Abstract
Since its initial formalization nearly twenty years ago, the concept of lipid rafts has generated a tremendous amount of attention and interest, and nearly as much controversy. The controversy is perhaps surprising because the notion itself is intuitive: compartmentalization in time and space is a ubiquitous theme at all scales of biology, and therefore the partitioning of cellular membranes into lateral subdivision should be expected. Nevertheless, the physicochemical principles responsible for compartmentalization and the molecular mechanisms by which they are functionalized remain nearly as mysterious today as they were two decades ago. Herein, we review recent literature on this topic with a specific focus on the major open questions in the field, including: (1) what are the best tools to assay raft behavior in living membranes; (2) what is the function of the complex lipidome of mammalian cells with respect to membrane organization; (3) what are the mechanisms that drive raft formation and determine their properties; (4) how can rafts be modulated; (5) how is membrane compartmentalization integrated into cellular signaling. Despite decades of intensive research, this compelling field remains full of fundamental questions.
Graphical abstract
Introduction
The lipid raft model was originally proposed to explain the robust sorting of select lipid and protein cargo to the apical plasma membrane (PM) in polarized epithelial cells1, 2. For the next twenty years, the intuitive appeal of a simple mechanism for functional compartmentalization of cellular membranes lead to the application of this idea to nearly all areas of cell biology3. Most notable among these is signal transduction at and through the PM, with lateral domains serving as core regulators by mediating the interaction between membrane proteins4, 5. However, despite the many cellular roles attributed to lipid rafts, the true nature of membrane organization in living cells remains unknown.
The continuing mystery of lipid rafts remains unresolved because of inherent limitations in methodology. It now seems clear that the simplistic conception of distinct, stable, long-lived membrane domains in unperturbed plasma membranes is inconsistent with most available evidence. Rather, cell membrane lateral organization is best conceptualized as being only somewhat heterogeneous on length scales of tens of nanometers and timescales of milliseconds. Although such length/time scales are well within range of biological relevance, few analytical techniques have the appropriate spatial and temporal resolution to accurately probe these features. Here, we review some of the major lines of inquiry into raft phenomena from their first applications to their modern incarnations and highlight the key methodologies that may yield answers to the many remaining fundamental questions that continue to define the field.
A spectrum of membrane models
The key physical insight behind the lipid raft hypothesis was the proposal that biomimetic model membranes could separate into coexisting liquid phases6-9. The early studies in this field used spectroscopic measurements of hydrated bilayers10-13 and microscopic visualization of lipid monolayers formed at air-water interfaces14 to suggest the formation of a novel liquid lipid phase in membranes composed of biological lipids. This conclusion was later confirmed by microscopic observation and characterization of coexisting liquid domains in both free and supported lipid bilayers15, 16, which were shown to have different compositions, degrees of molecular packing / order, and different single molecule diffusivities 15, 17-19. Such observations facilitated detailed mapping of the phase diagrams of biomimetic membrane systems, which revealed that the coexistence between a liquid-disordered (Ld) phase and a liquid-ordered (Lo) phase - distinguished by its higher molecular packing, lower diffusivity, and more extended (i.e. ordered) lipid acyl chains - was observable across a wide range of compositions and temperatures20-24. More recently, computational modeling has added a tool to the arsenal of probing biomimetic membranes25, allowing detailed molecular-level analysis of bilayer organization26, 27 and physical interactions between lipids28 and proteins29. Although previously limited to relatively small and simplified membranes, advances in course-graining and computing technologies now allow simulations of large and complex systems30.
These biophysical investigations provided the necessary physicochemical underpinnings for lipid-driven lateral domains in biological membranes. But direct translation from simple models to cellular membranes was, and continues to be, hampered by their fundamental differences. Certainly important are the limited compositional complexity of model membranes and their lack, or paucity, of integral proteins, which comprise a large fraction of cell membranes (~20% by area)31. As detailed below, eukaryotic membranes are composed of hundreds of lipids species whose relative abundances are dynamically regulated. Further, cell membranes are often asymmetric, with the two opposing leaflets containing distinct lipid compositions. In contrast, biomimetic membranes are usually constructed from less than five different lipid components and are rarely asymmetric although recent advances have introduced technologies that should facilitate investigation into this key membrane property32-34. Finally, most model membranes lack key aspects that are fundamental to cell membrane functions, including but not limited to connection to a dynamic actin cortex and the transport of membrane components via both active and passive mechanisms. Recent progress has begun to address some of these issues35-37 but the vast divide between model and cell membranes will likely remain a major challenge for bottom-up investigations for decades to come.
Liquid-liquid phase separation can also observed in lipids extracted from biological samples, including erythrocytes38 and luminal membranes of renal epithelia15, lending credence to its physiological relevance. However, the vital observations validating the capacity of intact cell membranes to form coexisting liquid phases was made in plasma membranes isolated from living cells, either as Giant Plasma Membrane Vesicles (GPMVs)39-42 or Plasma Membrane Spheres (PMS)43. These intact PMs detach from mammalian cells by blebbing44 and separate into coexisting liquid domains39 of different compositions39, 45-47, orders18, 47, and diffusivities48. In GPMVs, this behavior is typically induced by lowering the temperature; in PMS by crosslinking a raft component (the ganglioside GM1 using cholera toxin). Phase separation laterally sorts the endogenous components of the PM, often in agreement with previous insights into lipid raft composition. Specifically, ordered domains enrich for GPI-anchored proteins39, 45, 46, glycosphingolipids45, palmitoylated transmembrane proteins46, and proteins with long transmembrane domains49, and exclude putative non-raft proteins like the Transferrin receptor43, 46 and those with isoprenoid membrane anchors50. Altogether, these observations comprise a critical confirmation that complex biological membranes can form lipid-driven ordered domains that preferentially recruit raft components. This capability was recently confirmed in internal organelles of living yeast cells51.
GPMVs can be conceptualized as an intermediate membrane model system between fully synthetic microscopic membranes (i.e. Giant Unilamellar Vesicles – GUVs) and living cell membranes (Fig 1). The major disadvantage of the GPMV system compared to synthetic membranes is lack of control over their composition and the inter-cell and inter-preparation variability inherent to biology. Conversely, in terms of lipid and protein content, GPMVs are self-evidently more suited to comparison with living membranes. In that context, comparing features between GPMVs and GUVs can illustrate features unique to cell-derived membranes (Fig 1). A notable example is the difference in physical properties between coexisting domains in phase-separated vesicles: while both GUVs and GPMVs separate into two phases of different orders, the relative difference in order between the phases is much lower in the cell-derived GPMVs than the synthetic membranes18. The disparity is largely attributable to the Ld phase, which is much more packed/ordered (and therefore more similar to the Lo phase) in GPMVs than in GUVs. This discrepancy is likely due to the compositional details of the two membranes: lipids with two unsaturated acyl chains, which disrupt packing and are commonly used components of GUVs, are not as abundant in mammalian cell membranes52-54, where the majority of phospholipids contain a saturated and monounsaturated chain (Fig 2). Moreover, the abundant protein content of GPMVs likely affects membrane packing47.
Figure 1. Comparison between synthetic (GUVs) and biological (GPMVs) membrane model systems.
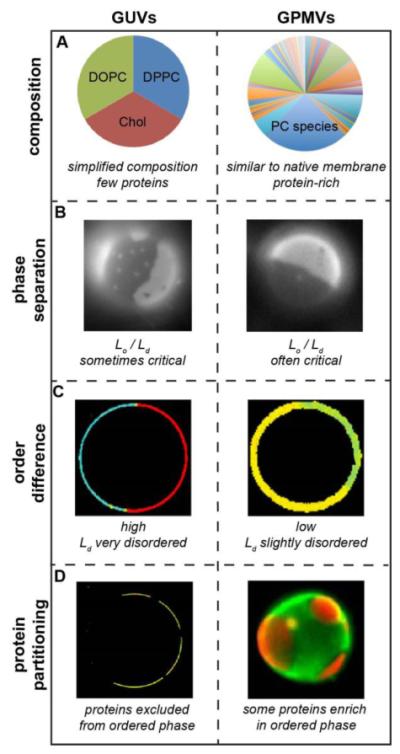
(A) Synthetic and biological model membranes differ dramatically with respect to their lipid and protein composition. Synthetic model membranes like GUVs are typically composed of a small number of different lipid components and few, if any, proteins. Shown is a canonical “raft mixture” containing equimolar saturated (DPPC) and unsaturated (DOPC) phospholipid, and cholesterol (Chol). In contrast, biological membrane models like GPMVs contain hundreds of different lipids and likely thousands of proteins. Shown is a representative distribution of phosphatidylcholine species in a PM sample, which contains a few major species (e.g. POPC is ~25 mol% of all PC) and over 100 minor ones54. (B) Despite these differences, GUVs and GPMVs are morphologically similar and both separate into coexisting ordered and disordered phases, visualized by staining vesicles with lipidic dyes that preferentially partitioning into one of the phases93. Notably, GPMVs often show near-critical behavior, also achievable in GUVs at specific compositions. (C) Despite these superficial similarities, these are notable difference in domain properties between GPMVs and GUVs. For example, coexisting domains in GPMVs are much more similar than in GUVs with respect to lipid packing. Images shown are pseudocolored maps of Laurdan generalized polarization, a widely used proxy for lipid packing (for experimental details, see references18, 160). (D) The relatively high disparity possibly results in most components being excluded from GUV ordered domains, whereas a number of lipids and proteins partition preferentially to this phase in GPMVs. Images show the partition of a peptide based on the transmembrane protein LAT. Yellow pseudocolor represents co-partitioning with a disordered phase marker in GUVs, while distinct red staining of one phase versus green in the other shows that the LAT-RFP protein partitions away from the disordered phase marker in GPMVs (for details, see references46, 57).
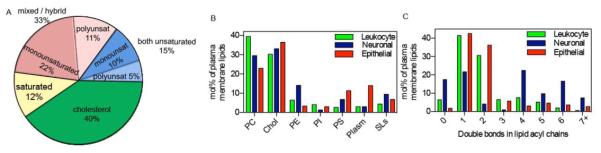
Figure 2. Sample lipidomes from mammalian plasma membranes.
(A) The complex composition of mammalian membranes can be represented in classes that are analogous to those often used for model membrane studies. In contrast to commonly used synthetic membrane preparations, mammalian PMs contain a high proportion of “mixed” lipid species with one saturated and one unsaturated acyl chain. Shown here is a representative lipidome of mesenchymal cell plasma membranes from unpublished studies in the IL lab. Further, it should be appreciated that most lipidomic features are cell / tissue specific. Shown are comparisons of (B) lipid classes and (C) unsaturation between the PMs of three different cell types: leukocytes54, epithelial cells52, and neurons82.
The difference in interdomain order between GUVs and GPMVs potentially resolves an important discrepancy in protein and lipid partitioning between the two systems. Namely, nearly all fluorescent lipids tested thus far show greater ordered phase affinity in GPMVs compared to GUVs55. The same is often true for proteins, including a GPI-anchored protein56 and a palmitoylated protein57 which prefer the disordered phase in synthetic membranes, in striking contrast to similar or identical proteins efficiently partitioning to the ordered phase in GPMVs39, 46. One explanation is that the highly disordered Ld domain and un-naturally packed Lo domain of GUVs combine to reduce order phase partitioning for nearly all probes. Finally, this interdomain order sorting has recently been shown to affect the bioactivity of membrane molecules, as shown for binding between the membrane glycolipid GM1 and its ligand cholera toxin, which was highly sensitive to membrane order and the difference between coexisting phases55, 58.
The advantage of GPMVs over live cell membranes is that they facilitate investigation into raft-associated phenomena like protein partitioning59 and regulators of phase separation60 in a controlled setting. However, it is important to emphasize that GPMVs are not the same as living cell membranes in many important ways, including: (1) their composition is likely altered during isolation, as was shown for degradation of phosphoinositides61 and some aspects of GPMVs are known to be affected by the chemicals used for preparation47, highlighting potential caveats associated the method of isolation; (2) GPMVs are not strictly asymmetric with respect to phosphatidylserine exposure39; (3) they are at equilibrium; (4) they are not coupled to an assembled actin cytoskeleton. These caveats do not negate the practical or conceptual value of GPMVs, but are important in translating insights from this biomimetic membrane model to physiological settings.
Lipidomic complexity: the known unknowns
One of the initial clues suggesting the existence of raft domains in cellular membranes was the coordinated sorting of lipids and proteins in epithelial cells, which maintain a highly specialized apical membrane enriched in canonical raft components1, 62. The isolation and characterization of specific trafficking vesicles destined for the apical versus the basolateral membrane63 confirmed a membrane-mediated sorting step at the late Golgi64. Such apically directed cargo was resistant to solubilization by non-ionic detergents (e.g. Triton X-100), and this detergent resistant membrane (DRM) residue was also enriched in specific lipid subsets, namely glycosphingolipids and cholesterol2. These observations led to the hypothesis of glycosphingolipid-enriched entities that facilitate membrane sorting in both polarized epithelia and unpolarized cells65, 66.
Early studies necessarily focused on broad classes of lipids, without much insight into the detailed compositions of the source membranes or their putative microdomains. Such insights were provided by mass spectrometric analysis of individual lipid species, which detailed the compositional enrichments in DRMs, confirming their selectivity for sphingomyelin (SM) and long, saturated phospholipids67, 68. Comprehensive information on membrane lipid composition has only become available recently, with the implementation of shotgun lipidomics. These studies have revealed the remarkable complexity and plasticity of eukaryotic lipidomes (Fig 2). A study in S. cerevisiae identified 250 individual lipid species with significant quantitative changes in nearly all of these species induced by single gene knockouts in biosynthetic pathways69. Such wholesale remodeling of the yeast lipidome appears to be a general phenomenon, as subtle variations in growth temperature, phase, and carbon source yield wholly unique lipidomic signatures70. These observations suggest deeply integrated cellular processes that sense changes in cellular metabolism and/or membrane properties and respond by tuning compositions across the spectrum of lipid sub-types. The mechanisms and purposes of these sense-and-respond modules remain generally unknown.
Comparative, lipidome-wide analyses enabled by lipidomics provide especially powerful tools to identify subtle lipid sorting signatures. A technical tour-de-force study isolated secretory vesicles leaving the Golgi Apparatus by immunopurification targeting a putative marker protein for raft domains. The lipid composition of these vesicles was clearly different from their parent organelle (the trans-Golgi), enriched in raft lipids including the major yeast sterol (ergosterol) and glycosphingolipids, providing clear evidence of lipid sorting in the late secretory pathway71. These vesicles were later shown to also be different from their destination organelle, the plasma membrane72. These observations validated the substantial literature implicating raft domains in membrane sorting processes62.
Similar themes are observable in mammalian cells. A model epithelial cell line that develops a discrete apical PM in culture on porous filter substrates (Madine-Darby Canine Kidney; MDCK) revealed clear remodeling of the lipidome during cellular polarization, which could be largely reversed by inducing an in vitro model of an epithelial-to-mesenchymal transition (EMT)53. These distinct lipid profiles are manifested in biophysical changes, as EMT induced by several independent means dramatically reduced the stability of domain formation in siolation GPMVs73. Notably, these effects could be partially reversed by altering cellular lipidomes via dietary lipids73. EMT is thought to be involved in several cancer phenotypes, including migration and escape from apoptosis, so the observations that EMT is associated with distinctive lipidomic and biophysical characteristics that are tunable by dietary pertubations may have important translational implications for cancer diagnosis and treatment. With respect to lipid sorting in mammalian membranes, much attention has been paid to viruses. Viral proteins (most notably influenza hemagglutinin) were some of the first to be associated with raft domains74, leading to the hypothesis that viruses may “hijack” raft domains for host cell entry, subcellular sorting, and/or assembly and budding of nascent virions75, 76. Evidence for this hypothesis was provided by comparison of viral lipidomes with those of their host cells. The lipid composition of the human immunodeficiency virus (HIV) membrane is clearly differentiated from that of the host cells77, and crucially also the host cell PM, which is the parent organelle for the budding viral particles78. Viral membranes are highly enriched in cholesterol and sphingolipids, convincingly implicating raft mediated sorting during viral propagation. These observations were confirmed in studies of the influenza virus52. Finally, lipidomic analysis has proven to be a powerful tool for diagnosing disease in vivo. For example, brain tissue from Alzheimer’s disease patients and rodent models suggested compositional signatures associated with disease79. Most remarkably, this signature could be identified in peripheral blood, where a biomarker study found a small set of phospholipids in blood plasma that was 90% predictive for symptomatic disease onset80.
Although these are still early days of lipidomic investigations, the data gathered thus far has revealed an unexpected complexity and diversity of lipid compositions in cellular membranes. Fig 2A shows a sample lipidome from primary human mesenchymal stem cells grouped into broad categories based on their acyl chains. These categories contain dozens, sometimes hundreds, of different species, distinguished by their hydrophilic headgroups, but also the lengths and unsaturations of their constituent fatty acids. These features can vary dramatically between PMs of various cell types (Fig 2B-C). Moreover, these lipidomic characteristics are not stable and constant, but rather affected by both endogenous processes (e.g. cell cycle81) and exogenous cues, including dietary fats54, 73. A dramatic recent example is remodeling of the lipid composition of neuronal synapses during post-natal development in the rat82. Over the first few weeks of life, synaptic membranes gradually become highly enriched in cholesterol and sphingolipids, in addition to continuous accumulation of lipids with ω-3 polyunsaturated fatty acids (PUFAs). These changes lead to a stabilization of membrane domains in reconstituted membranes, suggesting a mechanism for localized recruitment of raft domains to the neuronal synapse82.
The unexpected complexity and diversity of lipid compositions in cellular membranes prompt exciting but difficult questions regarding their biological purpose. In early descriptions of membrane physiology83, the active processes (e.g. transport, signal transduction, etc.) were carried out by proteins, with the lipids reduced to subservient roles of barriers and solvents. However, these passive functions can be accomplished by very simple membranes, composed even of single lipid species. Signaling lipids (e.g. ceramide, PIPs, glycosphingolipids) could perhaps account for a few more functionally important subtypes, but it is difficult to make the teleological case for the hundreds of lipid species produced in mammalian cells. Some recent studies of species-specific lipid functions begin to shed some light. A study combining photo-crosslinking, fluorescence spectroscopy, and bioinformatics showed remarkably specific binding between a single lipid (SM with an 18-carbon acyl chain) and a transmembrane domain of a single-pass membrane protein (P24)84. Other examples of species-level selectivity include preferential phosphorylation of polyunsaturated phosphoinosidites by PI3Kinase85.
Crucially, membrane behavior is dictated not just by the behaviors of individual lipids, but also by the emergent properties of lipid collectives. These include bulk physical characteristics like permeability, fluidity, spontaneous curvature, and stiffness, but also lateral domains resulting from preferred interactions between some lipid species. Although there is an extensive knowledge base about the compositional determinants of physical behavior in biomimetic membranes86, most such work has investigated simple membrane models. Thus, it remains an open question whether biological complexity introduces novel features to lipid collectives and how these may be regulated by compositional modulation. Our recent advances reveal that the collective behaviors of cell-derived membranes are indeed susceptible to external modulation, both by dietary lipids54 and exogenous amphiphiles60, 87, and it remains to be addressed how these modulations affect membrane physiology.
Some things remain universal
Even though cell plasma membranes have hundreds to thousands of individual components, they separate into only two microscopically discernable liquid phases39. In one sense, this is remarkable – the number of components dictates the number of potential degrees of freedom in the system, so in principle it is thermodynamically possible that a complex membrane could support hundreds of liquid phases88. In reality, maintaining even two liquid phases in coexistence requires a delicate balance. This is because liquids are defined by relatively weak intermolecular interactions that maintain fluidity, yet relatively large intermolecular interactions are required to overcome the major entropic cost to demixing. In three dimensions, most liquids are miscible with either organic or aqueous solvents and it is rare to observe more than two phases in coexistence. While there are exceptions89-91, they include compounds that vary dramatically in molecular weight (e.g. for the case of polymers) or atomic composition (e.g. metals, or compounds containing silicon or fluorine). In membranes, it is widely believed that cholesterol helps to manage this delicate balance92 by preventing strong and stable interactions between acyl chains that would lead to a gel phase while also permitting the preferential interactions that drive demixing.
The experimental observation of only two liquid phases in both model and biological membranes provides an opportunity to greatly simplify some aspects of even compositionally complex membranes. The phases themselves remain compositionally complex, but many of their bulk physical properties are both robust and simply described. For example, at any given set of equilibrium conditions, phases have well-defined average composition and acyl chain order, and individual components have strictly defined partition coefficients. Furthermore, even complex membranes have discrete miscibility transition temperatures, often referred to as Tmix or Tmisc. Above this transition temperature, membranes contain only a single liquid phase, while below it, two coexisting liquid phases are observed. The value of the transition temperature sets an important energy scale in the system – the temperature above which entropic interactions overwhelm enthalpic ones on macroscopic length-scales.
Beyond simply having a miscibility transition, GPMVs appear to have compositions that reside near a miscibility critical point at growth temperatures93. A critical point is a special composition and temperature in the thermodynamic phase diagram where the free energy cost of assembling / disassembling a macroscopic domain becomes small compared to the thermal energy94. As a result, systems near critical points exhibit large and dynamic fluctuations in composition. Micron-sized composition fluctuations are observed in GPMVs isolated from a variety of cell types very close to their miscibility transition temperature93 (Fig 3A). Above this temperature, called the critical temperature or TC, vesicles contain structure at and below a characteristic length, called the correlation length, which decreases as temperature rises. Below TC, GPMVs contain coexisting liquid domains that have dynamic fluctuating boundaries that become more stable as temperature is decreased. Together, fluctuations on either side of TC mean that GPMVs transition gradually between a single liquid phase and two coexisting liquid phases when temperature is varied slowly (e.g. at intervals of 0.2°C) (Fig 3A). Similar behavior is also observed in synthetic GUVs with compositions carefully chosen to pass through a critical point at the miscibility transition temperature95, 96. This is in contrast to model membranes with compositions that pass through the miscibility transition far from a critical point, which transition sharply from being uniform to containing circular domains 21, 92.
Figure 3. Critical fluctuations in GPMVs and the 2D Ising model.
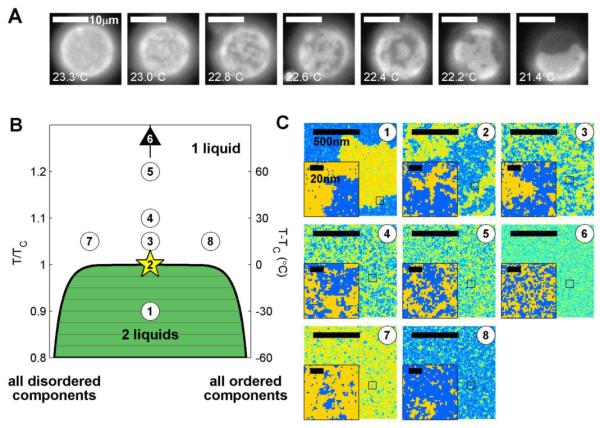
(A) Membrane heterogeneity emerges over an extended temperature range in GPMVs, consistent with their passing through a critical point at the miscibility transition temperature. (B) Miscibility phase diagram for the conserved order parameter 2D Ising model or binary fluid which contains only two components, referred to here as ordered or disordered components. The left axis shows temperature in units of the critical temperature (TC) while the right axis converts this to °C assuming that TC is 300K (27°C). (C) Simulation snap-shots from an equilibrated 2D Ising model for the conditions indicated in part B. Simulations were conducted using standard methods as described previously35. Insets are enlarged areas of the main image in the location indicated by a black box and indicate that structure on the 20nm scale is present even for conditions far removed from the critical point. Condition 6 is for an equal fraction of ordered and disordered components at a temperature of 2×TC or T-TC = 300°C, which is well outside of the temperature range plotted in B.
The experimental observation that GPMVs are close to a miscibility critical point provides the means to make even more broadly sweeping simplifications regarding the heterogeneity of these membranes. This is because many features of critical systems are universal, implying that they do not depend on the molecular details that determine the conditions of the critical point, but only the relative proximity to this point and their dimensionality94. This universality allows quantitative description of fluctuating domains (e.g. characteristic size and lifetime) by defining only a few parameters: namely the distance of the membrane from the critical point, both in composition and temperature. Crucially, both of these quantities can be derived from the macroscopic behavior of membranes. They can then be used to map the GPMV phase diagram onto a much simpler system that also exhibits critical behavior, namely the two dimensional (2D) Ising model35 (Fig 3B). In the simplest case, temperature is mapped by expressing in units of TC (in Kelvin). For example, if TC is 22°C or 295°K, then 37°C is expressed as 1.05×TC. Composition is mapped onto the 2D Ising model by measuring the surface fraction of phases at a known temperature below the transition97. This value can be converted into composition using the lever rule and the known position of the 2D Ising model phase boundary. The surface fraction of phases is equal at the critical composition. Under this condition, the characteristic size of compositional heterogeneities varies as the inverse temperature difference from the critical temperature94 (Fig 3C). This reasoning was used to predict roughly 20-nm domains at physiological temperature in GPMVs isolated from mammalian cells93. In principle, this approach can be used to generate predictions regarding structure in complex biomembranes even if they are not poised exactly at a critical point, as long as they are ‘close enough’.
How close is close enough? It seems that the answer may be: not that close. As stated above, the temperature scale is set by the critical temperature itself, which is typically between 275°K (2°C) and 300°K (27°C) for GPMVs isolated from mammalian cells, depending on the preparation method and cell type47, 48, 60, 93, 98. Thus, the physiological temperature (37°C) is between 10 and 35°C above the critical temperature, or 1.03×TC and 1.13×TC, respectively. Even at the high end, this differences is within 15% of Tc, well within the range where 2D Ising model scaling laws apply. Experiments using AFM to probe supported membranes have shown the applicability of 2D Ising model predictions to nearly 10°C from TC by directly measuring correlation lengths of detected domains in supported membranes99. Experiments in both synthetic GUVs and isolated GPMVs also showed consistency with 2D Ising model predictions over a broader temperature range (up to >30°C above TC), where an extended phase-like domain was stabilized through adhesion to a supported membrane97. This same study also found that Ising model predictions applied when synthetic GUV membranes were prepared with compositions far from a critical composition. This is likely because the shape of the high temperature edge of the 2D Ising model phase diagram is quite flat, meaning that a broad range of compositions reside close to this high temperature boundary at their transition (Fig 3B). In the 3D Ising model, as with 3D systems in general, the phase boundary has a different shape near the critical point, making fluctuations more dependent on composition than in 2D systems like membranes94. Finally, even vesicles that do not undergo a macroscopic phase transition can exhibit critical behavior, as long as their compositions are close to a phase boundary at some temperature97.
A system does need to be close to the critical point to observe the micron-sized critical composition fluctuations necessary for detection by conventional fluorescence microscopy (Fig 3A). These microscopic fluctuations are three orders of magnitude larger than the characteristic length-scale of the system, roughly the size of a typical lipid (~1nm). To obtain such large structures, the system has to be within 0.1% of the critical temperature (roughly 0.3C), and similarly close in membrane composition. However, such large fluctuations are not required for functional significance. Indeed, structures on length-scales that are relevant to interactions between proteins (~10 nm) require much less stringency. Heterogeneity on this length-scale is observed even 20% (60°C) above Tc and over a large swath of compositional space (Fig 3C). Therefore, since a miscibility transition has been observed in isolated plasma membranes under observed under laboratory conditions, it would be surprising if cell plasma membranes did not exhibit composition fluctuations on physiologically relevant scales.
Several recent theoretical approaches have suggested alternate mechanisms to produce sub-micron phase-like domains in membranes. Some of these require the presence of asymmetry in the membrane100, 101, or through incorporation of hybrid components that stabilize small domains while preventing their coalescence into larger structures102. These mechanisms produce so-called modulated phases that resemble emulsions such as milk, but are constrained to two dimensions. These processes could potentially work in concert with composition fluctuations to make structures more robust100, 103, 104. Much work remains to be done to uncover which exact physical means by which cells organize their plasma membranes, but a range of plausible and exciting possibilities exist.
Tuning domains
An outstanding question is how membrane domains are regulated and how such regulation may contribute to cell physiology. The primary means for functionalization of domains is thought to be preferential recruitment of protein and lipid components to generate compartments of specific composition. An additional control parameter is the domains themselves, namely their physical properties like size and/or lifetime. Variation of the spatial and temporal scales of membrane domains could be key for tuning the output of raft-associated signaling processes105-107. Unfortunately, there remains a dearth of reliable techniques for probing domains in situ, and for controllably modulating their properties.
The original method for perturbing rafts and raft-related processes in cells was cholesterol depletion via cyclodextrin (CD), a polysaccharide that extracts cholesterol with reasonable efficiency and specificity. This idea is deceptively simple – since cholesterol is required for rafts, its depletion should disrupt raft assembly and functionality. Consistent with this supposition, cholesterol depletion reduces detergent resistance of many proteins74, and affects polarized membrane traffic, signaling108, activation of immune cells 109, and dozens of other cellular processes. It is probably inaccurate to attribute all these effects directly to disruption of lipid rafts, because acute cholesterol depletion is inherently pleiotropic. Cholesterol is highly abundant in the PM (up to 40 mol%52, 54) and affects nearly all bulk membrane physical characteristics. For example, cholesterol is essential for PM barrier function110, and its depletion almost certainly increases membrane permeability and perturbs ion polarity. Self-evidently, such perturbations are extremely disruptive, and often cytotoxic111. Notably, even the effects of cholesterol modulation on membrane phase separation are not easily predictable. In two different cell lines, similar depletion protocols yielded opposing effects on the miscibility transition temperature, increasing in one case48 and decreasing in another54. These results reflect the clearly non-monotonic behavior of phase separation with varying cholesterol concentration, even in simple models21.
Recently, several approaches have taken advantage of GPMVs to introduce alternative methods of modulating raft domains. As described above, treatments that affect miscibility transition temperature in GPMVs may reflect modulators of raft properties in situ. One emerging theme from these studies is that exogenous amphiphiles are potentially powerful regulators of GPMV phase behavior. These include bile acids87, menthol112, hexadecanol113, and synthetic detergents54, which robustly enhance phase separation, and a broadly potent range of anesthetics60, which all suppress phase separation. These exogenous perturbations tend to be somewhat greater in magnitude than those induced by ectopic protein binding114 or expression115. One possible rationalization for these effects is that phase separation is dependent on the relative difference between coexisting domains, with treatments that increase this difference stabilizing phase separation, and vice versa. A defining difference between Lo and Ld domains is their membrane packing / order, and it has been demonstrated that agents that increase this order difference promote phase separation in GPMVs47, 54, 87 and may also affect protein-ligand interactions58. An attractive and related hypothesis is that perturbations partition to the Lo/Ld membrane interface, thereby promoting mixing and domain dispersion60, 116.
An emerging concept takes advantage of cellular metabolism and homeostasis to tune membrane domains via physiologically relevant conditions. For example, supplementation of cultured cells with dietary fatty acids, most notably the ω-3 polyunsaturated docosahexaenoic acid (DHA), leads to their robust incorporation into membrane lipids and subsequent homeostatic lipidomic remodeling. The combined result of these effects is a robust stabilization of phase separation54. As in the case of bile acids87, polyunsaturated lipids appear to disorder the Ld phase leading to enhanced interdomain order differences54. Similarly dramatic effects are induced by modulations of cell cycle and cell density of living cells98, with the time-scales of these responses suggesting adaptation to culture conditions. Currently, neither the cellular mechanisms nor the functional consequences of these responses are understood.
Measuring domains
A fundamental challenge remaining for the raft field is unambiguous identification and characterization of these domains and their properties in living cells. This hurdle persists because putative raft molecules do not segregate into microscopically visible domains in live cells. This observation prompts two exclusive conclusions: either there are no structures; or organization exists, but at a length-scale below the resolution of optical microscopy. Evidence for the latter conclusion was produced by early studies that took advantage of antibodies to cross-link and cluster raft proteins on the surface of the cells. Such crosslinking produces large lateral patches in the plasma membrane that are selective for putative raft components, including GPI-anchored proteins and GM1117. Patching could be disrupted by cholesterol depletion, supporting the conclusion that this phenomenon is related to previously unresolvable membrane rafts. Remarkably, patches induced by extracellular crosslinking of strictly outer leaflet components (e.g. GPI-anchored protein) could recruit strictly intracellular signaling molecules118 and activate signaling119, implying domain-mediated transmembrane communication.
Despite the tantalizing clues to underlying structure provided by these patching experiments, they do not reveal the unperturbed structure of the membrane. For this task, probes and techniques capable of resolution on the scale of tens of nanometers are required. Two such techniques are electron microscopy (EM) and fluorescence spectroscopy, in the form of Förster resonance energy transfer (FRET). An example of the former is immunogold localization microscopy of the Ras family of oncogenes in intact plasma membrane sheets, which showed that nearly identical Ras isoforms exhibited wholly distinct lateral organizations dependent on their post-translational lipid modification120, 121. Namely, the isoform that bears two palmitate residues (H-Ras), which are known raft targeting moieties122, segregate into separate domains from the isoform bearing a single isoprene (K-Ras), which prevents raft association. These EM observations have been validated by FRET analysis of the same constructs, and these studies were some of the first to formulate a signal transduction mechanism for such nanoscopic membrane organization123. Approximately coincident with this work on Ras were investigations into the nanoscale organization of lipidated intracellular proteins124 and GPI-APs125, 126 using FRET in live cells. These experiments convincingly demonstrated sub-resolution domains on the cell surface and, more recently, probed the cellular determinants of their formation and regulation. These studies implicate actively remodeled cortical actin127, 128 as an organizer of the inner leaflet via its interactions with specific inner leaflet lipids, which in turn couple this organization to the outer leaflet via interactions between lipid acyl chains129.
Detecting rafts through diffusion
A large number of past studies investigating domain organization in intact cell membranes have probed the mobility of membrane components130-133. A common assertion is that both plasma membrane lipids and proteins show anomalous diffusion in cells, with this behavior sometimes attributed to the partitioning of the diffusing components into raft domains. Further evidence is provided by co-diffusion of distinct membrane components whose only connectivity is hypothesized to be mediated by membrane domains134. In many cases, the implication of raft domains in anomalous diffusion is supported by changes to confinement / diffusion behavior when membranes are perturbed, usually by reducing cholesterol, but sometimes through varying other physical properties such as temperature or structure of the diffusing components. A prominent study used STED-FCS to observe the average time it takes single molecules to diffuse through an illumination area whose size was experimentally tuned135. This study observed slowly diffusing components, which were present even in very small spot sizes, suggesting single molecule partitioning into very small (i.e. <20 nm) membrane domains. As a point of reference, even a small domain 20-nm in diameter would still contain ~450 lipids (assuming a mean molecular area per lipid of 0.7 nm2), potentially large enough for collective behavior to play an important part in its organization (e.g. as in Fig 3).
Relying on similar methodology, several studies have questioned the existence of rafts based on diffusion measurements. A follow-up to the STED-FCS paper136 indicated that the trapping behaviors observed in live cells were not observed in model membranes interrogated with the same molecules and instruments, prompting the inference that the anomalous diffusion observed in cells was not due to phase separation of lipids. Similarly, numerous reports suggest that raft and non-raft membrane peptides exhibit qualitatively similar confinement behaviors130, 137, 138. Another study showed that diffusion varied smoothly with temperature for a range of different probe molecules, concluding that there was no evidence for an in situ phase transition in live cells139. Yet another found that probe localization and diffusion was not affected by the presence of macroscopic raft-like domains generated by binding raft proteins to a patterned surface140.
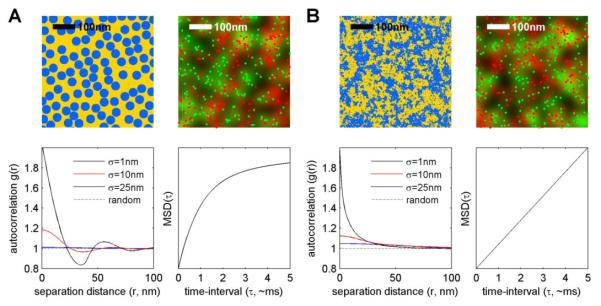
An important insight from this apparent controversy is the difficulty of defining expectations in these measurements. In other words, how would the presence of membrane heterogeneity impact diffusion? The effects could potentially be large if the local viscosity varies dramatically across the membrane, or if the membrane contains small but well-defined domains and the energy cost for components to leave these domains is large (Fig 4A). This type of behavior certainly occurs in model membranes with phase-separated domains that differ dramatically in composition, but may not be expected in biological membranes where the contrast between domains is thought to be subtle. In the furthest extreme, it is possible to have lateral heterogeneity that does not impact single molecule mobility. This is the case for systems near a critical point, which can support large composition fluctuations that have slow relaxation times even though single component motion is nearly Brownian (Fig 4B)35, 141, 142. Given this range of possibilities, it is not surprising that it has been difficult to draw definitive conclusions regarding membrane heterogeneity solely from diffusion studies.
Figure 4. Predicted experimental observations for two types of membrane heterogeneity.
(A) Some models of membrane heterogeneity propose that lipids are organized into small but clearly defined structures. (B) An alternate model is that membrane domains are more akin to super-critical fluctuations. For each model, several predictions can be made. (Images at top left) Possible configurations of ordered (blue) and disordered (yellow) membrane components in the two models presented. In each case, the characteristic length of structure is 20nm. (Images at top right) Green and red spots are a subset of components chosen randomly from the blue regions to mimic the finite concentration of a fluorescent probe. In these cases the label is included at 0.2% per color, or 2000 probes per μm2. These spots are also blurred with a Gaussian function with standard deviation of 25nm to represent the typical localization precision of a SR measurement as indicated in the background image. Note that both under sampling and blurring acts to obscure the structures from which these images were generated which are clearly visible in the top left panels. (Plots at bottom left) Pair cross-correlation functions between the differently colored spots above for several localization precisions. These curves were tabulated from images like those shown at the top right as described previously145, and indicate differently colored points are co-clustered (correlated) when g(r)>1, depleted (anti-correlated) when g(r)<1, and randomly co-distributed (uncorrelated) when g(r) = 1. Note that finite localization precision diminishes observations of the underlying structure even when localization precision is smaller than the characteristic size of structures. (Plots at bottom right) The predicted shape of mean squared displacement (MSD) vs time interval (τ) curves for both models, as discussed in the main text. Namely, single molecules that partition with the blue spots at the left will appear confined, while single molecules that partition with the blue fluctuations at the right are unconfined136.
Another important caveat is that confined motion of proposed raft components may often be convoluted by interactions with other cellular structures, including but not limited to the actin cortex (which is altered by acutely reduced cholesterol levels143), focal adhesions, and caveolae. Further, complex membrane topology can give rise to the appearance of lateral heterogeneity, since protein mobility in cells is typically detected as a 2D projection of a 3D structure144. Membrane topology is also likely sensitive to cholesterol depletion, since this lipid comprises a large fraction of the plasma membrane.
Detecting rafts using super-resolution microscopy
The advent of super-resolution microscopy brought great promise to resolving the lingering question of the existence and properties of rafts. The main justification for the inability to observe rafts in cells by diffraction limited microscopy was that rafts are small, in the 10-100 nm range, well below the diffraction limit of visible light. Both stimulated emission (STED) and point localization based (STORM/ PALM/PAINT) super-resolution (SR) methods provide access to these length-scales. Nonetheless, over a decade since the invention of these methods has failed to generate a definitive result that confirms or rules out the existence of raft structures. Why is this?
First, quantitative super-resolution measurements are difficult. An important potential artifact is over-counting of individual labeled molecules, which leads to apparent self-clustering when a single color is visualized145, 146. This experimental pitfall can be overcome by imaging two distinguishable sets of molecules to measure their co-distribution 145, 147-149. This too is not without experimental complexities: fluorescent impurities and spectral overlap can give rise to misidentification and artifactual co-localization150, 151. Although the currently available number and types of fluorophores limit these approaches, probe development is an active area of research. Second, the small size of putative raft domains still poses potential complications because the spatial resolution (practically, 15-50 nm) offered by SR methods may still not be enough to observe lipid heterogeneity directly without additional membrane perturbations to increase the size and lifetime of putative domains (Fig 4). Finally, a key drawback of SR imaging is low temporal resolution, which is a major issue for the likely highly dynamic nature of domains in vivo. Indeed, most SR experiments are conducted in chemically fixed cells or membrane sheets133, 152, 153, and these preparations can be prone to artifacts because lipids and even some membrane proteins are not immobilized by most fixation protocols154. Experiments in live cells are possible, but require experimental and analytical methods that anticipate the fast dynamics expected for raft structures155, 156.
Because of the above, direct imaging of rafts may be beyond current technical possibilities. Instead, less intuitive image quantitation methods that rely on averaging over many domains may be required to reveal the presence of heterogeneity in intact cells. Several approaches that do not rely in image reconstruction have had some success in this area. A recent advance definitively correlated three distinct measures of raft association – DRMs association, ordered phase portioning in GPMVs, and surface patching in live cells – across more than a dozen probes, along with probing the lifetime of the small entities in unperturbed cells, using multi-color single particle tracking156. This study has provided some of the strongest evidence to date to link classical raft observations with novel model membranes and modern imaging techniques. It is also possible to quantify heterogeneity in mobile systems by tabulating steady-state cross-correlations between differently colored probes155. If raft structures are well defined but small, with strong partitioning of ‘raft’ components, it is reasonable to expect that the improvement in resolution given by current methods will eventually lead to decisive observations of rafts in resting cells. However, the lack of positive data of this type does not exclude the presence of more subtle structures, as the ~20-nm domains predicted by the critical fluctuation hypothesis may still be below the practical detection limits of super-resolution imaging. Most likely, any decisive measurement of raft heterogeneity will also show that the observed structures vary with perturbations in agreement with theoretical predictions. For example, it would be informative to correlate the effects of membrane perturbations (like those described above) in GPMVs to their effects in live cells. If there is indeed a correlation between the properties of isolated and in situ PMs, the former could become a reliable proxy for the latter.
From structure to function: how to functionalize membrane domains?
The most intuitive way for lateral membrane domains to affect cell physiology is by regulating the interactions between membrane components. The simple analogy is to a reaction compartment that concentrates certain components while excluding negative regulators of a given reaction. In reality, raft and non-raft domains in biological membranes are relatively similar so most components do not partition exclusively into either of the domains; however, this does not preclude compartments of distinct composition. An important aspect of raft functionalization is dynamic regulation of their composition. One attractive possibility for such regulation is protein modification that promotes or inhibits raft affinity. The most well characterized instance is palmitoylation, a reversible saturated fatty acid modification believed to impart raft affinity to both transmembrane and peripheral proteins46, 122. Another attractive possibility is protein binding to lipids with inherent raft affinity, such as glycosphingolipids157 or cholesterol. It is also possible that the local lipid environment directly influences protein functions by setting membrane hydrophobic thickness, lateral pressure profiles, or through the interactions of certain lipid headgroup or chain types with the protein surface59.
In addition to these possibilities, the observations of critical behavior in isolated cell plasma membranes suggest that cells tune their membrane composition to reside near a miscibility critical point in order to accomplish some biological functions. Obvious questions arise as to how cells would sense and maintain near-critical compositions and, more importantly, why? One possibility is that critical composition fluctuations can mediate weak but long-range forces between membrane proteins158. Two proteins that prefer the same local lipid environment will experience a long-range attraction because of their preference for the same local lipids. Conversely, proteins that prefer different local lipid environments will feel repulsion, because they would require unfavorable lipid interactions to approach in close proximity. Interestingly, the magnitude of the repulsive interaction is generally larger than the attractive one, suggesting that this mechanism is better suited for keeping proteins apart rather than gathering them together.
Another immediate consequence of criticality that could be exploited by cells is that critical membranes are highly susceptible, meaning they are poised to remodel in response to small inputs. For three-dimensional systems in the vicinity of a liquid-gas critical point, susceptibility takes the form of a high compressibility, meaning it takes very little work to compress or expand a super-critical fluid because it takes very little energy to convert a low density gas to a higher density liquid, and vice versa. In a two dimensional multi-component membrane, high susceptibility means that the membrane can easily remodel with only a slight perturbation from outside the membrane plane. This perturbation could take the form of a subtle bias for a specific membrane environment over an extended area, as in interactions between the membrane and cortical actin35. Another possibility clustering of a small number of components that prefer a local environment, e.g. via adhesion or through binding of a clustering ligand97. In both cases, the slight bias for a specific membrane composition in a defined location would lead to global membrane remodeling, even for components that do not directly interact with the organizing components.
Finally, a recent theoretical study describes a mechanism by which fluctuations could impact the functional states of single membrane proteins, especially large transmembrane proteins that directly interact with many boundary lipids159. If two internal states of a single protein have preferences for distinct local lipid environments, then the availability of these environments will impact the population of these states. An example might be a receptor that strongly partitions into a raft environment only after it becomes activated upon binding a ligand. In this case, the receptor is more likely to become activated if the membrane already contains raft domains large enough to provide the boundary lipids preferred by the active state, thereby lowering the concentration of ligand needed for activation. In principle, this mechanism could provide a tool for cells to tune the activity of a subset of membrane proteins in parallel. Further, this mechanism may directly couple protein function to its localization. For example, a protein reliant on membrane domains may remain inactive within the early secretory pathway (i.e. the endoplasmic reticulum, where cholesterol concentrations are likely insufficient for domains62), but to become activated upon transfer to the plasma membrane. While this concept still requires experimental validation, it suggests new mechanisms through which lipid rafts could impact broad cellular functions.
Conclusion
The recent years have seen a revitalization of interest in the raft field. This enthusiasm has been spurred by methodological and conceptual advances that have bolstered arguments for the existence of membrane domains and established novel principles that reconcile evidence for domains with their persistent escape from direct detection. Although it is clear that biological membranes have the capacity for lateral organization, the specific manifestations of that organization remain unknown. Domain properties such as composition, size, and lifetimes remain unclear, partly because they are likely to differ widely between various cell types, cell states, and membranes within cells. Even more nebulous are the specific mechanisms by which these domains are integrated into cellular physiology. Ultimately, to quote Winston Churchill, rafts remain a riddle, wrapped in a mystery, inside an enigma, which will likely continue to frustrate and inspire researchers for decades to come.
Highlights.
- review of the current state of the lipid raft field
- highlights existing knowledge and open questions
- focus on novel techniques to probe key open questions
Acknowledgements
IL is supported by the Cancer Prevention and Research Institute of Texas (R1215) and the National Institutes of Health (grant 1R01GM114282). SV is supported by the National Institutes of Health (R01GM110052) and a CAREER award from the National Science Foundation (MCB-1552439).
Abbreviations
- PM
plasma membrane
- SM
sphingomyelin
- PIPs
phosphoinositide phosphates (phosphorylated phosphoinositide lipids)
- GUVs
giant unilamellar vesicles
- GPMVs
giant plasma membrane vesicles
- Lo / Ld
liquid-ordered / liquid-disordered membrane phases
- DRM
detergent resistant membrane
- Tmix / Tmisc / Tc
mixing / miscibility / critical transition temperature
- GPI-AP
glycophosphatidylinositol anchored protein
- SR
super resolution microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 5.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 6.McConnell HM, Vrljic M. Liquid-liquid immiscibility in membranes. Annu Rev Biophys Biomol Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 9.Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zuckermann MJ. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 10.Chapman D, Penkett SA. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966;211:1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- 11.Shimshick EJ, McConnell HM. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973;53:446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- 12.Vist MR, Davis JH. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 13.Huang TH, Lee CW, Das Gupta SK, Blume A, Griffin RG. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 1993;32:13277–13287. doi: 10.1021/bi00211a041. [DOI] [PubMed] [Google Scholar]
- 14.Rice PA, McConnell HM. Critical Shape Transitions of Monolayer Lipid Domains. Proceedings of the National Academy of Sciences. 1989;86:6445. doi: 10.1073/pnas.86.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich C, et al. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samsonov AV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veatch SL, Polozov IV, Gawrisch K, Keller SL. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser HJ, et al. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korlach J, Schwille P, Webb WW, Feigenson GW. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proceedings of the National Academy of Sciences. 1999;96:8461. doi: 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veatch SL, Keller SL. Organization in lipid membranes containing cholesterol. Phys Rev Lett. 2002;89:268101. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 21.Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feigenson GW, Buboltz JT. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys J. 2001;80:2775–2788. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh SL, Amazon JJ, Feigenson GW. Toward a better raft model: modulated phases in the four-component bilayer, DSPC/DOPC/POPC/CHOL. Biophys J. 2013;104:853–862. doi: 10.1016/j.bpj.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heberle FA, Buboltz JT, Stringer D, Feigenson GW. Fluorescence methods to detect phase boundaries in lipid bilayer mixtures. Biochimica Et Biophysica Acta-Molecular Cell Research. 2005;1746:186–192. doi: 10.1016/j.bbamcr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett WD, Tieleman DP. Computer simulations of lipid membrane domains. Biochim. Biophys. Acta-Biomembr. 2013;1828:1765–1776. doi: 10.1016/j.bbamem.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Sodt AJ, Pastor RW, Lyman E. Hexagonal Substructure and Hydrogen Bonding in Liquid-Ordered Phases Containing Palmitoyl Sphingomyelin. Biophys. J. 2015;109:948–955. doi: 10.1016/j.bpj.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risselada HJ, Marrink SJ. The molecular face of lipid rafts in model membranes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17367–17372. doi: 10.1073/pnas.0807527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman DG, Feigenson GW. Multiscale Modeling of Four-Component Lipid Mixtures: Domain Composition, Size, Alignment, and Properties of the Phase Interface. J. Phys. Chem. B. 2015;119:4240–4250. doi: 10.1021/jp511083z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer LV, et al. Lipid packing drives the segregation of transmembrane helices into disordered lipid domains in model membranes. Proc Natl Acad Sci U S A. 2011;108:1343–1348. doi: 10.1073/pnas.1009362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingolfsson HI, et al. Lipid organization of the plasma membrane. J Am Chem Soc. 2014;136:14554–14559. doi: 10.1021/ja507832e. [DOI] [PubMed] [Google Scholar]
- 31.Dupuy AD, Engelman DM. Protein area occupancy at the center of the red blood cell membrane. Proc Natl Acad Sci U S A. 2008;105:2848–2852. doi: 10.1073/pnas.0712379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng HT, Megha, London E. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J Biol Chem. 2009;284:6079–6092. doi: 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Q, London E. Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PloS one. 2014;9:e87903. doi: 10.1371/journal.pone.0087903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q, London E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys J. 2015;108:2212–2222. doi: 10.1016/j.bpj.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machta BB, Papanikolaou S, Sethna JP, Veatch SL. Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophys J. 2011;100:1668–1677. doi: 10.1016/j.bpj.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrig J, Petrov EP, Schwille P. Near-critical fluctuations and cytoskeleton-assisted phase separation lead to subdiffusion in cell membranes. Biophys J. 2011;100:80–89. doi: 10.1016/j.bpj.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honigmann A, et al. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife. 2014;3:e01671. doi: 10.7554/eLife.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller SL, Pitcher WH, Huestis WH, McConnell HM. Red Blood Cell Lipids Form Immiscible Liquids. Physical Review Letters. 1998;81:5019. [Google Scholar]
- 39.Baumgart T, et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sezgin E, et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- 41.Levental KR, Levental I. Giant plasma membrane vesicles: models for understanding membrane organization. Current topics in membranes. 2015;75:25–57. doi: 10.1016/bs.ctm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Levental KR, Levental I. Isolation of giant plasma membrane vesicles for evaluation of plasma membrane structure and protein partitioning. Methods in molecular biology. 2015;1232:65–77. doi: 10.1007/978-1-4939-1752-5_6. [DOI] [PubMed] [Google Scholar]
- 43.Lingwood D, Ries J, Schwille P, Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci U S A. 2008;105:10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott RE, Perkins RG, Zschunke MA, Hoerl BJ, Maercklein PB. Plasma membrane vesiculation in 3T3 and SV3T3 cells. I. Morphological and biochemical characterization. J Cell Sci. 1979;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim Biophys Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A. 2010;107:22050–22054. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levental I, Grzybek M, Simons K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc Natl Acad Sci U S A. 2011;108:11411–11416. doi: 10.1073/pnas.1105996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levental I, et al. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem J. 2009;424:163–167. doi: 10.1042/BJ20091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Rohrer B, Levental K, Simons K, Levental I. Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A. 2014;111 doi: 10.1073/pnas.1404582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 51.Toulmay A, Prinz WA. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J Cell Biol. 2013;202:35–44. doi: 10.1083/jcb.201301039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerl MJ, et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol. 2012;196:213–221. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampaio JL, et al. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci U S A. 2011;108:1903–1907. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levental KR, et al. Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys J. 2016;110(8):1800–1810. doi: 10.1016/j.bpj.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sezgin E, et al. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim Biophys Acta. 2012;1818:1777–1784. doi: 10.1016/j.bbamem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Kahya N, Brown DA, Schwille P. Raft partitioning and dynamic behavior of human placental alkaline phosphatase in giant unilamellar vesicles. Biochemistry. 2005;44:7479–7489. doi: 10.1021/bi047429d. [DOI] [PubMed] [Google Scholar]
- 57.Shogomori H, et al. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- 58.Sezgin E, et al. Adaptive lipid packing and bioactivity in membrane domains. PloS one. 2015;10:e0123930. doi: 10.1371/journal.pone.0123930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorent JH, Levental I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem Phys Lipids. 2015;192:23–32. doi: 10.1016/j.chemphyslip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 60.Gray E, Karslake J, Machta BB, Veatch SL. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys J. 2013;105:2751–2759. doi: 10.1016/j.bpj.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keller H, Lorizate M, Schwille P. PI(4,5)P2 degradation promotes the formation of cytoskeleton-free model membrane systems. Chemphyschem. 2009;10:2805–2812. doi: 10.1002/cphc.200900598. [DOI] [PubMed] [Google Scholar]
- 62.Diaz-Rohrer B, Levental KR, Levental I. Rafting through traffic: Membrane domains in cellular logistics. Biochim. Biophys. Acta-Biomembr. 2014;1838:3003–3013. doi: 10.1016/j.bbamem.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 63.Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- 65.Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fridriksson EK, et al. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 68.Han X, et al. IgE receptor-mediated alteration of membrane-cytoskeleton interactions revealed by mass spectrometric analysis of detergent-resistant membranes. Biochemistry. 2009;48:6540–6550. doi: 10.1021/bi900181w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ejsing CS, et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Natl Acad Sci U S A. 2009;106:2136–2141. doi: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klose C, et al. Flexibility of a eukaryotic lipidome--insights from yeast lipidomics. PloS one. 2012;7:e35063. doi: 10.1371/journal.pone.0035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klemm RW, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K. Generic sorting of raft lipids into secretory vesicles in yeast. Traffic. 2011;12:1139–1147. doi: 10.1111/j.1600-0854.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 73.Tisza MJ, et al. Motility and stem cell properties induced by the epithelial-mesenchymal transition require destabilization of lipid rafts. Oncotarget. 2016 doi: 10.18632/oncotarget.9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. Embo J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 76.Manes S, et al. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brugger B, et al. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci U S A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lorizate M, et al. Comparative lipidomics analysis of HIV-1 particles and their producer cell membrane in different cell lines. Cellular microbiology. 2013;15:292–304. doi: 10.1111/cmi.12101. [DOI] [PubMed] [Google Scholar]
- 79.Chan RB, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mapstone M, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nature medicine. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atilla-Gokcumen GE, et al. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–439. doi: 10.1016/j.cell.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tulodziecka K, et al. Remodeling of the postsynaptic plasma membrane during neural development. Mol Biol Cell. 2016 doi: 10.1091/mbc.E16-06-0420. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 84.Contreras FX, et al. Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature. 2012;481:525–529. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- 85.Clark J, et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods. 2011;8:267–272. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends in cell biology. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Y, et al. Bile acids modulate signaling by functional perturbation of plasma membrane domains. J. Biol. Chem. 2013;288:35660–35670. doi: 10.1074/jbc.M113.519116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibbs JW. Scientific papers. Dover Publications; New York: 1961. [Google Scholar]
- 89.Kittsley SL, Goeden HA. 8 Liquid Phases in Stable Equilibrium. Journal of the American Chemical Society. 1950;72:4841–4842. [Google Scholar]
- 90.Arce A, Earle MJ, Katdare SP, Rodriguez H, Seddon KR. Mutually immiscible ionic liquids. Chem Commun. 2006:2548–2550. doi: 10.1039/b604595b. [DOI] [PubMed] [Google Scholar]
- 91.Eckelmann J, Luning U. Mixing Liquids-Mission Impossible? A Colorful Demonstration on Immiscible Systems. J Chem Educ. 2013;90:224–227. [Google Scholar]
- 92.Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 93.Veatch SL, et al. Critical fluctuations in plasma membrane vesicles. ACS Chem Biol. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 94.Sethna JP. Statistical mechanics : entropy, order parameters, and complexity. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 95.Honerkamp-Smith AR, et al. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophys J. 2008;95:236–246. doi: 10.1529/biophysj.107.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honerkamp-Smith AR, Machta BB, Keller SL. Experimental observations of dynamic critical phenomena in a lipid membrane. Phys Rev Lett. 2012;108:265702. doi: 10.1103/PhysRevLett.108.265702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao J, Wu J, Veatch SL. Adhesion stabilizes robust lipid heterogeneity in supercritical membranes at physiological temperature. Biophys J. 2013;104:825–834. doi: 10.1016/j.bpj.2012.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray EM, Diaz-Vazquez G, Veatch SL. Growth Conditions and Cell Cycle Phase Modulate Phase Transition Temperatures in RBL-2H3 Derived Plasma Membrane Vesicles. PloS one. 2015;10:e0137741. doi: 10.1371/journal.pone.0137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Connell SD, Heath G, Olmsted PD, Kisil A. Critical point fluctuations in supported lipid membranes. Faraday discussions. 2013;161:91–111. doi: 10.1039/c2fd20119d. discussion 113-150. [DOI] [PubMed] [Google Scholar]
- 100.Wolff J, Komura S, Andelman D. Budding of domains in mixed bilayer membranes. Phys Rev E Stat Nonlin Soft Matter Phys. 2015;91:012708. doi: 10.1103/PhysRevE.91.012708. [DOI] [PubMed] [Google Scholar]
- 101.Shlomovitz R, Schick M. Model of a raft in both leaves of an asymmetric lipid bilayer. Biophys J. 2013;105:1406–1413. doi: 10.1016/j.bpj.2013.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brewster R, Safran SA. Line active hybrid lipids determine domain size in phase separation of saturated and unsaturated lipids. Biophys J. 2010;98:L21–23. doi: 10.1016/j.bpj.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palmieri B, Safran SA. Hybrid lipids increase the probability of fluctuating nanodomains in mixed membranes. Langmuir. 2013;29:5246–5261. doi: 10.1021/la4006168. [DOI] [PubMed] [Google Scholar]
- 104.Palmieri B, Safran SA. Hybrid lipids increase nanoscale fluctuation lifetimes in mixed membranes. Phys Rev E Stat Nonlin Soft Matter Phys. 2013;88:032708. doi: 10.1103/PhysRevE.88.032708. [DOI] [PubMed] [Google Scholar]
- 105.Barua D, Goldstein B. A mechanistic model of early FcepsilonRI signaling: lipid rafts and the question of protection from dephosphorylation. PloS one. 2012;7:e51669. doi: 10.1371/journal.pone.0051669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicolau DV, Jr., Burrage K, Parton RG, Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki KG. Lipid rafts generate digital-like signal transduction in cell plasma membranes. Biotechnology journal. 2012;7:753–761. doi: 10.1002/biot.201100360. [DOI] [PubMed] [Google Scholar]
- 108.Chen X, Resh MD. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J Biol Chem. 2002;277:49631–49637. doi: 10.1074/jbc.M208327200. [DOI] [PubMed] [Google Scholar]
- 109.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haines TH. Do sterols reduce proton and sodium leaks through lipid bilayers? Progress in lipid research. 2001;40:299–324. doi: 10.1016/s0163-7827(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 111.Mahammad S, Dinic J, Adler J, Parmryd I. Limited cholesterol depletion causes aggregation of plasma membrane lipid rafts inducing T cell activation. Biochim Biophys Acta. 2010;1801:625–634. doi: 10.1016/j.bbalip.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Raghunathan K, Ahsan A, Ray D, Nyati MK, Veatch SL. Membrane Transition Temperature Determines Cisplatin Response. PloS one. 2015;10:e0140925. doi: 10.1371/journal.pone.0140925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Machta BB, et al. Conditions that Stabilize Membrane Domains Also Antagonize n-Alcohol Anesthesia. Biophys J. 2016;111:537–545. doi: 10.1016/j.bpj.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson SA, et al. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim Biophys Acta. 2010;1798:1427–1435. doi: 10.1016/j.bbamem.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Podkalicka J, Biernatowska A, Majkowski M, Grzybek M, Sikorski AF. MPP1 as a Factor Regulating Phase Separation in Giant Plasma Membrane-Derived Vesicles. Biophys J. 2015;108:2201–2211. doi: 10.1016/j.bpj.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meerschaert RL, Kelly CV. Trace membrane additives affect lipid phases with distinct mechanisms: a modified Ising model. European biophysics journal : EBJ. 2015;44:227–233. doi: 10.1007/s00249-015-1017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harder T, Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur J Immunol. 1999;29:556–562. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 119.Hiscox S, Hallett MB, Morgan BP, van den Berg CW. GPI-anchored GFP signals Ca2+ but is homogeneously distributed on the cell surface. Biochem Biophys Res Commun. 2002;293:714–721. doi: 10.1016/S0006-291X(02)00280-2. [DOI] [PubMed] [Google Scholar]
- 120.Prior IA, et al. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 121.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 123.Tian T, et al. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol. 2007;9:905–914. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- 124.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 125.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 126.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 127.Goswami D, et al. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gowrishankar K, et al. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 2012;149:1353–1367. doi: 10.1016/j.cell.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 129.Raghupathy R, et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161:581–594. doi: 10.1016/j.cell.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kenworthy AK, et al. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kusumi A, Ike H, Nakada C, Murase K, Fujiwara T. Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin Immunol. 2005;17:3–21. doi: 10.1016/j.smim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 132.Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82:274–284. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Owen DM, Williamson DJ, Magenau A, Gaus K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nature communications. 2012;3:1256. doi: 10.1038/ncomms2273. [DOI] [PubMed] [Google Scholar]
- 134.Brameshuber M, et al. Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J Biol Chem. 2010;285:41765–41771. doi: 10.1074/jbc.M110.182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eggeling C, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 136.Mueller V, et al. STED nanoscopy reveals molecular details of cholesterol-and cytoskeleton-modulated lipid interactions in living cells. Biophys J. 2011;101:1651–1660. doi: 10.1016/j.bpj.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lommerse PH, et al. Single-molecule diffusion reveals similar mobility for the Lck, H-ras, and K-ras membrane anchors. Biophys J. 2006;91:1090–1097. doi: 10.1529/biophysj.105.079053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Edwald E, Stone MB, Gray EM, Wu J, Veatch SL. Oxygen depletion speeds and simplifies diffusion in HeLa cells. Biophys J. 2014;107:1873–1884. doi: 10.1016/j.bpj.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee IH, et al. Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J Phys Chem B. 2015;119:4450–4459. doi: 10.1021/jp512839q. [DOI] [PubMed] [Google Scholar]
- 140.Sevcsik E, et al. GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane. Nature communications. 2015;6:6969. doi: 10.1038/ncomms7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burns MC, Nouri M, Veatch SL. Spot size variation FCS in simulations of the 2D Ising model. J phys D. 2016;49:14001. doi: 10.1088/0022-3727/49/21/214001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McConnell H. Single molecule diffusion in critical lipid bilayers. J Chem Phys. 2012;137:215104. doi: 10.1063/1.4768956. [DOI] [PubMed] [Google Scholar]
- 143.Kwik J, et al. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adler J, Shevchuk AI, Novak P, Korchev YE, Parmryd I. Plasma membrane topography and interpretation of single-particle tracks. Nat Methods. 2010;7:170–171. doi: 10.1038/nmeth0310-170. [DOI] [PubMed] [Google Scholar]
- 145.Veatch SL, et al. Correlation functions quantify super-resolution images and estimate apparent clustering due to over-counting. PloS one. 2012;7:e31457. doi: 10.1371/journal.pone.0031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sengupta P, et al. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods. 2011;8:969–975. doi: 10.1038/nmeth.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shelby SA, Holowka D, Baird B, Veatch SL. Distinct stages of stimulated FcepsilonRI receptor clustering and immobilization are identified through superresolution imaging. Biophys J. 2013;105:2343–2354. doi: 10.1016/j.bpj.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grover JR, et al. Roles played by capsid-dependent induction of membrane curvature and Gag-ESCRT interactions in tetherin recruitment to HIV-1 assembly sites. J Virol. 2013;87:4650–4664. doi: 10.1128/JVI.03526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grover JR, Veatch SL, Ono A. Basic motifs target PSGL-1, CD43, and CD44 to plasma membrane sites where HIV-1 assembles. J Virol. 2015;89:454–467. doi: 10.1128/JVI.02178-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stone MB, Veatch SL. Far-red organic fluorophores contain a fluorescent impurity. Chemphyschem. 2014;15:2240–2246. doi: 10.1002/cphc.201402002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim D, Curthoys NM, Parent MT, Hess ST. Bleed-through correction for rendering and correlation analysis in multi-colour localization microscopy. Journal of optics. 2013;15 doi: 10.1088/2040-8978/15/9/094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lillemeier BF, et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saka SK, et al. Multi-protein assemblies underlie the mesoscale organization of the plasma membrane. Nature communications. 2014;5:4509. doi: 10.1038/ncomms5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanaka KA, et al. Membrane molecules mobile even after chemical fixation. Nat Methods. 2010;7:865–866. doi: 10.1038/nmeth.f.314. [DOI] [PubMed] [Google Scholar]
- 155.Stone MB, Veatch SL. Steady-state cross-correlations for live two-colour super-resolution localization data sets. Nature communications. 2015;6:7347. doi: 10.1038/ncomms8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Komura N, et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat Chem Biol. 2016;12:402–410. doi: 10.1038/nchembio.2059. [DOI] [PubMed] [Google Scholar]
- 157.Coskun U, Grzybek M, Drechsel D, Simons K. Regulation of human EGF receptor by lipids. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1105666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Machta BB, Veatch SL, Sethna JP. Critical Casimir forces in cellular membranes. Phys Rev Lett. 2012;109:138101. doi: 10.1103/PhysRevLett.109.138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kimchi O, Machta BB. Allosteric Regulation by a Critical Membrane. arXiv:1607.06836. 2016 S., V. [Google Scholar]
- 160.Sezgin E, et al. Adaptive lipid packing and bioactivity in membrane domains. PloS one. 2015;10:e0123930. doi: 10.1371/journal.pone.0123930. [DOI] [PMC free article] [PubMed] [Google Scholar]