Abstract
Children are at risk for cognitive difficulties following the diagnosis and treatment of a brain tumor. Longitudinal studies have consistently demonstrated declines on measures of intellectual functioning, and recently it has been proposed that specific neurocognitive processes underlie these changes, including working memory, processing speed, and attention. However, a fine-grained examination of the affected neurocognitive processes is required to inform intervention efforts. Radiation therapy (RT) impacts white matter integrity, likely affecting those cognitive processes supported by distributed neural networks. This study examined working memory and attention in children during the early delayed stages of recovery following surgical resection and RT. The participants included 27 children diagnosed with pediatric brain tumor, treated with (n = 12) or without (n = 15) RT, who completed experimental and standardized measures of working memory and attention (n-back and digit span tasks). Children treated with radiation performed less well than those who did not receive radiation on the n-back measure, though performance at the 0-back level was considerably poorer than would be expected for both groups, perhaps suggesting difficulties with more basic processes such as vigilance. Along these lines, marginal differences were noted on digit span forward. The findings are discussed with respect to models of attention and working memory, and the interplay between the two.
Keywords: Pediatric, Brain tumor, Working memory, Attention, Radiation therapy
Pediatric brain tumors are the most common solid tumor diagnosis of childhood (Ries et al., 2006). The incidence of pediatric brain tumors is estimated to be 5.26 cases per 100,000 children per year (Ostrom et al., 2013). Although pediatric brain tumors remain the second leading cause of death by disease in children, the overall five-year survival rate exceeds 73%, largely due to improvements in multimodal treatment regimens. Progress in survival rates has allowed for a shift of focus toward the management of morbidity associated with the disease process and its treatment. In fact, a substantial body of literature on developmental late effects of central nervous system (CNS)-directed therapy, namely radiation therapy (RT), attests to the notion that these children are at risk for decreased behavioral, social-emotional, and cognitive functioning (e.g., Merchant, Kiehna, Li, Xiong, & Mulhern, 2005; Ris, Packer, Goldwein, Jones-Wallace, & Boyette, 2001), with significant implications for educational and vocational outcomes.
Investigations of neuropsychological late-effects in children receiving RT for the treatment of a brain tumor have mostly relied on omnibus measures of neurobehavioral development, such as Full Scale IQ. Declines in IQ are generally thought to reflect the failure of these children to make normal gains relative to their peers rather than a loss of previously acquired information or skills. Longitudinal research indicates that cognitive decline begins to emerge on standardized tests as early as one year after treatment has ended (Moxon-Emre et al., 2014; Palmer et al., 2001; Ris & Noll, 1994). Research examining specific cognitive deficits underlying declines in IQ revealed deficits in foundational cognitive processes such as attention, working memory, and processing efficiency (e.g., Reddick et al., 2003). Disruptions to foundational cognitive processes such as attention and working memory are likely to have effects on higher-level cognitive functions such as problem-solving, complex executive functions, and academic achievement.
Working memory is commonly defined as a brain system that provides for the temporary storage and manipulation of information necessary for complex cognitive tasks such as language comprehension, problem-solving and reasoning, and learning (Baddeley, 2000). Attention and working memory are increasingly viewed as intimately related or overlapping constructs. Attentional control is a core component of a number of working memory models (e.g., Baddeley, 1993, 2000; Cowan et al., 2005; Engle & Kane, 2004). In general, attentional control capabilities serve to ensure that task goals are maintained in an active state and to prevent attentional capture from other distracting stimuli.
For the most part, studies of working memory in pediatric brain tumor survivors have employed simple span tasks such as Digit Span from the Wechsler Scales of Intelligence (Wechsler, 1991, 2003) or Numbers Reversed from the Woodcock–Johnson (Woodcock, McGrew, & Mather, 2001; but see Conklin et al., 2012; Jacola et al., 2014). In this study, we focus on a recent working memory paradigm: the n-back letter task. In this n-back task, participants are shown a sequence of letters, and for each one they have to decide whether it matches the letter that preceded it by n places in the series. One of the major virtues of this task is that it recruits two types of processes: first, it requires that participants store one or more letters in working memory, the number depending on the value of n in the task; and second, it requires that participants update the content of their working memory upon presentation of each new letter—dropping the oldest item, adding the newest, and keeping track of the order of presentation of each (Jonides et al., 1997). In this respect, the task fits the modal definition of working memory. However, several smaller steps are also involved in the successful performance of the task, including encoding, storage, rehearsal, matching, temporal ordering, inhibition, and response (for a review, see Jonides et al., 1997). Neuroimaging studies have suggested that a network of structures is recruited by this task (Owen, McMillan, Laird, & Bullmore, 2005). The posterior parietal cortex is thought to play a role in the storage of information in working memory; rehearsal of verbal information involves the supplementary motor area, premotor cortex, Broca’s area, insula cortex, and right cerebellum, while the dorsolateral prefrontal cortex is involved in temporal coding and inhibitory control. To date, few studies have employed measures based on articulated models of cognitive constructs, such as the n-back task, in children with a pediatric brain tumor treated with and without radiation. Moreover, given that successful task performance requires a number of neurocognitive processes supported by a network of brain structures, it may be particularly sensitive to the effects of radiation, known to cause significant disruptions to white matter integrity and connectivity.
RT is a primary method of treatment for pediatric brain tumor, and it is a well-established cause of change in cerebral white matter. White matter damage has been documented following treatment for medulloblastoma in particular, in specific regions of brain such as the cerebral hemispheres (Khong et al., 2003; Mabbott, Noseworthy, Bouffet, Rockel, & Laughlin, 2006) and cerebellum (Law et al., 2011), as well as in the connections between the two (Law et al., 2015). The effects of RT on the CNS have been classified according to the time period over which symptoms develop following RT exposure (Ball, Prenger, & Ballard, 1992; New, 2001). Acute injury from RT appears to be secondary to transient vasogenic edema and occurs in the several weeks following treatment; early delayed injury or subacute reactions are typically transient, occurring in one to six months following RT treatment, impacting deep gray and white matter; and late radiation injury, which is typically progressive and often irreversible, occurs months to years after treatment. Such late radiation injury has been associated with leukoencephalopathy, brain atrophy, necrosis, and neurocognitive deterioration (Monje & Palmer, 2003).
The cognitive difficulties observed in brain tumor survivors are likely due to a multitude of factors, including irradiation, the disease itself, and the effects of surgery (Archibald et al., 1994). Although a number of neurocognitive deficits are associated with the late effects of RT, available data on cognitive difficulties in pediatric brain tumor patients following acute or early delayed injury is sparse, and the findings are mixed. Palmer et al. (2013) reported average range attention and working memory within the first month following surgical intervention, but studies utilizing continuous performance tests have found increased inattention and slower reaction times over the course of treatment (Kiehna, Mulhern, Li, Xiong, & Merchant, 2006; Merchant et al., 2002). Moreover, one such study noted mild inattentiveness even prior to receiving RT with steadily increasing scores up to five years thereafter, implicating a role for local tumor effects and initial surgical problems compounded by focal irradiation (Kiehna et al., 2006). Early identification of any adverse treatment effects would suggest the need for early intervention and evaluation of its contribution to late effects associated with RT.
The primary objective for the current study is to compare the performance of children treated with and without RT on measures of attention and working memory in the early delayed stage of injury. As a corollary or secondary aim, the current study endeavors to speak to the sensitivity of experimental and clinical measures of attention and working memory within this population (see Table 1). We predicted that children treated with RT would perform significantly worse on the experimental n-back measure but not on the standardized span measures.
Table 1.
Operationalization of Cognitive Processes.
| Working Memory | Attention | |
|---|---|---|
| Experimental | N-back load conditions (1-back and 2-back conditions) | 0-back condition |
| Standardized | Digit Span Backward | Digit Span Forward |
Method
Participants
This study is part of a longitudinal project on cognitive, behavioral, and social-emotional outcomes following pediatric brain tumor treated with or without radiation. The participants in the study consisted of children aged 6–17 years, recently diagnosed with a brain tumor, who were recruited from neuro-oncology clinics at four urban medical centers: Cincinnati Children’s Hospital Medical Center in Cincinnati, Ohio; Dayton Children’s Hospital in Dayton, Ohio; Nationwide Children’s Hospital in Columbus, Ohio; and Kennedy Krieger Institute in Baltimore, Maryland. During the course of recruitment, 271 potential participants who had recently undergone surgical resection of a brain tumor were screened.
Of these 271 children, 158 did not meet eligibility criteria (reviewed in Robinson et al., 2015) due to the following reasons: severe pre-existing conditions or ineligible tumor types (e.g., glioblastoma multiforme, intrinsic brain stem gliomas; n = 30); severe post-surgical complications, rendering them too ill to participate in baseline evaluations or including acute imaging abnormalities (n = 93); history of neurofibromatosis type 1, as this genetic disorder is independently associated with cognitive and functional difficulties outside of the occurrence of tumors (n = 35). Of the remaining participants, 44 were eligible but declined participation for various reasons including family stress and living out of state. The remaining 69 participants were enrolled in the study, but 6 died prior to data collection and 4 dropped out, yielding 59 participants in total.
In the current study, children from this larger group were excluded for the following reasons: if they were younger than 6 years of age, as this was the lower age limit for the measures of interest (n = 11); if the n-back measure, a primary measure of interest, was not administered (n = 11); or if there were technical difficulties leading to invalid administration or missing data for the n-back (n = 10). Data collected during the baseline evaluation are included in this study, which was collected at similar time points for both groups (6.12 months post-surgery for the RT group and 6.93 months for the no RT group), t(25) < 1, p > .60.
The resulting sample consisted of 12 children treated with RT following surgical resection (RT group) and 15 children who were treated with surgical resection without RT (no RT group). Table 2 shows the distributions of demographic and disease-related variables across these two groups. The sociodemographics of both groups are comparable, with the majority of children being male and the mean education of the caregivers being some college. Groups did not significantly differ on lesion size or tumor location, with the majority of children having supratentorial tumors. Of those with supratentorial tumors in the no RT group, 20% of children were classified as having a primary tumor location in the frontal region and 40% each in the extrafrontal region and along the midline. For the RT group, 43% of tumors were classified as located along the midline, and 28.5% each in the frontal and extrafrontal regions. Children with post-surgical hydrocephalus and/or post-surgical shunt placement were included in the present study, as this is a common complication in brain tumor patients, and the composition of groups was similar. Notably, only children treated with cranial radiation were also treated with chemotherapeutic agents.
Table 2.
Demographic and Tumor-Related Variables.
| Variable | RT (n = 12) | No RT (n = 15) |
|---|---|---|
| Gender (% male) | 91.70 | 66.67 |
| Age at time of surgery (years), M (SD) | 11.65 (2.88) | 11.87 (3.47) |
| Age at time of assessment (years), M (SD) | 12.21 (2.92) | 12.47 (3.48) |
| Time since surgery (months) | 6.17 (3.99) | 6.93 (3.65) |
| Socioeconomic status (%) | ||
| <US$40,000 | 25.00 | 20.00 |
| US$40,001–US$60,000 | 16.67 | 20.00 |
| US$60,001–US$80,000 | 16.67 | 20.00 |
| US$80,001–US$100,000 | 8.30 | 13.30 |
| >US$100,000 | 41.70 | 26.70 |
| Education of primary caregiver (%) | ||
| Some high school | 8.30 | 0.00 |
| High school graduate | 16.70 | 26.70 |
| Some college | 33.33 | 33.30 |
| College graduate | 16.70 | 33.30 |
| Partial graduate school | 8.30 | 0.00 |
| Graduate degree | 16.70 | 6.70 |
| Type of tumor (%) | ||
| Medulloblastoma/PNET | 33.33 | 0.00 |
| Low grade astrocytoma | 8.30 | 20.00 |
| Germ cell tumor | 16.70 | 0.00 |
| Astrocytoma | 0.00 | 0.00 |
| Pineal tumor | 8.30 | 0.00 |
| Ependymoma | 8.30 | 0.00 |
| Craniopharyngioma | 0.00 | 13.30 |
| Choroid plexus tumor | 0.00 | 13.30 |
| Ganglioglioma | 0.00 | 6.70 |
| Other | 16.70 | 40.00 |
| Location of tumor (%) | ||
| Supratentorial | 58.30 | 66.70 |
| Right | 8.30 | 13.30 |
| Left | 25.00 | 26.70 |
| Midline | 25.00 | 26.70 |
| Infratentorial | 41.70 | 33.30 |
| Size of primary lesion, M (SD) | 1243.44 (1079.48) | 1221.65 (1380.98) |
| Shunt placement (% no) | 91.67 | 86.67 |
| Chemotherapya (% yes) | 75.00 | 0.00 |
| Type of radiation | ||
| Focal | 41.70 | - |
| Cranial/Craniospinal | 58.30 | - |
| Dose | ||
| 2340 cGy | 42.86 | - |
| 3600 cGy | 42.86 | - |
| 3960 cGy | 14.28 | - |
| Total radiation | ||
| Focal/boost dose, M (SD) | 4680.00 (1388.45) | - |
| Cranial/craniospinal, M (SD) | 2365.71 (780.66) | - |
Note.
Denotes a significant difference.
Measures
Working Memory
The n-back tasks with varying memory load were presented in central vision with a laptop computer. The task had two levels of memory load: 1-back and 2-back. In addition, a 0-back condition imposed a minimal memory load while controlling for attention to task. Across the task, there were 96 trials at the 0-back level, and 48 trials at each of the 1-back and 2-back levels. Each letter appeared one at a time on-screen for two seconds, onset to onset. The child responded by pressing a button with the preferred hand when a match occurred, or in the 0-load condition when the designated target appeared.
There were 15 to 17 targets and 31 to 33 distracters in each level of the task. The child was given explicit verbal and visual instructions at each level of the task (0-back, 1-back, and 2-back) immediately before training, practice, and experimental trials for that level. After the experimenter verified that the child understood the task, he or she completed training trials on the task and received feedback on his or her performance. Next, he or she engaged in a brief practice session at the specified memory level. Following the completion of the practice trials, the child received feedback on his or her performance and then completed the experimental trials for the specified level. The task was administered in ascending order, with a 0-back condition preceding each of the memory load conditions (1-back and 2-back). Each child included in this study completed all trials at all levels.
Verbal Working Memory
Digit span backward from the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003) assessed verbal working memory. The children repeated a sequence of dictated numbers in reverse order of presentation. This task consisted of eight span items, each containing two trials, and ranging from two to eight numbers in each item. Raw scores were converted to scaled scores. This measure has good reliability, r = 0.80.
Brief Auditory Attention
Digit span forward from the WISC-IV (Wechsler, 2003) assessed brief auditory attention. The children repeated a sequence of dictated numbers in the identical order of presentation. This task consisted of eight span items, each containing two trials, and ranging from two to nine numbers in each item. Raw scores were converted to scaled scores. This measure has good reliability, r = 0.83.
Procedure
The participants were recruited during regular neuro-oncology clinic follow-up visits. Informed consent was obtained from parents in accordance with the institutional review boards at all participating institutions. The neurocognitive measures included in the study were collected during an initial evaluation occurring 2 to 17 months following surgical resection. The participants were assessed in a single session lasting approximately three to four hours. Diagnostic and treatment-related variables were extracted from the participants’ medical records, and tumor characteristics were confirmed by a board-certified neuroradiologist. The participants received US$75 for completing the evaluation.
Statistical Analyses
Exploratory analyses revealed non-normal distributions for the n-back task, as is often the case with proportion or percentage scores. As such, percentage scores for correct targets and false alarms underwent an arcsine transformation. Group differences on the n-back were examined using one-way or mixed analyses of covariance (ANCOVAs), co-varying for age. The remaining group differences were examined with independent samples t-tests. Bivariate correlations assessed the relationship between performance on the n-back and digit span measures. Additional details regarding analyses are included in the results section below.
Results
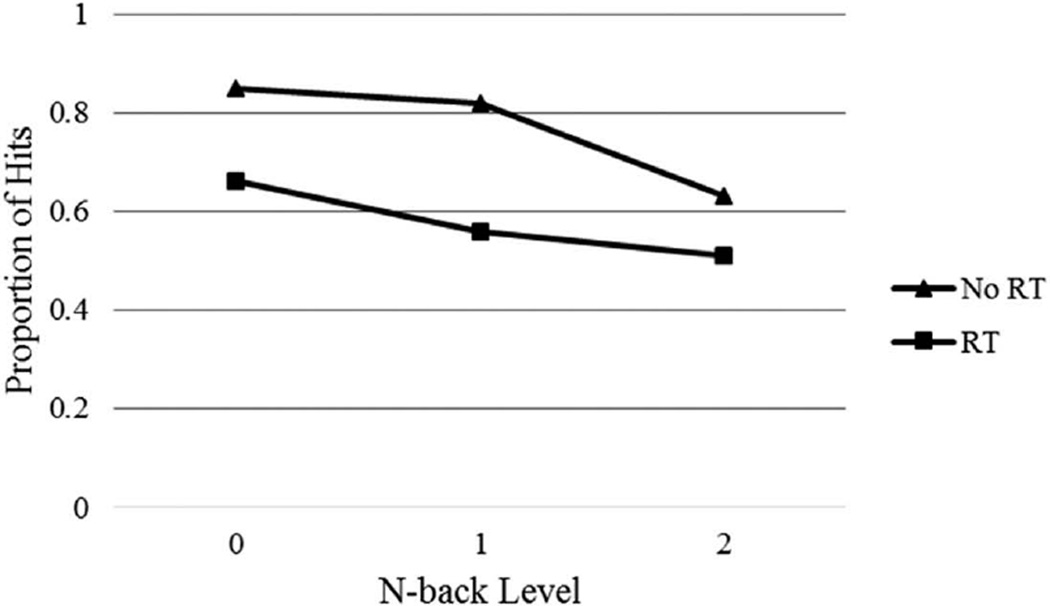
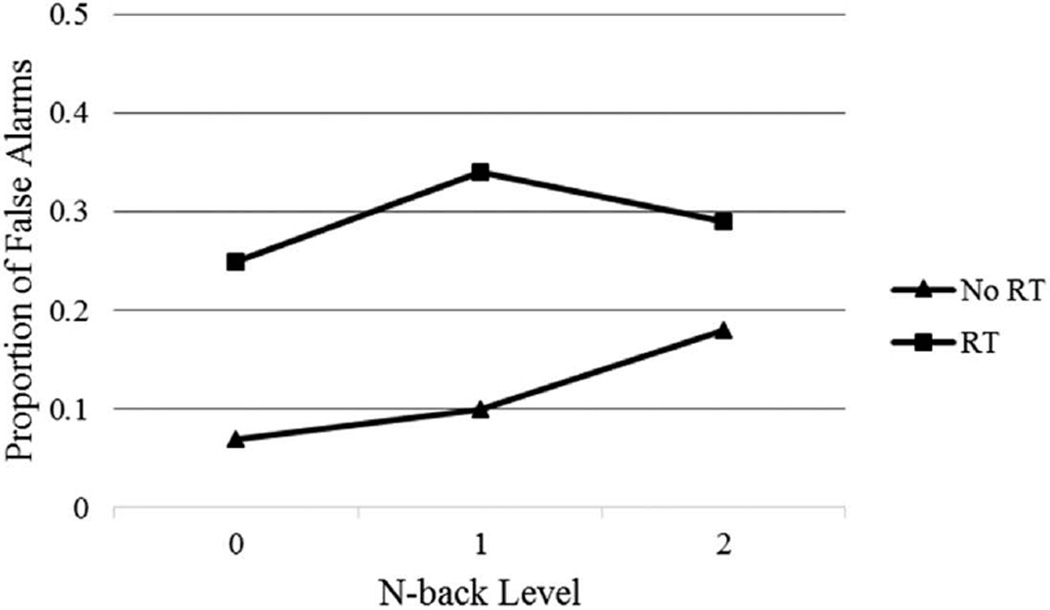
Two group (RT versus no RT) by three n-back level (0-back, 1-back, and 2-back) mixed ANCOVAs were performed on the proportion of correct targets identified and false alarms committed, co-varying for age at assessment, to compare the performance of pediatric brain tumor participants treated with and without RT on the n-back task (see Figures 1 and 2).
Figure 1.
Proportion of hits (correct detections) by n-back level for RT and no RT groups.
Figure 2.
Proportion of false alarms by n-back level for RT and no RT groups.
For correct targets, the main effect of group was significant, such that pediatric brain tumor participants treated with RT performed less well than participants treated with no RT, F(1, 24) = 7.86, p = .01, η2 = .25. There was also a significant main effect of n-back level, F(2, 48) = 6.12, p < .01, η2 = .20, such that all participants performed less well on the 2-back condition compared to the 0-back and 1-back conditions. The interaction between group and n-back levels was not significant, F(2, 48) = 1.26, p = .29, η2 = .05. For false alarms, the main effect of group was significant such that pediatric brain tumor participants treated with RT committed more false alarms compared to participants treated with no RT, F(1, 24) = 6.17, p < .05 η2 = .21. There was no main effect for n-back level, F(2, 48) < 1, p = .56, η2 = .02, and the interaction between group and n-back level was not significant, F(2, 48) = 2.35, p = .11, η2 = .09.
To investigate group differences on measures of verbal working memory and brief auditory attention, independent samples t-tests were conducted using standardized scores. Pediatric brain tumor participants treated with RT (M = 7.73, SD = 3.69) performed marginally worse than participants treated with no RT (M = 10.42, SD = 3.58) on a measure of brief auditory attention, t(21) = −1.77, p < .10, η2 = .13. The two groups did not differ significantly on a measure of verbal working memory, t (25) = −1.51, p = .35 (RT, M = 9.09, SD = 2.34; no RT, M = 10.17, SD = 3.01).
Standardized scores for verbal working memory and brief auditory attention did not significantly correlate with the proportion of correct targets or false alarms at any level of n-back, ranging from r = |.002| to |.160|, with the exception of marginal correlations for the proportion of correct targets at the 0-back level and brief auditory attention, r = .39, p = .073, and verbal working memory, r = .37, p = .088.
Discussion
The present study evaluates the association between RT and working memory and attention in pediatric brain tumor in the early delayed stage of injury. This study has several novel features. First, although long-term or late effects of RT are well-documented, few studies have examined core cognitive processes in the early delayed stage of injury from RT. Second, although working memory and attention have been examined in this population, both standardized and experimental measures have rarely been employed within a single study. Finally, while the n-back is a commonly-used experimental measure of working memory in typically developing children and in other populations with acquired brain injury, to our knowledge this is the first time it has been used in this population of pediatric brain tumor.
Significant differences between children treated with and without RT were observed on the experimental n-back measure, but not for the standardized digit span measures. After controlling for age, children with pediatric brain tumor treated with RT identified fewer correct targets and made more false alarms than children who were not treated with RT. Although the n-back is commonly regarded as a measure of working memory, the pattern of performance on this measure suggests that children treated with RT had more basic difficulties with vigilance or sustained attention. This group of children identified approximately 66% of correct targets in the least demanding version, or the 0-back level, where they had to match each letter in a series against a constant target that was specified prior to the beginning of the series. Typically developing children and children with other forms of acquired brain injury such as traumatic brain injury (TBI) typically have high accuracy rates at or above 95% (Levin et al., 2002).
Our findings from the n-back task are consistent with studies demonstrating difficulties with sustained attention during the early phases of recovery from RT. The few studies that have been conducted suggest that children with pediatric brain tumor demonstrate subtle or mild inattentiveness, even prior to RT (Kiehna et al., 2006; Merchant et al., 2002), with difficulties increasing up to five years post-treatment (Kiehna et al., 2006). Taken together, previous and current findings suggest that local tumor effects, initial surgical intervention, and focal irradiation may combine to contribute to potentially long-lasting attentional problems that are present early on in the treatment of pediatric brain tumor patients, likely having significant implications for learning. Additionally, in this particular study, only those children receiving RT were also treated with chemotherapy, which may have also impacted performance on the n-back.
Adequate sustained attention relies on basic processes of arousal or physiological reactivity as well as on attentional processes or allocation of processing resources to relevant stimuli. To maintain and manipulate information on-line, one must first attend to it. However, top-down processes from working memory have been shown to modulate the deployment of visual attention and the gating of information into awareness (reviewed in Soto, Hodsoll, Rotshtein, & Humphreys, 2008). Such top-down processing is generally consistent with models of working memory and suggests that attention and working memory are intimately related. A number of theories posit that the core component of interest within the construct of working memory is attentional control capabilities (consistent with Baddeley’s concept of the central executive). Such attentional control abilities are needed to ensure that task goals are maintained in an active state and to prevent attentional capture from other distracting stimuli. As such, individuals with increased working memory capacity tend to have greater attentional control capabilities than individuals with low or decreased working memory capacity, and thus are better at actively maintaining information in the presence of distraction. Based on marginal differences in simple span measures, children treated with RT may have had fewer resources to maintain information in the service of the goal, or target letter in the case of the n-back task, when faced with competing or interfering information.
No significant correlation was found between the two measures of working memory, the n-back task and or the digit span backward task, for either group. These findings are generally in keeping with the limited literature assessing the validity of the n-back (for brief reviews, see Jaeggi, Buschkuehl, Perrig, & Meier, 2010; Kane, Conway, Miura, & Colflesh, 2007). This measure has most recently been conceptualized as a continuous recognition measure that presents stimulus sequences, such as letters, and for each item in the sequence individuals judge whether it matches the one presented n items ago. The n-back has face validity as a working memory task because participants must maintain and update a dynamic rehearsal set while responding to each item; however, the n-back measure has actually received little empirical validation as a working memory measure. The limited research comparing the n-back with other putative working memory tasks is mixed (Jaeggi et al., 2010; Kane et al., 2007). A composite of 2-back through 5-back performance correlated more strongly with simple short-term memory span than with complex working memory span (Jaeggi et al., 2010; Roberts & Gibson, 2002). With respect to related tasks, the n-back shares more variance with Stroop performance or executive control than it does short-term memory span (Kwong-See & Ryan, 1995), and is also correlated with teachers’ attention ratings (Aronen, Vuontela, Steenari, Salmi, & Carlson, 2005). Consistent with findings to date, training on the n-back task has been shown to result in improved performance on simple span measures but not complex span measures (Jaeggi, Buschkuehl, Jonides, & Perrig, 2008). Taken together, the broader findings suggest some skepticism as to whether or not the n-back should be regarded as a measure of working memory.
Research to date suggests that a network of structures are recruited in the service of n-back performance in particular (for a review, see Owen et al., 2005). Moreover, this network has been shown to increase its overall magnitude of activation with increases in task load rather than recruiting new areas. Core areas activated during the n-back typically include the posterior parietal cortex thought to be involved in the storage of information in working memory, the dorsolateral prefrontal cortex for the temporal coding and inhibition processes, and the superior parietal and anterior cingulate areas for attentional processes. As the working memory demands of the n-back task increase when moving from 0-back to 1-back to 2-back, so too should the demands on the prefrontal cortex. Among children with a brain tumor and compromised white matter, task performance may not be related to compromise in a single region of the brain but rather to alterations or disruptions throughout the network.
Limitations and Future Directions
This study has several methodological limitations. First, the study sample is quite small and may have limited the potential for detecting significant findings. Secondly, a primary consideration is the diagnostic heterogeneity comprising both the no RT and RT groups, with the majority of participants having supratentorial tumors, although this was not a distinguishing variable between groups. Additionally, the sample was predominantly male and the majority of the children in the RT group received chemotherapy, introducing a potential confound for radiation. Finally, a healthy comparison group was not included in this study, making it difficult to interpret n-back performance.
Given that our data were collected in the early delayed stage of injury, future studies may examine whether demographic factors and tumor-related variables become increasingly important with respect to predicting late effects of brain tumor and its treatment. To this end, among children with TBI, another form of acquired brain injury, a decline in working memory as measured by the n-back task can be seen between one and two years post-injury (Levin et al., 2004), potentially because of degenerative brain changes that may also be at play in children with a pediatric brain tumor. Although our study focused on behavioral data alone, future studies may look at examining relationships between task performance and white matter tract integrity. Recent studies have demonstrated some associations between standardized measures of working memory and compromise to cerebrocerebellar microstructure in pediatric brain tumor patients.
Conclusion
In this study, children with pediatric brain tumor showed decrements in performance relative to children treated with no RT on an experimental measure of working memory following subacute reactions to treatment. On closer analysis, the decrements appeared to be related to the more basic processes of vigilance and sustained attention. Similarly, marginal findings were observed on a standardized measure of brief attention. These findings suggest that experimental measures with multiple processing demands may be more sensitive to the effects of pediatric brain tumors. Early attentional difficulties during the early delayed phase of radiation effects have important implications, particularly given research suggesting that attentional difficulties present in the few months following RT may remain relatively stable well into the future. Identification of such difficulties earlier in recovery may have important implications for children treated with RT, particularly given the importance of attentional processes for learning outcomes.
Acknowledgments
Funding
This work was supported by the National Cancer Institute [grant number R01 CA112182].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Archibald YM, Lunn D, Ruttan LA, Macdonald DR, Del Maestro RF, Barr HWK, Cairncross JG. Cognitive functioning in long-term survivors of high-grade glioma. Journal of Neurosurgery. 1994;80(2):247–253. doi: 10.3171/jns.1994.80.2.0247. [DOI] [PubMed] [Google Scholar]
- Aronen ET, Vuontela V, Steenari M-R, Salmi J, Carlson S. Working memory, psychiatric symptoms, and academic performance at school. Neurobiology of Learning and Memory. 2005;83:33–42. doi: 10.1016/j.nlm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory or working attention? In: Baddeley AD, Weiskrantz L, editors. Attention: Selection, awareness, and control A tribute to Donald Broadbent. New York, NY: Oxford University Press; 1993. pp. 152–170. [Google Scholar]
- Baddeley AD. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Ball WS, Prenger EC, Ballard ET. Neurotoxicity of radio/chemotherapy in children: Pathologic and MR correlation. American Journal of Neuroradiology. 1992;13(2):761–776. [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Ashford JM, Howarth RA, Merchant TE, Ogg RJ, Santana VM, Xiong X. Working memory performance among childhood brain tumor survivors. Journal of the International Neuropsychological Society. 2012;18(6):996–1005. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, Conway AR. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51(1):42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B, editor. The psychology of learning and motivation. New York, NY: Academic Press; 2004. pp. 145–199. [Google Scholar]
- Jacola LM, Ashford JM, Reddick WE, Glass JO, Ogg RJ, Merchant TE, Conklin HM. The relationship between working memory and cerebral white matter volume in survivors of childhood brain tumors treated with conformal radiation therapy. Journal of Neurooncology. 2014;119:197–205. doi: 10.1007/s11060-014-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith E, Lauber EJ, Awh E, Minoshima S, Koeppe R. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway AR, Miura TK, Colflesh GJ. Working memory, attention control, and the N-back task: A question of construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33(3):615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Khong PL, Kwong DL, Chan GC, Sham JS, Chan FL, Ooi GC. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: A pilot study. American Journal of Neuroradiology. 2003;24(4):734–740. [PMC free article] [PubMed] [Google Scholar]
- Kiehna EN, Mulhern RK, Li C, Xiong X, Merchant TE. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. Journal of Clinical Oncology. 2006;24(33):5283–5290. doi: 10.1200/JCO.2005.03.8547. [DOI] [PubMed] [Google Scholar]
- Kwong-See ST, Ryan EB. Cognitive mediation of adult age differences in language performance. Psychology and Aging. 1995;10(3):458–468. doi: 10.1037//0882-7974.10.3.458. [DOI] [PubMed] [Google Scholar]
- Law N, Bouffet E, Laughlin S, Laperriere N, Brière M-E, Strother D, Mabbott D. Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: Impact on working memory. Neuroimage. 2011;56(4):2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Law N, Smith ML, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Mabbott D. Executive function in paediatric medulloblastoma: The role of cerebrocerebellar connections. Journal of Neuropsychology. 2015 Aug;2015 doi: 10.1111/jnp.12082. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Chang C-C, Zhang L, Schachar R, Ewing-Cobbs L, Max JE. Working memory after traumatic brain injury in children. Annals of Neurology. 2002;52(1):82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank PR, Ewing-Cobbs L, Dennis M, Hunter JV. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18(2):240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: Correlation with IQ. Neuro-oncology. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. International Journal of Radiation Oncology* Biology* Physics. 2005;63(5):1546–1554. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Kiehna EN, Miles MA, Zhu J, Xiong X, Mulhern RK. Acute effects of irradiation on cognition: Changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. International Journal of Radiation Oncology* Biology* Physics. 2002;53(5):1271–1278. doi: 10.1016/s0360-3016(02)02828-6. [DOI] [PubMed] [Google Scholar]
- Monje ML, Palmer T. Radiation injury and neurogenesis. Current Opinion in Neurology. 2003;16(2):129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, Mabbott D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. Journal of Clinical Oncology. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- New P. Radiation injury to the nervous system. Current Opinion in Neurology. 2001;14(6):725–734. doi: 10.1097/00019052-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-oncology. 2013;15(suppl2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Armstrong C, Onar-Thomas A, Wu S, Wallace D, Bonner MJ, Gajjar A. Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. Journal of Clinical Oncology. 2013;31(28):3494–3500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. Journal of Clinical Oncology. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Mulhern RK. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- Ries L, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Hayat M, editors. SEER cancer statistics review, 1975–2003. National Cancer Institute. 2006 Retrieved from http://seer.cancer.gov/csr/1975_2003.
- Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain-tumor patients: Review and methodological critique. Journal of Clinical Experimental Neuropsychology. 1994;16:21–42. doi: 10.1080/01688639408402615. [DOI] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyette JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A children’s cancer group study. Journal of Clinical Oncology. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Roberts R, Gibson E. Individual differences in sentence memory. Journal of Psycholinguistic Research. 2002;31:573–598. doi: 10.1023/a:1021213004302. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Wolfe KR, Yeates KO, Mahone EM, Cecil KM, Ris MD. Predictors of adaptive functioning and psychosocial adjustment in children with pediatric brain tumor: A report from the brain radiation investigative study consortium. Pediatric Blood & Cancer. 2015;62(3):509–516. doi: 10.1002/pbc.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends in Cognitive Sciences. 2008;12(9):342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3rd. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III tests of cognitive abilities (WJ-III) Itasca, IL: Riverside; 2001. [Google Scholar]