Abstract
Background & Aims
Proton-pump inhibitors (PPIs) might reduce the risk of serious warfarin-related upper gastrointestinal bleeding, but the evidence of their efficacy for this indication is limited. A gastroprotective effect of PPIs would be particularly important for patients who take warfarin with antiplatelet drugs or nonselective non-steroidal anti-inflammatory drugs (NSAIDs), which further increase the risk of gastrointestinal bleeding.
Methods
This retrospective cohort study of patients beginning warfarin treatment in Tennessee Medicaid and the 5% National Medicare Sample identified 97,430 new episodes of warfarin treatment with 75,720 person-years of follow up. The study endpoints were hospitalizations for upper gastrointestinal bleeding potentially preventable by PPIs and for bleeding at other sites.
Results
Patients who took warfarin without PPI co-therapy had 119 hospitalizations for upper gastrointestinal bleeding per 10,000 person-years of treatment. The risk decreased by 24% among patients who received PPI co-therapy (adjusted hazard ratio [HR], 0.76; 95% CI, 0.63–0.91). There was no significant reduction in the risk of other gastrointestinal bleeding hospitalizations (HR, 1.07; 95% CI, 0.94–1.22) or non-gastrointestinal bleeding hospitalizations (HR, 0.98; 95% CI, 0.84–1.15) in this group. Among patients concurrently using antiplatelet drugs or NSAIDs, those without PPI co-therapy had 284 upper gastrointestinal bleeding hospitalizations per 10,000 person-years of warfarin treatment. The risk decreased by 45% (HR, 0.55; 95% CI, 0.39–0.77) with PPI co-therapy. PPI co-therapy had no significant protective effect for warfarin patients not using antiplatelet drugs or NSAIDs (HR, 0.86; 95% CI, 0.70-1.06). Findings were similar in both study populations.
Conclusions
In an analysis of patients beginning warfarin treatment in Tennessee Medicaid and the 5% National Medicare Sample, PPI co-therapy was associated with reduced risk of warfarin-related upper gastrointestinal bleeding; the greatest reduction occurred in patients also taking antiplatelet drugs or NSAIDs.
Keywords: warfarin, proton-pump inhibitor, antiplatelet drugs
Warfarin, an oral anticoagulant that inhibits the production of vitamin K-dependent clotting factors,1 is one of the most frequently prescribed medications in the U.S., with more than 30 million prescriptions annually.2 However, warfarin use is limited by frequent major bleeding; in the U.S., there are 29,000 warfarin-related bleeding hospitalizations each year.3 Serious warfarin-related bleeding commonly occurs in the gastrointestinal tract, accounting for 26% to 40% of such bleeds in large atrial fibrillation trials.4-7 Of these, many are upper gastrointestinal,8,9 which are thought to frequently originate from asymptomatic lesions.10
Proton-pump inhibitors (PPIs) reduce gastric acid production, promote ulcer healing, and prevent ulcer recurrence.11 These drugs might prevent warfarin-related upper gastrointestinal bleeding by decreasing the occurrence of spontaneous ulcers or promoting ulcer healing before serious bleeding occurs. However, evidence is limited as to whether or not PPIs prevent clinically important gastrointestinal bleeding during warfarin treatment.12,13 Thus, current guidelines for oral anticoagulant therapy do not recommend PPI co-therapy.14-18
A gastroprotective effect for PPIs would be particularly important for warfarin patients taking either antiplatelet drugs or non-selective nonsteroidal anti-inflammatory agents (NSAIDs), which further increase the risk of serious gastrointestinal bleeding.19 Antiplatelet drugs often are indicated for patients taking oral anticoagulants, who frequently have coronary artery disease treated with low-dose aspirin and who may require a P2Y12 inhibitor for an acute coronary syndrome or following a percutaneous coronary intervention.20 PPIs might be particularly beneficial for warfarin patients with concurrent antiplatelet drug or NSAID use, given that they decrease the risk of upper gastrointestinal ulcers and bleeding induced by low-dose aspirin,21 clopidogrel,22-24 and NSAIDs.21,25 Although guidelines recommend PPIs for most warfarin patients taking antiplatelet drugs or NSAIDs,19,26,27 there are no controlled studies of the efficacy of PPI co-therapy in this high-risk group.
Thus, we conducted a retrospective cohort study of patients beginning warfarin treatment to assess whether the risk of hospitalization for upper gastrointestinal bleeding was reduced with PPI co-therapy. We also studied the effect of PPI co-therapy in warfarin patients concurrently receiving antiplatelet drugs/NSAIDs.
Methods
Sources of Data
The cohort of patients beginning warfarin treatment came from two populations: Tennessee Medicaid enrollees28,29 (1996-2011) and the longitudinal 5% National Medicare Sample (2011-2013).30 Each population had computerized files documenting members’ periods of enrollment and medical care encounters for pharmacy, hospital, outpatient, and nursing home providers, an efficient means to identify the cohort and obtain study data.29 We studied two independent populations both to increase sample size and to examine the consistency of our findings.
Study medication use was identified from pharmacy files that recorded filled prescriptions. These included the dispensing date, drug, quantity, dose, and days of supply. Throughout much of the study period, Tennessee Medicaid had a relatively generous drug benefit, which covered over-the-counter medications such as low-dose aspirin when these were obtained with a prescription. Because Medicare reimbursement policies were more restrictive, the pharmacy files did not include information on low-dose aspirin and over-the-counter NSAIDs. Although some PPIs are available over-the-counter, they only are recommended at low doses and for 14 day-courses up to three times a year. (http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm).
Cohort
The cohort included persons 30 years of age or older in either population whose first prescription for warfarin was filled during the study period. They could not have had any oral anticoagulant prescription in the preceding year (Appendix Table 1). They had to have complete demographic information, full pharmacy benefits and, to assure regular contact with medical care, at least one outpatient visit and one filled prescription in the prior year. Patients could not have end-stage renal disease, serious gastrointestinal illness predisposing to bleeding (e.g., esophageal varices or gastrointestinal cancer), or a bleeding-related hospitalization in the past year (Appendix Table 1).
Patients entered the cohort on the day they filled their first warfarin prescription. Patients left the cohort on the first of: the last day of the study, 365 days with no filling of a warfarin prescription, filling of a prescription for a different oral anticoagulant, loss of enrollment, failure to meet the cohort eligibility criteria, a bleeding-related hospitalization, or death. Patients who left the cohort could reenter if they subsequently met the eligibility criteria.
Medication Exposure
Because the effect of study medications on the risk of bleeding is thought to be acute, each day of study followup was classified according to probable study medication use, as identified from filled prescriptions (Appendix §2). The exposure period was based upon the dispensed days of supply. The definitions of the period of use accounted for the residual effects of some study drugs on the risk of bleeding.
Warfarin treatment during followup was defined as the period during which patients were likely to have increased risk of warfarin-related bleeding complications. This period began on the date the prescription was filled, and, given the long half-life of warfarin, ended 3 days after the end of the days of supply (Appendix §2). All cohort followup and all study analyses were restricted to periods of warfarin treatment.
There were three possible categories of concomitant PPI use during warfarin treatment (Appendix §2). PPI co-therapy consisted of person-days on which the patient was likely to be taking the PPI and thus for which a gastroprotective effect was most plausible. It was defined as the interval between the filling of a PPI prescription through the end of days of supply. Former co-therapy consisted of person-days for patients who had had a PPI prescription but for which there should be no PPI-related gastroprotection. Analysis of this person-time permitted assessment of confounding by unmeasured factors associated with receiving a PPI prescription. No co-therapy consisted of person-days with no filled PPI prescription in the past year.
Concurrent antiplatelet drug or NSAID use indicated whether or not these drugs were used during warfarin treatment. For aspirin and other antiplatelet drugs, concurrent use was defined as present for the interval between the prescription fill and 7 days following the end of the days of supply, given the irreversible anti-platelet effects of these medications (Appendix §2). For NSAIDs, concurrent use was present for the interval between the prescription fill and the end of the days of supply.
Endpoints
The primary study endpoint was hospitalization for upper gastrointestinal bleeding potentially preventable by PPI co-therapy (Appendix §3). This included bleeding related to esophagitis, peptic ulcer disease, and gastritis, but excluded bleeding unlikely to be affected by PPIs (e.g., Mallory Weiss tear). We also examined hospitalizations for other gastrointestinal bleeding (predominantly lower gastrointestinal or gastrointestinal hemorrhages for which upper/lower site not indicated) and for bleeding at other sites (Appendix §3), which should not be affected by PPI co-therapy.
We identified bleeding-related hospitalizations from hospital admissions with a previously validated algorithm (Appendix §3)31 The positive predictive value was 99% for all bleeding-related hospitalizations, 98% for all gastrointestinal bleeding, and 80% for upper gastrointestinal bleeding (Appendix Table 2). The lower positive predictive value for upper gastrointestinal bleeding resulted from occasional use of diagnosis codes that did not specify the gastrointestinal bleeding site.
Analysis
We analyzed the occurrence of endpoints during warfarin treatment according to PPI co-therapy. Adjusted relative risk was estimated with the hazard ratio (HR), with the reference category no PPI co-therapy. HRs were calculated from Cox regression models. Because patients at higher risk of upper gastrointestinal bleeding are more likely to receive PPIs, the models included 61 time-dependent covariates plausibly related to bleeding risk (Appendix Table 3). These included the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization. Separate models were fit for bleeding-related hospitalizations at upper gastrointestinal, non-gastrointestinal, and other sites.
The absolute effect of PPI co-therapy on the risk of bleeding-related hospitalizations in the study cohort was described with the adjusted rate difference. These were estimated as I0*(HR-1), where I0 was the unadjusted incidence in patients without PPI co-therapy and HR the hazard ratio for PPI co-therapy. Confidence intervals were calculated analogously.
We analyzed the effect of PPI co-therapy for patients with concurrent antiplatelet drug or NSAID use (Appendix §2) as well as for those with a history in the past year of risk factors for upper gastrointestinal bleeding (peptic ulcer disease, gastritis, blood in stool/GI bleeding, abdominal pain, or anemia). PPI effect estimates were obtained from single degree of freedom contrasts.
The study was powered (α=.05, 1-β=.80) to detect a 22% reduction in the PPI co-therapy HR for the entire cohort. For warfarin patients with and without concurrent antiplatelet drugs or NSAIDs, the detectable reductions were 38% and 27%.
All statistical analyses were performed with SAS 9.4.
Results
The cohort included 97,430 new episodes of warfarin treatment with 75,720 person-years of active treatment, of which 52,442 were from Tennessee Medicaid and 23,278 from the Medicare sample. There were 14,658 person-years of warfarin treatment with PPI co-therapy, 52,407 person-years with no co-therapy and 8,654 person-years with non-current co-therapy. The indication was atrial fibrillation for more than one-half of the warfarin treatment and deep-vein thrombosis for more than one-fourth (Table 1).
Table 1.
Characteristics of new episodes of warfarin treatment according to PPI co-therapy a.
| No PPI Co-therapy | PPI Co-therapy | |
|---|---|---|
| Person-years warfarin treatmentb | 52,407 | 14,658 |
| Tennesee Medicaid | 71.8% | 63.4% |
| Age, years, mean | 67.7 | 68.2 |
| Female sex | 61.7% | 66.3% |
| Nursing home residence past year | 11.7% | 13.3% |
| Warfarin indication | ||
| Atrial fibrillation | 52.7% | 53.2% |
| Deep-vein thrombosis | 24.7% | 28.7% |
| Other cardiovascular | 14.6% | 11.2% |
| Other or unknown | 7.9% | 6.9% |
| History risk factors for upper GI bleeding | 35.1% | 57.4% |
| Peptic ulcer disease | 3.9% | 15.6% |
| Gastritis | 3.1% | 10.6% |
| Blood in stool/GI bleedingc | 2.6% | 6.1% |
| Abdominal pain | 15.9% | 29.1% |
| Anemiad | 19.2% | 31.2% |
| GERD/dyspepsia | 22.7% | 57.3% |
| Any antiplatelet drug or NSAID | 35.4% | 41.2% |
| Aspirin | 9.9% | 12.1% |
| NSAID | 23.0% | 24.7% |
| P2Y12 or other antiplatelet druge | 9.3% | 14.7% |
| Coxib, current use | 3.4% | 8.2% |
| Smoking | 14.0% | 18.4% |
| AMI, revascularization, angina | 13.7% | 17.5% |
| Stroke or TIA | 23.7% | 25.2% |
| Heart failure | 37.5% | 43.1% |
| Digoxin | 27.9% | 21.8% |
| Loop diuretic | 47.6% | 54.7% |
| Insulin | 13.9% | 17.4% |
| Oral hypoglycemic | 23.8% | 27.3% |
| Alcohol abuse and related illnesses | 1.8% | 1.9% |
| Hospitalization | 54.4% | 63.9% |
| ED visit, any, past 90 days | 27.3% | 32.6% |
Weighted according to person-years of warfarin treatment. Unless otherwise noted, all values are proportions. Unless otherwise stated, disease/medication variables reflect diagnosis/prescription fill during the preceding 365 days. Abbreviations: PPI, proton-pump inhibitor; GI, gastrointestinal; GERD, gastroesophageal reflux disease; NSAID, non-selective nonsteroidal anti-inflammatory drug; Coxib, Cycloxygenase-2 selective nonsteroidal anti-inflammatory drug; TIA = transient ischemic attack; ED, emergency department.
Not shown are 8,654 person-years of followup for non-current PPI co-therapy.
Outpatient or inpatient not meeting endpoint definition (admission not related to bleeding with secondary diagnosis, such as blood in stool, indicating bleeding).
Diagnosis or prescription for iron.
Dipyridamole, cilostazole
Warfarin patients with PPI co-therapy had greater prevalence of risk factors for upper gastrointestinal bleeding than did those without PPI co-therapy (57.4% vs 35.1%, Table 1). These included history of peptic ulcer disease (15.6% vs 3.9%), abdominal pain (29.1% vs 15.9%), and anemia (31.2% vs 19.2%). Those with PPI co-therapy were more likely to have had an antiplatelet drug or NSAID prescription in the past year (41.2% vs 35.4%), including aspirin, non-selective NSAIDs, and P2Y12 inhibitors/other antiplatelet drugs. Findings were similar in both the Tennessee Medicaid and Medicare populations (Appendix Table 4).
There were 3,848 bleeding-related hospitalizations during warfarin treatment, or 508 hospitalizations per 10,000 person-years. Of these, 964 (127 per 10,000 person-years) were upper gastrointestinal, 1,619 (214 per 10,000) were other gastrointestinal sites, and 1,265 (167 per 10,000) were other sites. Warfarin patients from each study population had similar incidence of hospitalizations for bleeding at any site (Medicaid: 494 per 10,000, Medicare: 541 per 10,000) or at upper gastrointestinal sites (Medicaid:130 per 10,000, Medicare 120 per 10,000).
Warfarin patients without PPI co-therapy had 119 hospitalizations for upper gastrointestinal bleeding per 10,000 person-years of treatment (Table 2). The risk decreased by 24% (adjusted hazard ratio [HR] = 0.76, 95% confidence interval [CI] = [0.63-0.91]) with PPI co-therapy, a reduction of 29 (10 to 44) hospitalizations per 10,000 person-years. PPI co-therapy was not significantly associated with reduced risk of other gastrointestinal (HR = 1.07 [0.94-1.22]) or non-gastrointestinal (HR = 0.98 [0.84-1.15]) bleeding hospitalizations. Former PPI co-therapy was not associated with reduced risk of hospitalization for upper gastrointestinal bleeding (HR = 1.00 [0.82-1.21]). Findings were similar in each of the study populations (Appendix Table 5).
Table 2.
Hospitalizations for bleeding during warfarin treatment according to site of the bleeding and PPI co-therapy. HR = adjusted hazard ratio, RD = adjusted rate difference per 10,000, CI = confidence interval. Both the HR and RD are adjusted for the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization (Appendix Table 3 has detailed list of covariates).
| No PPI Co-therapy | Former PPI Co-therapy | PPI Co-therapy | |
|---|---|---|---|
| Person-years | 52,407 | 8,654 | 14,658 |
| Upper gastrointestinal | |||
| Bleeding hospitalizations | 624 | 159 | 181 |
| Rate/10,000 person-years | 119.1 | 183.7 | 123.5 |
| HR (95% CI) | 1.00 ( 0.82- 1.21) | 0.76 ( 0.63- 0.91) | |
| RD (95% CI) | -0.3 ( -21.0 to 24.7) | -28.7 ( -44.0 to -10.3) | |
| p-value | 0.9799 | 0.0035 | |
| Other gastrointestinal | |||
| Bleeding hospitalizations | 936 | 280 | 403 |
| Rate/10,000 person-years | 178.6 | 323.5 | 274.9 |
| HR (95% CI) | 1 | 1.10 ( 0.95- 1.27) | 1.07 ( 0.94- 1.22) |
| RD (95% CI) | 17.1 ( -9.5 to 47.9) | 13.1 ( -10.6 to 40.1) | |
| p-value | 0.2195 | 0.2927 | |
| Other major bleeds | |||
| Bleeding hospitalizations | 801 | 205 | 259 |
| Rate/10,000 person-years | 152.8 | 236.9 | 176.7 |
| HR (95% CI) | 1.13 ( 0.95- 1.33) | 0.98 ( 0.84- 1.15) | |
| RD (95% CI) | 19.1 ( -7.4 to 50.4) | -3.0 ( -24.8 to 22.4) | |
| p-value | 0.1676 | 0.8014 |
Warfarin patients without PPI co-therapy who also had concurrent antiplatelet drug or NSAID use had 283 hospitalizations for upper gastrointestinal bleeding per 10,000 person years of warfarin treatment (Figure 1). The risk decreased by 45% (HR = 0.55 [0.39-0.77]) with PPI co-therapy, a reduction of 128 (66 to 173) hospitalizations per 10,000 person-years. The gastroprotective effect of PPI co-therapy did not differ significantly according to specific antiplatelet drug or NSAID (Table 3). In contrast, for warfarin patients without concurrent antiplatelet drug or NSAID use, the HR for PPI co-therapy was 0.86 (0.70-1.06), which differed significantly from that for patients with concurrent antiplatelet drug or NSAID use (p = .0109).
Figure 1.
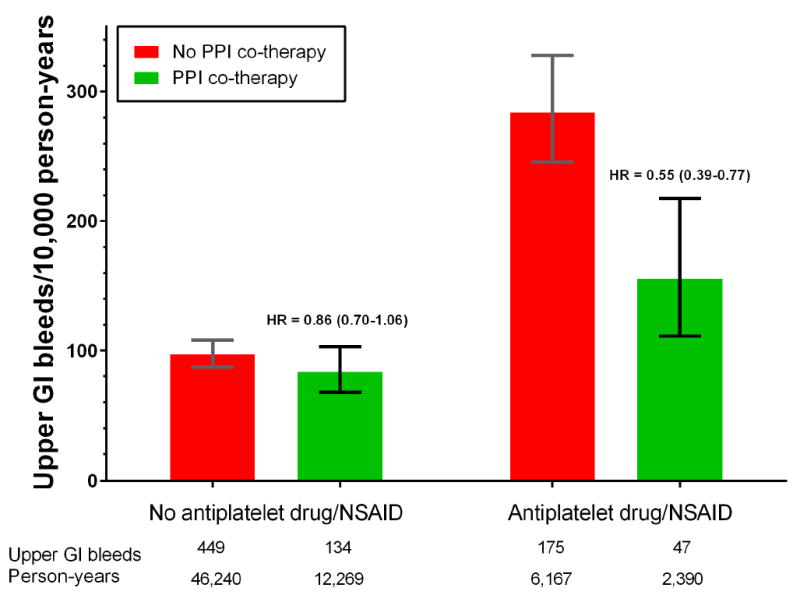
Incidence of warfarin-related upper gastrointestinal bleeding according to PPI co-therapy. Vertical bars indicate 95% confidence intervals. Stratified by concurrent use of antiplatelet drugs or nSAIDs. Incidence for patients with no PPI co-therapy (I0) is unadjusted (confidence intervals calculated assuming Poisson distribution); that for patients with PPI co-therapy calculated as I0*HR, where HR is the adjusted hazard ratio for PPI co-therapy (confidence intervals calculated analogously). HRs adjusted for the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization (See Appendix Table 3 for detailed list of covariates). Numbers below the x-axis are the number of hospitalizations for upper gastrointestinal bleeding and the person-years of warfarin treatment.
Table 3.
Hospitalizations for upper gastrointestinal (UGI) bleeding during warfarin treatment according to PPI co-therapy and concurrent use of specific antiplatelet drugs or NSAIDs. HR = adjusted hazard ratio, RD = adjusted rate difference per 10,000, CI = confidence interval. Both the HR and RD are adjusted for the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization (Appendix Table 3 has detailed list of covariates).
| No PPI Co-therapy | PPI Co-therapy | HR (95% CI) | p-value | RD (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Person -years | UGI Bleeds | Rate/10,000 | Person-years | UGI Bleeds | Rate/10,000 | ||||
| No concurrent antiplatelet drug or NSAID | 46,240 | 449 | 97.1 | 12,269 | 134 | 109.2 | 0.86 ( 0.70 - 1.06 ) | .1608 | -13.5 ( -29.3 to 6.0 ) |
| Any concurrent antiplatelet drug or NSAID | 6,167 | 175 | 283.8 | 2,390 | 47 | 196.7 | 0.55 ( 0.39 - 0.77 ) | .0004 | -128.2 ( -172.4 to -66.4 ) |
| Aspirin only | 1,783 | 47 | 263.5 | 648 | 12 | 185.3 | 0.51 ( 0.27 - 0.97 ) | .0388 | -129.5 ( -193.0 to -9.0 ) |
| NSAID only | 2,346 | 71 | 302.7 | 745 | 15 | 201.2 | 0.56 ( 0.32 - 0.98 ) | .0423 | -134.1 ( -206.9 to -6.1 ) |
| P2Y12/other antiplatelet only | 1,626 | 42 | 258.4 | 749 | 13 | 173.5 | 0.54 ( 0.29 - 1.01 ) | .0536 | -119.0 ( -183.9 to 2.5 ) |
| Multiple drugs | 412 | 15 | 363.9 | 248 | 7 | 282.8 | 0.63 ( 0.25 - 1.54 ) | .3089 | -136.1 ( -271.5 to 197.6 ) |
In the absence of PPI co-therapy, the incidence of upper gastrointestinal bleeding hospitalizations during warfarin treatment increased with both a history of risk factors for upper gastrointestinal bleeding and concurrent antiplatelet drug or NSAID use (Figure 2). For patients with no history of risk factors, hospitalizations for upper gastrointestinal bleeding during warfarin treatment increased from 68 to 224 per 10,000 person-years with concurrent antiplatelet drugs or NSAIDs, 156 (109 to 204) additional hospitalizations per 10,000 person-years. For patients with a history of risk factors, hospitalizations for upper gastrointestinal bleeding increased from 152 to 392 per 10,000 person-years with concurrent antiplatelet drug or NSAID use, 240 (155 to 325) additional hospitalizations per 10,000 person-years.
Figure 2.
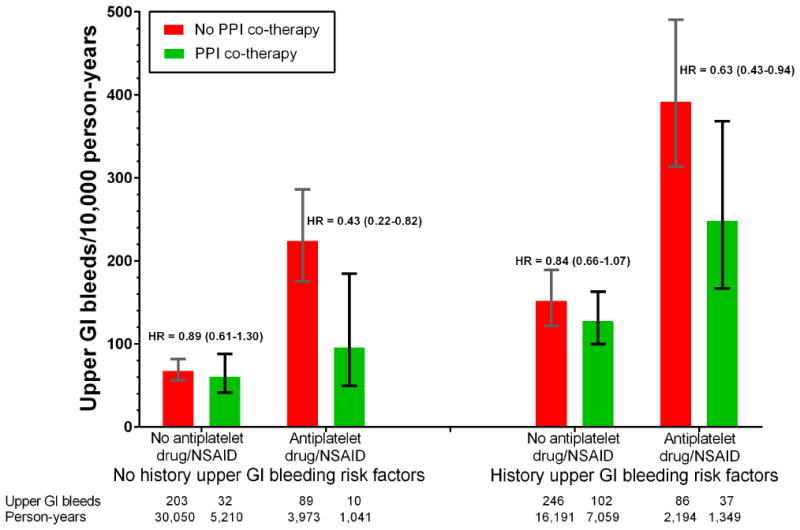
Incidence of warfarin-related upper gastrointestinal bleeding according to PPI co-therapy. Vertical bars indicate 95% confidence intervals. Stratified by concurrent use of antiplatelet drugs or NSAIDs and history of risk factors for upper gastrointestinal bleeding (peptic ulcer, gastritis, abdominal pain, blood in stool/GI bleeding, anemia in past year). Incidence for patients with no PPI co-therapy (I0) is unadjusted (confidence intervals calculated assuming Poisson distribution); that for patients with PPI co-therapy calculated as I0*HR, where HR is the adjusted hazard ratio for PPI co-therapy (confidence intervals calculated analogously). HRs adjusted for the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization (See Appendix Table 3 for detailed list of covariates). Numbers below the x-axis are the number of hospitalizations for upper gastrointestinal bleeding and the person-years of warfarin treatment.
The gastroprotective effect of PPI co-therapy for warfarin patients was only significant for those with concurrent antiplatelet drug or NSAID use, irrespective of the history of risk factors for upper gastrointestinal bleeding (Figure 2). For patients with concurrent antiplatelet drug or NSAID use but with no history of risk factors, the HR was 0.43 (0.22-0.83), whereas for those with a history of risk factors, the HR was 0.64 (0.43-0.94). In contrast, absent concurrent antiplatelet drug or NSAID use, the gastroprotective effect of PPI co-therapy was smaller and not statistically significant for those either with or without a history of risk factors for upper gastrointestinal bleeding.
We analyzed the effects of PPI co-therapy in warfarin patients according to concurrent antiplatelet drug or NSAID use and history of upper gastrointestinal bleeding risk factors for the individual study populations. Findings in each population were similar to those from the primary analyses (Appendix Tables 6-7).
In the primary analysis, all covariates could change during warfarin treatment, since PPI co-therapy could be initiated for gastrointestinal conditions that developed after cohort entry. However, some covariates could be on the causal pathway between PPI co-therapy and reduced risk of upper gastrointestinal bleeding hospitalization. We performed a sensitivity analysis in which 1) the only time-dependent covariates were PPI co-therapy, concurrent antiplatelet drug or NSAID use, and other drugs associated with gastrointestinal bleeding and 2) change in clinical status from baseline was limited by restricting followup to one year. The HR for PPI co-therapy was 0.77 (0.61-0.96) for all warfarin patients and 0.55 (0.36-0.84) for those with concurrent antiplatelet drug or NSAID use.
We also performed an analysis in which patients could not reenter the cohort. The HR for PPI co-therapy was 0.75 (0.62-0.91) for all warfarin patients and 0.53 (0.37-0.75) for those with concurrent antiplatelet drug or NSAID use.
Discussion
We sought to determine if PPI co-therapy could reduce the risk of serious upper gastrointestinal bleeding in patients treated with warfarin, given the frequency of this life-threatening complication and the absence of specific recommendations in current guidelines.14-18 In this retrospective cohort study in two populations of patients initiating warfarin treatment, PPI co-therapy was associated with a statistically significant 24% reduction in the incidence of hospitalizations for upper gastrointestinal bleeding, or 29 fewer hospitalizations per 10,000 person-years of warfarin treatment. In contrast, the protective effect was not present for hospitalizations for bleeding at other sites, nor was it present for patients who had stopped PPI co-therapy. Findings were similar in both the Tennessee Medicaid and Medicare 5% sample populations.
Our findings were generally consistent with those of previous smaller investigations of oral anticoagulants. Three case-control studies of the effects of gastroprotective co-therapy during treatment with a variety of medications that increase bleeding risk, including oral anticoagulants, have reported a non-significant protective effect for PPIs.12,32,33 A recent study by Chan and colleagues13 reported that PPI co-therapy during dabigatran treatment was associated with a 47% (9% - 69%) reduction in gastrointestinal bleeding. However, these previous studies did not present data for the high risk group of patients for whom the incidence of warfarin-related bleeding is elevated because they also take antiplatelet drugs or NSAIDs.19
Antiplatelet drugs often are indicated for warfarin patients with coronary artery disease, acute coronary syndrome or percutaneous coronary intervention.20 We found that in the absence of PPI co-therapy, the incidence of hospitalizations for upper gastrointestinal bleeding increased nearly three-fold with concurrent antiplatelet drug or NSAID use. However, for these high-risk patients, PPI co-therapy was associated with a 45% decreased risk, or 128 fewer hospitalizations per 10,000 person-years of warfarin treatment. Even for patients without a history of risk factors for upper gastrointestinal bleeding, concurrent antiplatelet drug or NSAID use more than tripled the incidence of upper gastrointestinal bleeding hospitalizations, but PPI co-therapy in this group was associated with a 57% decreased risk.
We did not find a significant protective effect of PPI co-therapy for warfarin patients without concurrent antiplatelet drug or NSAID use. The benefits of PPIs may be limited to patients who also take antiplatelet drugs or NSAIDs, for whom they promote healing of or prevent bleeding in otherwise asymptomatic lesions. However, the magnitude of the gastroprotective effect of PPI co-therapy in other patients may be lower than that which our study was powered to detect. The point estimate of a 14% protective effect, with an upper 95% CI bound of 30%, indicate that our data cannot rule out clinically important gastroprotection for these patients. Further studies are needed to clarify the role of PPI co-therapy for warfarin patients without concurrent antiplatelet drug or NSAID use.
There is a theoretical concern that PPI co-therapy could increase warfarin concentration, given that in vitro some PPIs inhibit metabolism by hepatic cytochrome P450 2C19 (CYP2C19),34 a minor pathway for warfarin metabolism. However, there are conflicting data on the clinical importance of this finding. A study of 82 Japanese patients prescribed either lansoprazole (n=41) or rabeprazole (n=41) at the initiation of warfarin treatment following cardiac surgery found greater risk of bleeding in the group receiving lansoprazole, the more potent CYP2C19 inhibitor.35 However, a subsequent study of 305 warfarin patients in Denmark reported that initiation of a PPI was not associated with a change in the international normalized ratio (INR).36 Our findings suggest that in practice, any adverse effect of PPI co-therapy on warfarin metabolism is outweighed by gastroprotective effects.
The limitations of this observational study generally should lead to underestimation of a PPI gastroprotective effect. Information on over-the-counter medications was likely to be incomplete, particularly for the Medicare population. This would result in misclassification of both aspirin and NSAID use, which should cause underestimation of PPI effects, given that both medications are strong risk factors for gastrointestinal bleeding and were positively correlated with PPI co-therapy in the study cohort. There also will be some misclassification of PPI co-therapy, which if non-differential, would bias to the null. Although we measured an extensive set of covariates, patients starting warfarin treatment could have had unrecorded risk factors for upper gastrointestinal bleeding, including Helicobacter pylori infection. Given the strong correlation we observed between the recorded risk factors and increased likelihood of PPI co-therapy, confounding by unmeasured risk factors also should lead to underestimation of the PPI effect. The concordance of the findings in the two study populations and the absence of an effect of PPI co-therapy on the risk of bleeding at either other gastrointestinal or non-gastrointestinal sites suggest that the influence of these factors was limited.
The study cohort came from two distinct populations: Tennessee Medicaid, a low-income population with a high proportion of patients enrolled because of illness; and the Medicare 5% sample, a national sample of Medicare enrollees restricted to those with fee-for-service plans and part D coverage. The incidence of bleeding hospitalizations for all sites and for upper gastrointestinal sites was very similar in the two populations. The magnitudes of PPI protective effect were comparable for both the entire population and for subgroups defined according to antiplatelet drug or NSAID use or history of risk factors for upper gastrointestinal bleeding. This suggests that our findings were not due to the specific characteristics of either the study populations or sources of data.
In summary, for patients initiating warfarin treatment in Tennessee Medicaid and the Medicare 5% sample, PPI co-therapy was associated with a statistically significant 24% reduction in the incidence of hospitalizations for upper gastrointestinal bleeding during warfarin treatment. A significant reduction in risk was found only among warfarin patients with concurrent antiplatelet drug or NSAID use. Although we cannot rule out a small gastroprotective effect among other warfarin patients, no significant associations were found for this group.
Acknowledgments
Support. Supported in part by a grant from the National Heart, Lung, and Blood Institute (HL114518). Dr. Chung was funded by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR064768) and the Rheumatology Research Foundation Career Development K Supplement. We gratefully acknowledge the Tennessee Bureau of TennCare and the Department of Health, which provided study data.
Appendix
This appendix provides additional details for the study of warfarin and proton-pump inhibitors (PPIs) and should be read in conjunction with the primary manuscript (MS).
1. Cohort
Tennessee Medicaid
The study Medicaid database included enrollment, pharmacy, hospital, outpatient, and nursing home files and was augmented with linkage to death certificates1;2 and a statewide hospital discharge database.1;3 Files for the period 1995 through 2011 were used.
Medicare 5% National Sample
The longitudinal 5% national sample of Medicare enrollees is selected on the basis of the 8th and 9th digits of the health insurance number.4 Study files included the beneficiary summary file (enrollment/demographics), Part D events file (filled prescriptions), MEDPAR file (inpatient/SNF), outpatient standard analytic file (institutional outpatient), Medicare Carrier file (non-institutional outpatient), and hospice file. Files for the period 2010 through 2013 were used.
Qualifying episodes warfarin use
The cohort included patients beginning warfarin treatment during the period 1/1/1996 through 12/31/2011 for Tennessee Medicaid and 1/1/2011 through 12/31/2013 for the Medicare 5% National Sample. To enter the cohort, patients had to meet study inclusion/exclusion criteria (Appendix Table 1) on the day the prescription was filled (t0). Appendix Table 1 shows the numbers of potential cohort members who successively met the inclusion/exclusion criteria. Because a person who left the cohort could subsequently reenter (see below), we also show the number of new episodes of warfarin treatment.
Overlap
From 2006 on, Tennessee Medicaid did not provide pharmacy benefits to persons eligible for Medicare, as these could be obtained through Medicare part D. Such enrollees were excluded from the Medicaid cohort because they did not meet criterion 1. Thus, the two cohorts should not overlap.
Appendix Table 1.
Qualifying episodes of warfarin use.
| Criterion | Medicaid Episodes (persons) | Medicare Episodes (persons) |
|---|---|---|
|
| ||
| 1. Filled prescription for warfarin during study period for a patient 30 years of age or older. For Tennessee Medicaid, the patient must be enrolled for the prior year in a category with full pharmacy benefits. For the Medicare 5% sample, the patient must be enrolled in Medicare parts A, B, and D but not in part C (Medicare Advantage) in the month the warfarin prescription was filled and in the preceding 12 calendar months. | 92,856 (83,807) | 118,832 (116,016) |
|
| ||
| 2. New treatment episode. No other filled prescription for any oral anticoagulant in the past year. | 64,015 (58,503) | 46,868 (46,021) |
|
| ||
| 3. At least one outpatient visit (excluding laboratory claims) and one filled prescription(excluding the warfarin prescription) in the past year to assure that patients have had regular contact with medical care. | 61,618 (56,471) | 46,096 (45,273) |
|
| ||
| 4. No end-stage renal disease in the past year, in order to exclude patients with use of warfarin for dialysis. | 59,157 (54,297) | 44,108 (43,335) |
|
| ||
| 5. No serious upper GI illness in the past year predisposing to frequent GI bleeding or altering the pathology of peptic ulcer disease. This excludes persons who may have frequent care for bleeding from other causes, such as esophageal varices or gastrointestinal cancer. | 57,178 (52,516) | 41,963 (41,243) |
|
| ||
| 6. No prior bleeding-related hospitalization in the past year, defined as a hospital stay meeting the study endpoint definition. We excluded these patients because it would be difficult to determine if subsequent bleeding hospitalizations represented a new event or continued care for bleeding that began prior to warfarin treatment. The numbers of exclusions for each type of bleeding hospitalization are shown in the columns. | 56,114 (51,584) | 41,316 (40,618) |
| a. Upper gastrointestinal | 336 (301) | 164 (161) |
| b. Other gastrointestinal | 417 (362) | 313 (300) |
| c. Other bleed. | 311 (269) | 170 (164) |
Patients entered the cohort on the date of the warfarin fill. Patients left the cohort on the first of the following dates:
End of warfarin use: one year with no filled warfarin prescription;
The last day of the study, 12/31/2011 for Medicaid and 12/31/2013 for Medicare;
Day prior to filling of a prescription for a non-study oral anticoagulant;
Last day of enrollment, including either loss of enrollment or transition to a category without full pharmacy benefits. For Medicare, transfer to Medicare Part C was considered loss of enrollment.
Day prior to failure to meet inclusion/exclusion criteria;
Day of a study endpoint;
Date of death.
Patients who left the cohort could reenter if they subsequently met the study criteria. This included patients with an endpoint if they subsequently had at least one year with no endpoint. Because a single person could have multiple endpoints, which could violate statistical independence assumptions, we performed a sensitivity analysis that excluded such patients.
2. Study Medication Use during Followup
The study analysis required identifying periods of exposure to warfarin, PPIs, and antiplatelet drugs/NSAIDs. Because these medications are thought to alter the risk of bleeding only while the patients are taking the drugs, we tracked study medication exposure during followup on a day-by-day basis.
Filled prescriptions provided a surrogate measure for drug use during followup. Periods of drug exposure were defined according to the date the prescription was filled and the dispensed days of supply. The maximum days of supply per prescription was 31 days for Tennessee Medicaid and 90 days for Medicare. Because medication regimens often change during a hospital stay, we ended the prescription days of supply when the patient was admitted to the hospital. Study medication use would resume if and when the patient refilled the medication after hospital discharge.
Some of the study drugs can affect the risk of bleeding for a few days following cessation of use. Thus, the definitions of the exposure periods varied slightly according to drug, as described below.
Warfarin treatment
The risk of warfarin-related bleeding should only be present while patients are taking the drug. Thus, all study analyses were restricted to periods of warfarin treatment during followup, defined as the interval from the date the prescription was filled through 3 days after the end of the days of supply. Although some warfarin effects can persist for up to 5 days (e.g., surgery is not recommended within 5 days of use), our assessment of the literature is that much of the risk for increased bleeding is gone by day 3.
PPI co-therapy
All warfarin treatment was classified into three categories according to concomitant use of PPIs (dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole).:
PPI co-therapy was the period during which gastroprotective effects were most plausible, defined as the interval from the filling of the prescription through the end of the dispensed days of supply.
No co-therapy indicated person-time for which no PPI prescription had been filled in the past 365 days.
Former PPI co-therapy indicated person-time for persons who had received a PPI prescription but should not have gastroprotection. This category permits assessment of confounding by unmeasured factors associated with being prescribed a PPI. It was defined as a) the period between the end of current co-therapy and the beginning of no co-therapy; or b) the first day for a new course of therapy (none past 365 days), given the implausibility of a gastroprotective effect on that day and the possibility that the PPI was started to treat a gastrointestinal bleed. Non-current use also includes person-time during which PPI exposure may be misclassified because patients were taking the drug on an as-needed schedule.
Concurrent antiplatelet drug/NSAID use
The study antiplatelet drugs were aspirin, P2Y12 inhibitors, and other antiplatelet drugs (dipyridamole and cilostazole). NSAIDs comprised the non-selective, nonsteroidal anti-inflammatory drugs other than aspirin. Concurrent antiplatelet drug/NSAID use during followup was dichotomized as either present or absent. For aspirin and other antiplatelet drugs, concurrent use was present for the interval from the filling of the prescription through 7 days following the end of the days of supply, given that these medications irreversibly inhibit platelets. The period of concurrent use for non-aspirin NSAIDs was the interval from the filling of the prescription through the end of the days of supply.
Medication use examples
Appendix Figure 1 provides examples of the medication use definitions. For convenience, the examples only depict the first 180 days of cohort followup. There are seven hypothetical patients with warfarin treatment, each illustrating a common pattern of warfarin use.
Patient starts warfarin therapy and has no other study medications. The entire followup period is included in the analysis as warfarin treatment.
Patient receives two 60 day courses of warfarin therapy separated by 60 days. The interval between the warfarin courses is not considered warfarin treatment and is excluded from the analysis. Thus, patient 2 would contribute 63+63 = 126 person-days of warfarin treatment to study analyses. The remaining 54 days of person-time for that patient would not be considered.
Patient is started on warfarin and a PPI. The entire 180 days of followup will be classified as warfarin treatment with PPI co-therapy.
A patient begins warfarin, a PPI, and a P2Y12 inhibitor. The entire 180 days of followup will be classified as warfarin treatment with concurrent antiplatelet drug/NSAID use and PPI co-therapy.
A patient begins warfarin and a P2Y12 inhibitor, but there is no PPI prescription within the past year. The entire 180 days of followup will be classified as warfarin treatment with concurrent antiplatelet drug/NSAID use. A PPI is started at day 61. Days 1 through 60 will be classified as no PPI co-therapy and days 62 through 180 as PPI co-therapy. Day 61 is classified as former PPI co-therapy
Patient receives 90 days of warfarin therapy with no history of PPI use in the past year. Shortly after therapy begins, an NSAID is started. Subsequent to the NSAID, a PPI prescription is filled. The first 30 days of followup is classified as warfarin treatment with neither PPI co-therapy nor concurrent antiplatelet drug/NSAID use. The next 30 days is warfarin treatment with concurrent antiplatelet drug/NSAID use, but with no PPI co-therapy. The remaining person-time is warfarin treatment with both concurrent antiplatelet drug/NSAID use and PPI co-therapy.
Patient has intermittent warfarin and PPI therapy. The gaps in warfarin therapy are not included in the study analysis. The periods where warfarin and PPI use overlap are warfarin treatment with PPI co-therapy, the other periods of warfarin therapy are warfarin treatment with former PPI co-therapy.
Figure 1.

Medication use patterns for 7 hypothetical warfarin patients: 1) Long-term warfarin use, no other study medications 2) Two 60 day warfarin courses separated by 60 days; 3)Warfarin with PPI; 4) Warfarin + PPI + P2Y12; 5) Warfarin + P2Y12, PPI starts after warfarin; 6) 90 day warfarin course, NSAID after warfarin start and subsequently PPI; 7) Intermittent warfarin and PPI therapy.
3. Serious Bleeding Endpoints
Serious bleeding endpoints were hospitalizations with diagnoses and procedures indicating that the hospitalization was primarily related to a major bleed. These hospitalizations were classified according to the probable site of the bleeding:
Gastroduodenal;
Esophageal, other than gastroesophageal reflux disease;
Upper gastrointestinal, unspecified as to esophageal or gastroduodenal;
Upper gastrointestinal, angiodysplasia;
Lower gastrointestinal;
Unspecified gastrointestinal, possibly esophageal, upper, or lower;
Multiple gastrointestinal;
Genitourinary;
Cerebral;
Other specified site;
Unspecified site;
Multiple sites.
The upper GI sites were classified as those that should (1 and 3) or should not (2 and 4) be affected by PPI co-therapy. The primary study endpoint, upper gastrointestinal bleeding, was the composite of sites 1 and 3. The other GI site endpoint was the composite of sites 2, 4, 5, 6, 7.
We identified bleeding-related hospitalizations and assigned a bleeding site using a previously validated algorithm5 with minor modifications. The primary modification was of the method for distinguishing upper and lower GI bleeding when the hospital discharge diagnosis was 578.1 or 578.9, which can indicate either an upper or lower site. Previously, we relied upon procedure codes: for example, if there was a procedure code for upper GI endoscopy, but no diagnosis compatible with upper GI bleeding, we would assign an upper site. We modified this definition because an endoscopy coupled with discharge diagnoses not indicating upper GI bleeding may reflect a negative diagnostic evaluation. The revised algorithm is available from the authors on request.
We assessed the performance of the modified algorithm for the primary study endpoint of upper gastrointestinal bleeding (UGIB). This utilized the 239 completed chart adjudications for bleeding hospitalizations from our previous validation study.5 The performance of algorithms for identifying any gastrointestinal bleeding did not change: Positive predictive value (PPV) = 102/103 = 99.0%; sensitivity = 102/103 = 99.0%. UGIB performance was based on 103 cases of gastrointestinal bleeding identified by both algorithms and with completed chart adjudication (Appendix Table 2)
Appendix Table 2.
Performance of modified algorithm for upper gastrointestinal bleeds.
| Published algorithm | |||
|---|---|---|---|
| Upper GI Bleed | No Upper GI Bleed | All | |
| Computer UGIB | 34 | 10 | 44 |
| No Computer UGIB | 5 | 54 | 59 |
| All | 39 | 64 | 103 |
| Revised Algorithm | |||
| Upper GI Bleed | No Upper GI Bleed | All | |
| Computer UGIB | 33 | 8 | 41 |
| No Computer UGIB | 6 | 56 | 62 |
| All | 39 | 64 | 103 |
For the published algorithm, the PPV was 77.3% and the sensitivity 87.1%. For the revised algorithm, the PPV was 80.5% and the sensitivity was 84.6%.
We also assigned a date of the bleeding onset. For 92% of the cases of the primary endpoint, this was the date of the hospital admission. When there was evidence that the bleeding began earlier (e.g., hospitalization for bleeding peptic ulcer with preceding day ED visit with hematemesis diagnosis) the date was reset: 6% to the day prior to hospital admission, 1% 2-7 days prior, and 1% 8-30 days prior.
4. Study Covariates
Appendix Table 3 shows all of the study covariates, according to PPI co-therapy status.
Appendix Table 3.
Study covariates to PPI Co-therapy
| No PPI Cotherapy | PPI Cotherapy | |
|---|---|---|
| Person-years warfarin treatment | 52,407 | 14,658 |
|
| ||
| Demographic characteristics | ||
|
| ||
| Age, years | 67.7 | 68.2 |
| Female sex | 61.7% | 66.3% |
| Year 1996-1999 | 20.4% | 2.2% |
| Year 2000-2002 | 24.4% | 17.1% |
| Year 2003-2005 | 19.7% | 41.0% |
| Year 2006-2011 | 12.1% | 8.8% |
| Year 2012-2013 | 23.4% | 30.9% |
| Medicaid, disability enrollment | 51.1% | 49.2% |
| Medicaid, other enrollment | 20.7% | 14.2% |
| Medicare | 28.2% | 36.6% |
| Nursing home residence past year | 11.7% | 13.3% |
| Warfarin first 30 days of treatment | 10.3% | 8.8% |
|
| ||
| Warfarin indication | ||
|
| ||
| Indication: Atrial fibrillation | 52.7% | 53.2% |
| Indication: Deep-vein thrombosis | 24.7% | 28.7% |
| Indication: Other cardiovascular | 14.6% | 11.2% |
| Indication: Other or unknown | 7.9% | 6.9% |
|
| ||
| Gastrointestinal disease | ||
|
| ||
| Peptic ulcer disease past 365 days | 3.9% | 15.6% |
| Gastritis | 3.1% | 10.6% |
| Other upper GI | 3.0% | 12.8% |
| Lower GI disease | 15.2% | 26.9% |
| Blood stool/GI bleeding past 90 days | 0.8% | 1.9% |
| Blood stool/GI bleeding past 91-365 days | 1.8% | 4.2% |
| Abdomen_Pain_30 | 2.2% | 4.4% |
| Epigastric/abdominal pain past 90 days | 3.7% | 7.2% |
| Epigastric/abdominal pain past 91-365 days | 12.2% | 21.9% |
| GERD/dyspepsia past 365 days | 22.7% | 57.3% |
| Lower GI symptoms | 5.0% | 10.5% |
|
| ||
| Risk factors for warfarin-related bleeding | ||
|
| ||
| Non-GI warfarin-related bleeding | 9.2% | 12.0% |
| Anemia past 30 days | 3.5% | 5.4% |
| Anemia past 90 days | 8.0% | 13.8% |
| Anemia past 91-365 days | 9.4% | 15.5% |
| Abnormal coagulation profile past 365 days | 5.9% | 7.9% |
| Transfusion | 7.1% | 11.0% |
|
| ||
| Medications affecting gastrointestinal bleeding | ||
|
| ||
| H2RA, any | 25.7% | 19.5% |
| Aspirin, current use | 4.0% | 5.8% |
| NSAID, current use, not new start | 4.7% | 5.6% |
| NSAID, current use, new start | 0.3% | 0.3% |
| Coxib, current use | 3.4% | 8.2% |
| Clopidogrel, current use | 2.8% | 5.2% |
| Other antiplatelet drug, current use | 0.9% | 1.3% |
| Other anticoagulant, current use | 0.6% | 0.9% |
| Corticosteroid (systemic), current use | 3.6% | 6.8% |
| SSRI, current use | 14.6% | 27.7% |
| Antibiotic, current use | 8.6% | 12.5% |
|
| ||
| Cardiovascular comorbidity | ||
|
| ||
| AMI, revascularization, angina | 13.7% | 17.5% |
| Stroke or TIA | 23.7% | 25.2% |
| Heart failure | 37.5% | 43.1% |
| Diabetes | 34.9% | 40.7% |
| Peripheral vascular disease | 11.4% | 13.3% |
| Renal failure | 7.6% | 12.4% |
| Smoking | 14.0% | 18.4% |
| Hypovolemia | 7.1% | 12.0% |
| Alcohol abuse and related illnesses | 1.8% | 1.9% |
| Liver disease | 3.0% | 5.2% |
|
| ||
| Medical care utilization | ||
|
| ||
| GI hospitalization past 90 days | 0.9% | 1.7% |
| GI hospitalization past 91-365 days | 2.3% | 4.8% |
| Other hospitalization past 90 days | 22.9% | 25.3% |
| Other hospitalization past 91-365 days | 30.5% | 36.8% |
| Hospital days: GI 10 or more | 0.7% | 1.7% |
| ED visit, GI, past year | 5.1% | 9.4% |
| ED visit, any, past 90 days | 27.3% | 32.6% |
5. Additional Findings
Appendix Table 4.
Characteristics of new episodes of warfarin treatmenta according to study population and PPI co-therapy.
| a. Tennessee Medicaid | ||
|---|---|---|
| No PPI Co-therapy | PPI Co-therapy | |
| Person-years warfarin treatmentb | 37,626 | 9,289 |
| Tennessee Medicaid | 100.0% | 100.0% |
| Age, years | 64.1 | 63.8 |
| Female sex | 62.4% | 66.2% |
| Nursing home residence past year | 12.6% | 12.6% |
| Warfarin indication | ||
| Atrial fibrillation | 46.6% | 46.4% |
| Deep-vein thrombosis | 26.2% | 30.7% |
| Other cardiovascular | 18.7% | 15.3% |
| Other or unknown | 8.4% | 7.7% |
| History risk factors for upper GI bleeding | 34.0% | 56.6% |
| Peptic ulcer disease | 4.3% | 16.6% |
| Gastritis | 3.4% | 11.1% |
| Blood in stool/GI bleedingc | 2.7% | 6.3% |
| Abdominal pain | 16.5% | 30.7% |
| Anemiad | 16.7% | 26.7% |
| GERD/dyspepsia | 21.8% | 55.3% |
| Any antiplatelet drug/NSAID | 40.4% | 47.9% |
| Aspirin | 13.7% | 19.1% |
| Non-selective NSAID | 27.0% | 28.6% |
| P2Y12 or other antiplatelet druge | 8.4% | 14.9% |
| Coxib, current use | 4.3% | 11.8% |
| Smoking | 13.0% | 16.8% |
| AMI, revascularization, angina | 14.9% | 19.2% |
| Stroke or TIA | 23.4% | 23.0% |
| Heart failure | 38.0% | 42.6% |
| Digoxin | 33.0% | 26.9% |
| Loop diuretic | 50.9% | 58.2% |
| Insulin | 15.7% | 19.1% |
| Oral hypoglycemic | 24.8% | 29.3% |
| Alcohol abuse and related illnesses | 2.3% | 2.5% |
| Hospitalization | 55.5% | 64.6% |
| ED visit, any, past 90 days | 28.2% | 33.8% |
| b Medicare 5% Sample | ||
| No PPI Co-therapy | PPI Co-therapy | |
| Person-years warfarin treatmentb | 14,781 | 5,369 |
| Tennessee Medicaid | 0.0% | 0.0% |
| Age, years | 77.0 | 75.9 |
| Female sex | 60.1% | 66.6% |
| Nursing home residence past year | 9.2% | 14.5% |
| Warfarin indication | ||
| Atrial fibrillation | 68.3% | 65.1% |
| Deep-vein thrombosis | 20.8% | 25.4% |
| Other cardiovascular | 4.2% | 4.1% |
| Other or unknown | 6.7% | 5.4% |
| History risk factors for upper GI bleeding | 37.9% | 58.7% |
| Peptic ulcer disease | 3.1% | 13.8% |
| Gastritis | 2.2% | 9.6% |
| Blood in stool/GI bleedingc | 2.3% | 5.7% |
| Abdominal pain | 14.5% | 26.4% |
| Anemiad | 25.7% | 39.0% |
| GERD/dyspepsia | 24.8% | 60.9% |
| Any antiplatelet drug/NSAID | 22.8% | 29.7% |
| Aspirin | 0.0% | 0.0% |
| Non-selective NSAID | 12.9% | 17.9% |
| P2Y12 or other antiplatelet druge | 11.6% | 14.3% |
| Coxib, current use | 1.1% | 1.8% |
| Smoking | 16.7% | 21.2% |
| AMI, revascularization, angina | 10.5% | 14.6% |
| Stroke or TIA | 24.5% | 29.0% |
| Heart failure | 36.2% | 43.8% |
| Digoxin | 15.0% | 13.1% |
| Loop diuretic | 39.3% | 48.6% |
| Insulin | 9.3% | 14.6% |
| Oral hypoglycemic | 21.4% | 23.9% |
| Alcohol abuse and related illnesses | 0.5% | 0.9% |
| Hospitalization | 51.7% | 62.7% |
| ED visit, any, past 90 days | 25.0% | 30.6% |
Weighted according to person-years of warfarin treatment. Unless otherwise noted, all values are proportions. Unless otherwise stated, disease/medication variables reflect diagnosis/prescription fill during the preceding 365 days. Abbreviations: PPI, proton-pump inhibitor; GI, gastrointestinal; GERD, gastroesophageal reflux disease; NSAID, non-selective nonsteroidal anti-inflammatory drug; Coxib, Cycloxygenase-2 selective nonsteroidal anti-inflammatory drug; TIA = transient ischemic attack; ED, emergency department.
Not shown are 8,654 person-years of followup for non-current PPI co-therapy.
Outpatient or inpatient not meeting endpoint definition (admission not related to bleeding with secondary diagnosis, such as blood in stool, indicating bleeding).
Diagnosis or prescription for iron.
Dipyridamole, cilostazole
Appendix Table 5.
Hospitalizations for bleeding during warfarin treatment according to study population, site of the bleeding and PPI co-therapy. HR = adjusted hazard ratio, RD = adjusted rate difference per 10,000, CI = confidence interval. Both the HR and RD are adjusted for the covariates shown in Appendix Table 3.
| a. Tennessee Medicaid | |||
|---|---|---|---|
| No PPI Cotherapy | Former PPI Co-therapy | PPI Co-therapy | |
| Person-years | 37,626 | 5,527 | 9,289 |
| Upper gastrointestinal | |||
| Bleeding hospitalizations | 452 | 107 | 125 |
| Rate/10,000 | 120.1 | 193.6 | 134.6 |
| HR (95% CI) | 1.05 ( 0.83- 1.33) | 0.78 ( 0.63- 0.98) | |
| RD (95% CI) | 6.1 ( -20.2 to 39.2) | -25.9 ( -45.0 to -2.1) | |
| p-value | . | 0.6779 | 0.0349 |
| Other gastrointestinal | |||
| Bleeding hospitalizations | 607 | 158 | 234 |
| Rate/10,000 | 161.3 | 285.9 | 251.9 |
| HR (95% CI) | 1.10 ( 0.91- 1.34) | 1.06 ( 0.89- 1.26) | |
| RD (95% CI) | 16.6 ( -14.6 to 54.5) | 9.5 ( -17.9 to 42.1) | |
| p-value | . | 0.3201 | 0.5224 |
| Other major bleeds | |||
| Bleeding hospitalizations | 601 | 128 | 176 |
| Rate/10,000 | 159.7 | 231.6 | 189.5 |
| HR (95% CI) | 1.06 ( 0.86- 1.31) | 1.00 ( 0.82- 1.21) | |
| RD (95% CI) | 9.7 ( -22.1 to 48.9) | -0.4 ( -28.2 to 33.3) | |
| p-value | . | 0.5785 | 0.9791 |
| b. Medicare 5% Sample | |||
| No PPI Co-therapy | Former PPI Co-therapy | PPI Co-therapy | |
| Person-years | 14,781 | 3,127 | 5,369 |
| Upper gastrointestinal | |||
| Bleeding hospitalizations | 172 | 52 | 56 |
| Rate/10,000 | 116.4 | 166.3 | 104.3 |
| HR (95% CI) | 0.90 ( 0.64- 1.26) | 0.72 ( 0.52- 0.99) | |
| RD (95% CI) | -11.7 ( -41.5 to 30.0) | -33.1 ( -56.2 to -1.2) | |
| p-value | . | 0.5361 | 0.0429 |
| Other gastrointestinal | |||
| Bleeding hospitalizations | 329 | 122 | 169 |
| Rate/10,000 | 222.6 | 390.1 | 314.7 |
| HR (95% CI) | 1.10 ( 0.88- 1.38) | 1.10 ( 0.90- 1.34) | |
| RD (95% CI) | 22.6 ( -26.6 to 84.2) | 21.2 ( -23.2 to 75.5) | |
| p-value | . | 0.3965 | 0.3743 |
| Other major bleeds | |||
| Bleeding hospitalizations | 200 | 77 | 83 |
| Rate/10,000 | 135.3 | 246.2 | 154.6 |
| HR (95% CI) | 1.28 ( 0.96- 1.70) | 0.95 ( 0.72- 1.25) | |
| RD (95% CI) | 37.4 ( -5.4 to 94.4) | -7.2 ( -38.0 to 33.4) | |
| p-value | . | 0.0928 | 0.6986 |
Appendix Table 6.
Hospitalizations for upper gastrointestinal bleeding during warfarin treatment according to study population, PPI co-therapy and concurrent use of antiplatelet drugs/NSAIDs. HR = adjusted hazard ratio, RD = adjusted rate difference per 10,000, CI = confidence interval.
| a. Tennessee Medicaid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No PPI Co-therapy | PPI Co-therapy | HR (95% CI) | RD (95% CI) | ||||||
| Person- years |
Hospital- izations |
Rate /10,000 |
Person- years |
Hospital- izations |
Rate /10,000 |
||||
| No concurrent antiplatelet drug/NSAID | 32,519 | 310 | 95.3 | 7,430 | 87 | 117.1 | 0.92 ( 0.71 - 1.19 ) | -7.6 ( -27.7 to 18.6 ) | |
| Any concurrent antiplatelet drug/NSAID | 5,107 | 142 | 278.0 | 1,859 | 38 | 204.4 | 0.56 ( 0.39 - 0.82 ) | -121.3 ( -170.4 to -49.8 ) | |
| Aspirin only | 1,783 | 47 | 263.5 | 648 | 12 | 185.3 | 0.51 ( 0.27 - 0.97 ) | -129.5 ( -193.0 to -9.0 ) | |
| NSAID only | 1,989 | 58 | 291.6 | 540 | 11 | 203.5 | 0.57 ( 0.30 - 1.10 ) | -124.5 ( -204.9 to 30.2 ) | |
| P2Y12/other antiplatelet only | 941 | 23 | 244.3 | 437 | 8 | 183.0 | 0.57 ( 0.25 - 1.29 ) | -104.5 ( -182.2 to 70.5 ) | |
| Multiple drugs | 393 | 14 | 355.8 | 233 | 7 | 299.9 | 0.69 ( 0.27 - 1.71 ) | -111.9 ( -258.0 to 252.5 ) | |
| b. Medicare 5% Sample | |||||||||
| No PPI Co-therapy | PPI Co-therapy | HR (95% CI) | RD (95% CI) | ||||||
| Person- years |
Hospital- izations |
Rate /10,000 |
Person- years |
Hospital- izations |
Rate /10,000 |
||||
| No concurrent antiplatelet drug/NSAID | 13,722 | 139 | 101.3 | 4,838 | 47 | 97.1 | 0.78 ( 0.55 - 1.11 ) | -22.4 ( -45.8 to 10.9 ) | |
| Any concurrent antiplatelet drug/NSAID | 1,060 | 33 | 311.4 | 531 | 9 | 169.4 | 0.49 ( 0.23 - 1.03 ) | -160.0 ( -239.7 to 8.2 ) | |
| Aspirin only | |||||||||
| NSAID only | 357 | 13 | 364.4 | 205 | 4 | 195.1 | 0.50 ( 0.16 - 1.55 ) | -181.8 ( -305.3 to 199.4 ) | |
| P2Y12/other antiplatelet only | 684 | 19 | 277.7 | 312 | 5 | 160.2 | 0.49 ( 0.18 - 1.33 ) | -140.7 ( -227.0 to 92.2 ) | |
| Multiple drugs | 19 | 1 | 532.5 | 14 | 0 | 0 | |||
Appendix Table 7.
Hospitalizations for upper gastrointestinal bleeding during warfarin treatment according to study population, PPI co-therapy, history of upper gastrointestinal bleeding risk factorsa, and concurrent use of antiplatelet drugs/NSAIDs. HR = adjusted hazard ratio, RD = adjusted rate difference per 10,000, CI = confidence interval.
| a. Tennessee Medicaid | ||||||||
|---|---|---|---|---|---|---|---|---|
| No PPI Co-therapy | PPI Co-therapy | HR (95% CI) | RD (95% CI) | |||||
| Person- years |
Hospital- izations |
Rate /10,000 |
Person- years |
Hospital- izations |
Rate /10,000 |
|||
| No history risk factors | ||||||||
| No concurrent antiplatelet drug/NSAID | 21,468 | 141 | 65.7 | 3,211 | 22 | 68.5 | 1.02 ( 0.64 - 1.61 ) | 1.1 ( -23.4 to 39.9 ) |
| Concurrent antiplatelet drug/NSAID | 3,375 | 78 | 231.1 | 823 | 8 | 97.2 | 0.42 ( 0.20 - 0.87 ) | -134.4 ( -184.7 to -29.5 ) |
| History risk factors | ||||||||
| No concurrent antiplatelet drug/NSAID | 11,050 | 169 | 152.9 | 4,219 | 65 | 154.1 | 0.87 ( 0.64 - 1.19 ) | -19.3 ( -54.6 to 28.6 ) |
| Concurrent antiplatelet drug/NSAID | 1,733 | 64 | 369.4 | 1,036 | 30 | 289.6 | 0.69 ( 0.44 - 1.08 ) | -115.4 ( -207.3 to 28.5 ) |
| b. Medicare 5% Sample | ||||||||
| No PPI Co-therapy | PPI Co-therapy | HR (95% CI) | RD (95% CI) | |||||
| Person- years |
Hospital- izations |
Rate /10,000 |
Person- years |
Hospital- izations |
Rate /10,000 |
|||
| No history risk factors | ||||||||
| No concurrent antiplatelet drug/NSAID | 8,581 | 62 | 72.2 | 1,999 | 10 | 50.0 | 0.71 ( 0.36 - 1.39 ) | -21.3 ( -46.2 to 27.8 ) |
| Concurrent antiplatelet drug/NSAID | 599 | 11 | 183.7 | 218 | 2 | 91.8 | 0.50 ( 0.11 - 2.24 ) | -92.7 ( -163.6 to 228.2 ) |
| History risk factors | ||||||||
| No concurrent antiplatelet drug/NSAID | 5,140 | 77 | 149.8 | 2,840 | 37 | 130.3 | 0.81 ( 0.54 - 1.22 ) | -28.5 ( -69.4 to 33.2 ) |
| Concurrent antiplatelet drug/NSAID | 461 | 22 | 477.4 | 313 | 7 | 223.3 | 0.48 ( 0.20 - 1.13 ) | -250.6 ( -381.6 to 59.7 ) |
Risk factors: Peptic ulcer, gastritis, abdominal pain, blood in stool/GI bleeding, anemia
Reference List
- 1.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 2.Piper JM, Ray WA, Griffin MR, Fought R, Daugherty JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990;132:561–571. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- 3.Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–1594. doi: 10.1056/NEJMp038145. [DOI] [PubMed] [Google Scholar]
- 4.Baillargeon J, Holmes HM, Lin YL, Raji MA, Sharma G, Kuo YF. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. The Am J Med. 2012;125:183–189. doi: 10.1016/j.amjmed.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacol Drug Safety. 2011;20:560–566. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
IRB. The Vanderbilt and Tennessee Department of Health Institutional Review Boards approved the study.
Authorship. Funding agencies had no role in study conduct or reporting. The listed authors were entirely responsible for study design, data analysis, manuscript preparation, and publication decisions. The first manuscript draft was written by the primary author, who vouches for the data and the analysis.
Conflict of interest. There are no conflicts of interest for any author to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hankey GJ, Eikelboom JW. Dabigatran etexilate: A new oral thrombin inhibitor. Circulation. 2011;123:1436–1450. doi: 10.1161/CIRCULATIONAHA.110.004424. [DOI] [PubMed] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wysowsky DK, Nourjah P, Swartz L. Bleeding complications with warfarin use. Arch Intern Med. 2007;167(13):1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 4.Olsson SB Investigators ESCobotSI. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362(9397):1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;10(1056):1, 5. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;10(1056):1–5. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 9.Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: rocket af trial. J Am Coll Cardiol. 2015;66(21):2271–2281. doi: 10.1016/j.jacc.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Cryer B. Reducing the risks of gastrointestinal bleeding with antiplatelet therapies. N Engl J Med. 2005;352(3):287–289. doi: 10.1056/NEJMe048330. [DOI] [PubMed] [Google Scholar]
- 11.Brunner G, Creutfeldt W. Omeprazole in the long-term treatment of patients with acid-related disease resistant to ranitidine. Scand J Gastroenterol. 1989;24(Suppl 166):101–105. doi: 10.3109/00365528909091254. [DOI] [PubMed] [Google Scholar]
- 12.Lin KJ, Hernandez-Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterol. 2011;141(1):71–79. doi: 10.1053/j.gastro.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586–595. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 15.Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M Committee. CAFG. Canadian Cardiovascular Society Atrial Fibrillation Guidelines 2010 Prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol. 2011;27:74–90. doi: 10.1016/j.cjca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinicial Practice Guidelines (8th Edition) Chest. 2008;133:257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 17.Garcia DA, Witt DM, Hylek E, et al. Delivery of optimized anticoagulant therapy: Consensus statement from the anticoagulation forum. Ann Pharmacother. 2008;42:979–988. doi: 10.1345/aph.1L098. [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64(21):1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 Expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008 Oct;15:1–16. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 20.Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv. 2011;4(5):522–534. doi: 10.1161/CIRCINTERVENTIONS.111.965186. [DOI] [PubMed] [Google Scholar]
- 21.Rostom A, Wells G, Tugwell P, Welch V, Dube C, McGowan J. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database of Systematic Reviews. 2001;1 doi: 10.1002/14651858.CD002296. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152(6):337–345. doi: 10.1059/0003-4819-152-6-201003160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 24.Vaduganathan M, Bhatt DL, Cryer BL, et al. Proton-pump inhibitors reduce gastrointestinal events regardless of aspirin dose in patients requiring dual antiplatelet therapy. J Am Coll Cardiol. 2016;67:1661–1671. doi: 10.1016/j.jacc.2015.12.068. [DOI] [PubMed] [Google Scholar]
- 25.Ray WA, Chung CP, Stein CM, Smalley WE, Arbogast PG, Griffin MR. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterol. 2007;133(3):790–798. doi: 10.1053/j.gastro.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 26.Abraham NS, El-Serag HB, Johnson ML, Richardsos P, Ray WA, Smalley W. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterol. 2005;129(4):1171–1178. doi: 10.1053/j.gastro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Abraham NS, Hlatky M, Antman E, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol. 2010;105:2533–2549. doi: 10.1038/ajg.2010.445. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–1594. doi: 10.1056/NEJMp038145. [DOI] [PubMed] [Google Scholar]
- 30.Baillargeon J, Holmes HM, Lin YL, Raji MA, Sharma G, Kuo YF. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. The Am J Med. 2012;125:183–189. doi: 10.1016/j.amjmed.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacol Drug Safety. 2011;20:560–566. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 33.Masso-Gonzalez EL, Garcia-Rodriguez LA. Proton pump inhibitors reduce the long-term risk of recurrent upper gastrointestinal bleeding: an observational study. Aliment Pharmacol Therap. 2008;28:629–637. doi: 10.1111/j.1365-2036.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 35.Hata M, Shiono M, Akiyama K, et al. Incidence of drug interaction when using proton pump inhibitor and warfarin according to cytochrome P450 2C19 genotype in Japanese. Thorac Cardiovasc Surg. 2015;63:45–50. doi: 10.1055/s-0034-1383814. [DOI] [PubMed] [Google Scholar]
- 36.Henriksen DP, Stage TB, Hansen MR, Rasmussen L, Damkier P, Pottegard A. The potential drug-drug interaction between proton pump inhibitors and warfarin. Pharmacoepidemiol Drug Saf. 2015;24:1337–1340. doi: 10.1002/pds.3881. [DOI] [PubMed] [Google Scholar]


