Abstract
Objective
Hypoxic brain injury is the largest contributor to disability and mortality after cardiac arrest. We aim to identify electroencephalogram (EEG) characteristics that can predict outcome on cardiac arrest patients treated with targeted temperature management (TTM).
Methods
We retrospectively examined clinical, EEG, functional outcome at discharge, and in-hospital mortality for 373 adult subjects with return of spontaneous circulation after cardiac arrest. Poor outcome was defined as a Cerebral Performance Category score of 3–5. Pure suppression-burst (SB) was defined as SB not associated with status epilepticus (SE), seizures, or generalized periodic discharges.
Results
In-hospital mortality was 68.6% (N=256). Presence of both unreactive EEG background and SE was associated with a positive predictive value (PPV) of 100% (95% Confidence Interval: 0.96–1) and a false-positive rate (FPR) of 0% (95% CI: 0–0.11) for poor functional outcome. A prediction model including demographics data, admission exam, presence of status epilepticus, pure SB, and lack of EEG reactivity had an area under the curve of 0.92 (95% CI:0.87–0.95) for poor functional outcome prediction, and 0.96 (95% CI: 0.94–0.98) for in-hospital mortality. Presence of pure SB (N=87) was confounded by anesthetics use in 83.9% of the cases, and was not an independent predictor of poor functional outcome, having a FPR of 23% (95% CI: 0.19–0.28).
Conclusions
An unreactive EEG background and SE predicted poor functional outcome and in-hospital mortality in cardiac arrest patients undergoing TTM. Prognostic value of pure SB is confounded by use of sedative agents, and its use on prognostication decisions should be made with caution.
Keywords: cardiac arrest, prognosis, EEG, status epilepticus, burst-suppression, EEG background reactivity
1. Introduction
Sudden cardiac arrest (CA) is the leading cause of death in North America in adults over the age of 40, with about 360,000 cases of non-traumatic out-of-hospital cardiac arrest (OHCA) each year.[1] Over the past decade, bundles of care including targeted temperature management (TTM) has become the standard treatment of patients who remain comatose after resuscitation, yielding significant improvement in survival rates and improved neurological function.[2] Despite the advancements in care with implementation of TTM, prognostication remains difficult, and a significant number of patients have withdrawal of life-sustaining therapies prior to formal prognostication, or are labeled with indeterminate outcome.[3] Moreover, the role of several well-established markers of poor prognosis has been challenged, hindering the determination of patient characteristics that indicate potential for neurological recovery.[4]
Electroencephalogram (EEG) is a widely used tool for neurological prognostication in cardiac arrest. [5–9] It can provide real-time continuous monitoring of brain physiology, and is both non-invasive and convenient to use in unstable patients. Clinical and subclinical seizures along with other epileptiform patterns or presence of a suppression-burst (SB) background have been shown to be robust predictors of poor neurological function in cardiac arrest.[6, 7, 9, 10] More recent data, however, indicates that good neurological outcome can be present despite the presence of these patterns.[11, 12] Other EEG features have emerged as powerful predictive factors for neurological recovery, and more attention has been given to other aspects of EEG background, in particular EEG background reactivity (EBR).[6, 12, 13]
The aim of this study is to estimate the association of epileptiform patterns and EEG background features with functional outcome of comatose cardiac arrest subjects treated with TTM.
2. Methods
2.1 Patients and Temperature Targeted Management
Adult subjects that remained comatose after successful resuscitation from either in-hospital (IHCA) or out-of-hospital cardiac arrest (OHCA) were prospectively included on a quality improvement database from January 2009 to June 2013. At the time of this study, all patients receiving TTM had a goal temperature of 33°C. Patients that did not undergo TTM to a goal temperature of 33°C, or who had continuous EEG monitoring for less than ten hours, were excluded. During the study period, our institution’s TTM protocol included induction with intravenous infusion of cold saline followed by application of external cooling pads for 24 hours of hypothermia maintenance.[14] Neuromuscular paralysis is frequently employed during induction of TTM, however, it is not routinely continued through hypothermia and rewarming phases. Sedation is performed primarily with propofol infusion (25–60 mcg/kg/h), however, midazolam (0.1 mg/kg/h) and fentanyl (25–100 mcg/h) can be utilized at the treating physician’s discretion. The University of Pittsburgh Institutional Review Board deemed this retrospective analysis of quality improvement data including demographic, clinical and EEG data exempt from requirements for informed consent.
2.2 EEG monitoring and classification
Our long-term continuous EEG monitoring protocol is initiated during TTM and continues until completion of rewarming.[14] Digital EEG was recorded using 22 electrodes according to a 10–20 system. EEG monitoring was performed for clinical indications and was interpreted by board certified electroencephalographers during standard care. EEG reports were retrospectively reviewed and categorized based on the presence of malignant EEG patterns (MEP), pure SB, or non-malignant EEG patterns as reported previously.[12] Malignant EEG patterns included: status epilepticus (SE), seizures, or generalized periodic discharges (GPD). EEG nomenclature was based on the “ACNS standardized critical care EEG terminology,” and the “Guidelines for the evaluation and management of status epilepticus” were used for the SE definition.[12, 15, 16] Suppression-burst was defined following ACNS criteria, and was described as “present” if SB was present for over 30 minutes and if “more than 50% of the record consisted of attenuation or suppression with bursts alternating with attenuation or suppression”.[15] In cases of SB in the absence of MEP, records were categorized as containing a “pure” SB pattern. Further sub-classification of the bursts regarding presence of embedded “highly epileptiform” discharge was not performed. If SB was present in the same day in which propofol or midazolam were administered, SB was categorized as “likely confounded by medication.” EEG recordings in which neither MEP or SB were present were considered “non-malignant”, even if epileptiform discharges or focal lateralized periodic discharges (LPD) were present. Chronic post-hypoxic myoclonus (i.e. Lance-Adams syndrome) was defined as action myoclonus in a conscious patient that develops 48 hours or more after cardiac arrest or respiratory arrest, and was not included as part of the MEP category.[17] The routine EEG monitoring protocol in our institution includes once daily neurological assessments performed by EEG technicians. These assessments include auditory and noxious stimulation in case patients are unresponsive. Noxious stimulation consisted of unilateral or bilateral fingernail compression performed at least once. Electroencephalogram background reactivity was reviewed in the first 72 hours of EEG monitoring and was defined as “change in EEG background frequency or amplitude after a noxious or auditory stimulus”. EBR was tested once a day and was scored as “reactive,” “unreactive,” or “not tested” by a board certified neurophysiologist during the hospital stay as per local protocol.[15] In cases in which EBR was not specifically attested, adjudication was performed by two board-certified neurophysiologist (M.E.B and A.P.), and discrepancies were resolved by consensus. Best EBR scoring during entire duration of EEG monitoring duration was used for final EBR categorization. EEG background reactivity testing associated with stimulus induced rhythmic, periodic or ictal discharges (SIRPDS), isolated muscle artifact without association with synchronized epileptiform discharges, or SB were not scored as reactive.
2.3 Data collection and neurological evaluation
Clinical and demographics data were collected, and a subset of records was reviewed separately to confirm data reliability. Discrepancies were resolved by consensus. We stratified patients by gender, location of cardiac arrest (in- or out-of-hospital), and initial cardiac rhythm, which was dichotomized as shockable (ventricular fibrillation or ventricular tachycardia) or non-shockable (including asystole, pulseless electrical activity, and unknown). Based on their initial neurological examination and Sequential Organ Failure Assessment (SOFA) score on admission, subjects were categorized using the validated Pittsburgh Cardiac Arrest Service Category (PCAC): PCAC I: awake and following commands, PCAC II: coma with preserved brainstem reflexes, PCAC III: coma with preserved brainstem reflexes and severe cardiopulmonary failure, and PCAC IV: coma with loss of some or all brainstem reflexes.[18, 19] Patient outcomes consisted of level of neurologic function at discharge and discharge disposition, and were scored by one of the PCAS physicians or a trained technician using a standard algorithm.[18] Neurologic function at discharge was graded retrospectively using the Glasgow-Pittsburgh Cerebral Performance categories (CPC) scale. “Good” functional outcome was defined as a CPC score of 1 or 2 and “poor” as CPC of 3 to 5. Subjects discharged home or to a rehabilitation institution were consider having “good disposition,” and those discharged to a skilled-nursing facility, long-term acute care facility, hospice, or who were deceased at discharge were categorized as having “poor disposition.” In order to qualify for rehabilitation referral, patients have to be able to tolerate more than three hours of physical therapy per day.
In our institution, a Post Cardiac Arrest Service attending sees almost all patients successfully resuscitated from cardiac arrest. Neurologic prognostication consists of serial examinations, computerized tomography of the brain, continuous EEG, and in select cases, somatosensory evoked potentials and magnetic resonance imaging of the brain. We have previously reported on the lack of specificity of these tests, therefore, no single test result is utilized for withdrawal of care.[10, 18, 20–22]
2.4 Statistical analysis
Univariate comparison of good and poor outcome groups was performed using Pearson χ2 for categorical variables and independent t-tests for continuous variables. The variables included in the multivariate logistic regression were age, gender, OHCA, shockable rhythm, PCAC IV, SE, GPD, Pure SB, and unreactive EEG background. Parameter optimization and selection of the maximally predictive subset of parameters that were included in the final predictive models (“feature selection”) were carried out using penalized multinomial logistic regression with elastic-net regularization used in combination with 10-fold cross validation.[23] Performance of the final multimodal algorithm for outcome prediction was evaluated using receiver operating curve (ROC) analysis. We compared the area under the ROC curve between multimodal algorithms with and without addition of EEG background reactivity information. The Bayesian credible interval was estimated using 1,000 rounds of bootstrapping. Specifically, we created 1,000 bootsrapped samples of the differences between AUC curves, and calculated the interval containing 95% of the probability density. For these calculations, we defined differences in AUC values as statistically significant when the 95% credible interval for AUC differences did not include zero. False-positive rates (FPR), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were obtained with their 95% confidence intervals (CI). Statistical significance was determined at the α level of 0.05. Statistical analysis was conducted with SPSS, version 22.0.1 software package (SPSS, Chicago, IL, USA) and MATLAB, version 17, (Natick, MA, USA).
3. Results
Patient population
A total of 885 subjects with return of spontaneous circulation (ROSC) after cardiac arrest were screened during the study period, and 373 fulfilled inclusion criteria. Demographics and clinical characteristics of the 373 subjects included in the final analysis are presented in Table 1.
Table 1. Baseline characteristics.
CPC: Cerebral Performance Category; OHCA: out-of-hospital cardiac arrest; PCAC: post cardiac arrest service category; IQR: interquartile range
| Survivors (N=117) |
Non-survivors (N=256) |
p | CPC 1–2 (N=39) |
CPC 3–5 (N=334) |
p | Good disposition (N=70) |
Poor disposition (N=303) |
p | Total (N=373) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Mean ± SD) | 56.1 ± 15.2 | 58 ± 16.7 | 0.29 | 51.6 ± 13.4 | 58 ± 16.4 | 0.02 | 57 ± 25.1 | 58 ± 16.7 | 0.1 | 57.4 ± 16.2 |
| Female | 44 (37.6 %) |
104 (40.6 %) |
0.58 | 13 (33.3 %) |
135 (40.4 %) |
0.39 | 27 (38.6%) |
121 (39.9%) |
0.83 | 148 (39.7 %) |
| OHCA | 85 (72.6 %) |
206 (80.5 %) |
0.09 | 35 (89.7 %) |
256 (76.6 %) |
0.06 | 61 (87.1%) |
230 (75.9%) |
0.04 | 291 (78%) |
| Shockable rhythm | 61 (52.1 %) |
53 (20.7 %) |
<0.001 | 26 (66.7 %) |
88 (26.3 %) |
<0.001 | 45 (64.3%) |
69 (22.8%) |
<0.001 | 114 (30.6 %) |
| CPCAC | <0.001 | <0.001 | <0.001 | |||||||
| Category II | 52 (45.4 %) |
41 (16%) |
243 (60%) |
70 (21.2 %) |
34 (48.7%) |
59 (19.5%) |
93 (24.9 %) |
|||
| Category III | 40 (33.6 %) |
36 (14.1 %) |
12 (30%) |
64 (19.1%) |
20 (28.7%) |
56 (18.5%) |
76 (20.4 %) |
|||
| Category IV | 25 (21%) |
179 (69.9 %) |
4 (10%) |
200 (59.7 %) |
16 (22.6%) |
188 (62%) |
204 (54.7 %) |
|||
| Length of stay, days (Median; IQR) | 18; (11, 24) |
4; (3,6) |
<0.001 | 12; (9,19) |
5; (3, 12) | 0.001 | 15; (11,23) |
4; (5,9) | <0.001 | 6; (3,13) |
The most common cause for exclusion was EEG monitoring duration of less than 10 hours in 298 subjects. An additional 214 subjects were excluded from the final analysis for the following reasons: withdrawal of life-sustaining therapies within 24 hours of admission (N=37), rearrested without ROSC within few 6 hours of arrival (N=33); intracerebral hemorrhage (N=19) and gastrointestinal hemorrhage (N=12). Thirty-nine were excluded due to other reasons such as: trauma, acute hemorrhage, coagulopathy, and need for immediate surgery; eleven patients awoke before 24 hours of TTM; and EBR could not be ascertained in two subjects. A specific reason for not initiating TTM was not documented in 61 cases.
EEG monitoring
EEG monitoring duration was of 24 hours or more in 302 (81%) of subjects. Median EEG monitoring duration was 41 hours. Malignant EEG patterns were present for 148 subjects (39.7%), with SE being the most frequent pattern among MEP (78.4%). Among subjects who had seizures or SE, one hundred (84.7%) subjects had them diagnosed within the first day of EEG monitoring. Suppression-burst was present in 215 (57.6%) subjects, and in 87 (40.5%) was categorized as pure-SB. No EBR was present in 266 (71.3%) subjects throughout the time of EEG monitoring. Electroencephalogram patterns distribution by outcome are shown on Table 2.
Table 2. EEG patterns distribution by outcome.
CPC: Cerebral Performance Category; SE: status epilepticus; GPD: generalized periodic discharges; SB: suppression burst
| Survivors (N=117) |
Non-survivors (N=256) |
CPC 1–2 (N=39) |
CPC 3–5 (N=334) |
Good disposition (N=70) |
Poor disposition (N=303) |
Total (N=373) |
|
|---|---|---|---|---|---|---|---|
| MEPs | 19 | 129 | 2 | 146 | 8 | 140 | 148 (39.7%) |
| - SE | 9 | 107 | 0 | 116 | 4 | 112 | 116 (30.9%) |
| - GPD | 10 | 20 | 2 | 28 | 4 | 26 | 30 (8%) |
| - Discrete seizure | 0 | 2 | 0 | 2 | 0 | 2 | 2 (0.5%) |
| Pure-SB | 22 | 65 | 9 | 78 | 15 | 72 | 87 (23.3%) |
| Non-malignant | 76 | 62 | 28 | 110 | 47 | 91 | 138 (37%) |
| -Epileptiform discharges | 11 | 18 | 0 | 29 | 4 | 25 | 29 (7.8%) |
| Unreactive background | 20 | 246 | 9 | 257 | 7 | 259 | 266 (71.3%) |
Clinical outcome
One hundred and seventeen subjects survived to hospital discharge, and 39 had good functional outcome at hospital discharge. Among survivors, 70 (59.8%) were discharged home or to a rehabilitation facility, and twelve (10.3%) had severe disability such as coma or vegetative state. All subjects with an unreactive EEG associated with MEP had poor functional outcome and poor disposition, however four survived to hospital discharge. Twenty subjects survived despite an unreactive EEG, with nine subjects having good functional outcome and seven being discharged to rehabilitation or home. Of note, eighteen out of these twenty subjects had an anesthetic agent given during the time of EEG monitoring.
Eight subjects with MEP and 15 with pure-SB were discharged to home or to a rehabilitation facility. The specific EEG patterns in the MEP group discharged to rehabilitation were: four subjects with SE and four with GPD, with four of these subjects having SB associated with the MEP. Six of the 12 subjects with severe disability (coma or vegetative state) had a reactive EEG background, including subjects with MEP (N=3) and pure-SB (N=1). The remaining two subjects with PSV and a reactive background had unspecific generalized slowing. Status epilepticus had a FPR of 0% (95% CI 0–0.12) for poor functional outcome, and 8% (CI 0.04–0.15) for in-hospital mortality (Table 3). In case of an unreactive background in association with SE, the predictive value (PPV) for poor functional outcome and poor disposition was of 100%, with a false-positive rate (FPR) of 0% (0–0.11). The PPV for mortality was 99% (95% CI: 0.94–0.99) and FPR 1% (95% CI 0.01–0.05).
Table 3. Predictive values of clinical and electrophysiologic features stratified by outcome.
CI: confidence interval; OHCA: out-of-hospital cardiac arrest; PCAC: post cardiac arrest service category; SE: status epilepticus; SB: suppression burst
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | FPR (95%CI) | |
|---|---|---|---|---|---|
| Mortality | |||||
| OHCA | 0.8 (0.75–0.85) | 0.27 (0.2–0.37) | 0.71 (0.65–0.76) | 0.39 (0.29–0.5) | 0.73 (0.63–0.8) |
| Shockable rhythm | 0.21 (0.16–0.26) | 0.48 (0.39–0.57) | 0.46 (0.37–0.56) | 0.22 (0.17–0.27) | 0.52 (0.43–0.61) |
| PCAC IV | 0.7 (0.64–0.75) | 0.79 (0.7–0.85) | 0.88 (0.82–0.92) | 0.54 (0.47–0.62) | 0.21 (0.15–0.3) |
| SE | 0.42 (0.36–0.49) | 0.92 (0.85–0.96) | 0.92 (0.86–0.96) | 0.42 (0.36–0.49) | 0.08 (0.04–0.15) |
| Unreactive EEG background | 0.96 (0.93–0.98) | 0.83 (0.75–0.89) | 0.92 (0.88–0.95) | 0.91 (0.83–0.95) | 0.17 (0.11–0.25) |
| SE + unreactive EEG background | 0.42 (0.36–0.48) | 0.99 (0.95–0.99) | 0.99 (0.94–0.99) | 0.44 (0.38–0.5) | 0.01 (0.01–0.05) |
| Pure-SB | 0.25 (0.2–0.31) | 0.81 (0.73–0.88) | 0.75 (0.64–0.83) | 0.33 (0.28–0.39) | 0.19 (0.12–0.27) |
| Poor outcome (CPC3–5) | |||||
| OHCA | 0.77 (0.72–0.81) | 0.1 (0.03–0.25) | 0.88 (0.84–0.91) | 0.05 (0.02–0.13) | 0.9 (0.75–0.97) |
| Shockable rhythm | 0.26 (0.22–0.31) | 0.33 (0.2–0.5) | 0.77 (0.68–0.84) | 0.05 (0.03–0.09) | 0.67 (0.69–0.78) |
| PCAC IV | 0.6 (0.54–0.65) | 0.9 (0.75–0.97) | 0.98 (0.95–0.99) | 0.21 (0.15–0.28) | 0.1 (0.03–0.25) |
| SE | 0.35 (0.3–0.41) | 1 (0.88–1) | 1 (0.96–1) | 0.175 (0.11–0.2) | 0 (0–0.12) |
| Unreactive EEG background | 0.77 (0.72–0.81) | 0.77 (0.6–0.88) | 0.97 (0.93–0.98) | 0.28 (0.2–0.38) | 0.23 (0.12–0.4) |
| SE + unreactive EEG background | 0.32 (0.27–0.38) | 1 (0.89–1) | 1 (0.96–1) | 0.15 (0.11–0.2) | 0 (0–0.11) |
| Pure-SB | 0.23 (0.19–0.28) | 0.77 (0.6–0.88) | 0.9 (0.8–0.95) | 0.1 (0.07–0.15) | 0.23 (0.12–0.4) |
| Poor disposition | |||||
| OHCA | 0.76 (0.71–0.81) | 0.13 (0.06–0.24) | 0.79 (0.74–0.83) | 0.11 (0.05–0.2) | 0.87 (0.76–0.94) |
| Shockable rhythm | 0.23 (0.18–0.28) | 0.36 (0.25–0.48) | 0.61 (0.51–0.69) | 0.1 (0.06–0.14) | 0.64 (0.52–0.75) |
| SE | 0.38 (0.32–0.43) | 0.94 (0.85–0.98) | 0.97 (0.91–0.99) | 0.26 (0.21–0.32) | 0.06 (0.02–0.15) |
| Unreactive EEG background | 0.85 (0.8–0.89) | 0.9 (0.8–0.96) | 0.97 (0.94–0.99) | 0.59 (0.49–0.68) | 0.1 (0.04–0.2) |
| SE + unreactive EEG background | 0.36 (0.3–0.41) | 1 (0.93–1) | 1 (0.96–1) | 0.26 (0.21–0.32) | 0 (0–0.07) |
| SE + unreactive EEG background | 0.36 (0.3–0.41) | 1 (0.93–1) | 1 (0.96–1) | 0.26 (0.21–0.32) | 0 (0–0.07) |
| Pure-SB | 0.24 (0.19–0.29) | 0.79 (0.67–0.87) | 0.83 (0.73–0.9) | 0.19 (0.15–0.24) | 0.21 (0.13–0.33) |
Among the 256 subjects not surviving to hospital discharge, 195 (76.2%) were deemed to have a poor prognosis by the treating team and had life-sustaining therapies withdrawn on a median of day 4 (IQR 3,6). Life-sustaining therapies were withdrawn due to other reasons in five subjects: underlying terminal illnesses (two), pre-existing advance directive (two), and family or surrogate representation of patient’s wishes (one). Twenty-nine subjects were medically unstable and died from multi-organ failure, recurrent arrest, or intractable shock. Brain death was diagnosed in 27 (10.5%) subjects.
Multimodal model logistic regression
We performed ROC curve analysis to examine whether outcome prediction was enhanced by inclusion of information about the presence vs. absence of an unreactive background EEG. When EBR information was included, there was improvement of the AUC from 0.87 (95% CI 0.83–0.91) to 0.96 (95% CI: 0.94–0.98) for mortality and from 0.87 (95% CI: 0.8–0.91) to 0.94 (95% CI: 0.9–0.96) for poor discharge disposition (Figure 1). These AUC improvements were statistically significant (Figure S1). The effect of EBR in the model for poor functional outcome prediction was not significant, with AUC change from 0.91 (95% CI:0.87–0.95) to 0.92 (95% CI:0.87–0.95) (Figure 1 and Figure S1).
Figure 1. Improved performance of multimodal outcome prediction modeling utilizing a receiver operating characteristic curve analysis with inclusion of EEG background reactivity data in the model.
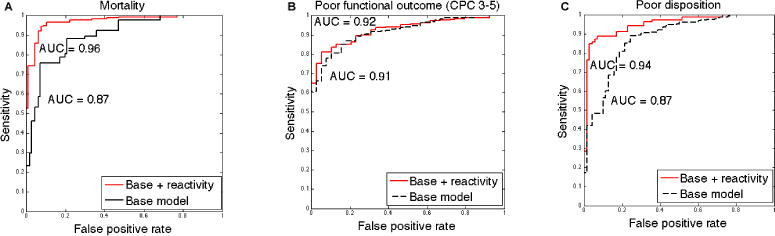
Receiver operating characteristic (ROC) curve analysis using a multimodal outcome prediction base model that included presence of seizures and burst-suppression on EEG, neurological exam, initial cardiac rhythm, and location of cardiac arrest. Including EBR data in the model was associated with AUC improvement from: (A) Mortality: 0.87 to 0.96; (B) Poor functional outcome (CPC 3–5): 0.91 to 0.92; (C) Poor disposition: AUC of 0.87 to 0.94.
Unreactive background, presence of status epilepticus, and a non-shockable rhythm were independent predictors of mortality, poor functional outcome, and poor discharge disposition (Table 4). At a false positive rate of 5%, including EBR in the model was associated with increased sensitivity for mortality from 46% to 79%, poor functional outcome 61% to 73%, and poor discharge disposition 42% to 85%.
Table 4. Multivariate analysis of characteristics associated with poor outcome (CPC 3–5), mortality, and poor disposition.
SE: standard error; PCAC: post cardiac arrest service category; OHCA: out-of-hospital cardiac arrest.
| Predicted outcome | Mortality | CPC 3–5 | Poor disposition | |||
|---|---|---|---|---|---|---|
| Estimated Coefficient (SE) | p | Estimated Coefficient (SE) | p | Estimated Coefficient (SE) | p | |
| Age | 0.52 (0.22) | 0.02 | ||||
| OHCA | −0.59 (0.25) | 0.02 | −0.62 (0.2) | 0.002 | ||
| Shockable rhythm | −0.48 (0.2) | 0.02 | −0.51 (0.2) | 0.01 | −0.68 (0.18) | <0.001 |
| CPCAC IV | 0.81 (0.22) | <0.001 | 0.96 (0.3) | 0.001 | ||
| Status Epilepticus | 0.78 (0.26) | 0.002 | 46.3 (<0.01) | 0.99 | 0.66 (0.3) | 0.03 |
| Unreactive background | 2.03 (0.21) | <0.001 | 0.55 (0.21) | 0.008 | 1.74 (0.21) | <0.001 |
Suppression-burst relationship with anesthetic agent administration
A SB EEG pattern was present within the same day of administration of a sedative agent in 197 (91.6%) subjects with SB (83.9% among pure SB), and therefore was considered as “likely confounded by medication.” All subjects with a SB background who did not receive anesthetic agents during EEG monitoring had an unreactive EEG and did not survive.
4. Discussion
In a cohort of 373 comatose cardiac arrest subjects treated with TTM, an unreactive EEG background and presence of SE independently predict in-hospital mortality and poor functional outcome. Addition of EBR in the multimodal prediction model strongly enhanced prediction for in-patient mortality and discharge disposition, with little effect on functional recovery as measured by the CPC at hospital discharge. These findings support previous reports underscoring the relevance of specific EEG features as biomarkers in prognostication after hypoxic brain injury and provide tighter confidence intervals. [5–7, 9, 10, 12, 13]
Our report supports previous literature indicating that an unreactive background EEG predicts poor functional outcome and mortality. EEG reactivity is considered a surrogate marker of thalamocortical network integrity, and therefore an unreactive background is suggestive of severe disruption of these connections, making substantial neurological recovery unlikely.[24, 25] The multimodal outcome prediction model including EBR greatly enhanced mortality and disposition prediction performance, however there was only a small effect on improving prediction of poor functional outcome at discharge. A limitation of this study is that CPC scores at hospital discharge might be prematurely pessimistic predictions. Recovery after resuscitation from cardiac arrest is a dynamic process with many patients experiencing initially poor outcome scores.[26] Many of these patients continue to improve after discharge from the hospital and require between 6–12 months to achieve full recovery.[27]
In the present study, a third of subjects (30.9%) developed SE and more than 80% of these cases had SE start during the maintenance or rewarming phases of TTM, indicating that early EEG monitoring is warranted for seizure detection and treatment. Most cases with MEP, GPD or epileptiform discharges had poor functional outcome, confirming previous reports that status epilepticus and other epileptiform patterns predict poor functional outcome.[8, 13] This association persists despite intensive treatment with anesthetics and anti-epileptic medications in our institution, underscoring the need for novel approaches to these malignant patterns.[10, 28] Alternatively, these patterns may represent severe neuronal injury that is not amenable to treatment.
Importantly, 20 subjects (7.8%) in our series survived despite an unreactive EEG background, with nine of them experiencing good functional outcome. Detailed review of this subgroup indicated that 18 out of the 20 subjects were managed with anesthetic agents during most, if not the entirety, of EEG monitoring. Use of sedatives might attenuate EEG response to reactivity testing and lead to apparently unreactive recordings. Likewise, over 90% of the cases with a SB pattern had anesthetic agents given during EEG monitoring. The degree and type of noxious stimulation used on reactivity testing might have not been sufficient to elicit reactivity in some subjects.[29] A pure SB pattern in this study was neither associated with poor functional outcome nor mortality, having FPR of 23% and 19%, respectively. These data contrast with several reports indicating that a SB background nearly precludes good outcome.[6, 7, 30] It has been postulated that “identical bursts” comprise the vast majority of the SB attributed to direct hypoxic brain injury and that they behave differently from the otherwise “medication-induced” SB. [7, 31] The ability to make this distinction at the bedside is often limited, as a large number of patients are exposed to prolonged use of sedatives, with potential delay on the metabolism of these drugs by hypothermia.[32] Our findings expand our previous observation on the potential for confounding between use of sedatives and presence of a SB background in cardiac arrest survivors.[12]
The retrospective design and the fact that the physicians caring for these patients were not blinded to the EEG results are important limitations of this study. Unfavorable clinical and electrographic markers might have being used on decision-making during goals of care meetings, and a self-fulfilling prophecy could have influenced our results. Nevertheless, the median length of stay of four days among non-survivors in our report indicates that withdrawal of life support before 72 hours was infrequent. Use of quantitative EEG could have enhanced our ability to identify identical bursts and also further characterize EEG background features and their relationship to anesthetic infusions.[7, 31]
5. Conclusions
The combination of status epilepticus and an unreactive background are strong predictors of poor functional outcome and mortality after cardiac arrest in the TTM era. Standardized reactivity testing during EEG monitoring and caution in prognosticating based on SB pattern during sedative agent administration is warranted. Prospective studies involving multiple centers using standardized criteria for EEG classification, reactivity testing, and withdrawal of life-sustaining therapies are warranted.
Supplementary Material
Acknowledgments
The authors thank Cindy Huynh for her assistance with manuscript review.
J.C.R. is supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (K12 RR024154), and by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship. M.B.W. has received support from NIH-NINDS (1K23NS090900), the Andrew David Heitman Neuroendovascular Research Fund, and the Rappaport Foundation. C.W.C. received support from the NHLBI Resuscitation Outcomes Consortium (5U01 HL077871).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
E.A, J.C.R., M.E.B., C.W.C, A.P. conceptualized and designed the study. E.A. (MGH neurocritical care fellow) and M.B.W. (faculty with the MGH Epilepsy Service) completed the statistical analysis. E.A, J.C.R., A.P. drafted the original manuscript. E.A., J.C.R., J.J.Z., M.E.B, A.P. contributed to data production and collection. E.A, J.C.R., J.J.Z., M.B.W., M.E.B., C.W.C, A.P. reviewed and revised the manuscript.
Conflict of Interest Statement:
E.A, J.C.R., J.J.Z., M.B.W., M.E.B., C.W.C, A.P. report no disclosures relevant to the manuscript
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–15. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 4.Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic-ischaemic coma in the therapeutic hypothermia era. Nat Rev Neurol. 2014;10:190–203. doi: 10.1038/nrneurol.2014.36. [DOI] [PubMed] [Google Scholar]
- 5.Westhall E, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Horn J, Ullen S, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–90. doi: 10.1212/WNL.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeijer J, Beernink TM, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, van Putten MJ. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85:137–43. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–14. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 9.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–75. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 10.Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–22. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72:744–9. doi: 10.1212/01.wnl.0000343006.60851.62. [DOI] [PubMed] [Google Scholar]
- 12.Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A, Post Cardiac Arrest S Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–32. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crepeau AZ, Rabinstein AA, Fugate JE, Mandrekar J, Wijdicks EF, White RD, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–44. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 14.Rittenberger JC, Guyette FX, Tisherman SA, DeVita MA, Alvarez RJ, Callaway CW. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79:198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 16.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 17.Thomke F, Marx JJ, Sauer O, Hundsberger T, Hagele S, Wiechelt J, et al. Observations on comatose survivors of cardiopulmonary resuscitation with generalized myoclonus. BMC Neurol. 2005;5:14. doi: 10.1186/1471-2377-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82:1399–404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppler PJ, Elmer J, Calderon L, Sabedra A, Doshi AA, Callaway CW, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderon LM, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC, Post Cardiac Arrest S Combining NSE and S100B with clinical examination findings to predict survival after resuscitation from cardiac arrest. Resuscitation. 2014;85:1025–9. doi: 10.1016/j.resuscitation.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittenberger JC, Sangl J, Wheeler M, Guyette FX, Callaway CW. Association between clinical examination and outcome after cardiac arrest. Resuscitation. 2010;81:1128–32. doi: 10.1016/j.resuscitation.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011;82:1180–5. doi: 10.1016/j.resuscitation.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society. 1996;58:267–88. [Google Scholar]
- 24.Madhok J, Maybhate A, Xiong W, Koenig MA, Geocadin RG, Jia X, et al. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: prognostication of functional outcomes. Critical care medicine. 2010;38:1709–17. doi: 10.1097/CCM.0b013e3181e7dd29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno MA, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain: a journal of neurology. 2012;135:1308–20. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036–40. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raina KD, Rittenberger JC, Holm MB, Callaway CW. Functional Outcomes: One Year after a Cardiac Arrest. Biomed Res Int. 2015;2015:283608. doi: 10.1155/2015/283608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsetsou S, Novy J, Oddo M, Rossetti AO. EEG reactivity to pain in comatose patients: Importance of the stimulus type. Resuscitation. 2015;97:34–7. doi: 10.1016/j.resuscitation.2015.09.380. [DOI] [PubMed] [Google Scholar]
- 30.Snyder BD, Hauser WA, Loewenson RB, Leppik IE, Ramirez-Lassepas M, Gumnit RJ. Neurologic prognosis after cardiopulmonary arrest: III. Seizure activity. Neurology. 1980;30:1292–7. doi: 10.1212/wnl.30.12.1292. [DOI] [PubMed] [Google Scholar]
- 31.Sivaraju A, Gilmore EJ, Wira CR, Stevens A, Rampal N, Moeller JJ, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive care medicine. 2015;41:1264–72. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 32.Hostler D, Thomas EG, Emerson SS, Christenson J, Stiell IG, Rittenberger JC, et al. Increased survival after EMS witnessed cardiac arrest. Observations from the Resuscitation Outcomes Consortium (ROC) Epistry-Cardiac arrest. Resuscitation. 2010;81:826–30. doi: 10.1016/j.resuscitation.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


