Abstract
Myelination is a recent evolutionary addition that significantly enhances the speed of transmission in the neural network. Even slight defects in myelin integrity impair performance and enhance the risk of neurological disorders. Indeed, myelin degeneration is an early and well-recognized neuropathology that is age associated, but appears before cognitive decline. Myelin is only formed by fully differentiated oligodendrocytes, but the entire oligodendrocyte lineage are clear targets of the altered chemistry of the aging brain. As in neurons, unrepaired DNA damage accumulates in the postmitotic oligodendrocyte genome during normal aging, and indeed may be one of the upstream causes of cellular aging - a fact well illustrated by myelin co-morbidity in premature aging syndromes arising from deficits in DNA repair enzymes. The clinical and experimental evidence from Alzheimer's disease, progeroid syndromes, ataxia-telangiectasia and other conditions strongly suggest that oligodendrocytes may in fact be uniquely vulnerable to oxidative DNA damage. If this damage remains unrepaired, as is increasingly true in the aging brain, myelin gene transcription and oligodendrocyte differentiation is impaired. Delineating the relationships between early myelin loss and DNA damage in brain aging will offer an additional dimension outside the neurocentric view of neurodegenerative disease.
Keywords: Oligodendrocyte, DNA damage, Alzheimer’s disease, Progeriod syndromes, White matter, aging brain
1. Introduction
Research into neurological disorders, particularly the age-associated dementias, focuses heavily on the nerve cell. For example, the most common age-associated dementia is Alzheimer's disease (AD), whose hallmark pathologies of amyloid plaque formation and neurofibrillary tangles have typically been studied primarily for their impact on the neurons of the brain. Indeed, the very name, neurodegenerative disease, draws attention towards the striking loss of neuronal cell bodies and processes, and away from the roles of a variety of other brain cells in disease pathogenesis. With the failure of clinical trials based on this neurocentric outlook and the importance of developing alternative disease models, this perspective is beginning to broaden. Neuroinflammation and the role of the microglial cell are increasingly recognized as important players in AD (Heneka et al., 2015). Vascular problems and the participation of the endothelial cells is also gaining attention (Iadecola, 2013; Marchesi, 2011) as is the role of astrocytes (Rodriguez et al., 2009). In this review, we focus on the role of yet another cell type that is a likely contributor to the appearance of dementia, the oligodendrocyte (OL).
White matter (WM) degeneration and abnormalities in the aging brain have been well established over the years, if not fully appreciated. Evidence for the involvement of myelin abnormalities in the process of aging is compelling. Indeed the initial WM decline in the aging brain begins at about ~45 years of age (Bartzokis et al., 2001; Bartzokis et al., 2003; Sperling et al., 2014). This puts WM changes as a strong correlate of aging, and certainly as strong as the modest decline found in neuron numbers during normal healthy aging (Mortera and Herculano-Houzel, 2012). The changes may also be one of the earliest events in AD pathogenesis. The significance of the oligodendrocyte to aging and neurodegenerative disease is further emphasized by its unusual sensitivity to DNA damage, in particular oxidative lesions. Exploring such intriguing temporal correlations and the clinical relationship between WM abnormalities and cognitive decline (Provenzano et al., 2013), may reveal important and early facets of the underlying neurobiology of prodromal late life dementias such as Alzheimer's.
2. The life cycle of OLs and myelin formation
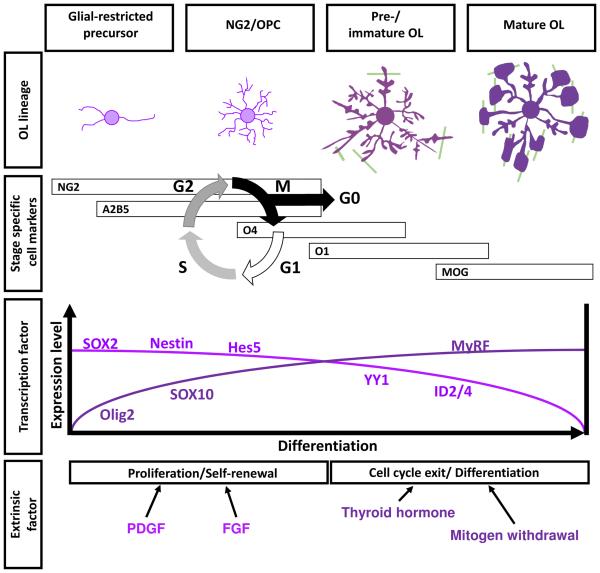
During human development, the development of the OL lineage begins at about nine weeks of gestation, when OL progenitor cells (OPC) first appear (Jakovcevski et al., 2009). These OPC, also known as NG2 cells, proliferate and become the dominant myelin-related cell type in the telencephalon until 18 weeks of gestation (Jakovcevski et al., 2009). Then, until about 27 weeks of gestation, the OPC differentiate into late OL progenitors or PreOLs by extending their cellular processes to enwrap multiple axons. The number of preOLs subsequently increases and as they mature, they begin to lose their OPC markers to become immature OL (Back et al., 2001). Myelin is formed by the wrapping of a 'finger' of OL cytoplasm around nearby neuronal axons. The cytoplasm within this 'finger' is expelled, the membranes of sequential wraps become adherent leading to the deposition of a multilayered lipid-rich myelin sheath.
The life cycle of the OPC is highly regulated by multiple transcription factors. Developmentally, the early stages of the OL lineage is specified by the expression of SOX10, Olig2 and Nkx2.2 (Liu et al., 2007b), which orchestrate the life cycle of OLs (Hernandez and Casaccia, 2015). The proliferation of OPC is promoted by mitogens, while differentiation is induced upon mitogen withdrawal and the availability of thyroid hormone, retinoic acid and cortisol (Barres et al., 1994; Schoonover et al., 2004). Cell cycle progression is inhibited by the cooperation between Olig2/SOX10 with the transcription inhibitors, ID2 and ID4 (Chen et al., 2012; Samanta and Kessler, 2004). During proliferation, these inhibitors are counteracted by the actions of E2F1 (Magri et al., 2014) or Yin Yang 1 (YY1) (He et al., 2007a).
These transcription factors are further controlled by the epigenetic remodeling of the chromatin structure by histone acetylation and methylation. Class I histone deacetylases (HDAC1 and 2), but not class II or class III, are particularly important, for the differentiation of OL (Liu et al., 2015; Shen et al., 2005). The absence or pharmacological inhibition of HDAC1/2 will prevent the differentiation of OPC into mOL in rodent (Marin-Husstege et al., 2002) and in human (Conway et al., 2012). Inhibition of HDAC1 in the OPC will maintain OL progenitor cells status through the activation of genes like Nestin, ID2, ID4 and Sox2 (Lyssiotis et al., 2007). Expression of these genes is the consequence of interactions between HDAC1/2 and OL-specific transcription factors. For instance, HDACs cooperate with YY1 to promote the chromatin remodeling required for cell cycle exit and OPC differentiation (He et al., 2007b). The histone acetylation and methylation marks on the chromatin have been hypothesized as the epigenetic memory that allows the OPC lineage to differentiate and myelinate in proper spatial and temporal order (Liu et al., 2007a; Shen et al., 2008a). In both developmental and disease models, the activities of HDAC1/2 dictate the timing of WM development as well as remyelination after injury (Liu et al., 2012b; Shen et al., 2005).
Myelin gene regulatory factor (MyRF) is expressed exclusively by postmitotic OLs (Cahoy et al., 2008). To induce differentiation, MyRF partners with SOX10 to induce transcription of myelin-related genes including myelin basic protein (MBP), proteolipid protein (PLP), myelin associated glycoprotein (MAG) and myelin OL glycoprotein (MOG) (Bujalka et al., 2013; Koenning et al., 2012), all of which contain MyRF binding motifs in their promoters. MyRF is thus essential for the function of postmitotic OLs (Emery et al., 2009; McKenzie et al., 2014).
Starting at 28 weeks of human gestation until birth, immature OLs begin to dominate the myelin forming cell population and start to produce vast quantities of glycolipid-rich membrane in order to form the premyelin sheath (Back et al., 2001). Myelin-related proteins including MBP, PLP, MAG and MOG are then inserted enabling the formation of the multiple compact layers of myelin (Back et al., 2002b). This is the situation of OL lineage at birth, but the development of OLs continues throughout adult life. In the adult brain, approximately 3-6% of the total cells are NG2-positive OPC in both rodent (Dawson et al., 2003; Peters, 2004) and humans (Scolding et al., 1998). These adult OPCs reside in the brain as a mitotically quiescent progenitor pool, but in response to injury they can re-enter the cell cycle. An additional layer of regulation is added by the local neuronal population as OPC can also be induced differentiate into mature OLs in response to neuronal activity (Dawson et al., 2003) – an event that is crucial for learning (McKenzie et al., 2014). As explained in latter sections, many transcriptional steps of this multi-stage process tie the OL development to the DNA repair mechanism, see Figure 1.
Figure 1.
The life cycle of the oligodendrocyte lineage
3. Myelination is uniquely expanded in human cortex
Myelination is a relatively recent evolutionary development that greatly enhances neural transmission in vertebrates. The conduction velocity of myelinated fibers is estimated to be at least 10-fold faster than that of unmyelinated fibers of the same diameter (Salami et al., 2003). Further, despite the classical dichotomy of white matter versus gray matter (GM), myelination is found in both (Krimer et al., 1997). Nonetheless in the human brain (the prefrontal lobe in particular) it is the volume of WM rather than GM that appears to grow disproportionately larger than in other primates. (Schoenemann et al., 2005). Correlated with this dramatic expansion in WM volume, the OLs of the human show an extensively delayed maturation relative to non-human primates (Miller et al., 2012). Indeed, it had been suggested that myelination is the last step in the entire maturation of the human brain, and does not reach its peak (by volume) until 45 to 47 years of age, after which it progressively declines (Bartzokis et al., 2001). Plus, the accumulated evidence makes it highly likely that the late maturation of myelination has considerable importance for brain function. For example intracortical myelination in the prefrontal cortex is crucial for higher cognitive performance in humans and other primates (Grydeland et al., 2013; Peters and Sethares, 2002).
The myelination of the prefrontal cortex is hit hardest by the aging process, showing more significant degeneration than any other region (Raz et al., 2005). The suggestion is that these types of association tracts are somehow unusually fragile. Thus, not only are they late evolutionary additions, they are the last to mature during brain development but the first to degenerate with age (Bender et al., 2016). Strikingly, at a systems neuroscience level, loss of function in these very same networks of association fibers – those connecting the heteromodal cortex, limbic and paralimbic regions – is tightly correlated with the advance of AD (Douaud et al., 2014). The thinking that has emerged out of the totality of this data is a “last in, first out” hypothesis of normal white matter aging (Raz, 2000, 2001).
While the death of neurons will inevitably stop the action potential activity in any neural network, network modeling exercises make it clear that even a slight defect in myelination (i.e. enough to cause a conduction delay of just 1 millisecond) will significantly impact the synchrony of a neural networks and cause cognitive impairment (Pajevic et al., 2014). As would be predicted from this modeling, cognitive functions gradually decline in primary demyelinating disease such as multiple sclerosis (Calabrese et al., 2009). It is a bit more of a speculation, but given its importance to cognitive function, it would not be unreasonable to assume that the myelin loss may underlie at least some of the cognitive signs of dementing illnesses such as Alzheimer's disease. And while it is more speculative still, since we know so little about the biology of dementing illnesses (Herrup, 2015), it cannot be ruled out that myelin defects (or cell biological deficiencies in the constituent OLs) actually serve to initiate the processes of neuronal cell death. Indeed, this could be viewed as an explanation of why, in spite of the presence of amyloid deposition, there is no tau pathology or neurodegeneration observed in older non-human primates (Heuer et al., 2012). In this view (Haroutunian et al., 2014; Raz, 2000), the initial cognitive decline as well as the enhanced neuronal vulnerability in degenerative diseases such as AD derives from the uniquely human extended dependence of the brain networks on an intact system of myelination. This line of thinking is fully consistent with the observation that it is the WM of the frontal lobes that is the most affected region in severe AD cases (Sjobeck et al., 2006). Therefore, it is the late maturation of myelination and its heightened sensitivity to the process of aging that arguably put humans at elevated risk for cognitive decline in aging.
4. Loss of myelination is a pathological hallmark of the aging and AD brain
The seminal observations in this area are the pioneering MRI studies by Bartzokis et al showing that the developmental curve of WM volume in healthy human brain resembles an inverted-U shape (Bartzokis et al., 2001). The WM volume in the frontal and temporal lobe increases at the beginning of adolescence and peaks at 45 years of age, before beginning a slow decline. Such a pattern of WM change is the inverse of that of the gray matter, which begins to reduce in the second decade of life (Bartzokis et al., 2001). These findings were confirmed by independent groups who observed the inverted U-shape myelination development not only in the cerebral WM, but also in cerebellum and hippocampus (Walhovd et al., 2005). Importantly, Bartzokis et al confirmed that such aging-associated decline in WM is significantly accelerated in AD subjects (Bartzokis et al., 2003).
The loss of WM in aging is accompanied with an increased appearance of WM hyperintensities (WMHs) in MRI. WMHs are discrete foci or confluent areas with enhanced signals detected by T2- weighted MRI scans, and are suggested to be WM lesions. These lesions are commonly located in the periventricular, or subcortical WM and can be found in healthy aging or in subjects with dementia attributed to a number of different causes (Barber et al., 1999; Holland et al., 2008). Higher densities and/or sizes of WMH are associated with attenuated level of global cognitive function, reduced speed of processing, poorer immediate-recent memory and loss of executive functioning. As might be expected from the foregoing, they are more frequently found in MCI and AD than in individuals with normal cognition (Gunning-Dixon and Raz, 2000).
In a longitudinal cohort study of MCI subjects, high WMH volume is predictive of entrorhinal cortex atrophy as well as the progression of MCI to AD within 3 years. It has been suggested from this data that WMHs, by interacting with tau abnormalities, may exert direct pathological influence on the development and progression of dementia (Tosto et al., 2015). The pathology underlying WMHs is traditionally associated with cerebrovascular changes and compromised integrity of the blood brain barrier, which together result in a region of poor perfusion and thus enhanced MRI signals (Black et al., 2009; Young et al., 2008). Nevertheless, postmortem histopathologcial studies of MRI-scanned brains shows patchy loss of myelin, or myelin pallor, in the regions where WMHs were visualized, but not in WM that appeared normal in the MRI (Erten-Lyons et al., 2013; Gouw et al., 2008). Thus while the WMH may have multiple origins, one of them is likely to be frank myelin pathology.
5. DNA damage in oligodendrocytes is commonly found in WM lesions
The cellular pathology underlying WMH is best illustrated by the pathology of the well-known demyelinating disease, multiple sclerosis (MS). MS is characterized by foci of demyelinating plaques which are commonly imaged as WMH in MRI studies (West et al., 2014). Postmorterm analysis of MS brain found that these WMH are plaques with loss of myelin, heavy astrogliosis and strong microglia activation (Moll et al., 2011; Schmierer et al., 2007). In these WMH forming, demyelinating lesions, oxidative DNA damage was found in the nuclei of OLs, which appeared to be undergoing apoptosis (Haider et al., 2011). DNA damage is clearly one of the more consistent cellular anomalies in OLs in demyelinating lesions. The relationship with age-related dementias has recently illustrated by a correlation between OL pathology in WMH and the appearance of AD (Al-Mashhadi et al., 2015; Wharton et al., 2015). In the postmortem AD brain, there is a significant reduction in the number of OLs; and the survivors suffer from enhanced levels of oxidative DNA damage. A subset of the OL in the WMH regions actually enter a senescent state in response to the resulting genomic stress with increased p53 expression and the appearance of a senescent phenotype (Al-Mashhadi et al., 2015). In our lab we also find a significant increase of γH2AX immunoreactivity in the Olig2-positive population of the AD brain, indicative of DNA damage in the OL lineage (Tse et al, in preparation). Importantly, we found DNA damage in the oligodendrocytes of both plaque-free subjects diagnosed with dementia as well as in AD subjects.
These observations begin to tie the problems of age related myelin loss to the genomic integrity of the oligodendrocytes, and suggest the hypothesis that OL may suffer from a long period of oxidative stress and DNA damage prior to the more extensive loss of myelination that is required to produce a WMH on imaging. This pattern is quite similar to that seen in neurons with compromised genomic integrity during brain aging where genomic instability is elegantly balanced by an array of DNA repair mechanisms (Chow and Herrup, 2015). This comparison naturally raises the question of whether, in OLs as in neurons, mutations in proteins that contribute to the DNA damage response and produce symptoms of premature aging also compromise the biology of the OL and might serve as a root cause of WM loss in the aging brain. In fact the clinical evidence from a number of inherited progeroid syndromes strongly supports the concept that WM degeneration is indeed a prominent pathology of many different DNA repair deficits.
6. Oligodendrocytes and myelin are vulnerable to genetic deficits in DNA repair
6.1 Defects in single strand break repair
Bulky lesions are dangerous modifications to the DNA; they disrupt transcription and often lead to single strand breaks (SSBs). These SSBs are usually repaired by processes known as base excision repair (BER), nucleotide excision repair (NER) or mismatch repair (MMR). BER is responsible for most oxidative or alkylation DNA damage. NER is most commonly used to repair SSB occur after UV irradiations. In mitotic cells, genomic errors formed during DNA replication are repaired by MMR. Mutations in the genes encoding these repair proteins lead to a variety of conditions, virtually all of which are characterized by neurological defects and premature aging.
6.1.1 Cockayne Syndrome
Cockayne Syndrome (CS) is a rare autosomal recessive disease with mutation in either of the two DNA repair genes, CSA or CSB (also known as ERCC8 and ERCC6, respectively) (Stevnsner et al., 2008). About 80% of the CS patients present with mutation in the CSB gene (Mallery et al., 1998), which participate in both the NER and BER pathways. First described by E.A. Cockayne, CS patients are characterized by deafness and a loss of motor skills, even though their social skills are well-preserved (Cockayne, 1936). In the CS brain, the loss of myelination in the cerebrum, midbrain, cerebellum and brain stem is a prominent pathology (Soffer et al., 1979). These lesions are characterized by patchy loss of myelinated fibers with a remarkable disappearance of OLs (Houston et al., 1982; Soffer et al., 1979). Compared to the WM, individuals with CS retain a cortex of normal thickness, which suggests that the gray matter is largely preserved (Houston et al., 1982). Given their importance in more classical age-associated dementias, it is noteworthy that neither amyloid plaques nor neurofibrillary tangles are found in these brains despite the presence of accelerated aging. The implication is that the loss of OL in CS is unlikely to be the consequence of amyloid or tau-dependent events and further that OL deficits can be associated with, and even a primary cause of, cognitive problems (Houston et al., 1982; Woody et al., 1991). WMHs are found scattered throughout the subcortical WM of the cerebrum of affected CS individuals (Adachi et al., 2006; Sugita et al., 1992). A larger cohort study with 19 CS patients, found WM atrophy to be the earliest neuroradiological finding – present as early as four months of age. Equally noteworthy, the extent of myelination never developed beyond the equivalent of a normal six month old infant in any affected individual (Koob et al., 2010). In a subtype of CS with mutations in ERCC1 and ERCC4 (or XP-F), myelination delay is not observed until later, but still as early as 3 years of age with visible WMHs (Kashiyama et al., 2013).
6.1.2 Xeroderma pigmentosum
Xeroderma pigmentosum (XP) is another progeroid syndrome that is closely related to CS. It was first described by De Sanctis and Caccion and the subjects are clinically characterized by skin sensitivity to sunlight, cognitive impairment in childhood and accelerated aging (Anttinen et al., 2008). Like CS, XP is caused by autosomal recessive mutations in one of a family of the seven DNA repair genes for NER (XP-A to XP-G), with XP-A and XP-C being the most common forms. It has long been recognized that the peripheral nerves of XP patients are poorly myelinated (Hakamada et al., 1982; Kanda et al., 1990), and estimates are that about 70% of XP patients also develop neurological deficits early in life (Kassubek et al., 2012). For example, patients with XP-A mutations develop neurological symptoms before eight years age, and their cognitive decline is proportional to the overall cerebral and cerebellar atrophy, where WMHs are found (Anttinen et al., 2008). Apart from WMHs, MRI-diffusion tensor imaging (DTI) of the WM tracts clearly showed that the volume and directionality of individual myelinated tracts were critically reduced in the corticospinal pathway, thalamus and corpus callosum, regardless of the XP mutation variants (Kassubek et al., 2012). Myelin pallors were found in the temporal lobe, frontal lobe of the cerebrum, basal ganglia as well as in the cerebellum (Lai et al., 2013).
Apart from individual cases of XP and CS, there are reports of rare cases of XP/CS complex disease, in which myelination is severely reduced in both the central and peripheral nervous systems in the presence of mutation in both CSA/CSB and XP. The loss of myelination was accompanied by OL abnormalities including hyperchromatic nuclei that resembled the morphology of proliferating cells (Lindenbaum et al., 2001; Rapin et al., 2000; Rapin et al., 2006).
6.1.3 Trichothiodystrophy
Trichothiodystrophy (TTD) is another progeroid syndrome that is related to both CS and XP. Genetically, the condition is associated with mutations in the XPD and XPB subunits of TFIIH as well as a third form caused by mutations in the trichothiodystrophy group A (TTDA) protein. Clinically, TTD presents as a syndrome consisting of brittle sulfate deficient hair, mental retardation and infertility. TTD differs from XP, as only half of the cases demonstrate UV sensitivity and none show increased risk to skin cancer (Chen et al., 1994; Yoon et al., 2005). But like CS and XP, hypomyelination and WMHs are found throughout the cerebrum in TTD patients as young as 2.5 years of age (Chen et al., 1994; Harreld et al., 2010; Toelle et al., 2001). Myelination is progressively lost in TTD with one 19 year old patient reported with completely unmyelinated WM in the cerebrum and cerebellum but remarkably intact cortical gray matter (Yoon et al., 2005).
6.1.4 Role of SSB repair in myelination – experimental evidence
Transgenic mouse models of CS, XP and TTD have been developed. These mice roughly recapitulate the human myelin phenotypes with a few exceptions. For example, the hypomyelination phenotype of CS was not reproduced in mice that carried only a CSB mutation (van der Horst et al., 1997), but when XP-C was simultaneously knocked out, the brains of the double mutants revealed a significant loss of myelination in the sensorimotor cortex and corpus callosum at birth, despite normal neuronal and axonal development (Revet et al., 2012). In ERCC1 (XP-F) knockout mice, WM abnormalities were found in corpus callosum, cerebellum and spinal tract, with vacuolation and splitting of myelin lamellae. Abnormal myelin structures were also found in peripheral nerves accompanied by slower conduction velocity (Goss et al., 2011; Lawrence et al., 2008). XP-D mutation in mice results in a TTD phenotype which is not only characterized by microcephaly, but also by hypomyelination in the striatum, corpus callosum, and thalamus (Compe et al., 2007). Taken as a group, these animal models suggest that the myelin abnormalities induced by deficits in DNA repair proteins are gene-dose dependent, quantitatively different in mouse and human brain and not restricted by the subtypes of DNA repair enzyme. They also strongly suggest that WM and the OL lineage are selectively susceptible to deficits in DNA repair of the ERCC family.
NER-associated enzymes play a role in transcription-coupled repair (TCR) as well as in transcription itself. For example, CSB, is not only required for strand repair during transcriptional repair, but is also an essential co-factor of RNA polymerase II (van den Boom et al., 2004; van der Horst et al., 1997). In turn, RNA polymerase II was found to bind to the promoter of genes essential for myelination, including MBP and PLP (Compe et al., 2007). CSB and p53 are both involved in aging but reciprocally regulate each other. This was shown by the finding that elevated levels of p53 suppress CSB from entering the nucleosome (Lake et al., 2011). Indeed, p53 activation was observed in OL of the CSA−/−CSB−/− and XP-D mutant mice (Jaarsma et al., 2011), which display prominent myelin deficits. Further there is evidence that activation of p53 in the OL lineage is associated with DNA damage and cellular senescence in the aging human brain (Al-Mashhadi et al., 2015). Therefore, age-related p53 activation may obstruct myelin gene expression through the suppression of CSB (Rufini et al., 2013).
Similarly, XP-B and XP-D that are commonly mutated in TTD, are not only members of NER pathway, but also subunits of transcription factor II H (TFIIH) (Compe and Egly, 2012; Hashimoto and Egly, 2009). Mutation of XP-D in mice results in a TTD phenotype which is not only characterized by microcephaly, but also by hypomyelination in the cerebrum and cerebellum (Compe et al., 2007). These myelin deficits in TTD mice are caused by the failure of TFIIH to stabilize transcription of MBP and PLP triggered by thyroid hormone – the endocrine trigger of myelin gene transcription in OLs. (Barres et al., 1994; Schoonover et al., 2004).
Like NER, BER can also repair global genomic damage; therefore, it would be predicted that deficits in BER-specific enzymes such as XRCC1 will cause an age related neurodegenerative phenotype. Experiments to examine myelin and OL pathology in these models are just beginning to emerge (Akbari et al., 2015; Sykora et al., 2013). Furthermore, evidence suggests that BER and NER act in competition during repair of oxidative DNA damage at guanine residues (Shafirovich et al., 2016), and NER-related enzymes, such as CSB, are also found to promote BER-mediated repair of the genome (Tuo et al., 2001). Deficits in either the BER or NER pathway may similarly lead to loss of OLs.
6.2 Defects in double strand break repair
As compelling as this evidence might be for the vulnerability of myelin to the NER capacity of its constituent cells, the evidence goes beyond this single DNA repair system. A second major type of DNA lesion that cells must defend against is complete rupture of the double helix. These are known as double strand breaks (DSBs) and they are particularly hazardous as the two ends, if not rapidly reconnected, can become separated leading to significant problems in genomic integrity. DSBs are best repaired by a process known as homologous recombination (HR) repair. This mechanism is only available during cell division, however, as it requires access to the paired sister chromatid to ensure sequence preservation. Neurons are non-mitotic cells; therefore they (and presumably mature oligodendrocytes) make use of an alternative DSB repair process is known as non-homologous end joining (NEHJ). In NEHJ, the broken ends of the helix are bound by a complex known as the MRN complex (Mre11, Rad50, NBS1). The ATM protein (ataxia-telangiectasia mutated) binds to the complex, triggering its autophosphorylation and activation, thus facilitating DNA repair (Lee and Paull, 2005). Mutations in three of the four proteins of the NEHJ process lead to syndromes with prominent neurological symptoms, and two of these are explicitly found to have significant myelin pathology as well.
6.2.1 Ataxia telangiectasia
Ataxia telangiectasia (A-T) is a hereditary disease with loss-of-function of the A-T-mutated (ATM) protein. It is clinically characterized by cerebellar atrophy and the gradual loss of motor function. A-T is closely associated with neuronal dysfunction. While this may be due in part to persistent DNA damage, additional neuron-specific ATM functions might be involved as well. Cerebellar Purkinje cell death is preceded by their aberrant cell cycle re-entry (Yang and Herrup, 2005). More recent studies suggest that the more direct cause of this unscheduled cell cycling might be the nuclear accumulation of histone deacetylase 4, HDAC4 (Li et al., 2012), stabilization of the EZH2 histone methyltransferase (Li et al., 2013) and/or the decrease in Purkinje cell DNA hydroxymethylation (Jiang et al., 2015). But neuronal degeneration is not the only pathology observed in A-T. WMHs are found in A-T patients as early as 17 months of age, well before any clinical symptoms of A-T are detected (Chung et al., 1994). Similarly, a reduction of large myelinated fibers was observed in the sural nerve of two A-T siblings (Serizawa et al., 1994). Additional MRI studies have identified hypomyelinated foci in the cerebrum (Ciemins and Horowitz, 2000), as well as in the cerebellum in A-T patients of different ages (Firat et al., 2005). Indeed, MRI-DTI study on a larger cohort of young patients found that the WM tracts projecting to the cortex, including corticomotor, corticospinal and somatosensory pathways, undergo degeneration (Sahama et al., 2015; Sahama et al., 2014). These clinical findings provide evidence that wide-spread defects in myelination and most likely OL function are part of the A-T phenotype that can be found in additional to the neuronal cell death.
6.2.2 Nijmegen Breakage Syndrome
Nijmegen Breakage Syndrome (NBS) is an autosomal recessive disease with a clinical presentation similar to A-T (Van de Kaa et al., 1994). Mutation of the gene encoding NBS1 is the genetic cause of the disease. Recall that NBS1 coupled with Mre11and Rad50 forms the MRN complex which can sense DNA DSBs in the genome. Clinically, NSB patients commonly present with microcephaly, but without the progressive cerebellar atrophy and ataxia that is seen in A-T (Van de Kaa et al., 1994). Importantly, agenesis of the corpus callosum was found in an NBS fetus at 33 weeks of gestation by ultrasound examination suggesting abortive proliferation and differentiation of OLs (Maraschio et al., 2001). Such hypoplasia of the corpus callosum is found in about 30% of NSB patients (Bekiesinska-Figatowska et al., 2004; Bekiesinska-Figatowska et al., 2000).
6.2.3 Role of DSB repair in myelination – experimental evidences
Like the progeroid syndromes, A-T and NSB were modeled in transgenic mice. In Nbs1−/− but not Atm−/− mice, a partial loss of the corpus callosum was observed (Stracker and Petrini, 2011). In Nbs1−/−;Atm−/− double mutants, the development of the cerebellum was severely delayed at two weeks postnatal, and the OL lineage in the cerebellar cortex was completely abolished (Dar et al., 2011). ATM and NSB are also implicated in the cell biology of OL differentiation. In CNS-specific Nbs1−/− ;Atm−/− double knockout mice, a near complete aplasia of the corpus callosum coupled with demyelination of the optic nerve are found (Baranes et al., 2009; Dar et al., 2011). In these mice, the development of the cerebellum is severely retarded at two weeks of age, despite the survival of some Purkinje cells (36%) and a few granule cells (5%). This loss of myelination is also seen in Nbs1−/− mutants accompanied by the loss of orientation of the WM tracts and the reduction of the density of MBP or GalC positive mature OLs, without any detectable changes in the gray matter (Assaf et al., 2008). Curiously, these WM pathologies are absent in single mutant Atm knockouts (Dar et al., 2011). These findings suggests that the MRN complex is not only a major regulator of ATM in DNA repair, but also maybe required in OL development (Stracker and Petrini, 2011).
Indeed, OPC derived from Nbs1-CNS-Δ−/− or Atm−/− failed to differentiate into mature OL in culture (Allen et al., 2001; Carlessi et al., 2009; Carlessi et al., 2013; Liu et al., 2014b). In vivo, as early as seven days postnatal, the number of proliferating OPC is significantly reduced and apoptotic OPC increased in the Nbs1-CNS -Δ−/− brain with a resulting loss of myelin proteins including MBP, MOG and PLP in the corpus callosum (Liu et al., 2014a, b). The mechanisms behind this relationship are still being sought, but it has been shown that the increased DNA damage and oxidative stress in NSB1-deficient OPC inhibits ATR-Chk1 and activates ATM-Chk2 (Liu et al., 2014a). As a result of ATM-Chk2 signaling, p53 is activated, potentially inducing defective proliferation of the OPC and increased apoptosis in mature OLs (Liu et al., 2014a, b). Importantly, the p53 activation in mature OLs is accompanied by the attenuation of HDAC1 and MyRF expression, which are crucial for myelin formation (Liu et al., 2014b). The overlapping function of various DNA repair enzymes in the OL developmental program indicates that their function in differentiation maybe attenuated when the OL lineage accumulates DNA damage during aging, as depicted in Figure 2.
Figure 2.
The relationship between DNA damage and myelin degeneration in brain aging
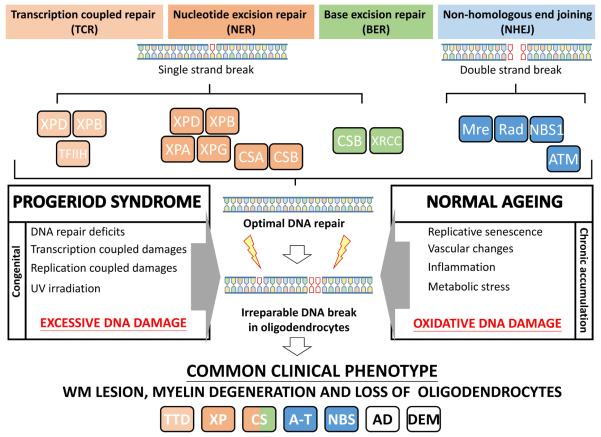
CS, XP, CS/XP, TTD, A-T and NSB are all naturally occurring genetic diseases with loss of function in different DNA repairing genes. The common findings of myelination deficits and neurological impairment among these diseases and in the AD brain suggests that DNA damage is a common cause of oligodendrocyte degeneration. A key remaining question therefore is what is it about the biology of the oligodendrocyte that makes it vulnerable to early degeneration in DNA repair deficiencies and in the aging brain? The answer may lie within the highly specialized developmental program of OL.
7. The aging OL is a selective target of global oxidative stress
7.1 Oxidative stress is the ultimate cause of DNA damage to OL
DNA damage of OLs in WM can be caused by the chronic oxidative stress of the AD brain (Al-Mashhadi et al., 2015). This linkage between oxidation and DNA damage is best illustrated, however, by the pathology of multiple sclerosis (MS). MS is a progressive and irreversible demyelination triggered by an excessive autoimmune reaction to largely unknown antigens. Apoptosis with DNA fragmentation of cells in the OL lineage is frequent in MS plaques (Ozawa et al., 1994) where high levels of reactive oxidative species (ROS) are generated by the inflammatory cells as they attack the myelin. The consequences of the excess ROS are predictable. OLs in the plaque suffer extensive oxidative damage to their DNA as detected by enhanced levels of 8-hydroxyguanine, the most common DNA oxidative lesion (Haider et al., 2011). The damage is apparent in both mitochondrial (Lu et al., 2000) and nuclear DNA (Vladimirova et al., 1998). Cortical demyelination distant from the plaques was also found in patients suffering from progressive, but not acute MS, arguing that chronic oxidative stress may allow the demyelinating damage to spread to intact myelinated regions over the years of the disease pathogenesis, as in the AD brain (Kutzelnigg et al., 2005).
The full pathological picture of the MS brain underscores the unique vulnerability of the cells of the OL lineage. In the MS cerebellum, for example, the neurons surrounding the demyelinating OLs are largely preserved. The interpretation is that the OL lineage is least able to repair the DNA damage caused by the oxidative chemistry of this autoimmune disease (Kutzelnigg et al., 2007). The high susceptibility of OL to oxidative stress is correlated with, and probably caused by, a remarkably low antioxidant content. Compared to astrocytes, OLs are estimated to endure a six fold higher oxidative stress during brain function (Thorburne and Juurlink, 1996), while only possessing 50% of the intracellular antioxidant glutathione (Juurlink et al., 1998). The relative paucity of glutathione would be expected to render the OL, especially the preOL, vulnerable to free radical damage and cell death (Back et al., 1998). This situation should also worsen with age as the overall level of glutathione producing enzyme, γ-glutamylcysteine synthetase, declines as the brain ages. Though the examples listed here are from MS, it is easy to imagine that with their high susceptibility to oxidative stress and the resulting DNA damage OL death would be a common early symptom in different neurodegenerative disease.
7.2 Oxidative stress in the Alzheimer’s brain
An example of such a disease is Alzheimer's disease (AD). While aging and the formation of plaques are proposed to be the prominent cause of AD (Hardy and Selkoe, 2002), WMHs are detectable well before any major plaque deposition (Grimmer et al., 2012). This suggests either that the myelin abnormalities are independent of amyloid plaque or that the OL lineage is vulnerable to the earliest stages of the amyloidosis. It is worth recalling that the β-amyloid peptide is known to possess oxidative properties and has been shown to cause OL apoptosis accompanied by oxidative injury and mitochondria dysfunction (Xu et al., 2001). The oxidative stress brought by excessive level of amyloid-β may be responsible for the significant loss of myelinated axons in the aggressive mouse models of AD (Behrendt et al., 2013; Desai et al., 2009). The genetically engineered mice generate a high level of amyloid-mediated oxidative stress that can kill OL in both the undifferentiated and differentiated state (Desai et al., 2010). However, these aggressive mouse models contain at least two copies of either presenilin-1 (PS1), amyloid precursor protein (APP) or tau transgenes with pathogenic mutations – a situation that is unlikely to be naturally found in subjects with sporadic AD.
It is worth remembering that as aggressive mouse as these mouse models may seem from the standpoint of amyloid pathology, they are in fact exhibiting only very early stages AD pathogenesis and cognitive decline, while the human subjects analyzed were in more advanced stages that the mouse never reaches. Given the failure of anti-amyloid therapy to slow cognitive decline, amyloid-independent mechanisms would seem to certainly be involved in the loss of OL and the resulting impact on the symptoms of AD. Indeed, the oxidative stress mediated by multiple age-associated changes, including but not limited to β-amyloid or its insoluble aggregates, had been proposed to be the fundamental pathological cause of AD (Butterfield et al., 2007; Markesbery, 1997). The amyloid-independent nature of this oxidative stress hypothesis is well exemplified by the adverse effect of chemotherapy on cognition (Ahles and Saykin, 2007; Ricard et al., 2009). Conventional chemotherapy drugs, such as adriamycin (doxorubicin), do not cross the blood brain barrier, but can nonetheless cause peripheral inflammation and transfer the subsequent oxidative stress in the brain (Joshi et al., 2010; Keeney et al., 2015).
AD is also characterized by a chronic innate immune response that can induce neuronal apoptosis through microglia-mediated inflammation and oxidative stress (Combs et al., 2001). Activated microglia have been shown to cause the death of OPC through nitric oxide-dependent oxidative stress, and these OPCs are unable to develop to the stage where they become MBP-expressing mOLs (Pang et al., 2010). This chronic inflammatory state is likely to induce excessive DNA damage in the OLs, who, as we have seen, are ill-prepared to deal with a sustained oxidative challenge. In fact, clinically the presence of inflammation in MS patients is significantly correlated with the cortical demyelination (Lucchinetti et al., 2011).
7.3 Age-associated vascular pathology meets high metabolic rate in myelinating OLs
Pathological changes of the vasculature are commonly found in myelin, especially in WMH, in the aging brain. These changes include arteriolar tortuosity, loss of endothelial intercellular junctions, loss of capillaries, and ultimately the breakdown of blood brain barrier (Black et al., 2009; Brown et al., 2007; Young et al., 2008). These changes in the vasculature, which begin around 50 years of age, coincide well with the appearance of WM pathology (Brown et al., 2007; Thore et al., 2007). Over time, the changes likely create an ischemic/hypoperfused microenvironment surrounding the WM. This presents a significant problem for the OL since, to make the lipid-rich myelin layers during differentiation and maintain them during adult life, a large amount of cholesterol is required and cholesterol synthesis is an energy intensive process. While the OLs synthesize cholesterol de novo via 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) mediated metabolism (Saher et al., 2005), the transfer of cholesterol from astrocyte to oligodendrocyte also contributes significantly, especially in aging when the levels of HMG-coA drop (Clement et al., 2009; Thelen et al., 2006). On top of the metabolic demands created by the production of cholesterol esters, the OLs also play a major role in providing trophic support for the neurons they interact with. Mature OLs provide glycolic support to the axons by transporting lactate via the monocarboxylate transporter-1 (MCT-1) expressed at the node of Ranvier (Funfschilling et al., 2012; Lee et al., 2012). Genetic knockdown of MCT-1 in OL causes neuronal loss as well as axonal injury (Lee et al., 2012). With the need to provide glycolytic support to the neuron (cell soma and axons) as well as to itself, it is not surprising to learn that the metabolic rate in OLs is consistently high. With the high energy demand of creating and maintaining myelin, providing metabolic supports to the neurons, a hypoperfused or frankly ischemic microenvironment will likely result in extreme metabolic stress to the resident OLs. Add to this, their heightened vulnerability to oxidative stress the cells of OL lineage quickly become primary targets of any hypoxic/ischemic microenvironment (Back et al., 2002a). And it is mostly likely the OLs that are just begin to differentiate and synthesize myelin that are the most vulnerable of all (Behrendt et al., 2013; Cai et al., 2006).
7.4 Stage-specific vulnerability of the OL lineage in the ageing brain
OL at different developmental stages reside simultaneously in the adult brain (Fig 1). While the postmitotic, myelinating OL are undoubtedly the primary cell-type to degenerate during demyelination, their immature OL predecessors are also subjected to the same hostile microenvironment of aging. In the adult brain, OPC (or NG2) cells are part of a proliferative OL population whose numbers remain relatively stable throughout aging. The adult OPCs remain mitotically competent, persistently undergoing self-renewal and differentiation into mature myelinating OL. They thus serve as a stable source of cell replacement for myelin segments that are lost during the life of the mature brain (Rivers et al., 2008). However, while the number of actively proliferating OPC remains constant in the ageing brain, the cell cycle time of each OPC division is significantly increased in old animals (Lasiene et al., 2009; Psachoulia et al., 2009). The OPCs that are generated in the old brain preferentially retain progenitor status rather than undergo OL differentiation (Boda et al., 2015). As a consequence, the time required for these OPC to differentiate into mature OL is more than doubled in the old brain (Zhu et al., 2011). Further, when these adult-born OPC do differentiate, the resulting premyelinating OL (or immature OL), are slow to transit through the specific stage of their lineage that is the most susceptible to age-associated insults such as free radical damage and vascular changes (see 7.3) (Back et al., 1998; Back et al., 2002a; Back et al., 2001; Back et al., 2002b). Thus, it would appear to be intrinsic vulnerabilities of the OPCs that are present only during the differentiation process that are the greatest contributors to the delayed or failed remyelination in the aging brain.
In summary, oxidative stress is highly likely to produce DNA damage during aging, and it is the actively differentiating, postmitotic OPC that show the greatest vulnerability to this oxidative-induced damage. Thus, the OL lineage is a clear target of the reactive oxygen species generated by inflammation, vascular changes, metabolic stress and other amyloid-dependent or -independent mechanisms in the aging brain (see Figure 2). These chronic oxidative stress will ultimately cause oxidative DNA damage in the OL genome.
8. Aging OLs and their lineage-specific dependence on components of the DNA repair pathway
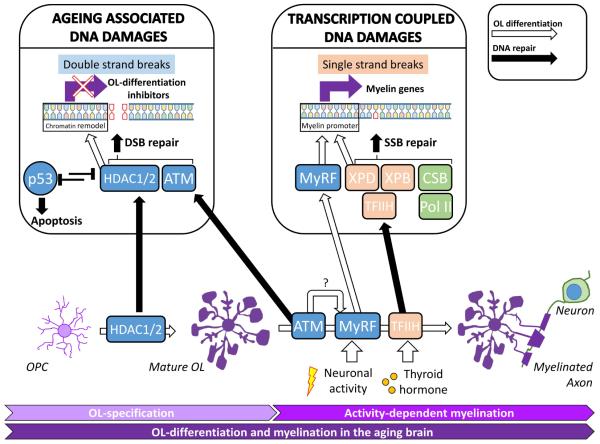
The aforementioned intrinsic properties of OL differentiation are predictably tied to the developmental program of myelination. Here we speculate that the special requirement for intact DNA repair pathways during OL development confers such additional cell type-specific risks to the OL lineage in the aging brain. These specific risks have resonance with dynamic aspects of OL development because of the possibility of activity-dependent, and DNA repair-dependent transcription of myelin-related genes for continuous myelin remodeling, and also because of the overlapping functions of proteins involved in both DNA repair and OL-differentiation, as illustrated in Figure 3.
Figure 3.
The overlapping functions of DNA repair proteins needed to maintain genomic integrity and drive oligodendrocyte differentiation
8.1 Activity-dependent transcription in OL may induce DNA damage
Mature oligodendrocytes are postmitotic and not subjected to DNA damage caused by the replication of the genome. But myelination is a highly dynamic process that is regulated extensively by neuronal activity (McKenzie et al., 2014; Wake et al., 2011). The myelination of the nervous system is not complete at birth. As cited above in humans it does not peak until well into the fifth decade of life. Not only does it continue throughout adulthood, studies also show that it requires neuronal activity. Only 25% of the OL processes and myelination are mature at birth in the vertebrate nervous system. This slow maturation takes on extra significance because the fine details of the structure of the adult myelin sheet are highly responsive to the electrophysiological activity of the neurons themselves. During in vivo imaging of the zebrafish nervous system, an increase of neuronal or synaptic activity increases the thickness of myelination – even at the level of a single OL (Mensch et al., 2015). A negative influence can also be seen as the myelin sheath is retracted from silent axons (Hines et al., 2015). Myelination in the PNS and the CNS has been shown to require neurotransmitters such as glutamate and ATP-induced calcium signaling, which then respond to the increased neuronal activity of axons that frequently fire by increasing their myelination (Stevens and Fields, 2000; Wake et al., 2011). Recently, the concept of activity-dependent myelination has been elegantly demonstrated by the impairment of myelination in the prefrontal cortex of socially isolated mice (Liu et al., 2012a). Importantly, such activity dependent OL plasticity was shown to be essential for motor-task learning in adult (McKenzie et al., 2014). Activity-dependent myelination is thus analogous in many ways to the mechanisms involved in the synaptic remodeling that underlies the processes of learning and memory that occur throughout life.
Given the significant anabolic requirements of myelin production, myelin remodeling comes at a significant energetic cost as well as enhanced risk of oxidative stress and mitochondrial dysfunction (Gilman et al., 2003; Mattson and Liu, 2002). In neurons, activity-dependent gene transcription is preceded by topoisomerase I or IIß-induced DNA double strand breaks (Madabhushi et al., 2015; Sordet et al., 2009). This transient DNA damage is normally efficiently repaired by ATM-dependent activity (Sordet et al., 2009); if not, the damage can lead to neurodegeneration (Alagoz et al., 2013; Katyal et al., 2014). Importantly, in the presence of oxidative stress, these topoisomerase-induced DNA lesions will be stabilized and thus induce apoptosis (Daroui et al., 2004; Sordet et al., 2004). Like neurons, OLs are transcriptionally activated in response to the environment (Liu et al., 2012a), and thus we might speculate that they too are likely to be prone to activity-dependent DNA damage, especially in the presence of oxidative stress, to which they are particularly vulnerable.
8.2 The competing demand of DNA repair and OL differentiation
A number of enzymes, while perhaps best known for their roles in DNA repair also play critical roles in the differentiation of the OL lineage (see section 6). These enzymes may help in the activity-dependent or thyroid dependent transcription of OL-specific genes. For example XP-B and XP-D are components of TFIIH that is required to stabilize thyroid hormone receptor on thyroxine-responsive myelin genes (Compe and Egly, 2012; Compe et al., 2007). As a second example, CSB is a co-factor of RNA polymerase II during TCR yet it is also essential for SOX10 mediated myelin gene transcription (Arter and Wegner, 2015; van den Boom et al., 2004). Additional examples include the DSB repairing enzymes ATM and NSB1. Both are required for DNA repair, but each is also necessary for successful OL differentiation. The molecular mechanism behind this dual function may be related to MyRF expression, but at present remains largely unknown (Liu et al., 2014a, b). In the aging brain with increased oxidative DNA damage in the OL, it is imaginable that the demand for the repair function of these enzymes associated with myelin transcription and OL differentiation is competing with the demand for their more native function in other types of DNA repair. We speculate such competition is potentially catastrophic for overall myelin maintenance in the brain.
Apart from transcription coupled DNA repair, OL differentiation and myelin-specific gene transcription requires chromatin remodeling by class I HDACs (Conway et al., 2012; Marin-Husstege et al., 2002). Importantly, the expression and activity of HDAC1 in WM is significantly reduced with age. As would be predicted, OL differentiation inhibitors – Sox2, ID4 and Hes5 – are re-expressed in the OL of old animals with low HDAC1 activity. The age-related down-regulation of the OL differentiation activator, Olig2, also fits this pattern (Shen et al., 2008a). Pharmacologically, treatment with HDAC inhibitor TSA also served to re-activate Sox2, ID4 and Hes5 in the OLs of the corpus callosum (Shen et al., 2008a; Shen et al., 2008b). These unscheduled, dedifferentiations in the OL lineage resulted in a poor remyelination after injury of older animals (Shen et al., 2008b). The lack of deacetylation of histone H3 in the OPC is one likely cause of the defective differentiation of myelinating OLs in old animals (Shen et al., 2005).
HDACs perform pleiotropic functions in differentiated cells. For example, HDAC1 and HDAC2 decline not only with aging but also in cells undergoing replicative senescence (Place et al., 2005; Wagner et al., 2001). They also control p53 activation and caspase-3 mediated apoptosis (Rebbaa et al., 2006). The age-associated loss of HDAC activity would then prevent OPC differentiation, and cause apoptosis in the myelinating OL. The exact cause of the reduction in HDAC1 and HDAC2 with age is unknown. One plausible reason is the increasing requirement their function in DNA damage repair (Di Micco et al., 2011). HDAC1 and HDAC2 are actively recruited to the DNA lesion site where they deacetylate lysine 56 of histone H3 (Miller et al., 2010). The deacetylation by HDAC1 and HDAC2 is postulated to prevent defective transcription at the DNA damage site and, importantly, to promote NHEJ of the DSB. In support of their importance for DNA repair, pharmacological inhibition of HDAC1 and HDAC2 can induce DNA damage in primary cells (Lee et al., 2010). HDAC1 and HDAC2 may further facilitate DNA repair by modulating the ATM-mediated response and activation of p53 (Thurn et al., 2013). Although a specific role for HDAC1 and/or 2 in the DNA damage response of OL remains to be formally demonstrated, it is likely that their expression is not only important for OL differentiation, but also OL DNA repair. Further experiments are required to determine whether the differentiation program and the DNA repair are subjected to the declining level of HDAC in the aging OL (Shen et al., 2008a; Shen et al., 2008b), and resulted in the loss of myelination.
Conclusions
In a sentiment often attributed to Don Cleveland, neurodegenerative diseases are, in reality, diseases "of the neighborhood" (Ilieva et al., 2009). The meaning behind this statement is that failure of nervous system function is as much a failure of the web of cell-cell interactions as it is the demise of any one specific cell type. The value of this perspective is that if we assume that it is the web that fails, then it is easier to imagine that the first step in the decline can be taken by any of the elements of the network. Indeed, for complex degenerative diseases of aging, this multifactorial origin idea fits comfortably with both the clinical and experimental literature. In this review we summarize the evidence that the oligodendrocyte and its myelin ensheathment of neuronal axons is a network element that is well worth considering in this context. Like other cells of the body, their function declines with age and the evidence suggests that their intrinsic cell biology makes them particularly sensitive to two of the strongest correlates of the aging process – DNA damage and oxidative lesions. Although the literature is less extensive for the oligodendrocyte, it would appear that just as in neurons inadequate genomic maintenance is a factor in the slow loss of myelin that is found with age and is greatly enhanced during the course of neurodegenerative diseases such as AD.
The importance of the genomic integrity of the oligodendrocyte is further emphasized by the clinical presence of myelin loss in a wide range of progeroid conditions resulting from loss of function of a wide variety of DNA repair enzymes. Different and overlapping DNA repair mechanisms are necessary to maintain genomic integrity of postmitotic cells, and some of these mechanisms are explicitly required for the proper maturation of myelinating oligodendrocytes. With the ever growing number of sources identified that induce oxidative stress during brain aging, excessive DNA damage in the OL would seem to be a nearly inevitable result. As the decline of brain WM volume naturally begins in the mid-40s, even slight defects in myelination may followed by a shifts in transmission speed and major asynchrony of the neural network. Although speculative, the timing of such early WM degeneration fits well with the “change of state” hypothesized to be the final step in the initiation of AD cellular pathology (Herrup, 2010). This role of the oligodendrocyte in cognition and aging has been appreciated for many years (Bartzokis et al., 2003; Haroutunian et al., 2014), but largely ignored because of our intense focus on the neuron as the ultimate source of cognitive failure and dementia. The time may be ripe to return to these older ideas and integrate them more completely in our descriptions of age-related diseases of the nervous system.
The life cycle of OL begins with glial-restricted precursor cells that are formed during embryogenesis. OL-restricted transcription factors Olig2 and SOX10 are expressed as these cells becoming specified for the OL lineage. The OPCs derived from this pool of cells are characterized by surface markers of NG2 and A2B5. The expression of transcription factors SOX2, Nestin and Hes5, needed to maintain the "stemness" of the cells, are downregulated along with OL differentiation. The inhibitors of OL differentiation, such as YY1 and ID2/4, are upregulated to promote cell cycle and proliferation in response to mitogens such as PDGF or FGF. Upon the withdrawal of mitogens and the stimulation by thyroid hormone, OPCs begin to withdraw from cell cycle, form contacts with surrounding axons and become O4/O1 positive immature OLs. Coupled with neuronal activity, the transcription factor MyRF is expressed to complete the differentiation program by binding to the promoter of various myelin genes to induce myelination. These mature OL are characterized by cell surface marker of MOG. The figure is highly simplified and does not include other important, OL-related signaling pathways such as Wnt, Sonic hedgehog and BMP4, and other transcription factors such as TCF7L2, Olig1, Nkx2.2/ Nkx6.2, Cdk5 or Brg. For a full review of the developmental program and its epigenetic control of OL differentiation, the readers are referred to comprehensive works of others (Hernandez and Casaccia, 2015; Jakovcevski et al., 2009; Mitew et al., 2014). Factors contributing to OL proliferation are shown in pink, OL differentiation are shown in deep purple.
Single strand breaks (SSB) and double strand breaks (DSB) are the main types of DNA damage in the genome and are repaired by different strategies. SSBs are commonly restored by TCR (pale orange box), NER (orange box) and BER (green box) through the XP, CS and XRCC families of DNA repair proteins. DSBs are detected by the Mre-Rad-NBS1 complex which activates ATM for NHEJ repairs (blue box). Genetic deficiency of these proteins results in inadequate DNA damage repair and compromised genomic integrity as in progeriod syndromes or congenital ataxia. Likewise, the chronic and accumulated assaults of replicative senescence, vascular pathology, inflammation, metabolic stress in the normal aging brain result in cumulative oxidative DNA damage that overwhelms the repair machinery. This irreparable DNA damage ultimately causes cell death of senesced OLs, myelin degeneration and WM lesions that are found in both progeriod syndrome and the aging brain. TTD, Trichothiodystrophy; XP, Xeroderma pigmentosum; CS, Cockayne Syndrome; A-T Ataxia telangiectasia; NBS, Nijmegen Breakage Syndrome; AD, Alzheimer’s disease; Dementia, all non-AD dementia without amyloid plaques Each genetic disease is color-coded to match the DNA repair mechanism in deficits.
DNA repair enzymes are involved both in the restoration of DNA breaks and OL differentiations. Left panel HDAC1/2 and ATM are recruited to the DNA lesions to facilitate NMEJs in age-associated DSBs (Lee and Paull, 2005; Miller et al., 2010). But HDAC1/2 and ATM are also known to promote OL maturation by promoting myelin gene expression (Shen et al., 2008a; Shen et al., 2008b), and OPC differentiation respectively (Allen et al., 2001; Carlessi et al., 2009; Carlessi et al., 2013; Liu et al., 2014b). ATM may affect MyRF expression but the underlying molecular mechanism remains unknown (Liu et al., 2014b). Right panel Activity-dependent and thyroid hormone dependent transcription are important to induce myelin gene transcription that requires the promoter binding by TFIIH complex and mRNA elongation by CSB-associated RNA polymerase II (Pol II) (Barres et al., 1994; Compe et al., 2007; Schoonover et al., 2004; van den Boom et al., 2004). During myelin remodeling, the intensive neuron-mediated myelin gene expression may constantly produce transcription-coupled DNA breaks that is also dependent on SSB repair function by TFIIH and CSB. The overlapping function of HDAC1/2, ATM, TFIIH and CSB in both OL differentiation, and their native function in DNA repair may be crucial for the continuous remodeling of myelin during normal brain function (McKenzie et al., 2014), but these competing functions are predictably exhausted by aging. Open arrow, pathway for OL differentiation; closed arrow, pathway for DNA repair.
Dedicated to the memory of Dr. George Bartzokis. We who have come late to this field may not have known him, but we recognize the strength of the conceptual foundation that he laid, and upon which many of the ideas of this review are built. We mourn his untimely passing in August, 2014
Highlights.
Myelin loss is a robust pathology for the aging brain and progeriod syndromes
White matter lesions are commonly tied with DNA damage in oligodendrocytes (OLs)
OLs are highly vulnerable to oxidative stress and DNA damage in the aging brain
DNA repair proteins are essential for genomic integrity and myelin formation in OLs
Acknowledgments
The present work is funded by GRF16101315 and GRF660813 from the Research Grant Council, Hong Kong SAR. Support was also received from the National Key Basic Research Program of China (2013CB530900) and the National Institutes of Health of the United States (NS71022)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Kawanami T, Ohshima F, Hosoya T. MR findings of cerebral white matter in Cockayne syndrome. Magn Reson Med Sci. 2006;5:41–45. doi: 10.2463/mrms.5.41. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M, Morevati M, Croteau D, Bohr VA. The role of DNA base excision repair in brain homeostasis and disease. DNA Repair (Amst) 2015;32:172–179. doi: 10.1016/j.dnarep.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mashhadi S, Simpson JE, Heath PR, Dickman M, Forster G, Matthews FE, Brayne C, Ince PG, Wharton SB, Medical Research Council Cognitive, F. Ageing S. Oxidative Glial Cell Damage Associated with White Matter Lesions in the Aging Human Brain. Brain pathology. 2015;25:565–574. doi: 10.1111/bpa.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagoz M, Chiang SC, Sharma A, El-Khamisy SF. ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells. PloS one. 2013;8:e58239. doi: 10.1371/journal.pone.0058239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH, Barlow C. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes & development. 2001;15:554–566. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttinen A, Koulu L, Nikoskelainen E, Portin R, Kurki T, Erkinjuntti M, Jaspers NG, Raams A, Green MH, Lehmann AR, Wing JF, Arlett CF, Marttila RJ. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–1989. doi: 10.1093/brain/awn126. [DOI] [PubMed] [Google Scholar]
- Arter J, Wegner M. Transcription factors Sox10 and Sox2 functionally interact with positive transcription elongation factor b in Schwann cells. Journal of neurochemistry. 2015;132:384–393. doi: 10.1111/jnc.13013. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Galron R, Shapira I, Nitzan A, Blumenfeld-Katzir T, Solomon AS, Holdengreber V, Wang ZQ, Shiloh Y, Barzilai A. MRI evidence of white matter damage in a mouse model of Nijmegen breakage syndrome. Experimental neurology. 2008;209:181–191. doi: 10.1016/j.expneurol.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002a;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002b;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- Baranes K, Raz-Prag D, Nitzan A, Galron R, Ashery-Padan R, Rotenstreich Y, Assaf Y, Shiloh Y, Wang ZQ, Barzilai A, Solomon AS. Conditional inactivation of the NBS1 gene in the mouse central nervous system leads to neurodegeneration and disorganization of the visual system. Experimental neurology. 2009;218:24–32. doi: 10.1016/j.expneurol.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O'Brien J. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer's disease, vascular dementia, and normal aging. Journal of neurology, neurosurgery, and psychiatry. 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of neurology. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Behrendt G, Baer K, Buffo A, Curtis MA, Faull RL, Rees MI, Gotz M, Dimou L. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61:273–286. doi: 10.1002/glia.22432. [DOI] [PubMed] [Google Scholar]
- Bekiesinska-Figatowska M, Chrzanowska KH, Jurkiewicz E, Wakulinska A, Rysiewskis H, Gladkowska-Dura M, Walecki J. Magnetic resonance imaging of brain abnormalities in patients with the Nijmegen breakage syndrome. Acta neurobiologiae experimentalis. 2004;64:503–509. doi: 10.55782/ane-2004-1532. [DOI] [PubMed] [Google Scholar]
- Bekiesinska-Figatowska M, Chrzanowska KH, Sikorska J, Walecki J, Krajewska-Walasek M, Jozwiak S, Kleijer WJ. Cranial MRI in the Nijmegen breakage syndrome. Neuroradiology. 2000;42:43–47. doi: 10.1007/s002340050011. [DOI] [PubMed] [Google Scholar]
- Bender AR, Volkle MC, Raz N. Differential aging of cerebral white matter in middle-aged and older adults: A seven-year follow-up. NeuroImage. 2016;125:74–83. doi: 10.1016/j.neuroimage.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- Boda E, Di Maria S, Rosa P, Taylor V, Abbracchio MP, Buffo A. Early phenotypic asymmetry of sister oligodendrocyte progenitor cells after mitosis and its modulation by aging and extrinsic factors. Glia. 2015;63:271–286. doi: 10.1002/glia.22750. [DOI] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. Journal of the neurological sciences. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Mitew S, Hill AF, Lu QR, Wegner M, Srinivasan R, Svaren J, Willingham M, Barres BA, Emery B. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS biology. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Archives of neurology. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- Carlessi L, De Filippis L, Lecis D, Vescovi A, Delia D. DNA-damage response, survival and differentiation in vitro of a human neural stem cell line in relation to ATM expression. Cell Death Differ. 2009;16:795–806. doi: 10.1038/cdd.2009.10. [DOI] [PubMed] [Google Scholar]
- Carlessi L, Fusar Poli E, De Filippis L, Delia D. ATM-deficient human neural stem cells as an in vitro model system to study neurodegeneration. DNA Repair (Amst) 2013;12:605–611. doi: 10.1016/j.dnarep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cleaver JE, Weber CA, Packman S, Barkovich AJ, Koch TK, Williams ML, Golabi M, Price VH. Trichothiodystrophy: clinical spectrum, central nervous system imaging, and biochemical characterization of two siblings. J Invest Dermatol. 1994;103:154S–158S. doi: 10.1111/1523-1747.ep12399493. [DOI] [PubMed] [Google Scholar]
- Chen XS, Zhang YH, Cai QY, Yao ZX. ID2: A negative transcription factor regulating oligodendroglia differentiation. Journal of neuroscience research. 2012;90:925–932. doi: 10.1002/jnr.22826. [DOI] [PubMed] [Google Scholar]
- Chow HM, Herrup K. Genomic integrity and the ageing brain. Nat Rev Neurosci. 2015;16:672–684. doi: 10.1038/nrn4020. [DOI] [PubMed] [Google Scholar]
- Chung EO, Bodensteiner JB, Noorani PA, Schochet SS., Jr. Cerebral white-matter changes suggesting leukodystrophy in ataxia telangiectasia. J Child Neurol. 1994;9:31–35. doi: 10.1177/088307389400900106. [DOI] [PubMed] [Google Scholar]
- Ciemins JJ, Horowitz AL. Abnormal white matter signal in ataxia telangiectasia. AJNR. American journal of neuroradiology. 2000;21:1483–1485. [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Gamerdinger M, Tamboli IY, Lutjohann D, Walter J, Greeve I, Gimpl G, Behl C. Adaptation of neuronal cells to chronic oxidative stress is associated with altered cholesterol and sphingolipid homeostasis and lysosomal function. Journal of neurochemistry. 2009;111:669–682. doi: 10.1111/j.1471-4159.2009.06360.x. [DOI] [PubMed] [Google Scholar]
- Cockayne EA. Dwarfism with retinal atrophy and deafness. Arch Dis Child. 1936;11:1–8. doi: 10.1136/adc.11.61.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compe E, Egly JM. TFIIH: when transcription met DNA repair. Nature reviews. Molecular cell biology. 2012;13:343–354. doi: 10.1038/nrm3350. [DOI] [PubMed] [Google Scholar]
- Compe E, Malerba M, Soler L, Marescaux J, Borrelli E, Egly JM. Neurological defects in trichothiodystrophy reveal a coactivator function of TFIIH. Nature neuroscience. 2007;10:1414–1422. doi: 10.1038/nn1990. [DOI] [PubMed] [Google Scholar]
- Conway GD, O'Bara MA, Vedia BH, Pol SU, Sim FJ. Histone deacetylase activity is required for human oligodendrocyte progenitor differentiation. Glia. 2012;60:1944–1953. doi: 10.1002/glia.22410. [DOI] [PubMed] [Google Scholar]
- Dar I, Yosha G, Elfassy R, Galron R, Wang ZQ, Shiloh Y, Barzilai A. Investigation of the functional link between ATM and NBS1 in the DNA damage response in the mouse cerebellum. The Journal of biological chemistry. 2011;286:15361–15376. doi: 10.1074/jbc.M110.204172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daroui P, Desai SD, Li TK, Liu AA, Liu LF. Hydrogen peroxide induces topoisomerase I-mediated DNA damage and cell death. The Journal of biological chemistry. 2004;279:14587–14594. doi: 10.1074/jbc.M311370200. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer's disease mice constitutes a novel therapeutic target. Am J Pathol. 2010;177:1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer's disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57:54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Sulli G, Dobreva M, Liontos M, Botrugno OA, Gargiulo G, dal Zuffo R, Matti V, d'Ario G, Montani E, Mercurio C, Hahn WC, Gorgoulis V, Minucci S, d'Adda di Fagagna F. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol. 2011;13:292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Groves AR, Tamnes CK, Westlye LT, Duff EP, Engvig A, Walhovd KB, James A, Gass A, Monsch AU, Matthews PM, Fjell AM, Smith SM, Johansen-Berg H. A common brain network links development, aging, and vulnerability to disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17648–17653. doi: 10.1073/pnas.1410378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, Tran H, Howieson DB, Wild K, Silbert LC. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81:977–983. doi: 10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat AK, Karakas HM, Firat Y, Yakinci C. Quantitative evaluation of brain involvement in ataxia telangiectasia by diffusion weighted MR imaging. Eur J Radiol. 2005;56:192–196. doi: 10.1016/j.ejrad.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman CP, Chan SL, Guo Z, Zhu X, Greig N, Mattson MP. p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults. Neuromolecular Med. 2003;3:159–172. doi: 10.1385/NMM:3:3:159. [DOI] [PubMed] [Google Scholar]
- Goss JR, Stolz DB, Robinson AR, Zhang M, Arbujas N, Robbins PD, Glorioso JC, Niedernhofer LJ. Premature aging-related peripheral neuropathy in a mouse model of progeria. Mechanisms of ageing and development. 2011;132:437–442. doi: 10.1016/j.mad.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain. 2008;131:3286–3298. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- Grimmer T, Faust M, Auer F, Alexopoulos P, Forstl H, Henriksen G, Perneczky R, Sorg C, Yousefi BH, Drzezga A, Kurz A. White matter hyperintensities predict amyloid increase in Alzheimer's disease. Neurobiology of aging. 2012;33:2766–2773. doi: 10.1016/j.neurobiolaging.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2- weighted MRI myelin mapping and diffusion tensor imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamada S, Watanabe K, Sobue G, Hara K, Miyazaki S. Xeroderma pigmentosum: neurological, neurophysiological and morphological studies. Eur Neurol. 1982;21:69–76. doi: 10.1159/000115457. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- Harreld JH, Smith EC, Prose NS, Puri PK, Barboriak DP. Trichothiodystrophy with dysmyelination and central osteosclerosis. AJNR Am J Neuroradiol. 2010;31:129–130. doi: 10.3174/ajnr.A1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Egly JM. Trichothiodystrophy view from the molecular basis of DNA repair/transcription factor TFIIH. Hum Mol Genet. 2009;18:R224–230. doi: 10.1093/hmg/ddp390. [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007a;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Sandoval J, Casaccia-Bonnefil P. Events at the transition between cell cycle exit and oligodendrocyte progenitor differentiation: the role of HDAC and YY1. Neuron glia biology. 2007b;3:221–231. doi: 10.1017/S1740925X08000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. The Lancet. Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Casaccia P. Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia. 2015;63:1357–1375. doi: 10.1002/glia.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. The case for rejecting the amyloid cascade hypothesis. Nature neuroscience. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des. 2012;18:1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nature neuroscience. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke; a journal of cerebral circulation. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CS, Zaleski WA, Rozdilsky B. Identical male twins and brother with Cockayne syndrome. Am J Med Genet. 1982;13:211–223. doi: 10.1002/ajmg.1320130212. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009 doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma D, van der Pluijm I, de Waard MC, Haasdijk ED, Brandt R, Vermeij M, Rijksen Y, Maas A, van Steeg H, Hoeijmakers JH, van der Horst GT. Age-related neuronal degeneration: complementary roles of nucleotide excision repair and transcription- coupled repair in preventing neuropathology. PLoS genetics. 2011;7:e1002405. doi: 10.1371/journal.pgen.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhang Y, Hart RP, Chen J, Herrup K, Li J. Alteration in 5- hydroxymethylcytosine-mediated epigenetic regulation leads to Purkinje cell vulnerability in ATM deficiency. Brain. 2015;138:3520–3536. doi: 10.1093/brain/awv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kanda T, Oda M, Yonezawa M, Tamagawa K, Isa F, Hanakago R, Tsukagoshi H. Peripheral neuropathy in xeroderma pigmentosum. Brain. 1990;113:1025–1044. doi: 10.1093/brain/113.4.1025. Pt 4. [DOI] [PubMed] [Google Scholar]
- Kashiyama K, Nakazawa Y, Pilz DT, Guo C, Shimada M, Sasaki K, Fawcett H, Wing JF, Lewin SO, Carr L, Li TS, Yoshiura K, Utani A, Hirano A, Yamashita S, Greenblatt D, Nardo T, Stefanini M, McGibbon D, Sarkany R, Fassihi H, Takahashi Y, Nagayama Y, Mitsutake N, Lehmann AR, Ogi T. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. American journal of human genetics. 2013;92:807–819. doi: 10.1016/j.ajhg.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Sperfeld AD, Pinkhardt EH, Unrath A, Muller HP, Scharffetter-Kochanek K, Ludolph AC, Berneburg M. The cerebro-morphological fingerprint of a progeroid syndrome: white matter changes correlate with neurological symptoms in xeroderma pigmentosum. PloS one. 2012;7:e30926. doi: 10.1371/journal.pone.0030926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal S, Lee Y, Nitiss KC, Downing SM, Li Y, Shimada M, Zhao J, Russell HR, Petrini JH, Nitiss JL, McKinnon PJ. Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nature neuroscience. 2014;17:813–821. doi: 10.1038/nn.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney JT, Miriyala S, Noel T, Moscow JA, St Clair DK, Butterfield DA. Superoxide induces protein oxidation in plasma and TNF-alpha elevation in macrophage culture: Insights into mechanisms of neurotoxicity following doxorubicin chemotherapy. Cancer Lett. 2015;367:157–161. doi: 10.1016/j.canlet.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob M, Laugel V, Durand M, Fothergill H, Dalloz C, Sauvanaud F, Dollfus H, Namer IJ, Dietemann JL. Neuroimaging in Cockayne syndrome. AJNR Am J Neuroradiol. 2010;31:1623–1630. doi: 10.3174/ajnr.A2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Hyde TM, Herman MM, Saunders RC. The entorhinal cortex: an examination of cyto- and myeloarchitectonic organization in humans. Cerebral cortex. 1997;7:722–731. doi: 10.1093/cercor/7.8.722. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Faber-Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain pathology. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Lai JP, Liu YC, Alimchandani M, Liu Q, Aung PP, Matsuda K, Lee CC, Tsokos M, Hewitt S, Rushing EJ, Tamura D, Levens DL, Digiovanna JJ, Fine HA, Patronas N, Khan SG, Kleiner DE, Oberholtzer JC, Quezado MM, Kraemer KH. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D) Acta Neuropathol Commun. 2013;1:4. doi: 10.1186/2051-5960-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake RJ, Basheer A, Fan HY. Reciprocally regulated chromatin association of Cockayne syndrome protein B and p53 protein. The Journal of biological chemistry. 2011;286:34951–34958. doi: 10.1074/jbc.M111.252643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging cell. 2009;8:201–213. doi: 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NJ, Sacco JJ, Brownstein DG, Gillingwater TH, Melton DW. A neurological phenotype in mice with DNA repair gene Ercc1 deficiency. DNA Repair (Amst) 2008;7:281–291. doi: 10.1016/j.dnarep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11- Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A, Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nature medicine. 2012;18:783–790. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K. EZH2- mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nature neuroscience. 2013;16:1745–1753. doi: 10.1038/nn.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum Y, Dickson D, Rosenbaum P, Kraemer K, Robbins I, Rapin I. Xeroderma pigmentosum/cockayne syndrome complex: first neuropathological study and review of eight other cases. Eur J Paediatr Neurol. 2001;5:225–242. doi: 10.1053/ejpn.2001.0523. [DOI] [PubMed] [Google Scholar]
- Liu A, Han YR, Li J, Sun D, Ouyang M, Plummer MR, Casaccia-Bonnefil P. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007a;27:7339–7343. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Chen X, Wang ZQ, Tong WM. DNA damage and oxidative injury are associated with hypomyelination in the corpus callosum of newborn Nbn(CNS-del) mice. Journal of neuroscience research. 2014a;92:254–266. doi: 10.1002/jnr.23313. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen X, Wang ZQ, Tong WM. Nbn gene inactivation in the CNS of mouse inhibits the myelinating ability of the mature cortical oligodendrocytes. Glia. 2014b;62:133–144. doi: 10.1002/glia.22593. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature neuroscience. 2012a;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Magri L, Zhang F, Marsh NO, Albrecht S, Huynh JL, Kaur J, Kuhlmann T, Zhang W, Slesinger PA, Casaccia P. Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J Neurosci. 2015;35:352–365. doi: 10.1523/JNEUROSCI.2606-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Kassis H, Jia LF, Hozeska-Solgot A, Zhang RL, Chen C, Cui YS, Zhang ZG. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience. 2012b;220:313–321. doi: 10.1016/j.neuroscience.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, Qiu M. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Developmental biology. 2007b;302:683–693. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. Journal of the neurological sciences. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14982–14987. doi: 10.1073/pnas.0707044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R, Gao F, Pfenning AR, Pan L, Yamakawa S, Seo J, Rueda R, Phan TX, Yamakawa H, Pao PC, Stott RT, Gjoneska E, Nott A, Cho S, Kellis M, Tsai LH. Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell. 2015;161:1592–1605. doi: 10.1016/j.cell.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri L, Swiss VA, Jablonska B, Lei L, Pedre X, Walsh M, Zhang W, Gallo V, Canoll P, Casaccia P. E2F1 coregulates cell cycle genes and chromatin components during the transition of oligodendrocyte progenitors from proliferation to differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1481–1493. doi: 10.1523/JNEUROSCI.2840-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery DL, Tanganelli B, Colella S, Steingrimsdottir H, van Gool AJ, Troelstra C, Stefanini M, Lehmann AR. Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. American journal of human genetics. 1998;62:77–85. doi: 10.1086/301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraschio P, Danesino C, Antoccia A, Ricordy R, Tanzarella C, Varon R, Reis A, Besana D, Guala A, Tiepolo L. A novel mutation and novel features in Nijmegen breakage syndrome. J Med Genet. 2001;38:113–117. doi: 10.1136/jmg.38.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nature neuroscience. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Moll NM, Rietsch AM, Thomas S, Ransohoff AJ, Lee JC, Fox R, Chang A, Ransohoff RM, Fisher E. Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Ann Neurol. 2011;70:764–773. doi: 10.1002/ana.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortera P, Herculano-Houzel S. Age-related neuronal loss in the rat brain starts at the end of adolescence. Front Neuroanat. 2012;6:45. doi: 10.3389/fnana.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117:1311–1322. doi: 10.1093/brain/117.6.1311. Pt 6. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide- activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Place RF, Noonan EJ, Giardina C. HDACs and the senescent phenotype of WI-38 cells. BMC Cell Biol. 2005;6:37. doi: 10.1186/1471-2121-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM, Alzheimer's Disease Neuroimaging, I. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron glia biology. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–1449. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I, Weidenheim K, Lindenbaum Y, Rosenbaum P, Merchant SN, Krishna S, Dickson DW. Cockayne syndrome in adults: review with clinical and pathologic study of a new case. J Child Neurol. 2006;21:991–1006. doi: 10.1177/08830738060210110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, editor. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings, The handbook of aging and cognition. 2nd Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US: 2000. pp. 1–90. [Google Scholar]
- Raz N. Ageing and the Brain, eLS. John Wiley & Sons, Ltd.; 2001. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Zheng X, Chu F, Mirkin BL. The role of histone acetylation versus DNA damage in drug-induced senescence and apoptosis. Cell Death Differ. 2006;13:1960–1967. doi: 10.1038/sj.cdd.4401895. [DOI] [PubMed] [Google Scholar]
- Revet I, Feeney L, Tang AA, Huang EJ, Cleaver JE. Dysmyelination not demyelination causes neurological symptoms in preweaned mice in a murine model of Cockayne syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4627–4632. doi: 10.1073/pnas.1202621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard D, Taillia H, Renard JL. Brain damage from anticancer treatments in adults. Curr Opin Oncol. 2009;21:559–565. doi: 10.1097/CCO.0b013e328330c669. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature neuroscience. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- Sahama I, Sinclair K, Fiori S, Doecke J, Pannek K, Reid L, Lavin M, Rose S. Motor pathway degeneration in young ataxia telangiectasia patients: A diffusion tractography study. Neuroimage Clin. 2015;9:206–215. doi: 10.1016/j.nicl.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahama I, Sinclair K, Fiori S, Pannek K, Lavin M, Rose S. Altered corticomotor- cerebellar integrity in young ataxia telangiectasia patients. Mov Disord. 2014;29:1289–1298. doi: 10.1002/mds.25970. [DOI] [PubMed] [Google Scholar]
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nature neuroscience. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Boulby PA, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8:242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA, Mariash CN, Anderson GW. Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology. 2004;145:5013–5020. doi: 10.1210/en.2004-0065. [DOI] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. Pt 12. [DOI] [PubMed] [Google Scholar]
- Serizawa M, Sakamoto M, Hirabayashi K, Fujiwara Y, Atsumi T. [Histological and radiobiological study on adult cases with ataxia telangiectasia] Rinsho Shinkeigaku. 1994;34:38–42. [PubMed] [Google Scholar]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Liu A, Li J, Wolubah C, Casaccia-Bonnefil P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiology of aging. 2008a;29:452–463. doi: 10.1016/j.neurobiolaging.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nature neuroscience. 2008b;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjobeck M, Haglund M, Englund E. White matter mapping in Alzheimer's disease: A neuropathological study. Neurobiology of aging. 2006;27:673–680. doi: 10.1016/j.neurobiolaging.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Soffer D, Grotsky HW, Rapin I, Suzuki K. Cockayne syndrome: unusual neuropathological findings and review of the literature. Ann Neurol. 1979;6:340–348. doi: 10.1002/ana.410060407. [DOI] [PubMed] [Google Scholar]
- Sordet O, Khan QA, Plo I, Pourquier P, Urasaki Y, Yoshida A, Antony S, Kohlhagen G, Solary E, Saparbaev M, Laval J, Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by staurosporine-mediated oxygen radicals. The Journal of biological chemistry. 2004;279:50499–50504. doi: 10.1074/jbc.M410277200. [DOI] [PubMed] [Google Scholar]
- Sordet O, Redon CE, Guirouilh-Barbat J, Smith S, Solier S, Douarre C, Conti C, Nakamura AJ, Das BB, Nicolas E, Kohn KW, Bonner WM, Pommier Y. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO reports. 2009;10:887–893. doi: 10.1038/embor.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevnsner T, Muftuoglu M, Aamann MD, Bohr VA. The role of Cockayne Syndrome group B (CSB) protein in base excision repair and aging. Mechanisms of ageing and development. 2008;129:441–448. doi: 10.1016/j.mad.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nature reviews. Molecular cell biology. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K, Takanashi J, Ishii M, Niimi H. Comparison of MRI white matter changes with neuropsychologic impairment in Cockayne syndrome. Pediatr Neurol. 1992;8:295–298. doi: 10.1016/0887-8994(92)90369-a. [DOI] [PubMed] [Google Scholar]
- Sykora P, Wilson DM, 3rd, Bohr VA. Base excision repair in the mammalian brain: implication for age related neurodegeneration. Mechanisms of ageing and development. 2013;134:440–448. doi: 10.1016/j.mad.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen KM, Falkai P, Bayer TA, Lutjohann D. Cholesterol synthesis rate in human hippocampus declines with aging. Neurosci Lett. 2006;403:15–19. doi: 10.1016/j.neulet.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66:337–345. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- Thurn KT, Thomas S, Raha P, Qureshi I, Munster PN. Histone deacetylase regulation of ATM-mediated DNA damage signaling. Mol Cancer Ther. 2013;12:2078–2087. doi: 10.1158/1535-7163.MCT-12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelle SP, Valsangiacomo E, Boltshauser E. Trichothiodystrophy with severe cardiac and neurological involvement in two sisters. Eur J Pediatr. 2001;160:728–731. doi: 10.1007/s004310100845. [DOI] [PubMed] [Google Scholar]
- Tosto G, Zimmerman ME, Hamilton JL, Carmichael OT, Brickman AM, Alzheimer's Disease Neuroimaging, I. The effect of white matter hyperintensities on neurodegeneration in mild cognitive impairment. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11:1510–1519. doi: 10.1016/j.jalz.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Muftuoglu M, Chen C, Jaruga P, Selzer RR, Brosh RM, Jr., Rodriguez H, Dizdaroglu M, Bohr VA. The Cockayne Syndrome group B gene product is involved in general genome base excision repair of 8-hydroxyguanine in DNA. The Journal of biological chemistry. 2001;276:45772–45779. doi: 10.1074/jbc.M107888200. [DOI] [PubMed] [Google Scholar]
- Van de Kaa CA, Weemaes CM, Wesseling P, Schaafsma HE, Haraldsson A, De Weger RA. Postmortem findings in the Nijmegen breakage syndrome. Pediatric pathology / affiliated with the International Paediatric Pathology Association. 1994;14:787–796. doi: 10.3109/15513819409037676. [DOI] [PubMed] [Google Scholar]
- van den Boom V, Citterio E, Hoogstraten D, Zotter A, Egly JM, van Cappellen WA, Hoeijmakers JH, Houtsmuller AB, Vermeulen W. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. The Journal of cell biology. 2004;166:27–36. doi: 10.1083/jcb.200401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, Morreau H, Beems RB, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- Vladimirova O, O'Connor J, Cahill A, Alder H, Butunoi C, Kalman B. Oxidative damage to DNA in plaques of MS brains. Multiple sclerosis. 1998;4:413–418. doi: 10.1177/135245859800400503. [DOI] [PubMed] [Google Scholar]
- Wagner M, Brosch G, Zwerschke W, Seto E, Loidl P, Jansen-Durr P. Histone deacetylases in replicative senescence: evidence for a senescence-specific form of HDAC-2. FEBS Lett. 2001;499:101–106. doi: 10.1016/s0014-5793(01)02524-8. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- West J, Aalto A, Tisell A, Leinhard OD, Landtblom AM, Smedby O, Lundberg P. Normal appearing and diffusely abnormal white matter in patients with multiple sclerosis assessed with quantitative MR. PLoS One. 2014;9:e95161. doi: 10.1371/journal.pone.0095161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton SB, Simpson JE, Brayne C, Ince PG. Age-associated white matter lesions: the MRC Cognitive Function and Ageing Study. Brain pathology. 2015;25:35–43. doi: 10.1111/bpa.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody RC, Harding BN, Brumback RA, Leech RW. Absence of beta-amyloid immunoreactivity in mesial temporal lobe in Cockayne's syndrome. J Child Neurol. 1991;6:32–34. doi: 10.1177/088307389100600106. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY. Amyloid-beta peptides are cytotoxic to oligodendrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:RC118. doi: 10.1523/JNEUROSCI.21-01-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: a unified disease mechanism. J Neurosci. 2005;25:2522–2529. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HK, Sargent MA, Prendiville JS, Poskitt KJ. Cerebellar and cerebral atrophy in trichothiodystrophy. Pediatr Radiol. 2005;35:1019–1023. doi: 10.1007/s00247-005-1495-6. [DOI] [PubMed] [Google Scholar]
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]