Abstract
Associative studies across a range of neurodevelopmental disorders have revealed a relationship between immune system function and behavioral deficits. These correlations are particularly evident in individuals with autism spectrum disorders (ASD), a developmental disorder characterized by social behavior deficits and noted for its high instances of immune system dysfunction. Mouse models provide a unique opportunity to explore causal links between immune and nervous system function and reveal how changes in these systems alter behavioral profiles. The BTBR T+ Itpr3tf/J (BTBR) mouse strain is characterized by both social behavior impairments and aberrant immune responses, affording the unique opportunity to investigate the causal relationship between behavior and immunity through direct manipulation of these systems. Using bone marrow from the highly social C57BL/6J (C57) mouse strain, BTBR mice were tested for changes in social approach behavior and repetitive grooming following irradiation and bone marrow transplant. BTBR recipient mice treated with allogeneic bone marrow from C57 donor mice, but not syngeneic BTBR bone marrow, displayed increased sociability as measured by the three-chamber social approach task and total time spent social sniffing. In addition, C57 recipient mice given allogeneic bone marrow from BTBR donors showed a significant increase in repetitive grooming behavior. These data provide evidence for a causal relationship between peripheral immune phenotype and social behavior in the BTBR mouse strain and further strengthen and expand on our existing understanding of the role of immune function in behavior.
Keywords: Behavior, Immune, Autism, ASD, Sociability, BTBR T+tf/J, Animal model, Translational, Bone marrow transplant
1. Introduction
Autism spectrum disorders (ASD) are characterized by impairments in social interaction and the presence of stereotyped behaviors and restricted interests or activities (APA, 2013). While the diagnosis is based exclusively on behavioral criteria, there is a large body of evidence that suggests a relationship between abnormal immune phenotype and ASD in both humans and animal models (for review see (Onore et al., 2012)). Observations of immune abnormalities in ASD include evidence of neuroinflammation (Gupta et al., 2014; Li et al., 2009; Morgan et al., 2010; Suzuki et al., 2013; Vargas et al., 2005), increased peripheral immune cell activation (Ashwood et al., 2011a; Careaga et al., 2015a; Enstrom et al., 2009; Enstrom et al., 2010), elevated plasma pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8 and IL-12p40 (Ashwood et al., 2011b), and decreased anti-inflammatory cytokines such as transforming growth factor (TGF)-β (Ashwood et al., 2008; Okada et al., 2007). While this information has contributed to our understanding of the potential relationship between immune phenotype and behavior, restrictions in experimental procedures and ethical considerations limit our ability to distinguish between a symptomatic or causative relationship between immune function and ASD. For this reason, mouse models provide a unique opportunity to explore causal links between immune and nervous system function and reveal how changes in these systems alter behavioral profiles associated with core features of ASD and other neurodevelopmental disorders.
The BTBR T+ Itpr3tf/J (BTBR) inbred mouse strain is a well characterized animal model exhibiting several behaviors relevant to the core features of ASD, including reduced social interactions, repetitive behavior, and atypical vocalizations (for review see (Careaga et al., 2015b)). Compared to the highly social C57BL/6J (C57) strain, BTBR mice exhibit heightened inflammatory phenotypes in the peripheral immune system and central nervous system, characteristics often reported in individuals with ASD (Careaga et al., 2015b). For example, BTBR mice show signs of increased microglia activation (Heo et al., 2011), a marker of neuroinflammation also reported in individuals with ASD (Morgan et al., 2010; Suzuki et al., 2013). In the peripheral immune system, macrophages derived from BTBR bone marrow show enhanced M1 polarization and exaggerated cytokine and chemokine production including elevations in IL-6, IL-12p40, CCL2, CCL3, and CCL4. These elevations in immune markers correlate with the severity of social impairments and repetitive grooming behaviors (Heo et al., 2011; Onore et al., 2012; Onore et al., 2013; Schwartzer et al., 2013). Together, these data suggest that the aberrant behaviors characteristic of the BTBR mouse may be a result of immune system dysregulation and give rise to the notion that altering the immune system profile may improve behavioral deficits.
While the asocial BTBR mouse strain is derived from a distinct lineage compared to C57 mice, these strains share a common MHC H2b haplotype allowing for bone marrow transplantation without tissue rejection and the ability to test the causal links between peripheral immune system phenotype and social behavior deficits. Previous work utilizing bone marrow transplants between C57 and BTBR strains revealed that BTBR-derived T-cells become highly activated following transplantation into C57 and BTBR recipients (Zhang et al., 2013). However, it remains unknown whether these changes in immune cell phenotype can alter autism-like behavioral states. To this end, we investigated whether altering the immune-phenotype via cross-strain bone marrow transplant could augment the behavioral profile of both BTBR and C57 strains.
2. Methods
2.1. Mice
Ten-week old male and female C57 (Jackson Laboratory, Sacramento, CA) and BTBR mice (Jackson Laboratory, Bar Harbor, Maine) were maintained by the Campus Laboratory Animal Services, at University of California, Davis at ambient room temperature on a 12 hour light/dark cycle with food and water available ad libitum. All procedures were performed with approval by the University of California, Davis Institutional Animal Care and Use Committee and in accordance with the guidelines provided by the National Institutes of Health for the ethical treatment of animals.
2.2. Preparation of bone marrow
Donor male and female C57 and BTBR mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Mice were thoroughly cleaned with 70% ethanol, dissected in a sterile laminar flow hood, and tissue collection was performed aseptically. The femur and tibia were collected from each mouse and the spinal cord was carefully removed and discarded. Bones were placed in a dish with 10mls of sterile, ice cold Hank's Buffered saline solution without calcium or magnesium. Femur and tibia bones were severed proximal to each joint with sterilized scissors, and bone marrow was flushed from the bone cavity with 10mLs of ice cold HBSS using a 25-guage needle. Bone marrow extracted from tibia and femur were combined and cells from each strain and sex were pooled, counted, and pelleted by centrifugation at 500 × g for 10 minutes. Pooled bone marrow cells were resuspended in sterile phosphate buffered saline (PBS) at 2.0 × 108 cells/mL for injection into irradiated recipient mice. Bone marrow was prepared less than 2 hours in advance and kept on ice prior to injection.
2.3. Preparation of recipient mice and bone marrow injection
Seven days prior to irradiation and transplantation, all recipient mice were placed on a prophylactic antibiotic supplemented diet of Uniprim® (275ppm trimethoprim and 1,365ppm of sulfonamide sulfadiazine). On the day of bone marrow transplantation, recipient mice received total body irradiation with a split dose of 11Gy from Caesium-137. Each dose of radiation delivered 5.5Gy, with a 3-hour interval between doses. Between 1-3 hours after the final dose of irradiation, each recipient mouse received 2 × 106 cells from either the same strain (i.e. syngeneic) or opposite strain (i.e. allogeneic) donors in 100μL of sterile saline solution via tail vein injection with a 25-gauge needle resulting in 4 treatment groups: C57 donor ➝ C57 recipient (C57-Syngeneic) (n=12), BTBR donor ➝ C57 recipient (C57-Allogeneic) (n=11), BTBR donor ➝ BTBR recipient (BTBR-Syngeneic) (n=13), C57 donor ➝ BTBR recipient (BTBR-Allogeneic) (n=15). All recipient mice received cell suspensions from same-sex donors. Following transplantation, recipient mice remained on a Uniprim® diet for an additional 14-day period followed by a two month recovery period before undergoing behavioral testing.
2.4. Three chamber social approach
Mice were assessed for social approach behavior as previously described (Yang et al., 2011). In brief, mice were habituated to a three-chamber arena with photo beam sensors located in the entrance of each chamber. Following habituation, mice underwent a 10-minute testing period where they were measured automatically based on photo beam breaks for the time spent exploring a chamber with a novel mouse compared to a chamber with a novel object. A sociability score was calculated as the time spent in the novel mouse chamber minus time spent in the novel object chamber. Mice were also video-recorded and analyzed for the total time spent sniffing the novel mouse and novel object using EthoVision XT 11.5 (Noldus Information Technology).
2.5. Grooming
One week following social approach, mice were placed in an empty clean Plexiglas cage and left undisturbed to habituate for 10 minutes. Following habituation, mice were video-recorded for 10 minutes and later scored for the total time self-grooming by two individuals blind to treatment conditions. Grooming was defined as time spent licking paws, washing the nose, face or scratching fur with any foot.
2.6. Tissue collection and processing
Following behavioral assessment, 6 mice from each recipient group were sacrificed by CO2 asphyxiation and cardiac perfused with 10mLs ice-cold PBS to remove circulating blood from tissues. Spleens were collected from individual mice to evaluate engraftment of bone marrow derived cells. Spleens were dissociated by passage through a 100μm nylon mesh in 10mLs of sterile ice cold PBS. Dissociated splenocytes were pelleted and resuspended in 2mLs of 1X ice-cold ammonium-chloride-potassium (ACK) lysis buffer to remove red blood cells and incubated on ice. After 1 minute, 5mLs of cold Roswell Park Memorial Institute media, supplemented with 10% FBS was added and the cells filtered through a 100μm nylon mesh and pelleted by centrifugation 500 × g at 4°C for 10 minutes. The pelleted splenocytes were resuspended in sterile PBS for flow cytometric analysis as described below.
2.7. Fluorescent labeling and flow cytometry analysis
Splenocytes were resuspended at room temperature in PBS at 5.00 × 106 cells/mL and divided into 100μl samples for fluorescent labeling using standardized protocols for flow cytometry. Cells were labeled with Aqua live-dead viability label for 20 minutes at room temperature. Following viability labeling, cells were centrifuged at 500 × g for 10 minutes and washed in 200μL staining buffer. Cells were then resuspended in 100μl of staining buffer and incubated with 25μg/mL Fc Block™ for 10 minutes. Cells were then cooled to 4°C by refrigeration and either left unlabeled, labeled with fluorescein isothiocyanate (FITC)-CD45.1, Alexaflour700 (Alexa-700)-CD45.2, phycoerythrin (PE)-CD3, or allophycocyanin (APC)-CD11b, (eBiosciences, San Diego, CA), or an equal concentration of their fluorescently conjugated corresponding isotype controls for 30 minutes at 4°C. The two strains differ in CD45 alleles, with BTBR expressing the CD45.1 allele and C57 expressing the CD45.2 allele, enabling clear distinction between cell populations following transplantation. Following labeling, cells were washed twice with 200μL staining buffer, resuspended in 300μL staining buffer and analyzed by flow cytometric analysis using a BD-LSRII (BD Biosciences). Flow cytometry data was analyzed with FlowJo software.
2.8 Statistics
In the three-chambered social approach task, mice were assessed for preference for the social or object chamber between each treatment group using mixed-measures analysis of variance with time in chamber as the within-subject variable and strain, treatment, and sex as the between-subjects variables. Additionally, a social preference score, calculated as the time spent in the chamber with the novel mouse minus the time in the chamber with the novel object, and total time spent grooming were analyzed across conditions using 3-way ANOVA with treatment, strain, and sex as between-subject variables. Total time spent sniffing the novel mouse and novel object were analyzed using pairwise planned comparisons. Cell engraftment was determined by the percentage of CD3+ or CD11b+ cells expressing CD45.1 or CD45.2 markers. For example, percentage of BTBR origin T-cells present in C57-Allogeneic mice was calculated as the number of CD3+CD45.1+ cells divided by the total number of CD3+ cells. Pairwise post hoc analysis was used with Bonferroni corrections for multiple comparisons when applicable. All comparisons were two-tailed and analyzed using R statistical software with alpha set at 0.05. Outliers were determined by the Tukey Fence method (±1.5 IQR).
3. Results
3.1. Social Approach
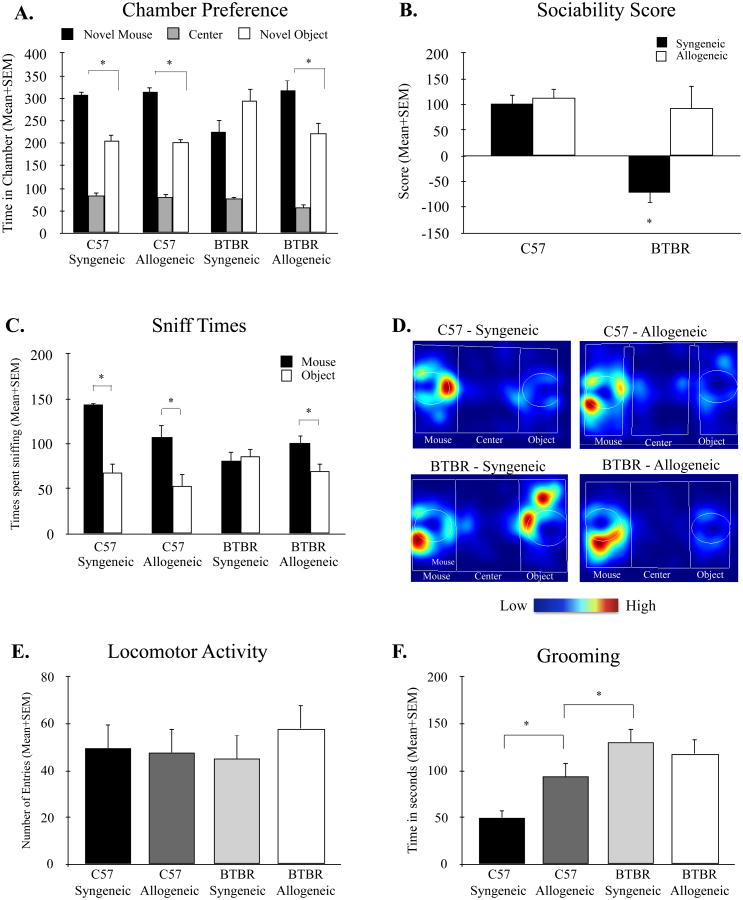
A main effects analysis for chamber preferences revealed that C57 mice displayed species typical preference for the chamber with the novel mouse compared to the novel object, F(1, 19) = 47.11, p < 0.001, and this preference was observed across treatment groups as indicated by a non-significant treatment by chamber interaction, F(1,18) = 0.10, p = 0.75. Conversely, in BTBR mice there was a significant treatment effect, F(1, 26) = 4.79, p < 0.05, as well as a treatment by chamber interaction, F(1, 26) = 5.82, p < 0.05. Specifically, while the BTBR-Syngeneic mice showed no preference for either novel mouse chamber or novel object chamber, t(12) = -1.35, p = 0.20, BTBR-Allogeneic mice displayed increased social preference for the chamber with the novel mouse, t(14) = 2.11, p = 0.05, indicating restoration of the characteristic social behavior deficits (Figure 1A). These differences were independent of sex, as indicated by no main effect for sex, F(1, 40) = 1.10, p = 0.30, and no sex by chamber by treatment interaction, F(1, 40) = 0.03, p = 0.85.
Figure 1.
Changes in social appraoch and grooming behavior following bone marrow transplant. (A) Both syngeneic and allogeneic C57 recipient mice showed species-typical social approach behavior indcicated by a significant preference for the chamber containing a novel mouse compared to the novel object. Conversley, syngeneic BTBR mice displayed characteristic deficits in social appraoch as measured by an absence of preference for either mouse or object chamber. However, BTBR recipient mice treated with bone marrow of allogeneic C57 donors showed significant prefrence for the novel mosue over the novel object, indicating a restoration of social behavior deficits. (B) No difference in sociability scores were observed between C57 mice reciveing bone marrow from either C57 or BTBR recipients. Conversley, while BTBR mice treated with syngeneic bone marrow showed low sociabilty scores, allogeneic recipients displayed high levels of sociabilty equal to that of C57 control mice. (C) C57 recipients of both syngeneic (C57 donor) and allogeneic (BTBR donor) conditions spent significantly more time sniffing and engaging with a novel mouse compared to the novel object. In contrast, BTBR mice who received syngeneic bone marrow from BTBR recipients spent equal time sniffing the novelm mouse and novel object. BTBR recipient mice treated with allogeneic bone marrow from the highly social C57 donor strain showed significant increases in the time spent sniffing the novel mouse compared to the novel object, indicating improvemetns in social engagement and preference for a social stimulus. (D) Representative heat maps showing the total time and location of the nose of each mouse was during the 10-minute social approach task. Warmer colors (red) indicate greater time the nose-point was detected. White circles represent the location of the novel mouse (left) and novel object (right) in each arena. (E) No differences in overall locomotor activity, as indicated by total number of chamber entries, was present across groups. (F) C57 mice treatd with BTBR bone marrow spent signficnatly more time grooming compared to syngeneic C57 control mice. This increase in grooming behavior was similar to that of BTBR mice treated with C57 bone marrow but significantly less than that of syngeneic BTBR mice. *p < 0.05, C57-Syngeneic n = 10, C57-Allogeneic n = 10, BTBR-Syngeneic n = 13, BTBR-Allogeneic, n=15.
To further quantify changes in sociability following bone marrow transfer, the social preference score was analyzed across groups. A 3-way analysis of variance revealed a main effect for strain across social scores, F(1,40) = 5.82, p < 0.05, with C57 mice displaying a high social score compared to BTBR mice regardless of treatment. Additionally, there was a treatment by strain interaction that approached significance, F(1,40) = 4.06, p = 0.051, with BTBR-Allogeneic mice displaying significantly increased sociability scores compared to BTBR-Syngeneic littermates, F(1, 26) = 5.82, p < 0.05. These differences in social approach behavior were not present between treatment groups in the C57 strain, F(1, 18) = 1.01, p = 0.75. BTBR-Syngeneic mice exhibited significantly reduced sociability scores (-70.85 ± 52.37) in comparison to the social C57-Syngeneic mouse group (100.44 ± 19.90) and C57-Allogeneic group (113.3 ± 17.68). However, this deficit was not present in the BTBR-Allogeneic group (93.4 ± 44.29) (Figure 1B). Together, these data indicate that transplanting C57 bone marrow into BTBR mice improves social approach behaviors.
Mice were also measured for the total time spent sniffing the novel mouse and novel object in each condition (Figure 1C-D). Both C57-Syngeneic and C57-Allogeneic groups spent significant more time sniffing the novel mouse compared to the time spent sniffing the novel object (p < 0.01). BTBR-Syngeneic mice showed no difference in the total time spent sniffing the novel object compared to the novel mouse, t(12) = -0.46, p = 0.65. However, when BTBR recipient mice received allogeneic bone marrow from C57 donor mice, a significant increase was observed in the total time spent sniffing the novel mouse compared to the time spent sniffing the novel object, t(14) = 2.25, p < 0.05, indicating improved social sniffing and more time engaging with a social stimulus. These data were independent of sex.
To determine whether changes in chamber preference time were a result of altered motor activity, a mixed-model ANOVA was performed on the total number of chamber entries made across conditions. There were no main effects in total number of entries between strains, F(1, 44) = 0.60, p = 0.44, or treatment, F(1, 44) = 2.90, p = 0.09, and no strain by treatment interaction, F(1, 41) = 2.31, p = 0.14. Mice in all conditions showed similar locomotor activity indicating that behavioral changes were not a result of altered motor function (Figure 1E)
3.2 Grooming
Analysis of total time spent grooming revealed a main effect for strain, F(1,40) = 12.56, p < 0.001, with BTBR mice spending significantly more time grooming (124.11 ± 10.20) compared to C57 mice (73.53 ± 9.84). These differences were present regardless of treatment condition, F(1,40) = 0.43, p = 0.51, or sex, F(1,42) = 0.01, p = 0.98. Interestingly, there was a treatment by strain interaction that approached significance F(1,40) = 3.797, p = 0.057. Simple main effects analysis revealed a significant treatment effect in C57 mice, F(1, 20) = 6.05, p < 0.05, with C57 mice who received cells from BTBR donors spending significantly more time grooming compared to the C57-Syngeneic group. This increase in grooming behavior observed in the C57-Allogeneic mice was similar to that of BTBR mice treated with C57 bone marrow, t(24) = 1.82, p = 0.08, but still less than BTBR-Syngeneic mice, t(21) = -5.06, p < 0.001 (Figure 1F).
3.3 Tissue Engraftment
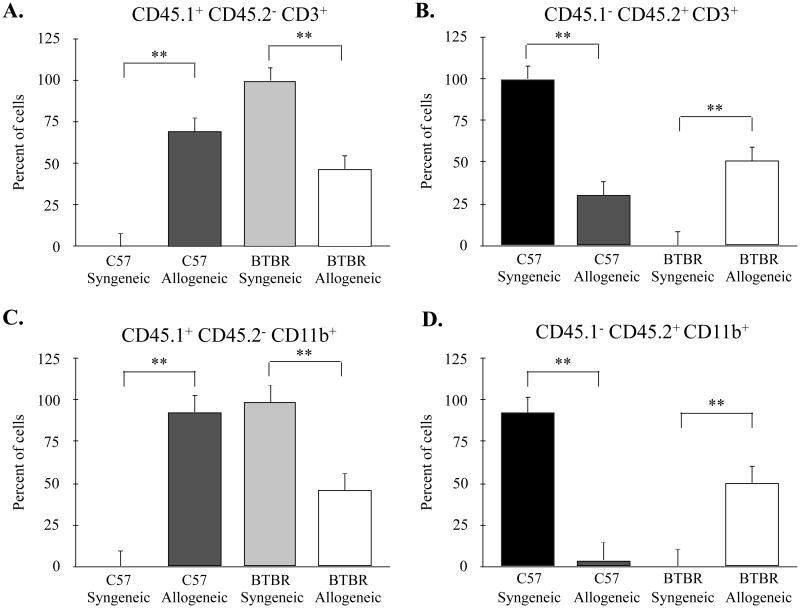
Bone marrow engraftment in recipients of CD45.1+ (BTBR) or CD45.2+ (C57) cells were assessed using flow cytometry. C57 recipients of bone marrow from BTBR donor mice exhibited on average 64.2 ± 12.9% engraftment of donor T-cells (CD45.1+ CD45.2- CD3+) and 92.14 ± 13.52% engraftment of donor myeloid cells (CD45.1+ CD45.2+ CD11b+). BTBR recipient mice treated with bone marrow from C57 donor mice generally exhibited lower levels of engraftment with an average of 50.97 ± 13.08% of CD45.1- CD45.2+ CD3+ and 50.34 ± 20.96% of CD45.1-CD45.2+ CD11b+ cells. Nonetheless, the frequencies of CD45.1+ and CD45.2+ cells in spleen were significantly different between recipients of syngeneic and allogeneic donors in both C57 (p < 0.001) and BTBR (p < 0.001) strains (Figure 2).
Figure 2.
Mean percentage of donor cell engraftment from splenoctyes. (A) Percentage of CD45.1+ (BTBR origin) CD3+ T-cells and (B) CD45.2 (C57 origin) CD3+ T-cells. (C) Percentage of CD45.1+ CD11b+ myeloid cells and (D) CD45.2+ CD11b+ cells. Significant differences in the presence of both CD45 alleles were detected between allogeneic and syngeneic recipients in spleen tissue of both strains. *p < 0.05. C57-Syngeneic n = 6, C57-Allogeneic n = 6, BTBR-Syngeneic n = 6, BTBR-Allogeneic, n = 6.
4. Discussion
Ongoing immune system function regulates important homeostatic mechanisms and behaviors including memory, sleep, motivation, and social interactions (Hart, 1988). Persistent immune alterations are thought to contribute to the behavioral sequel associated with many psychiatric and neurodvelopmental disorders (Dantzer, 2006; Pace and Miller, 2009). This neuro-immune association is evident in the BTBR mouse model, an inbred strain characterized by both elevated pro-inflammatory signaling and reduced social interactions (Careaga et al., 2015b; McFarlane et al., 2008; Onore et al., 2013). These parallels suggest that chronic inflammation may be a source of the reduced sociability and increased repetitive behavior seen in BTBR mice. To identify whether the ongoing inflammation in the BTBR mouse contributes to the characteristic ASD-like behavior deficits, bone marrow from the highly social and “immune-typical” C57 mouse was transplanted into the BTBR strain and recipient mice were screened for changes in social interactions and repetitive grooming behavior.
Following transplantation, BTBR mice treated with C57 bone marrow cells showed marked improvements in social approach and social sniffing behavior equal to that observed in the social C57 strain. Improved behavioral performance following bone marrow transplant has previously been reported in a model of maternal immune activation (MIA). Mice born to dams exposed to immune stimulation during pregnancy exhibit behavioral deficits and exaggerated pro-inflammatory responses (Malkova et al., 2012; Onore et al., 2014; Schwartzer et al., 2013). When offspring of MIA dams were treated with bone marrow of control offspring the behavioral alterations were ameliorated (Hsiao et al., 2012). Interestingly, while transplant of healthy bone marrow into MIA offspring improved behavioral deficits, these changes were not reversed in control saline offspring. That is, species-typical behaviors were not altered in control mice treated with bone marrow of MIA offspring. This lack of behavioral change was also observed in our social C57 recipient mice following exposure to BTBR bone marrow. The mechanism underlying why control bone marrow cell transfer to the asocial mice in either the MIA or BTBR models is sufficient to rectify social behavior but cells from the affected animal do not confer change are not clear. The changes, or lack thereof, may reflect cell-intrinsic epigenetic or genetic programming, or other factors in the peripheral immune environment may also be necessary to produce neuro-immune alterations in behavior. Importantly, the characteristic low sociability in BTBR mice are hypothesized to emerge from a combination of changes in nervous system (Fenlon et al., 2015), gastrointestinal-microbiota (Klein et al., 2016), metabolic (Smith et al., 2016; Zilkha et al., 2016), and immunological function (Careaga et al., 2015b; Onore et al., 2013). This suggests there may be several factors working synergistically to modulate the social behavior improvements observed in BTBR-Allogeneic recipients and underscores the complex nature of social behavior deficits in the BTBR mouse strain.
In addition to changes in social behavior, we found that C57 recipient mice treated with BTBR donor cells displayed increased grooming compared to species-typical controls. Our previous studies showed that the BTBR mouse exhibits an increased pro-inflammatory macrophage phenotype with elevations in inflammatory cytokine production correlating with excessive grooming behavior (Careaga et al., 2015b; Onore et al., 2013) but less so with social behavior suggesting that grooming behavior may be more acutely driven by peripheral cytokine levels than social behaviors (Onore et al., 2013). These data may suggest the presence of distinct immune mechanisms that differentially drive repetitive grooming behaviors from social behaviors within each strain or that cytokine production alone is sufficient to alter grooming behavior.
To further explore the discrepancy in behavioral restoration between repetitive and social behaviors in allogeneic recipient mice, splenocytes from recipient mice were assessed for the level of peripheral immune cell engraftment across conditions. BTBR-Allogeneic mice showed poorer donor cell engraftment compared to C57-Allogeneic mice. It is not clear why engraftment was lower in the BTBR strain but may relate to a number of factors including residual circulating resident cells that survived irradiation, cell proliferation kinetics of BTBR cells, or other factors that made irradiation sub-optimal in the BTBR strain. That is, diverse genetic differences in BTBR mice may contribute to multiple alterations in the immune environment. Asymmetric proliferation of immune cells of BTBR and C57 mice following irradiation in BTBR or C57 recipient strains has previously been reported (Zhang et al., 2013). In line with this research, we report greater levels of BTBR donor cell engraftment in C57-Allogeneic recipient mice compared to C57 donor cells present in BTBR-Allogeneic recipients. This asymmetric engraftment observed in the BTBR-Allogeneic condition may potentially have resulted in the lack of change in grooming behavior in BTBR receiving C57 cells. Although speculative, it is plausible that the presence of residual host cells in BTBR recipients of C57 donor cells was sufficient to overcome the beneficial effects C57 donor cells would have on grooming behavior. Considering the excessive production of pro-inflammatory cytokines in BTBR immune cells and their association with grooming intensity (Onore et al., 2013), the presence of even a minority of resident cells in BTBR recipients of allogeneic C57 donors may be sufficient to increase cytokine levels or other signaling factors that exacerbate grooming behavior while other factors less dependent on cytokine production may have permitted the restoration of BTBR social behavior in allogeneic recipients. These findings warrant further investigation to identify differences in specific immune cell subsets in different tissue compartments and the kinetics of cell engraftment and repopulation over time.
In summary, our findings reveal increased social approach behavior in BTBR mice receiving C57 bone marrow transplant, providing evidence for a causative relationship between immune phenotype and social behavior. These data offer new evidence that social behavior can be modulated by the manipulation of the immune system, even in adult mammals, and highlight the profound behavioral consequences of altered immune system function. Moreover our findings further support previous literature linking immunotherapy as a plausible mechanism for treating neurodevelopmental disorders and provides useful translational information about the relationship between behavior and immune function in neuropsychiatric diseases.
Acknowledgments
The authors would like to acknowledge Carolyn Chang, Duy Pham, Houa Yang, Milo Careaga, Nancy Nguyen, Jennifer Mead, Nicole Corley, and the staff of the UC Davis Imaging Research Center for their technical expertise. Special thank you to Dr. Robert Berman for his critical insight and support of this research. The authors further acknowledge support from NIH T32MH073124 (JS) and NIH R15HD082638 (JS), NARSAD Foundation, Jane Botsford Johnson Foundation, Peter Emch Foundation, Autism Speaks Foundation, UC Davis Dissertation Year Fellowship.
Footnotes
The authors declare no conflicts of interest.
References
- APA, A.P.A, editor. Diagnostic and statistical manual of mental disorders. 5th 2013. [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain, behavior, and immunity. 2011a;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011b;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, Ashwood P. Immune Endophenotypes in Children with Autism Spectrum Disorder. Biol Psychiatry. 2015a doi: 10.1016/j.biopsych.2015.08.036. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Schwartzer J, Ashwood P. Inflammatory profiles in the BTBR mouse: how relevant are they to autism spectrum disorders? Brain, behavior, and immunity. 2015b;43:11–16. doi: 10.1016/j.bbi.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain, behavior, and immunity. 2009;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain, behavior, and immunity. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenlon LR, Liu S, Gobius I, Kurniawan ND, Murphy S, Moldrich RX, Richards LJ. Formation of functional areas in the cerebral cortex is disrupted in a mouse model of autism spectrum disorder. Neural Dev. 2015;10:10. doi: 10.1186/s13064-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, West AB, Arking DE. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748. doi: 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heo Y, Zhang Y, Gao D, Miller VM, Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS One. 2011;6:e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MS, Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Vogel HJ, Shearer J. Metabolomic Modeling To Monitor Host Responsiveness to Gut Microbiota Manipulation in the BTBR(T+tf/j) Mouse. J Proteome Res. 2016;15:1143–1150. doi: 10.1021/acs.jproteome.5b01025. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, behavior, and immunity. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, brain, and behavior. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Sugiyama T, Kawai M, Minabe Y, Takei N, Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore CE, Careaga M, Babineau BA, Schwartzer JJ, Berman RF, Ashwood P. Inflammatory macrophage phenotype in BTBR T+tf/J mice. Frontiers in neuroscience. 2013;7:158. doi: 10.3389/fnins.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain, behavior, and immunity. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Rho JM, Teskey GC. Ketogenic diet restores aberrant cortical motor maps and excitation-to-inhibition imbalance in the BTBR mouse model of autism spectrum disorder. Behav Brain Res. 2016;304:67–70. doi: 10.1016/j.bbr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Y, Omata K, Matsumoto K, Tsuchiya KJ, Iwata Y, Tsujii M, Sugiyama T, Mori N. Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2011;Chapter 8:26. doi: 10.1002/0471142301.ns0826s56. Unit 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao D, Kluetzman K, Mendoza A, Bolivar VJ, Reilly A, Jolly JK, Lawrence DA. The maternal autoimmune environment affects the social behavior of offspring. J Neuroimmunol. 2013;258:51–60. doi: 10.1016/j.jneuroim.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Zilkha N, Kuperman Y, Kimchi T. High-fat diet exacerbates cognitive rigidity and social deficiency in the BTBR mouse model of autism. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.070. [DOI] [PubMed] [Google Scholar]