Abstract
Multiple aspects of organismal physiology influence the number and activity of stem cells and their progeny, including nutritional status. Previous studies demonstrated that Drosophila germline stem cells (GSCs), follicle stem cells (FSCs), and their progeny sense and respond to diet via complex mechanisms involving many systemic and local signals. AMP-activated protein kinase, or AMPK, is a highly conserved regulator of energy homeostasis known to be activated under low cellular energy conditions; however, its role in the ovarian response to diet has not been investigated. Here, we describe nutrient-dependent and -independent requirements for AMPK in Drosophila oogenesis. We found that AMPK is cell autonomously required for the slow down in GSC and follicle cell proliferation that occurs on a poor diet. Similarly, AMPK activity is necessary in the germline for the degeneration of vitellogenic stages in response to nutrient deprivation. In contrast, AMPK activity is not required within the germline to modulate its growth. Instead, AMPK acts in follicle cells to negatively regulate their growth and proliferation, thereby indirectly limiting the size of the underlying germline cyst within developing follicles. Paradoxically, AMPK is required for GSC maintenance in well-fed flies (when AMPK activity is presumably at its lowest), suggesting potentially important roles for basal AMPK activity in specific cell types. Finally, we identified a nutrient-independent, developmental role for AMPK in cyst encapsulation by follicle cells. These results uncover specific AMPK requirements in multiple cell types in the ovary and suggest that AMPK can function outside of its canonical nutrient-sensing role in specific developmental contexts.
Keywords: diet, AMPK, LKB1, germline stem cell, follicle growth, oogenesis, Drosophila
Graphical abstract
INTRODUCTION
Adult stem cell lineages are sensitive to many physiological cues, including nutritional status. In species ranging from model organisms to humans, diet influences circulating factors, including nutrients, metabolites, and hormones, that can impinge on local signaling networks or act on stem cells directly to modulate their behavior (Ables et al., 2012). Although nutrient-sensing pathways are typically ubiquitously expressed, they can exert distinct effects on different cell types within stem cell lineages (Ables et al., 2012). A complete understanding of how nutrient sensors modulate stem cells and their descendants therefore requires dissecting how their roles in specific cell types contribute to the regulation of each lineage as a whole.
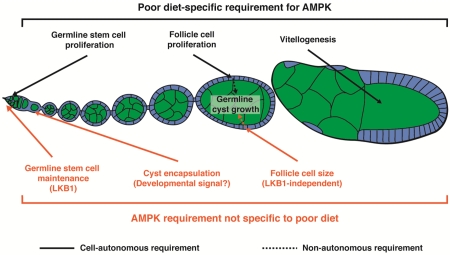
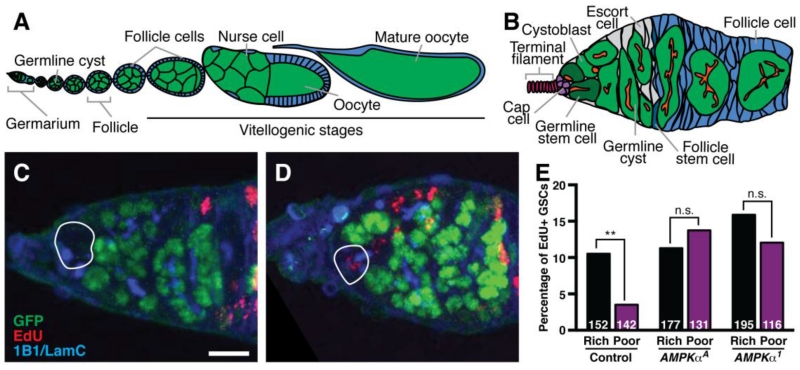
In the Drosophila melanogaster ovary, stem cell populations continuously maintain oogenesis and have a well-described response to diet (Ables et al., 2012). Each ovary comprises a set of ovarioles, which are chronologically ordered arrays of developing follicles (or egg chambers) (Fig. 1A). Each follicle consists of a 16-cell germline cyst encapsulated by follicle cells that arise from germline stem cells (GSCs) and follicle stem cells (FSCs), respectively, within an anterior structure called the germarium (Fig. 1B). On a nutrient-rich diet, GSCs, FSCs, and their progeny divide and grow robustly, and oogenesis proceeds with minimal cell death. When females are shifted to a nutrient-poor diet, proliferation and growth of stem cells and their descendants slows uniformly (Drummond-Barbosa and Spradling, 2001), and GSC numbers decrease (Hsu and Drummond-Barbosa, 2009). In addition, early dividing cysts die frequently and most follicles degenerate instead of progressing through vitellogenesis (Drummond-Barbosa and Spradling, 2001). Several nutrient-dependent pathways play an active role within the GSC and/or FSC lineages to maintain robust rates of oogenesis on a rich diet. For example, germline insulin signaling is required for GSC division, cyst growth and follicle progression through vitellogenesis (Hsu et al., 2008; LaFever and Drummond-Barbosa, 2005). Target of rapamycin (TOR) signaling maintains GSC numbers and promotes GSC proliferation cell autonomously, and stimulates follicle growth by acting in both the germline cyst and in surrounding follicle cells (LaFever et al., 2010). The steroid hormone ecdysone acts directly on the germline to promote GSC maintenance and proliferation, survival of early dividing cysts (Ables and Drummond-Barbosa, 2010), and follicle growth and vitellogenesis (Ables et al., 2015; Buszczak et al., 1999; Carney and Bender, 2000). The role of pathways involved in actively sensing and responding to nutrient deprivation, however, remains less well understood.
Fig. 1. AMPK is required in GSCs for downregulation of proliferation on a poor diet.
(A) Diagram of Drosophila ovariole showing progressively more developed follicles, which bud off from an anterior germarium. Each follicle consists of a germline cyst (green) surrounded by somatic follicle cells (blue). Follicle cells undergo mitotic proliferation until stage 6 and transition to endoreplication at stage 7 of oogenesis. The oocyte begins yolk uptake, or vitellogenesis, during stage 8 (Spradling, 1993). (B) Diagram of the germarium, which houses germline stem cells (GSCs; dark green) juxtaposed to a somatic niche comprising cap cells (purple), a subset of escort cells (grey), and terminal filament cells (magenta). GSCs give rise to cystoblasts, which develop into 16-cell germline cysts that are encapsulated by follicle cells (blue) derived from a pair of follicle stem cells (dark blue) to form a new follicle. The fusome (red) is a special cellular structure present in early germ cells that becomes progressively more branched as cysts divide. (C and D) Maximum intensity projections of mosaic germaria showing GFP-negative GSCs (outlined) without (C) or with (D) EdU incorporation at 7 days after clone induction. GFP (green) labels wild-type cell nuclei; 1B1 (blue) labels fusomes and cell membranes; Lamin C (LamC; blue) labels cap cell nuclear envelopes; EdU (red) labels nuclei in S phase. Scale bar, 10 μm. (E) Average percentage of GFP-negative GSCs in control and AMPK mutant mosaic germaria that have incorporated EdU. These data combine three independent experiments, and sample sizes are indicated inside bars. Error bars represent S.E.M. **, p<0.01 by Chi-square test.
AMP-activated protein kinase (AMPK) is a heterotrimeric complex that is activated in response to low energy levels to control a number of cellular processes, including metabolism, protein homeostasis, and the cell cycle. The complex consists of a catalytic subunit (α) and two regulatory subunits (β and γ), and a single gene encodes each subunit in Drosophila. Studies in culture show that AMPK activity increases with high cellular levels of AMP or ADP (when ATP levels are low) and with activation by upstream kinases, including liver kinase B1 (LKB1). Activated AMPK stimulates catabolism and inhibits anabolic processes, thereby maintaining energy homeostasis (Hardie et al., 2016). Previous work in Drosophila showed that AMPK controls follicle cell growth (Haack et al., 2013); however, other potential roles of AMPK in oogenesis and its requirement in the germline have not been investigated.
In this study, we uncovered both diet-dependent and -independent roles for AMPK in the Drosophila ovary using genetic mosaic analysis of available AMPKα alleles. We found that AMPK is required on a poor diet for slowing down GSC proliferation and follicle growth (through repression of follicle cell growth and proliferation). Surprisingly, AMPK is dispensable within the germline itself for follicle growth, in stark contrast to the germline requirement for insulin/TOR signaling in this process (LaFever et al., 2010). In addition, AMPK and LKB1 are intrinsically required for GSC maintenance in well-fed flies, suggesting that basal LKB1-dependent AMPK function can be important on a rich diet. Finally, we found that follicle cells intrinsically require AMPK to properly encapsulate germline cysts during follicle formation in a diet-independent fashion. This study underscores how widely acting nutrient-dependent pathways can have specific cellular requirements in multiple cell types within a tissue, some of which may be independent of their canonical role of tying cellular processes to the nutritional environment.
MATERIALS AND METHODS
Drosophila strains and culture conditions
Fly stocks were maintained on standard cornmeal/molasses/yeast/agar medium at 22-25°C. For experiments, females (in the presence of wild-type males) were transferred daily onto either standard medium supplemented with wet yeast paste (“rich diet”) or molasses/agar (“poor diet”) (Armstrong et al., 2014). All AMPKα mutant stocks were maintained with a Y chromosome carrying a duplication including the AMPKα locus, Dp(1;Y)2E, y1 (DGRC #106089). The AMPKD2 FRT19A and FRT82B LKB1X5 null alleles were gifts from Jongkyeong Chung (Seoul National University) (Lee et al., 2007; Lee et al., 2006). The AMPKα1 null allele is described in Haack et al. (2013), and the AMPKα1 FRT19A recombinant chromosome was generated by standard crosses. AMPKaA is a lethal mutation described in Haelterman et al. (2014). The FRT82B LKB14A4-2 null allele was a gift from Daniel St. Johnston (University of Cambridge) (Martin and St Johnston, 2003). Other genetic elements are described in FlyBase (http://www.flybase.org).
Genetic mosaic analysis
Females of genotype y w His2Av::GFP hs-FLP FRT19A/AMPKα* FRT19A and hs-FLP; FRT82B Ubi-GFP/LKB1* were generated through standard crosses. (AMPKα* and LKB1* represent null or wild-type alleles of the AMPKα or LKB1 genes, respectively.) Zero- to 3-day-old females were maintained on dry yeast and heat shocked twice daily at 37°C for 3 days to induce mitotic recombination (Xu and Rubin, 1993). For GSC maintenance assays, flies were kept on a rich diet for 3 days after the final heat shock, then either maintained on a rich diet or shifted to a poor diet for an additional 4 days prior to dissection and processing. AMPKα* and LKB1* homozygous clones were recognized by the absence of green fluorescent protein (GFP), and GSCs were identified based on their anterior location and typical fusome morphology (de Cuevas and Spradling, 1998; Hsu et al., 2008). To quantify GSC loss, we analyzed all germaria containing GFP-negative cystoblasts and/or cysts, and calculated the percentage of germaria that no longer contained GFP-negative GSCs (i.e. “GSC loss events”), as described (Laws and Drummond-Barbosa, 2015). To measure GSC and follicle cell proliferation, flies were maintained on a rich diet for 4 days following the last heat shock, then either switched to a poor diet or maintained on a rich diet for an additional 3 days. The frequency of EdU-positive, GFP-negative GSCs or follicle cells was calculated as a percentage of the total number of GFP-negative GSCs or follicle cells, respectively, for multiple single plane images of follicle epithelia, as described (Laws and Drummond-Barbosa, 2015).
To assess follicle growth, the size of follicles containing any number of GFP-negative follicle cells was compared to that of flanking follicles 7 days after the last heat-shock; a follicle was considered overgrown if larger than the follicle to its immediate posterior. Only follicles stage 10 or younger, and containing a single germline cyst were included in this analysis; the follicle cells analyzed are therefore part of follicle stem cell clones (Margolis and Spradling, 1995). This is a straightforward analysis because wild-type ovarioles always display progressively larger and more developed follicles from anterior to posterior, according to their chronological age; therefore, any significant deviation from this pattern is readily apparent in mosaic ovarioles. It is worth noting, however, that our analysis likely underestimates follicle overgrowth (see Fig. 3). Specifically, it is conceivable that in a significant number of ovarioles with mosaic AMPKα follicle cells, all follicles might have similar proportions of wild-type and mutant follicles cells (which are derived from wild-type and mutant follicle stem cells, respectively, in our experiments) relative to their neighbors and undergo similar overgrowth, retaining a normal pattern of relative follicle size.
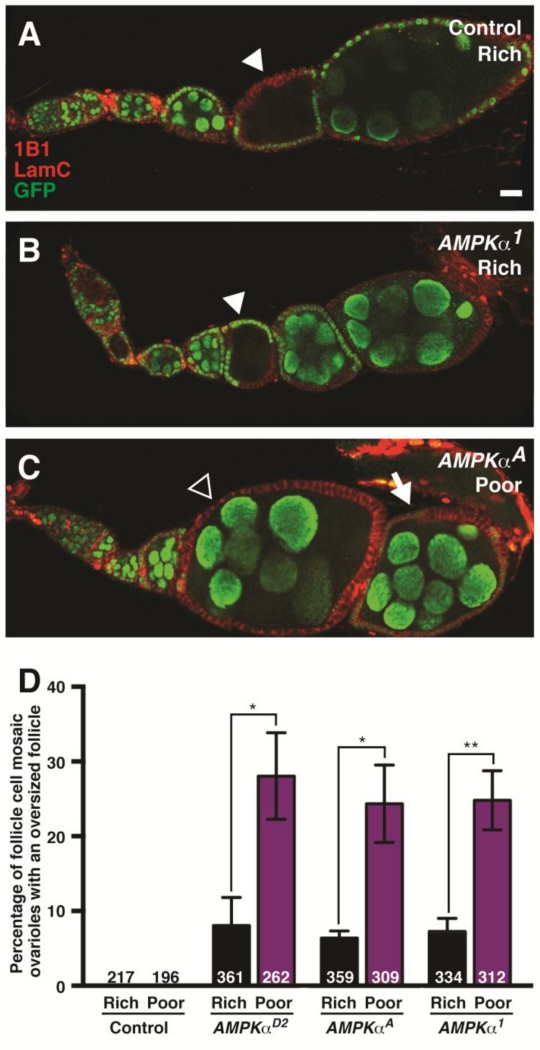
Figure 3. AMPK function is required in follicle cells, but not in the germline, for follicle growth.
(A and B) Control (A) and AMPKα mutant mosaic (B) ovarioles with GFP-negative germline cysts (arrowheads) that grow normally relative to flanking GFP-positive cysts. (C) AMPKα mutant mosaic ovariole showing overgrowth of a follicle containing a wild-type germline cyst surrounded by AMPKα mutant follicle cells (open arrowhead) relative to the posterior, older follicle, which contains fewer GFP-negative follicle cells (arrow). GFP (green) labels wild-type cell nuclei; 1B1 (red) labels fusomes and cell membranes; Lamin C (LamC; red) labels cap cell nuclear envelopes. Scale bar, 10 μm. (D) Quantification of follicle overgrowth in follicle cell mosaic ovarioles at 7 days after clone induction. This phenotype is markedly enhanced on poor relative to rich diets. Sample sizes are shown inside bars and represent results from three independent experiments. Error bars represent S.E.M. *p<0.05; **p<0.01 by Student’s t test.
Follicle cell size analysis was performed in follicles in the mitotic program (stages 2-6) or later endoreplicative stages, and egg chambers were staged based on size, nuclear morphology, and yolk uptake (Spradling, 1993). The average size of follicle cells was determined by measuring the respective areas encompassing the same number of GFP-positive and GFP-negative cells in a single follicle cell monolayer, then dividing each area by the number of cells. Budding defects in region 3 were visually identified; those with excessive accumulation of follicle cells in region 3 were considered defective. Follicles were scored as misencapsulated if they contained two germline cysts within the same follicle cell monolayer.
Immunofluorescence, EdU labeling, and microscopy
Adult ovaries were dissected in Grace’s Insect Medium (Lonza), teased apart, and fixed for 13 minutes in 5.3% formaldehyde (Ted Pella) in Grace’s. Samples were rinsed and washed four times in 0.1% Triton X-100 (Sigma) in phosphate-buffered saline (PBS), or PBT, and blocked for at least 3 hours at room temperature or overnight at 4°C in 5% bovine serum albumin (BSA; Sigma) and 5% normal goat serum (NGS; Jackson ImmunoResearch) in PBT unless otherwise noted. Samples were incubated at 4°C overnight with primary antibodies in blocking solution at the following concentrations: mouse anti-Hts (1B1) (Developmental Studies Hybridoma Bank [DSHB], 1:10); mouse anti-Lamin C (LC28.26) (DSHB, 1:100); chicken anti-GFP (1:2000, Abcam); rabbit anti-pAMPK (1:200, Cell Signaling). After primary antibody incubation, samples were washed for 2 hours in PBT and incubated for 2 to 4 hours in Alexa Fluor 488-, 568-, or 633-conjugated goat species-specific secondary antibodies (1:200, Invitrogen) in blocking solution. Samples were mounted in Vectashield with DAPI (Vector Laboratories). Confocal images were acquired using a Zeiss LSM 700 microscope, and analyzed using either Zeiss ZEN 2009 or ImageJ software, and equally and minimally enhanced via histogram using Adobe Photoshop CS4.
Although previous studies in Drosophila and human cell culture have used the aforementioned commercially available antibody against phosphorylated AMPKα (pAMPK) as a readout for AMPK activity (Castanieto et al., 2014; Lee et al., 2015; Vazquez-Martin et al., 2011), we detect a strong signal with this antibody in AMPKα mutant cells undergoing mitosis (Fig. S1), indicating that it is not a valid AMPK activity reporter in whole mount ovarian samples.
EdU incorporation assays were performed as described (Ables and Drummond-Barbosa, 2013). Briefly, ovaries were dissected in Grace’s medium at room temperature, and incubated in 100 μM EdU (Invitrogen) in Grace’s medium for 1 hour prior to being teased apart, fixed, and stained as above. EdU was detected with AlexaFluor-594 via Click-It chemistry using the manufacturer’s instructions (Invitrogen) following secondary antibody incubation.
RESULTS
AMPK controls GSC proliferation in response to diet
Downregulation of GSC division rates contributes to a reduction in egg production in response to a poor diet (Drummond-Barbosa and Spradling, 2001). Our previous work demonstrated an intrinsic requirement for insulin, TOR, and ecdysone signaling for robust GSC proliferation on a rich diet, consistent with their higher activity levels when nutrients are abundant (Ables and Drummond-Barbosa, 2010; Hsu et al., 2008; LaFever and Drummond-Barbosa, 2005; LaFever et al., 2010). It remains unknown, however, whether active repression of proliferation on a poor diet is also required. We therefore tested if AMPK, which becomes activated when resources are scarce (Towler and Hardie, 2007), is required for restricting GSC proliferation on a poor diet using genetic mosaic analysis of three independently generated AMPKα alleles: the lethal AMPKαA allele (Haelterman et al., 2014) and null AMPKαD2 and AMPKα1 alleles (Haack et al., 2013; Lee et al., 2007). We measured incorporation of the thymidine analog EdU, a marker for S phase, in control and AMPKα mutant mosaic ovarioles (Fig. 1C-E). The frequency of control mosaic GSCs in S phase decreased significantly when females were shifted from rich to poor diets (Fig. 1E). By contrast, the fraction of EdU-positive AMPKα mutant GSCs on either diet was statistically indistinguishable from that of control GSCs on a rich diet (Fig. 1E), showing that AMPKα mutant GSCs fail to downregulate proliferation on a poor diet. These results are consistent with a requirement for AMPK in actively repressing GSC proliferation in response to a poor diet.
AMPK is required for GSC maintenance in well-fed flies on a rich diet
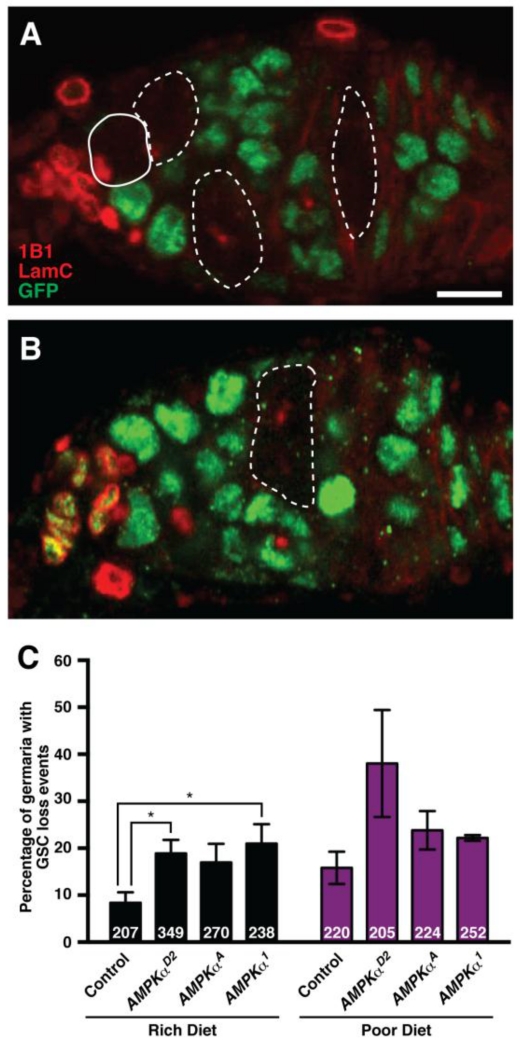
In females on a poor diet, GSCs are lost more frequently from the niche, in part due to reduced TOR (LaFever et al., 2010; Sun et al., 2010) and ecdysone signaling in GSCs (Ables and Drummond-Barbosa, 2010), as well as to the non cell-autonomous effects of reduced insulin pathway activity (Hsu and Drummond-Barbosa, 2009, 2011; Yang et al., 2013) and amino acid levels (Armstrong et al., 2014). If AMPK activity were required to promote GSC loss on a poor diet, AMPKα mutant GSCs would be maintained better than controls on a poor diet, but similarly on a rich diet. To test these predictions, we compared the incidence of GSC loss events in control and AMPKα mutant mosaic germaria on both diets (Fig. 2A-C). In control mosaic females, where all cells are wild type, we detect GSC loss events in about 10% of germaria with a mosaic germline on a rich diet (Fig. 2C). Contrary to our prediction, GSCs homozygous mutant for AMPKα are lost approximately twice as frequently as controls on a rich diet, suggesting that the basal levels of AMPK activity present under those conditions are required for normal GSC maintenance. On a poor diet, AMPKα mutant GSCs also appear to be lost at higher frequencies relative to control GSCs, although these differences do not reach statistical significance (Fig. 2C). In addition, cleaved Dcp-1-positive GSCs are never detected in AMPKα or control mosaic germaria (see below), suggesting that mutant GSCs differentiate instead of undergoing apoptosis. In any case, the frequent loss of AMPKα mutant GSCs on a poor diet indicates that AMPK activity is not required for poor diet-induced GSC loss. Given that AMPK is required independently of low cellular energy conditions for GSC maintenance, it is possible that its activity on a rich diet is maintained by LKB1, a known upstream activator of AMPK (Hardie et al., 2016). Indeed, homozygous LKB1 mutant GSCs are lost from the niche significantly more frequently than control GSCs in well-fed germline mosaic females (Fig. S2).
Figure 2. AMPK is required intrinsically for GSC maintenance on a rich diet.
(A and B) Genetic mosaic germaria showing GFP-negative cystoblasts and cysts (dashed outline) derived from a GFP-negative GSC (solid outline) at 7 days after clone induction. The presence of GFP-negative germline cyst(s) in the absence of a GFP-negative GSC indicates a GSC loss event (B). GFP (green) labels wild-type cell nuclei; 1B1 (red) labels fusomes and cell membranes; Lamin C (LamC; red) labels cap cell nuclear envelopes. Scale bar, 10 μm. (C) Quantification of GSC loss events in control and AMPKα mutant mosaic germaria showing a significant increase in loss of AMPKα mutant GSCs at 7 days after clone induction on a rich diet. Differences on a poor diet do not reach statistical significance. Sample sizes from four independent experiments are indicated inside bars. Error bars represent S.E.M. *p<0.05 by Student’s t test.
AMPK is not required in the germline for follicle growth
Insulin/TOR signaling has a well-described role in the control of germline cyst growth both through an intrinsic germline requirement and non-autonomously through the regulation of follicle cells (LaFever et al., 2010). Because AMPK restricts cellular growth in times of nutrient deprivation (Yuan et al., 2013) and is a known negative regulator of TOR signaling (Hindupur et al., 2015), we asked if AMPK function is required to repress germline cyst growth on a poor diet. Surprisingly, we found that AMPK is dispensable in the germline for follicle growth. In both control and AMPK mutant mosaic ovarioles (Fig. 3A and B; n=>100 follicles with GFP-negative germline for each), all follicles carrying GFP-negative germline cysts develop at normal rates compared to flanking GFP-positive follicles.
AMPK activity in follicle cells restricts germline cyst growth
In addition to being intrinsically regulated, germline cyst growth is indirectly controlled by follicle cells. For example, both insulin/TOR signaling and the transcriptional factor Myc are required in follicle cells to regulate underlying germline cyst growth (LaFever et al., 2010; Maines et al., 2004). We therefore tested if AMPK activity in follicle cells might regulate follicle growth instead, by taking advantage of the linear and chronologically ordered arrangement of developing follicles of each ovariole that is characteristic of wild-type females. We analyzed ovarioles containing control versus AMPKα mutant mosaic follicle cell layers seven days after clone induction, and quantified the fraction of follicle cell mosaic ovarioles that contained one or more follicles with abnormal growth relative to its posterior (i.e. older) neighbor. In contrast to control mosaics, in which this is never observed, approximately 10% of ovarioles with AMPKα mutant mosaic follicle cells contain follicles that grow at a faster rate compared to the older, immediately posterior follicles on a rich diet (Fig. 3C and D). These overgrown follicles contain large patches of AMPKα mutant follicle cells, corresponding to ~50% or more of the follicle layer (>200 ovarioles analyzed for each genotype). These results suggest that basal AMPK levels are required in follicle cells for appropriate follicle growth in well-fed flies. On a poor diet, the frequency of follicle overgrowth triples (Fig. 3D), indicating that AMPK activity in follicle cells is particularly critical to further restrict follicle growth when nutrients are limiting.
AMPK controls follicle cell proliferation and follicle cell size
The requirement for AMPK in follicle cells to limit follicle growth could reflect a role of AMPK in restricting follicle cell proliferation, size, or both. In late stage follicles, where follicle cells undergo endoreplication (Spradling, 1993), we often observe markedly larger GFP-negative, AMPKα mutant follicle cells next to patches of normal sized GFP-positive, wild-type follicle cells in mosaic ovarioles (Fig. 4A-A’). We therefore measured the sizes of neighboring GFP-negative and -positive follicle cells in control and AMPKα mutant mosaic ovarioles on both rich and poor diets. As expected, in control mosaic ovarioles, GFP-negative and -positive follicle cells have similar sizes (Fig. 4B-C). By contrast, in AMPKα mutant mosaics GFP-negative follicle cells are significantly larger than neighboring wild-type, GFP-positive follicle cells regardless of diet or of whether they are proliferating (Fig. 4B) or endoreplicating (Fig. 4C). We conclude that AMPK restricts follicle cell growth on both rich and poor diets. Unlike the case for GSC maintenance, however, LKB1 function is not required for follicle cell growth regulation because LKB1 mutant follicle cells are comparable in size to neighboring wild-type follicle cells throughout mosaic ovarioles (Fig. S3).
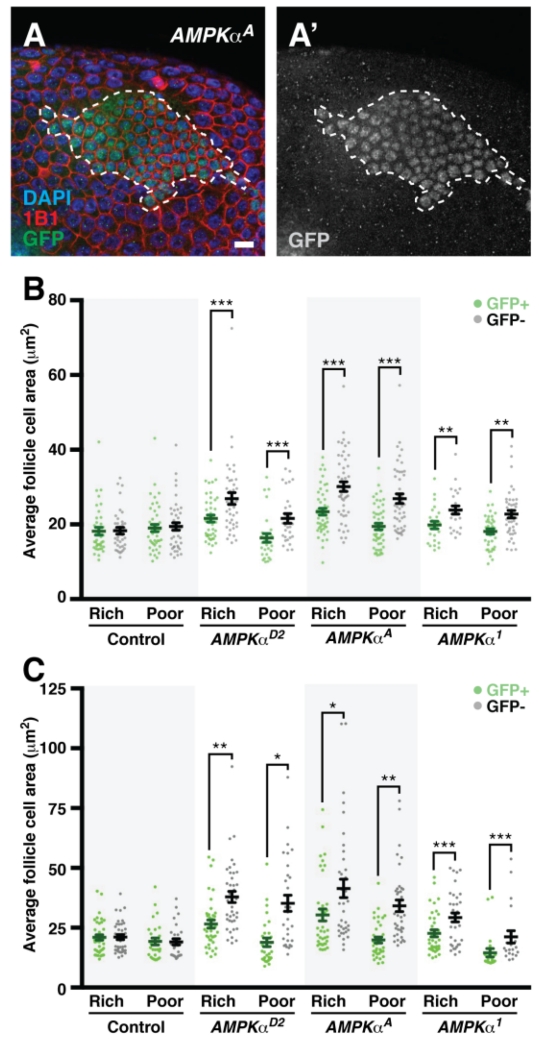
Fig. 4. AMPK cell-autonomously controls follicle cell growth during mitotic and endoreplicative cycles.
(A) AMPKα mutant mosaic follicle cell layer showing larger mutant follicle cells surrounding wild type, GFP-positive follicle cells (outlined). GFP (green in A), labels wild-type cell nuclei; 1B1 (red) marks cell membranes; DAPI (blue) marks nuclei. GFP channel alone is show in grayscale in A’. Scale bar, 10 μm. (B and C) Quantification of GFP-positive and -negative follicle cell size at 7 days after clone induction in mitotic (B) or endoreplicative (C) follicle stages. Error bars represent S.E.M. *p<0.05, **p<0.01; ***p<0.001 by Student’s t test.
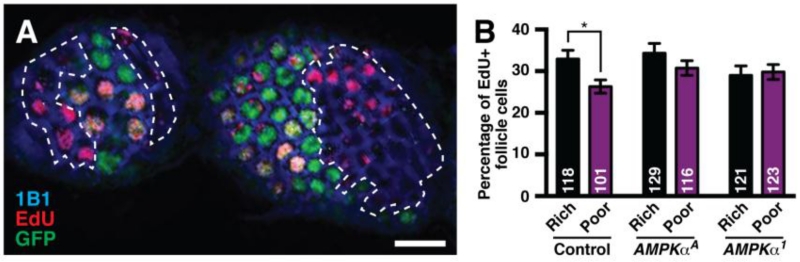
Since AMPK regulates follicle cell growth to similar extents on both rich and poor diets (Fig. 4B-C; compare differences between GFP-positive and GFP-negative follicle cells in AMPKα mosaics for each diet), this function of AMPK is not sufficient to explain the three-fold increase in oversized follicles with AMPKα mutant follicle cells on a poor diet (Fig. 3D). We therefore asked whether AMPK regulates follicle cell proliferation in a diet-dependent manner. In control mosaics, the frequency of EdU incorporation in GFP-negative follicle cells trends is lower on poor relative to rich diets (Fig. 5A-B). Unlike in controls, however, GFP-negative follicle cells in AMPKα mutant mosaics have similar frequencies of EdU incorporation on rich and poor diets (Fig. 5B), indicating that AMPK activity is required for follicle cells to downregulate proliferation on a poor diet. Taken together, these data support a model in which the regulation of follicle growth by AMPK is achieved through its intrinsic regulation of follicle cell growth and proliferation, which non-autonomously controls the growth of the underlying germline cyst.
Fig. 5. AMPK is required to restrict follicle cell proliferation on a poor diet.
(A) A genetic mosaic ovariole showing GFP-negative follicle cells with EdU incorporation. GFP (green) labels wild-type cell nuclei; 1B1 (blue) labels cell membranes; EdU (red) labels cells in S phase. GFP-negative follicle cell clones are outlined. Scale bar, 10 μm. (B) Percentage of control and AMPKα mosaic GFP-negative follicle cells in mitotic stages that incorporate EdU at 7 days after clone induction. Sample sizes from three independent combined experiments are indicated inside bars. Error bars represent S.E.M. *, p<0.05 by Student’s t test.
AMPK controls the vitellogenesis block under poor diet conditions
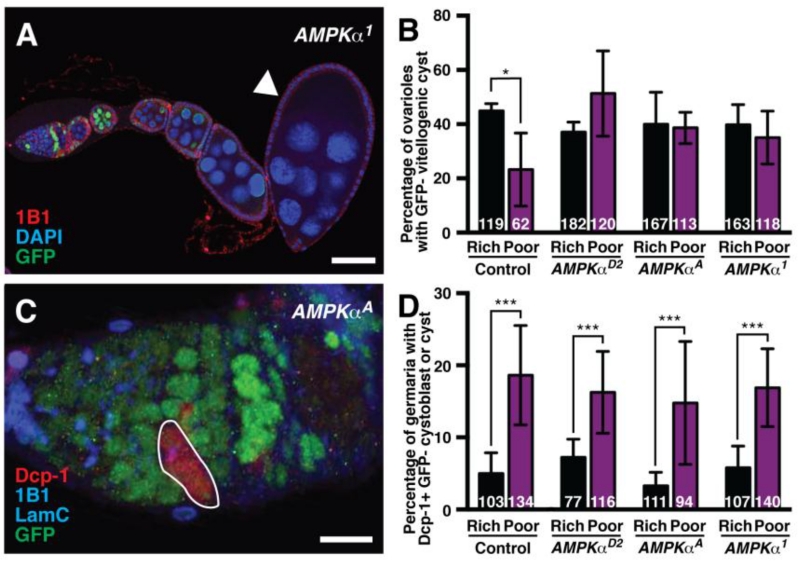
Vitellogenesis is blocked in wild-type flies cultured on a poor diet due to the degeneration of early vitellogenic follicles. To determine if AMPK is required for this block in vitellogenesis, we compared the frequency of GFP-negative vitellogenic follicles in control versus AMPKα mosaic ovarioles. Approximately 40% of control mosaic ovarioles on a rich diet have vitellogenic follicles containing GFP-negative germline cysts, whereas this frequency drops to ~20% on a poor diet (Fig. 6A-B), consistent with reduced progression through vitellogenesis. By contrast, the fraction of AMPKα mutant mosaic ovarioles displaying vitellogenic follicles is not reduced on a poor diet compared to that on a rich diet (Fig. 6B). These results suggest that AMPK function contributes to the nutrient-sensitive vitellogenesis checkpoint.
Fig. 6. AMPK is required for the inhibition of vitellogenesis, but not for early cyst death, in response to a poor diet.
(A) AMPKα mutant mosaic ovariole showing a vitellogenic GFP-negative germline cyst (arrowhead). GFP (green) labels wild-type cell nuclei; 1B1 (red) labels fusomes and cell membranes; DAPI (blue) labels nuclei. Scale bar, 50 μm. (B) Quantification of germline mosaic ovarioles containing vitellogenic GFP-negative germline cysts in control and AMPKα mosaic females. Error bars represent S.E.M. *, p<0.05 by Student’s t test. (C) Maximum intensity projection of an AMPKα mosaic germarium showing a GFP-negative, cleaved Dcp-1-positive germline cyst (outlined). GFP (green) labels wild-type cell nuclei; 1B1 (blue) labels fusomes and cell membranes; Lamin C (LamC; blue) labels cap cell nuclear envelopes; cleaved Dcp-1 (red) is an apoptosis marker. Scale bar, 10 μm. (D) Percentage of germaria containing GFP-negative, cleaved Dcp-1-positive cystoblasts and/or cysts in control and AMPKα mosaics. We did not observe any cleaved Dcp-1 positive GSCs in our experiments. Sample sizes from three independent experiments are indicated inside bars. Error bars represent S.E.M. ***p<0.001 by Chi-square test.
In addition to early vitellogenic follicle degeneration, switching females to a poor diet also induces the death of early germline cysts in the germarium (Drummond-Barbosa and Spradling, 2001). We therefore assessed whether AMPK was also required for early cyst death on a poor diet using cleaved Death caspase-1 (Dcp-1) as a marker (Florentin and Arama, 2012; Song et al., 1997) (Fig. 6C). We found, however, that Dcp-1-positive germline cysts were detected at similar frequencies in control and AMPKα mutant mosaic germaria on both rich and poor diets (Fig. 6D), indicating that the poor diet-induced increase in early cyst death is independent of AMPK activity.
AMPK controls follicle cell development independently of diet
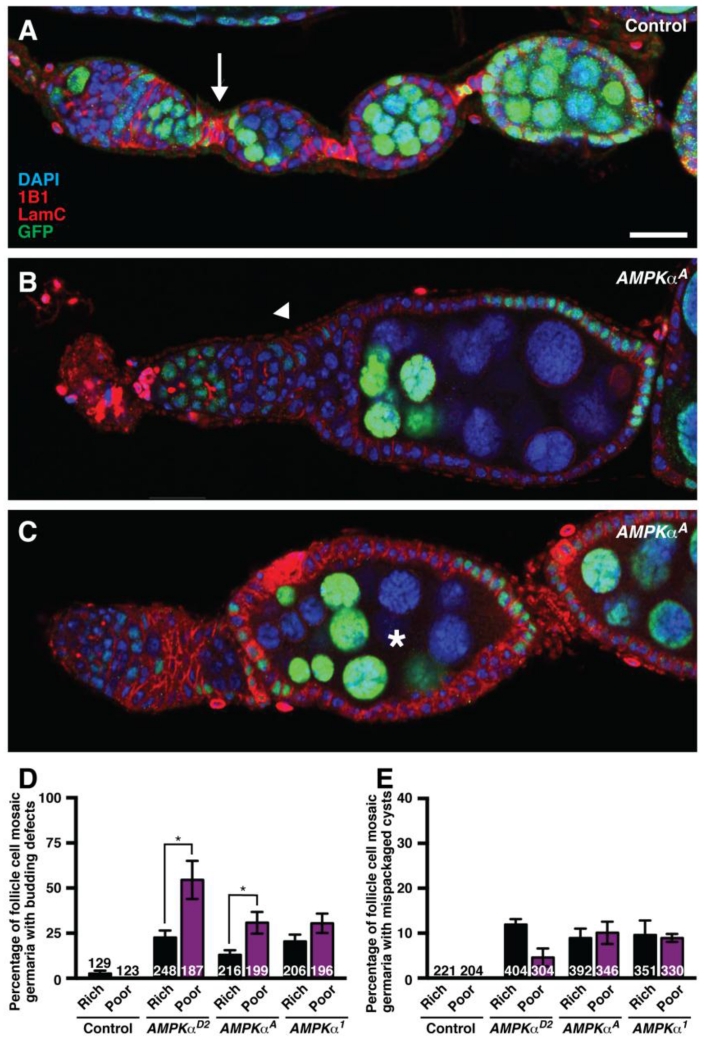
Interestingly, we found that AMPK regulates follicle cell encapsulation of cysts in the germarium, a developmental process that is not diet-dependent (Drummond-Barbosa and Spradling, 2001). In control mosaics, germline cysts bud off of the germarium, and a monolayer of follicle cells encapsulates each 16-cell germline cyst (Fig. 7A). By contrast, AMPKα mutant follicle cells frequently fail to properly execute this budding event, and mutant follicle cells form sacs containing multiple germline cysts (Fig. 7B). At later stages, follicles containing multiple germline cysts are also observed (Fig. 7C). We can rule out that these phenotypes are an indirect consequence of the role of AMPK in limiting follicle cell size because mosaic Tsc1 mutant ovarioles, where follicle cell overgrowth also occurs, do not display these defects (LaFever et al., 2010). We quantified these phenotypes on both rich and poor diets, and found that although initial follicle budding defects in the germarium are observed more frequently on a poor diet (Fig. 7D), misencapsulated follicles occur at the same frequency on rich and poor diets (Fig. 7E). The similar number of mispackaged follicles on rich and poor diets indicates that these encapsulation events are not dependent on diet. It is possible that the global slowdown in oogenesis on a poor diet (Drummond-Barbosa and Spradling, 2001) leads to the observation of more follicles in the process of budding - and therefore to the increased visualization of any defects in this process. Therefore, we conclude that AMPK is developmentally required for follicle encapsulation independently of diet.
Fig. 7. Follicle cell AMPK controls follicle encapsulation independently of diet.
(A) In control mosaic ovarioles, follicle cells envelop germline cysts to form follicles and also give rise to follicle cell stalks (arrow), which separate follicles for the remainder of oogenesis. (B) Example of AMPKα mutant follicle cells leading to abnormal follicle budding (arrowhead). (C) Example of AMPKα mutant mosaic follicle cells that have mispackaged multiple germline cysts with nurse cells of variable ploidy (asterisk). Given that the timing of our experiments is controlled such that we analyze exclusively stem cell clones, the presence of GFP-positive and GFP-negative nurse cells within the same follicle further indicates that distinct cysts (or parts thereof) were misencapsulated together. GFP (green) labels wild-type nuclei; 1B1 (red) labels fusomes and cell membranes; Lamin C (LamC; red) labels cap cell nuclear envelopes; DAPI (blue) labels nuclei. A single optical slice is shown in each panel; therefore, not all cells within the follicles are visible. Scale bar, 20 μm. (D and E) Graphs indicating the frequency of phenotypes shown in (B) and (C), respectively. Budding defects are more frequently observable on a poor diet (D); however, similar numbers of mispackaged follicles are generated regardless of diet (E) at 7 days after clone induction. Sample sizes represent data from four independent trials and are shown inside bars. Error bars, S.E.M. *p<0.05, Student’s t test.
DISCUSSION
Based on its role as a nutrient sensor in the literature (Gowans and Hardie, 2014; Hardie, 2014; Hardie and Ashford, 2014; Hardie and Hawley, 2001; Hardie et al., 2012; Hardie et al., 2016), AMPK activity is expected to be low when flies are fed a rich diet and to increase in response to a poor diet. This study demonstrates that AMPK controls multiple diet-dependent steps during oogenesis, but also reveals unexpected roles in GSC maintenance and early follicle formation. AMPK is required in the germline to restrict GSC proliferation and vitellogenesis on a poor diet, and in follicle cells (but not in the germline) for follicle growth, consistent with the presumably higher levels of AMPK activity under low energy conditions. Surprisingly, AMPK is required for GSC maintenance on a rich diet, suggesting that basal levels of AMPK in the absence of apparent energy stress are required for optimal self-renewal. Finally, germline cyst encapsulation by follicle cells, which is a diet-independent process, also requires AMPK function, suggesting that developmental signals might regulate AMPK.
A conserved role for AMPK in controlling germline and somatic cell proliferation in response to diet
AMPK is required to downregulate GSC and follicle cell proliferation on a poor diet. Similarly, the Caenorhabditis elegans AMPKα homologs, aak-1 and aak-2, suppress germline proliferation during nutrient-dependent developmental arrests, and double mutants have hyperplastic germlines (Fukuyama et al., 2012; Narbonne and Roy, 2006). Failure to maintain germline quiescence during these nutrient-dependent arrests is catastrophic, leading to precocious entry into meiosis and sterility in surviving animals (Fukuyama et al., 2012; Narbonne and Roy, 2006). AMPK also appears to be important in the somatic gonad of several mammals to repress proliferation based on studies in culture. Both bovine and rat ovarian follicles cultured with AMPK activators have a reduction in granulosa cell proliferation (Kayampilly and Menon, 2009; Tosca et al., 2010), and inhibition of AMPK in cultured rat follicles leads to increased granulosa cell proliferation (Kayampilly and Menon, 2009). While further work is necessary to determine whether these observations hold true in vivo, these studies suggest that AMPK function in the somatic gonad is highly conserved.
Unusual lack of germline requirement for AMPK in follicle growth regulation
Insulin/TOR and Myc signaling are required in the germline itself for follicle growth in addition to also being required in follicle cells (LaFever et al., 2010; Maines et al., 2004). By contrast, AMPK activity restricts follicle cell growth and, non-autonomously, the growth of underlying germline cyst; however, it is surprisingly dispensable in the germline for cyst growth. This is the first example, to our knowledge, of a diet-dependent regulator that controls germline growth and development exclusively non-autonomously, via follicle cells. Interestingly, oocyte-specific deletion of AMPK in mice results only in mild fertility defects (Bertoldo et al., 2015), suggesting that the unusual lack of requirement for AMPK in the germline for follicle growth might be shared across species.
TOR as a potential mediator of the diet-dependent role of AMPK in follicle cells
Given the variety of phenotypes resulting from loss of AMPK in different types of germline and somatic cells, it is likely that distinct downstream targets are involved in each cell type. TOR is a cellular integrator of nutritional information that acts downstream of AMPK in many systems (Hardie et al., 2016); for instance, TSC2, an upstream inhibitor of TOR, contains a stimulatory AMPK phosphorylation site conserved from Drosophila to mammals (Kim and Lee, 2015). It is therefore conceivable that TOR signaling mediates a subset of AMPK effects in the Drosophila ovary. Previous studies demonstrated that either loss or overactivation of TOR signaling results in GSC loss (LaFever et al., 2010; Sun et al., 2010), raising the possibility that AMPKα mutant GSC loss is a consequence of hyperactive TOR signaling. Our data demonstrating that AMPK is not required in the germline for follicle growth, however, suggest that TOR regulation in later germline cysts is AMPK-independent. In genetic mosaic ovarioles, Tor mutant follicle cells are smaller than neighboring wild-type cells (LaFever et al., 2010), whereas AMPK mutant follicle cells are larger than control cells based on this study and on a previously published study using another AMPKα null allele, AMPKα3 (Haack et al., 2013). These data are consistent with a model in which AMPK acts as an upstream inhibitor of TOR signaling to control follicle cell size. It is unlikely, however, that disrupted TOR signaling is responsible for the follicle cell budding defect in AMPK mutant follicle cells, as these defects are not observed in TOR pathway mosaic ovaries (LaFever et al., 2010; Sun et al., 2010). Therefore, while TOR signaling may mediate the effects of AMPK in several ovarian processes, it is not the sole downstream effector of AMPKα in this system.
Notch or hedgehog signaling may mediate the developmental functions of AMPK
Our data reveal a diet-independent role for AMPK in the encapsulation of germline cysts by follicle cells during follicle formation, suggesting non-canonical upstream regulation of AMPK by developmental signals in this context. Other examples of energy-independent activation of AMPK have been previously described. For instance, energy-independent AMPK activation occurs in response to reactivate oxygen species (Mungai et al., 2011), and CAMKKβ can promote AMPK activation without elevated AMP (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005). It will be interesting to investigate the specific signals regulating AMPK or acting downstream of it in the context of follicle formation. Hedgehog and Notch signaling have well-characterized roles in germline follicle budding (Forbes et al., 1996; Ruohola et al., 1991) and follicle cell specification (Chang et al., 2013; Nystul and Spradling, 2010), and are therefore logical candidates. A recent screen for Notch interactors in follicle cells uncovered interactions with multiple processes associated with metabolic state, including protein translation and degradation (Jia et al., 2015). Notch signaling is also regulated by autophagy, a process controlled by AMPK (Hardie et al., 2016). While it is clear that autophagy is upregulated in ovaries from nutrient-deprived flies, autophagy genes are also required for oogenesis under well-fed conditions (Barth et al., 2011). Follicle cell mutant clones of ATG1, a major autophagy-related gene, have fused egg chambers without stalk cells, reminiscent of both Notch and AMPK mutant phenotypes (Barth et al., 2012). Further, Notch controls the switch from mitosis to endoreplication in Drosophila (Deng et al., 2001; Lilly and Duronio, 2005; Lopez-Schier and St Johnston, 2001), and the large size of AMPK mutant follicle cells could reflect precocious entry into the endocycle. Future experiments should test a potential functional interaction between Notch signaling and AMPK activity in follicle cells.
Supplementary Material
HIGHLIGHTS.
AMPK restricts germline stem cell (GSC) proliferation on a poor diet
AMPK inhibits vitellogenesis on a poor diet, but does not control early cyst death
AMPK in follicle cells, but not in the germline, controls follicle growth
Basal AMPK activity promotes GSC maintenance on a rich diet
AMPK has a nutrient-independent, developmental role in follicle formation
ACKNOWLEDGEMENTS
K.M.L. and D.D.-B. designed experiments, analyzed and interpreted data, and wrote the manuscript; K.M.L. performed all experiments. We thank the Developmental Studies Hybridoma Bank for antibodies and the Bloomington Stock Center (supported by National Institutes of Health P40 OD018537), D. St. Johnston, and J. Chung for Drosophila stocks. We are grateful to members of the Drummond-Barbosa lab for critical reading of the manuscript. This work was supported by National Institutes of Health R01 GM069875 (D.D.-B.). K.M.L. was supported by National Institutes of Health T32 CA009110 and the Elsa Orent Keiles Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ables ET, Bois KE, Garcia CA, Drummond-Barbosa D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev Biol. 2015;400:33–42. doi: 10.1016/j.ydbio.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7:581–592. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Drummond-Barbosa D. Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals. Development. 2013;140:530–540. doi: 10.1242/dev.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables ET, Laws KM, Drummond-Barbosa D. Control of adult stem cells in vivo by a dynamic physiological environment: diet-dependent systemic factors in Drosophila and beyond. Wiley Interdiscip Rev Dev Biol. 2012;1:657–674. doi: 10.1002/wdev.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AR, Laws KM, Drummond-Barbosa D. Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila. Development. 2014;141:4479–4488. doi: 10.1242/dev.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth JM, Hafen E, Kohler K. The lack of autophagy triggers precocious activation of Notch signaling during Drosophila oogenesis. BMC Dev Biol. 2012;12:35. doi: 10.1186/1471-213X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth JM, Szabad J, Hafen E, Kohler K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2011;18:915–924. doi: 10.1038/cdd.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo MJ, Guibert E, Faure M, Rame C, Foretz M, Viollet B, Dupont J, Froment P. Specific deletion of AMP-activated protein kinase (alpha1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS One. 2015;10:e0119680. doi: 10.1371/journal.pone.0119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154:1203–1211. doi: 10.1093/genetics/154.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanieto A, Johnston MJ, Nystul TG. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. Elife. 2014;3 doi: 10.7554/eLife.04437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Jang AC, Lin CH, Montell DJ. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci U S A. 2013;110:E1734–1742. doi: 10.1073/pnas.1300725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Florentin A, Arama E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J Cell Biol. 2012;196:513–527. doi: 10.1083/jcb.201107133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Sakuma K, Park R, Kasuga H, Nagaya R, Atsumi Y, Shimomura Y, Takahashi S, Kajiho H, Rougvie A, Kontani K, Katada T. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol Open. 2012;1:929–936. doi: 10.1242/bio.2012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hardie DG. AMPK: a cellular energy sensor primarily regulated by AMP. Biochem Soc Trans. 2014;42:71–75. doi: 10.1042/BST20130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack T, Bergstralh DT, St Johnston D. Damage to the Drosophila follicle cell epithelium produces “false clones” with apparent polarity phenotypes. Biol Open. 2013;2:1313–1320. doi: 10.1242/bio.20134671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelterman NA, Jiang L, Li Y, Bayat V, Sandoval H, Ugur B, Tan KL, Zhang K, Bei D, Xiong B, Charng WL, Busby T, Jawaid A, David G, Jaiswal M, Venken KJ, Yamamoto S, Chen R, Bellen HJ. Large-scale identification of chemically induced mutations in Drosophila melanogaster. Genome Res. 2014;24:1707–1718. doi: 10.1101/gr.174615.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hindupur SK, Gonzalez A, Hall MN. The Opposing Actions of Target of Rapamycin and AMP-Activated Protein Kinase in Cell Growth Control. Cold Spring Harb Perspect Med. 2015;5:a019141. doi: 10.1101/cshperspect.a019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci U S A. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 2011;350:290–300. doi: 10.1016/j.ydbio.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Jia D, Soylemez M, Calvin G, Bornmann R, Bryant J, Hanna C, Huang YC, Deng WM. A large-scale in vivo RNAi screen to identify genes involved in Notch-mediated follicle cell differentiation and cell cycle switches. Sci Rep. 2015;5:12328. doi: 10.1038/srep12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150:929–935. doi: 10.1210/en.2008-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Lee JH. Identification of an AMPK phosphorylation site in Drosophila TSC2 (gigas) that regulate cell growth. Int J Mol Sci. 2015;16:7015–7026. doi: 10.3390/ijms16047015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137:2117–2126. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws KM, Drummond-Barbosa D. Genetic Mosaic Analysis of Stem Cell Lineages in the Drosophila Ovary. Methods Mol Biol. 2015;1328:57–72. doi: 10.1007/978-1-4939-2851-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IJ, Lee CW, Lee JH. CaMKKbeta-AMPKalpha2 signaling contributes to mitotic Golgi fragmentation and the G2/M transition in mammalian cells. Cell Cycle. 2015;14:598–611. doi: 10.4161/15384101.2014.991557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Park J, Lee SY, Lee S, Chung J. JNK pathway mediates apoptotic cell death induced by tumor suppressor LKB1 in Drosophila. Cell Death Differ. 2006;13:1110–1122. doi: 10.1038/sj.cdd.4401790. [DOI] [PubMed] [Google Scholar]
- Lilly MA, Duronio RJ. New insights into cell cycle control from the Drosophila endocycle. Oncogene. 2005;24:2765–2775. doi: 10.1038/sj.onc.1208610. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines JZ, Stevens LM, Tong X, Stein D. Drosophila dMyc is required for ovary cell growth and endoreplication. Development. 2004;131:775–786. doi: 10.1242/dev.00932. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006;133:611–619. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184:503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Song Z, McCall K, Steller H. DCP-1, a Drosophila cell death protease essential for development. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. [Google Scholar]
- Sun P, Quan Z, Zhang B, Wu T, Xi R. TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development. 2010;137:2461–2469. doi: 10.1242/dev.051466. [DOI] [PubMed] [Google Scholar]
- Tosca L, Rame C, Chabrolle C, Tesseraud S, Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–418. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011;10:1295–1302. doi: 10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yang SA, Wang WD, Chen CT, Tseng CY, Chen YN, Hsu HJ. FOXO/Fringe is necessary for maintenance of the germline stem cell niche in response to insulin insufficiency. Dev Biol. 2013;382:124–135. doi: 10.1016/j.ydbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.