory CR (Crestani et al., 2002; Jin, Fujii, and Graybiel, 2009; Shors et al., 2001). Thus, the dlPFC may underlie time-related processes that are critical for temporal conditioning. Prior work has demonstrated dlPFC activation during Pavlovian conditioning (Delgado et al., 2008; Dunsmoor, Bandettini, and Knight, 2008; Haritha et al., 2013; Knight, Waters, King, and Bandettini, 2010; LaBar et al., 1998; Wood, Ver Hoef, and Knight, 2012) as well as other tasks that rely upon processing sequences of stimuli over time (Barch, Braver, Nystrom, Forman, Noll, and Cohen, 1997; Cohen, Perlstein, Braver, Nystrom, Noll, Jonides, and Smith, 1997; Dormal, Dormal, Joassin, and Pesenti, 2012; Harrington, Haaland, and Knight, 1998; Owen, McMillan, Laird, and Bullmore, 2005; Rao, Mayer, and Harrington, 2001). Similar observations have been made in animal studies. Specifically, prior research has demonstrated the medial PFC (mPFC), the rodent homologue to human dlPFC (Weiss, Kronforst-Collins, and Disterhoft, 1996), is critical for timing-related processes especially during trace conditioning (Gilmartin and McEchron, 2005; Kronforst-Collins and Disterhoft, 1998). In fact, conditioned eyeblink responses mediated by cerebellar cortex in rodents are dependent on mPFC activity (Kalmbach et al., 2010). Consistent with the view that dlPFC supports temporal processes, we observed greater BOLD fMRI signal responses during temporal conditioning than control trials within the dlPFC. Thus, the observed dlPFC activity appears to represent an anticipatory process that relies upon time-related information about impending threats.
In addition to the dlPFC, the hippocampus may also be important for time-related learning processes. Previous research has frequently observed hippocampal activity during trace conditioning (Büchel et al., 1999; Cheng et al., 2008; Crestani et al., 2002; Haritha et al., 2013; Knight et al., 2004a; Shors et al., 2001; Solomon et al., 1986). Further, prior work has shown that disruption of hippocampal activity disrupts the adaptive timing of the CR (Kishimoto, Nakazawa, Tonegawa, Kirino, and Kano, 2006; Tam and Bonardi, 2012). These findings suggest the hippocampus is necessary for behavioral responses to events separated in time. In the present study, we observed greater conditioned fMRI signal responses within the hippocampus during temporal conditioning than the control procedure. Given the lack of a discrete CS in our procedure, the time between UCS presentations represents temporal information which must be encoded for an appropriately timed CR. Taken together, these data suggest the hippocampus may support a process that encodes the time between subsequent UCS presentations.
While the hippocampus and dlPFC appear to support temporal information processing, the amygdala may be the site where time and UCS-related information converge to mediate CR acquisition. Prior research has demonstrated CS (e.g., time in the present study) and UCS information projects to the amygdala where the CS-UCS association is formed (LeDoux et al., 1990; Romanski and LeDoux, 1992; 1993). Additionally, recent work indicates that projections from the PFC and hippocampus to the amygdala are important for CR acquisition during complex Pavlovian conditioning procedures (e.g., trace conditioning) (Ferry et al., 1999; Gilmartin, Kwapis, and Helmstetter, 2012). Further, projections from the amygdala to brain regions such as periaqueductal gray, hypothalamus, and other subcortical structures appear to mediate expression of the peripheral emotional response (Cheng et al., 2006; Cheng et al., 2003; Davis, Walker, Miles, and Grillon, 2010; Fendt and Fanselow, 1999; Helmstetter, 1992; Knight et al., 2005; LeDoux et al., 1990). Thus, the association between the passage of time and the UCS may be formed within the amygdala which then projects to other subcortical brain structures to elicit the CR.
The current study presented the UCS at both fixed (i.e., temporal conditioning) and random (i.e., control condition) intervals. Specifically, UCS presentations were predictable during fixed trials and were unpredictable during random trials. Interestingly, UCS predictability may influence fear and anxiety processes. Specifically, predictable UCS presentations support an association between the CS and UCS resulting in a phasic fear response. Conversely, unpredictable UCS presentations reinforce an association between context and the UCS resulting in sustained fear (i.e., anxiety) (Davis et al., 2010; Grillon, 2002; Grillon, Baas, Cornwell, and Johnson, 2006; Grillon and Davis, 1997; Grillon, Pellowski, Merikangas, and Davis, 1997; Rescorla and Wagner, 1972) . The present experiment was not designed or analyzed with the intent to investigate the role of predictability in phasic (i.e., fear) versus sustained (i.e., anxiety) responses. Thus, it is difficult to interpret the current results in relation to prior work that has attempted to distinguish fear and anxiety. Instead, the predictability of the UCS was used to support (i.e., fixed trials) or prevent (i.e., random trials) temporal conditioning. Thus, the current study demonstrates an association between time and the UCS, and identifies regions (e.g., dlPFC, hippocampus, and amygdala) that show temporal learning-related changes in brain activity, and should not be interpreted in terms of fear (phasic) versus anxiety (sustained) processes.
Conclusions
The present study investigated the neural substrates of Pavlovian temporal conditioning. The current findings suggest human brain regions including the amygdala, hippocampus, IPL, and dlPFC support temporal fear conditioning. These brain regions may form a neural network that supports temporal conditioning processes that are necessary for CR acquisition. To our knowledge, this is the first human neuroimaging study to investigate the neural substrates of temporal fear conditioning. Thus, the results of the current study elucidate the neural substrates of temporal fear learning in humans, and extend our knowledge of the mechanisms that underlie associative learning and memory.
Highlights.
Neural substrates of human temporal conditioning were investigated.
Learning-related changes were observed in the emotional response.
Dorsolateral PFC, hippocampus, and amygdala showed learning-related changes.
These regions appear to support temporal processes during fear conditioning.
Acknowledgements
The authors would like to thank Adam Goodman for helpful discussions on the writing of this manuscript. This research was supported by the National Institute of Mental Health of the National Institutes of Health under award number MH098348 (D. C. K.) and the University of Alabama at Birmingham, Office of Equity and Diversity’s CMFSDP Fellowship (N. G. H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balsam PD, Drew MR, Gallistel C. Time and associative learning. Comparative Cognition & Behavior Reviews. 2010;5:1. doi: 10.3819/ccbr.2010.50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam PD, Drew MR, Yang C. Timing at the start of associative learning. Learning and Motivation. 2002;33:141–155. [Google Scholar]
- Balsam PD, Fairhurst S, Gallistel CR. Pavlovian contingencies and temporal information. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:284. doi: 10.1037/0097-7403.32.3.284. [DOI] [PubMed] [Google Scholar]
- Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends in Neurosciences. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala–hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. The Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proceedings of the National Academy of Sciences. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral neuroscience. 2006;120:1187. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral neuroscience. 2003;117:3. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. 1997 doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proceedings of the National Academy of Sciences. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Schlesinger LS, Sorenson C. Temporal specificity of fear conditioning: Effects of different conditioned stimulus--unconditioned stimulus intervals on the fear-potentiated startle effect. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:295. [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mataix L, Martinez RCR, Schafe GE, LeDoux JE, Doyère V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Current Biology. 2013;23:467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormal V, Dormal G, Joassin F, Pesenti M. A common right fronto-parietal network for numerosity and duration processing: An fMRI study. Human brain mapping. 2012;33:1490–1501. doi: 10.1002/hbm.21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral neuroscience. 2007;121:635. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow M. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Ferry B, Di Scala G. Bicuculline administration into basolateral amygdala facilitates trace conditioning of odor aversion in the rat. Neurobiology of learning and memory. 1997;67:80–83. doi: 10.1006/nlme.1996.3743. [DOI] [PubMed] [Google Scholar]
- Ferry B, Wirth S, Di Scala G. Functional interaction between entorhinal cortex and basolateral amygdala during trace conditioning of odor aversion in the rat. Behavioral neuroscience. 1999;113:118. doi: 10.1037//0735-7044.113.1.118. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, Radua J. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychol Rev. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: advances and challenges. Current opinion in neurobiology. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of learning and memory. 2012;97:452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral neuroscience. 2005;119:1496. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle reflex in humans. Biological psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Haritha AT, Wood KH, Ver Hoef LW, Knight DC. Human trace fear conditioning: right-lateralized cortical activity supports trace-interval processes. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:225–237. doi: 10.3758/s13415-012-0142-6. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. The Journal of Neuroscience. 1998;18:1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behavioral neuroscience. 1992;106:518. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain research. 1993;612:253–257. doi: 10.1016/0006-8993(93)91669-j. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Fujii N, Graybiel AM. Neural representation of time in cortico-basal ganglia circuits. Proceedings of the National Academy of Sciences. 2009;106:19156–19161. doi: 10.1073/pnas.0909881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. Journal of neurophysiology. 2010;104:627–640. doi: 10.1152/jn.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Stimulus and temporal cues in classical conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:206. doi: 10.1037//0097-7403.26.2.206. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. The Journal of Neuroscience. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of Neuroscience. 2004a;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2004b;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA. Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45:208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49:843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Wood KH. Investigating the neural mechanisms of aware and unaware fear memory with fMRI. Journal of visualized experiments: JoVE. 2011 doi: 10.3791/3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiology of learning and memory. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBarbera J, Church RM. Magnitude of fear as a function of expected time to all aversive event. Animal Learning & Behavior. 1974;2:199–202. [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. The Journal of Neuroscience. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart RA. Temporal conditioning of GSR. Journal of experimental psychology. 1966;71:438. doi: 10.1037/h0022979. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papka M, Ivry RB, Woodruff-Pak DS. Selective disruption of eyeblink classical conditioning by concurrent tapping. Neuroreport. 1995;6:1493–1497. doi: 10.1097/00001756-199507310-00007. [DOI] [PubMed] [Google Scholar]
- Pendergrass VE, Kimmel H. UCR diminution in temporal conditioning and habituation. Journal of experimental psychology. 1968;77:1. doi: 10.1037/h0025815. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. The Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature neuroscience. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning: current research and theory. 1972 [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. The Journal of Neuroscience. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cerebral Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Roth WT, Dawson ME, Filion DL. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Chen G, Reynolds RC, Christidis PP, Hammett KR, Bellgowan PS, Cox RW. Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Human brain mapping. 2006;27:417–424. doi: 10.1002/hbm.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DH, Helmstetter FJ. Classical conditioning of autonomic fear responses is independent of contingency awareness. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:495. doi: 10.1037/a0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral neuroscience. 1986;100:729. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Tam SK, Bonardi C. Dorsal hippocampal involvement in appetitive trace conditioning and interval timing. Behavioral neuroscience. 2012;126:258. doi: 10.1037/a0027164. [DOI] [PubMed] [Google Scholar]
- Weiss C, Kronforst-Collins MA, Disterhoft JF. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage. 2012;60:787–799. doi: 10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]