Abstract
Intravital imaging enables to study dynamic tumour–stroma interactions within primary and metastatic sites, including the lung. The combination of optical windows with specific molecular probes targeting the tumour microenvironment will provide new insights into prometastatic stromal interactions and lead to novel therapeutic strategies for metastatic diseases.
Cellular Interactions in the Tumour Microenvironment
Malignant tumour cells frequently metastasise to distant organs, such as the bone, liver, brain, and lung, which dramatically reduces the survival rate of patients with cancer. To establish metastatic tumours, cancer cells egress from primary sites through local invasion and intravasation (i. e., entrance into lymphatic or blood vessels), since the elaboration of such vessels is enhanced during tumour growth. Following escape into the circulatory system, they need to extravasate (i.e., escape from lymphatic or blood vessels), become established, and proliferate at metastatic sites. During this process, metastatic cancer cells also need to escape from the immune surveillance of CD8+ T and natural killer (NK) cells. Recent studies have shown that all of these events are promoted by stromal cells, such as endothelial cells, fibroblasts, and tumour-infiltrating immune cells, especially neutrophils and macrophages [1]. Since stromal cells also contribute to chemotherapy resistance in malignant tumours [2], tumour–stroma interactions are considered as promising targets for improved anticancer therapies. Therefore, it has become essential to analyse the dynamic behaviour of stromal cells to understand how and why they interact with cancer cells in the tumour microenvironment, and the pro- or antitumoural consequences of these interactions.
Intravital Imaging Windows
Over the past few years, intravital optical imaging has been used to examine complex biological events in vivo with subcellular resolution. For example, the groups of Friedl and Condeelis, Segall, and van Rheenen were some of the first to image sub-cutaneous andmammary tumours via skin-fold chambers or optical windows [3,4]. The development of this technology has opened the possibility to study in vivo key processes that had been previously described in the tumour microenvironment, such as findings from the Sahai lab showing that cancer-associated fibroblasts are necessary to enable the collective invasion of tumour cells [5], or the paracrine loop between tumour-associated macrophages and cancer cells in tumour cell invasion and intravasation described by Condeelis and coworkers [6].
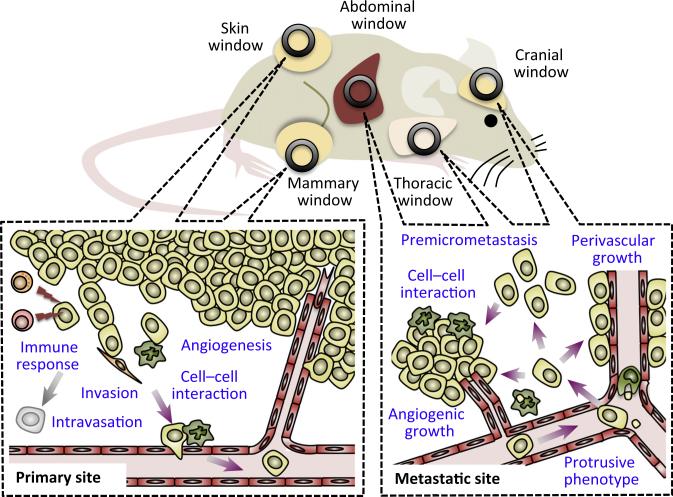
Although most imaging studies to date have focussed on primary tumours, Sipkins et al. imaged the bone marrow under the thin mouse skull to identify that leukaemia bone metastasis is initiated at a unique bone marrow vascular area where the endothelium secretes chemokine SDF-1/CXCL12 [7]. Furthermore, recent advances in optical windows have enabled researchers to image metastatic tumours in vital organs, such as the brain or liver (Figure 1). For instance, Kienast et al. imaged the development of metastatic tumours in the brain via cranial windows (i.e., windows implanted in the skulls of mice) to identify the rate-limiting steps in the formation of macrometastasis, such as perivascular growth by angiogenesis or vessel co-option [7]. Using an abdominal imaging window, Ritsma et al. visualised liver metastasis and observed that extravasated tumour cells proliferated and formed premicrometastases where individual cells were motile and lacked contact with neighbouring tumour cells [7]. Imaging subcutaneous tumours that are transferred with fluorescently labelled CD8+ T and NK cells has also evidenced the contacts between tumour cells and the cytotoxic immune cells aiming to eliminate them [7].
Figure 1. Imaging Windows to Study Tumour–Stroma Interactions in vivo.
Superficial primary tumours, such as subcutaneous tumours and mammary tumours, can be imaged through skin-fold chambers or optical windows inserted into the skin over the tumour. These studies have accelerated the study of the kinetics of angiogenesis during tumour development, the immune response from CD8+ T and NK cells against tumour cells, and the tumour cell invasion and intravasation guided by cancer-associated fibroblasts or tumour-associated macrophages. Metastatic tumours in the liver, brain, and lung can be imaged through optical windows (e.g., abdominal, cranial, and thoracic windows, respectively). Intravital microscopy through these windows has been used to analyse the dynamics of metastasised tumour cells (e.g., protrusion formation, premetastasis development, perivascular, or angiogenic growth) as well as their interaction with macrophages.
Imaging these processes in the lung, another major metastatic site, has been challenging because it is a vital organ in continuous motion and is enclosed within the thoracic cavity. Nevertheless, Entenberg et al. recently succeeded in imaging metastatic tumours in the breathing lung with subcellular resolution using a vacuum window and signal optimisation techniques [8]. The high resolution of this new technology allowed the authors to visualise how tumour cells exhibit protrusions to move inside the vessels immediately upon arrival at the capillary. The authors also showed myeloid cells (e.g., tumour-associated macrophages) interacting directly with tumour cells, which suggests their critical role in the formation of metastatic niches. Using a similar model, Headley et al. recently imaged how microparticles secreted by tumour cells are ingested by macrophages in the lung and how they upregulate a variety of adhesion and chemotactic receptors [9]. Although visualising the lung via these windows is currently limited to short imaging sessions (e.g., less than 24 h), these studies corroborate the power of intravital imaging to study the dynamics of tumour and stromal cells in lung metastatic sites and provide new insights into the cellular interactions that regulate the establishment of lethal meta-static tumours.
Activatable Molecular Probes
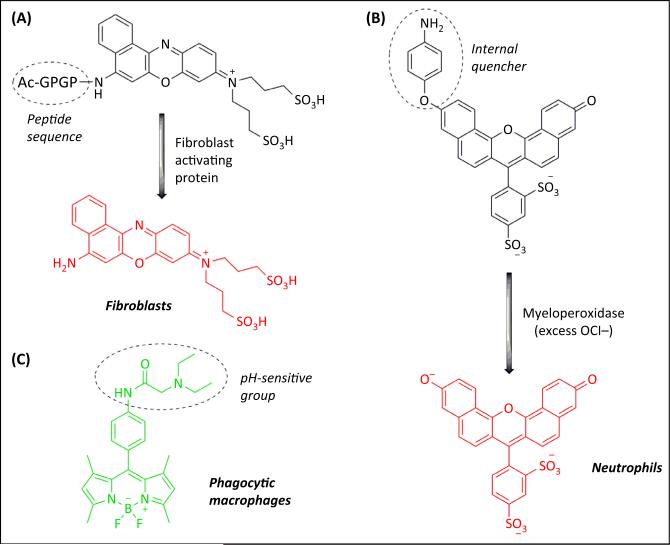
To be detected by intravital microscopy, the cells of interest (e.g., stromal or cancer cells) must be fluorescently labelled, although recent advances in coherent anti-Stokes Raman scattering (CARS) may provide a label-free optical imaging platform in the near future. This labelling can be readily achieved in transgenic animals where fluorescent proteins (e.g., eCFP, eGFP, RFP, and mCherry) are expressed under lineage-specific promoters. However, this approach often cannot discriminate between specific cell subpopulations [e.g., macrophages versus neutrophils (LysM promoter) or tissue-resident versus recruited macrophages (Csf1r promoter)], and often requires multiple genetic crosses that are expensive and time-consuming. Alternatively, specific cells can be isolated, labelled with fluorescent dyes (e.g., CFSE or CMTMR) and then adoptively transferred to track their fate in vivo. However, these labelling methods are short-lived (e.g., less than a week) and the transfer of large numbers of cells can lead to artefacts. Fluorescently labelled antibodies can be used to stain specific markers in vivo, but their tissue distribution, permeability, and immunogenicity are a cause for major concern. Another attractive strategy involves the administration of activatable molecular probes that emit a fluorescent signal only after they are activated by specific enzymes (e.g., matrix metalloproteases or myeloperoxidases) or in defined intracellular environments (e.g., low-pH organelles). Several activatable molecular probes have been designed to track tumour or stromal cells as well as to provide a direct readout of cellular or enzymatic activity (Figure 2). These include fluorescently labelled inhibitors or quenched peptides to image protease activity (e.g., matrix metalloproteases in cancer cells or fibroblast activation protein in cancer-associated fibroblasts [10]), fluorescent probes activated by myeloperoxidase for monitoring activated neutrophils and alveolar macrophages in the lungs [11], or pH-sensitive probes for imaging phagocytic macrophages in vivo [12].
Figure 2. Activatable Molecular Probes for Imaging Stromal Cells.
(A) Peptide sequences can be conjugated to quenched fluorophores to produce activatable molecular probes that emit fluorescence only after cleavage by specific proteins (e.g., fibroblast activating protein) [10]; (B) an internally-quenched fluorophore (SNAPF), which reacts with hypochlorite (OCl−) produced by the myeloperoxidase enzyme, allows the monitoring of activated neutrophils in the lung by fluorescence imaging [11]; (C) PhagoGreen is a pH-sensitive BODIPY fluorophore for imaging phagocytic macrophages in vivo [12].
Concluding Remarks
Activatable fluorescent probes are a powerful toolbox for monitoring distinct populations of stromal cells in vivo and for studying their unique functions in subsets of cells without the need for transgenic manipulation. Furthermore, molecular probes can be designed to target specific metabolites, which might enable researchers to monitor the metabolic activity of different cell types in the tumour microenvironment. However, the integration of molecular probes to intravital imaging is not straightforward because the cellular complexity of the tumour micro-environment demands probes with very high selectivity. To this end, the incorporation of other biomolecules (e.g., peptides, small proteins, or drug molecules) might be necessary to image specific subsets of cells. The optimisation of molecular probes must also address other important factors, such as their biodistribution and kinetics, to ensure that real-time intravital imaging can be performed at the site of action. Bearing these challenges in mind, we envisage that the combination of high-resolution optical imaging windows with molecular probes targeting key events in the metastatic cascade will become an excellent partnership to understand the biology behind the progression of metastasis as well as to evaluate new anticancer therapies in situ and in real time.
Acknowledgments
T.K. acknowledges funding from The Wellcome Trust (109657/Z/15/Z) and the United States Department of Defense Congressionally Directed Medical Research Programs (DOD#W81xWH-11-1-0703). J.W.P. is supported by The Wellcome Trust (101067/Z/13/Z), the Medical Research Council (G1002033), and NIH CA100324. M.V. acknowledges the support of the Medical Research Council and the Marie Curie Integration Grant (333487).
References
- 1.Kitamura T, et al. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander S, et al. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 4.Kedrin D, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 6.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbroek SI, van Rheenen J. Imaging hallmarks of cancer in living mice. Nat. Rev. Cancer. 2014;14:406–418. doi: 10.1038/nrc3742. [DOI] [PubMed] [Google Scholar]
- 8.Entenberg D, et al. In vivo subcellular resolution optical imaging in the lung reveals early metastatic proliferation and motility. Intravital. 2015;4:e1086613. doi: 10.1080/21659087.2015.1086613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Headley MB, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531:513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai KS, et al. Selective fluorescence probes for dipeptidyl peptidase activity-fibroblast activation protein and dipeptidyl peptidase IV. Bioconjug. Chem. 2007;18:1246–1250. doi: 10.1021/bc0603586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chagnon F, et al. Smart imaging of acute lung injury: exploration of myeloperoxidase activity using in vivo endoscopic confocal fluorescence microscopy. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L543–L551. doi: 10.1152/ajplung.00289.2014. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez-Romero AM, et al. Multicomponent reactions for de novo synthesis of BODIPY probes: in vivo imaging of phagocytic macrophages. J. Am. Chem. Soc. 2013;135:16018–16021. doi: 10.1021/ja408093p. [DOI] [PMC free article] [PubMed] [Google Scholar]