Abstract
Objective
Macrophage migration inhibitory factor (MIF) is a key regulator of both atherosclerosis and systemic lupus erythematosus (SLE), yet factors leading to its overproduction remain unclear. To explore regulation of MIF in SLE, we studied effects and potential mechanisms of type I interferon (IFN) and artesunate (ART), an anti-malarial agent extracted from Chinese herbs, on levels of MIF.
Methods
Serum and peripheral blood cells from SLE patients and healthy controls were measured for MIF levels by ELISA and type I IFN inducible gene expressions by real-time PCR respectively, and assessed for associations by Spearman correlation. ART was added to human umbilical vein endothelial cells (HUVECs) cultures with or without prior IFNα-1b stimulation and to SLE peripheral blood mononuclear cells (PBMC) cultures. Protein levels of STATs and phosphorylated (p-) STATs in HUVECs were determined by Western blotting.
Results
Serum MIF levels were elevated in SLE patients and positively associated with disease activity (r = 0.86, p < 0.0001), accumulated damage (r = 0.34, p < 0.05), and IFN scores in SLE PBMCs (r = 0.74, p = 0.0002). The addition of IFNα-1b promoted MIF production in a time and dose-dependent manner in HUVEC cultures. ART could inhibit expressions of IFN inducible genes (LY6E and ISG15) in both HUVEC and SLE PBMC cultures, and suppress MIF production and over-expression of p-STAT1, but not p-STAT3 or STAT5, induced by IFNα-1b stimulation. IFNγ-induced expression of p-STAT1 in HUVECs was not inhibited by ART.
Conclusion
MIF could be regulated by type I IFN in SLE patients. ART counteracts the effect of IFNα to inhibit MIF production by blocking STAT1 phosphorylation and thus may 3 have therapeutic potential for SLE-associated atherosclerosis.
Keywords: Systemic lupus erythematosus, artesunate, macrophage migration inhibitory factor, interferon, signal transducer and activator of transcription 1
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease characterized by the production of autoantibodies and end organ damage from immune complex deposition (1). In 1976, Urowitz et al. noted premature myocardial infarction among patients with SLE (2). Since this pioneering discovery, many follow-up studies have demonstrated that the incidence of coronary artery disease in women with SLE is much higher than age-matched general population, which could not be fully accounted for by traditional risk factors (3). Patients with SLE-associated atherosclerosis have an earlier occurrence, a longer duration of lupus and a higher damage-index score (4;5). However, the molecular mechanisms of accelerated atherosclerosis in SLE remain to be elucidated.
Macrophage migration inhibitory factor (MIF) is released upon pro-inflammatory activation and exerts an upstream role in regulating the innate immune response. High-expression MIF alleles are associated with disease risk in SLE (6), while anti-MIF treatment reduces functional and histological indices of glomerulonephritis in different strains of lupus-prone mice (7), indicating that MIF is a key regulator for developing disease manifestations.. Of note, a growing body of literature has suggested that MIF also plays an important role in the pathogenesis of atherosclerosis. MIF expression within atheromatous plaques is closely associated with progression and instability in human disease and inhibition of MIF activity prevents atherosclerosis in animal models (8;9). Thus, MIF may be a common mediator for accelerated atherosclerosis in SLE.
At present, it is unclear how MIF is modulated in SLE patients and there is a lack of preventive strategies targeting this process. Over the past decade, interferon-alpha (IFNα) or type I IFN has been identified to play a central role in the pathogenesis of SLE (10;11), and an association between type I IFN, vascular damage and progression of atherosclerosis has emerged (12). Artesunate (ART) is a semi-synthetic derivative of a Chinese herb named artemisinin that is commonly used as an anti-malarial agent in treating Chinese patients. Previously we have demonstrated that ART could inhibit the progression of lupus disease and reverse the pathologic lesion of nephritis in the MRL/lpr mouse model (13). Since ART could also exert an anti-angiogenic effect by decreasing the levels of vasoactive factors and inducing endothelial cell apoptosis, it is hypothesized that this drug may work on the regulation of atherosclerosis (14). In the current study, we showed for the first time that MIF level was associated with type I IFN gene expression signature in SLE patients. ART treatment down-regulated IFNα induced MIF production through the inhibition of phosphorylation of signal transducer and activator of transcription 1 (STAT1), and herein may have a potential role in ameliorating SLE-associated atherosclerosis.
Materials and methods
Patients and controls
The demographics of SLE patients and normal controls were shown in Table 1. Thirty-five SLE in-patients (35.2±12.8 years old, including 34 female and 1 male) were enrolled in the study and all of them fulfilled the classification criteria of SLE proposed by the American College of Rheumatology (15). SLE disease activity was assessed by the Systemic Lupus Erythematosus Disease Activity Index (16), and damage was measured by Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC) scores (17). The average dose of corticosteroids was 32.6 ± 24.0 mg/day (counted as the equivalent dose to methyl- prednisolone) at the time of blood draw. Twenty-one healthy donors (34.5±8.5 years old, 19 female and 2 male) were recruited as normal controls. The study was approved by the Ethics Committee at The Affiliated Drum Tower Hospital of Nanjing University Medical School. Informed consent was obtained from each patient and healthy donor.
Table 1.
Demographics of SLE patients and normal controls
| SLE patients (n = 35) | Normal controls (n = 21) | |
|---|---|---|
| Age, years | 35.2 ± 12.8 | 34.5 ± 8.5 |
| Female (n) | 34 | 19 |
| Reasons for admission (n) | ||
| Infection | 4 | |
| Lupus nephritis | 19 | |
| NPSLE+ | 3 | |
| Hematologic involvement | 5 | |
| Pulmonary involvement | 3 | |
| Framingham risk score$≥ 0.01 (n) | 16 | |
| SLEDAI score | 8.91 ± 4.05 | |
| SLICC score | 1.94 ± 1.63 |
Except where indicated otherwise, values were presented as mean ± SD.
NPSLE: Neuropsychiatric systemic lupus erythematosus.
Framingham risk score was calculated according to the literature (38).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Shanghai Fumeng Gene Biologicals (Shanghai, China). Cells were resuspended at 3 × 105 per well (1.5 × 105/ml) in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) with or without the presence of different concentrations of IFNα-1b (200 U/ml, 1000 U/ml, 2000 U/ml) and incubated at 37 °C with 5 % CO2 for 6, 12 or 24 hours. Then the culture medium was replaced with a medium containing ART at the concentration of 5 or 20 µmol/L, with dexamethasone sodium phosphate (100 ng/ml, 0.19 µM) or fludarabine (50 µM) applied as a positive control. 12 and 24 hours later, cells and supernatants were harvested for further measurements. To confirm the results from HUVECs, peripheral blood mononuclear cells (PBMCs) from SLE patients were cultured with ART (5 or 20 µM) or dexamethasone sodium phosphate (100 ng/ml) at 3 × 105 per well for 24 hours.
Gene expression measuring
A 2 to 3 ml blood sample or 1 × 105 HUVECs was collected, and total RNA was extracted immediately using Trizol reagent (Invitrogen, USA). RNA was reverse-transcribed and quantified by real-time polymerase chain reaction (Q-PCR) using the PrimeScript RT-PCR and SYBR® Premix Ex Taq™ kit (TaKaRa Biotechnology) for the detection of type I IFN inducible gene (MX1, OASL, OAS1, ISG15 and LY6E) expression in triplicates in the ABI 7500 FAST real-time PCR detection system (Applied Biosystems, USA). All primers were purchased from TaKaRa (Dalian, China) and the sequences were the same as previously described (18). Human ribosomal protein, large, P0 (RPLP0) was used as the house-keeping gene to normalize cellular RNA amounts. After the real-time PCR procedure, mean CT value of a target gene was obtained for each sample. Relative gene expression values were presented as 2−ΔΔCT, and IFN scores were calculated using MX1, OASL, OAS1, ISG15 and LY6E mRNA expression as previously described (19). Because these 5 genes exhibited highly correlated expressions among each other (19), in in vitro studies two of them (LY6E and ISG15) were measured to represent the status of IFN inducible gene expressions.
Protein analysis
HUVECs were harvested and resuspended in lysis buffer for 30 minutes, and then centrifuged at 14,000 g for 10 minutes at 4°C. After centrifugation, cell lysates were subjected to 10% sodium dodecylsulfate -polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, USA). After blocking with 5% skim milk, the membranes were incubated with primary antibodies at 4°C overnight, including antibodies against phosphorylated (p-) STAT1, STAT1, p-STAT3, STAT3, p-STAT5, STAT5 and GAPDH (Cell Signaling Technology). After washing with Tris-buffered saline with Tween 20, the membranes were incubated with horseradish peroxidase conjugated secondary antibody (1:5000–1:10000) for 30 minutes and the bands were visualized in a luminol-based detection system with enhanced chemiluminescence (Millipore, USA). Anti-GAPDH antibody (Cell Signaling Technology) was used as a loading control. MIF levels in serum or cultured supernatants were determined by enzyme-linked immunosorbent assay (ELISA) (R&D, USA) with samples diluted in 1: 10 or 1: 20.
Statistical analysis
All data were analyzed using Prism version 6.0 software (GraphPad, San Diego, CA). Comparisons between two groups were made by unpaired t test or Mann-Whitney tests when the data were not normally distributed, and results were correspondingly shown as mean ± SD or median (interquartile range, IQRs). Spearman correlation was used to depict relationships between two factors. To determine whether the parameters were significantly altered after the treatment of ART in vitro, one-way analysis of variance (ANOVA) was applied, followed by Dunnett's multiple comparisons test. A p < 0.05 was considered to be statistically significant.
Results
Increased serum MIF levels in SLE patients
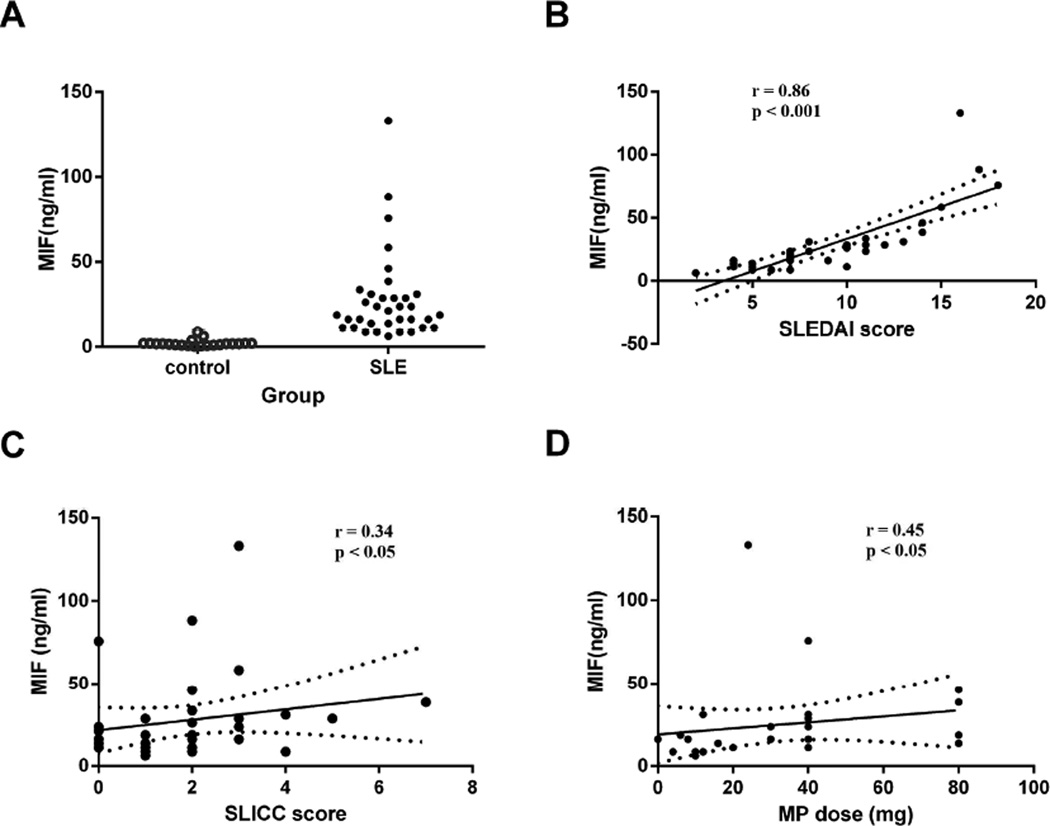
Compared to healthy controls, serum MIF levels were significantly elevated in SLE patients [18.7 (IQRs 12.5, 29.9) ng/ml vs. 1.8 (1.0, 2.0) ng/ml, p < 0.0001] (Figure 1A). Positive correlations were detected between MIF levels and SLEDAI scores (r = 0.86, p < 0.0001), SLICC scores (r = 0.34, p = 0.048), as well as the dose of methylprednisolone at the time of blood draw (r = 0.45, p = 0.016) (Figure 1B, C, and D; respectively), implying that MIF is an important contributor to both disease activity and accumulated damage in SLE, which is reflected in the treatment dosage of steroids.
Figure 1.
Serum MIF levels in SLE patients. A. Elevated MIF production in SLE patients compared with healthy individuals. Each symbol represents an individual sample. B. Associations of MIF levels with SLEDAI scores (r = 0.86, p < 0.001). C. Associations of MIF levels with SLICC scores (r = 0.34, p = 0.048). D. Associations of MIF levels with MP doses (r = 0.45, p = 0.016)
The disturbance of MIF was related to aberrant IFN inducible gene expression in SLE patients
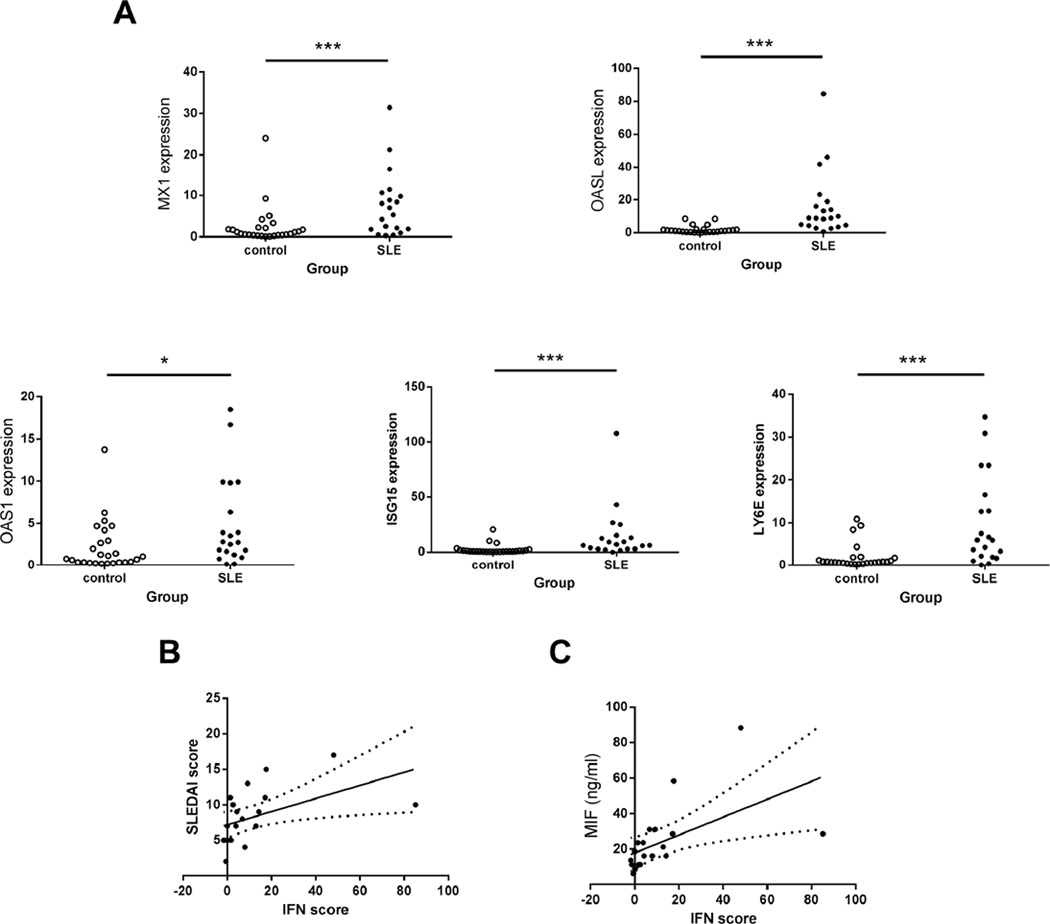
To explore potential factors that participated in upregulation of MIF in SLE, expressions of 5 type I IFN inducible genes (MX1, OASL, OAS1, ISG15 and LY6E) in PBMCs from SLE patients and healthy controls were measured. Q-PCR analysis demonstrated that significantly higher expressions of all 5 IFN inducible genes were present in patients with SLE (p all < 0.001, except for OAS1 where p < 0.05) (Figure 2A–E). As shown in Figure 2F, IFN scores that calculated according to the expression levels of 5 IFN inducible genes were positively correlated to SLEDAI scores (r = 0.69, p = 0.0007). The increase of IFN scores was robustly associated with the elevation of MIF levels (r = 0.74, p = 0.0002) (Figure 2G), supporting a link between circulating MIF levels of SLE patients and elevated levels of type I IFN inducible gene expression in SLE PBMC.
Figure 2.
Elevated IFN inducible genes expressions were related to both disease activities and MIF levels in SLE patients. A-E. Significantly higher expressions of 5 type I IFN inducible genes in SLE patients than in healthy donors. Each symbol represents an individual sample. *p < 0.05, ***p < 0.001. F. Association of SLEDAI scores with IFN scores (r = 0.69, p < 0.001). G. Associations of MIF levels with IFN scores (r = 0.74, p < 0.001).
IFNα could directly modulate MIF levels in vitro
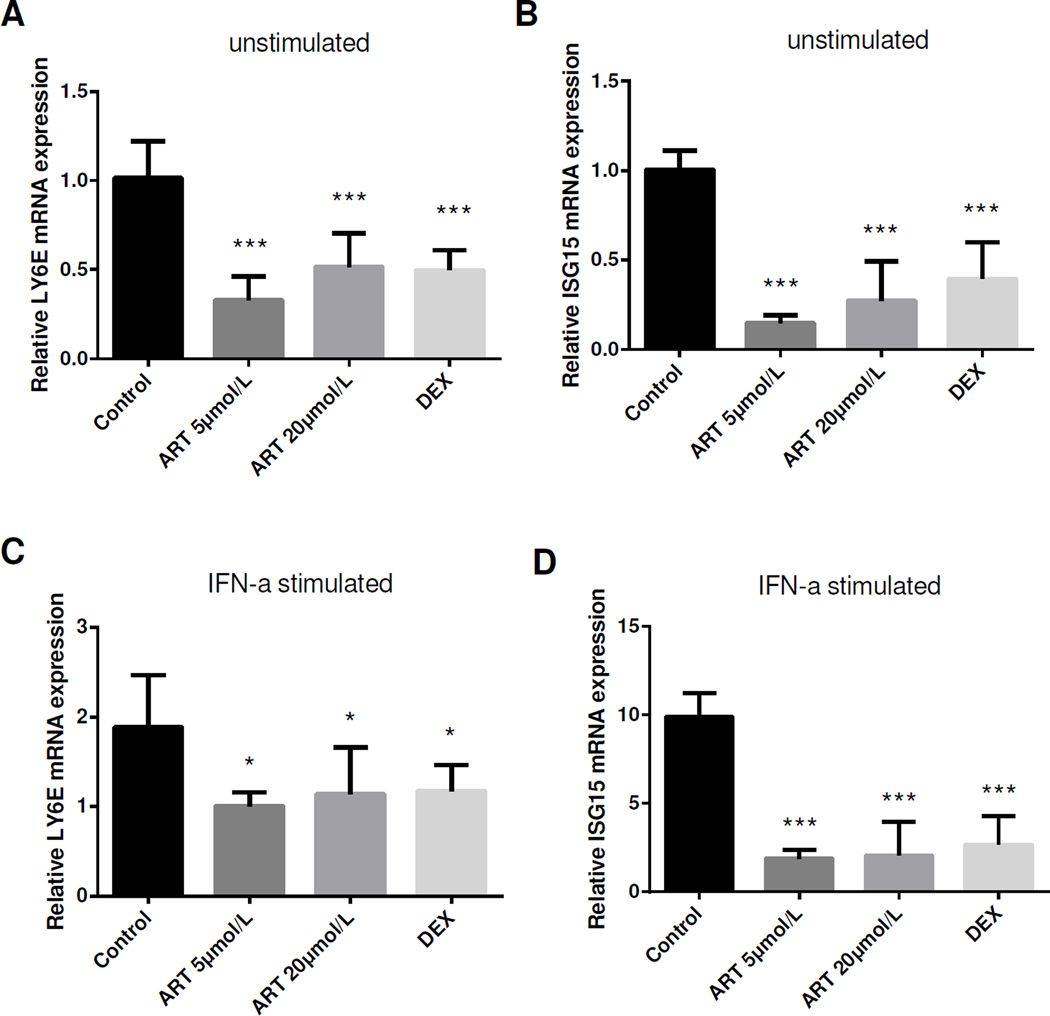
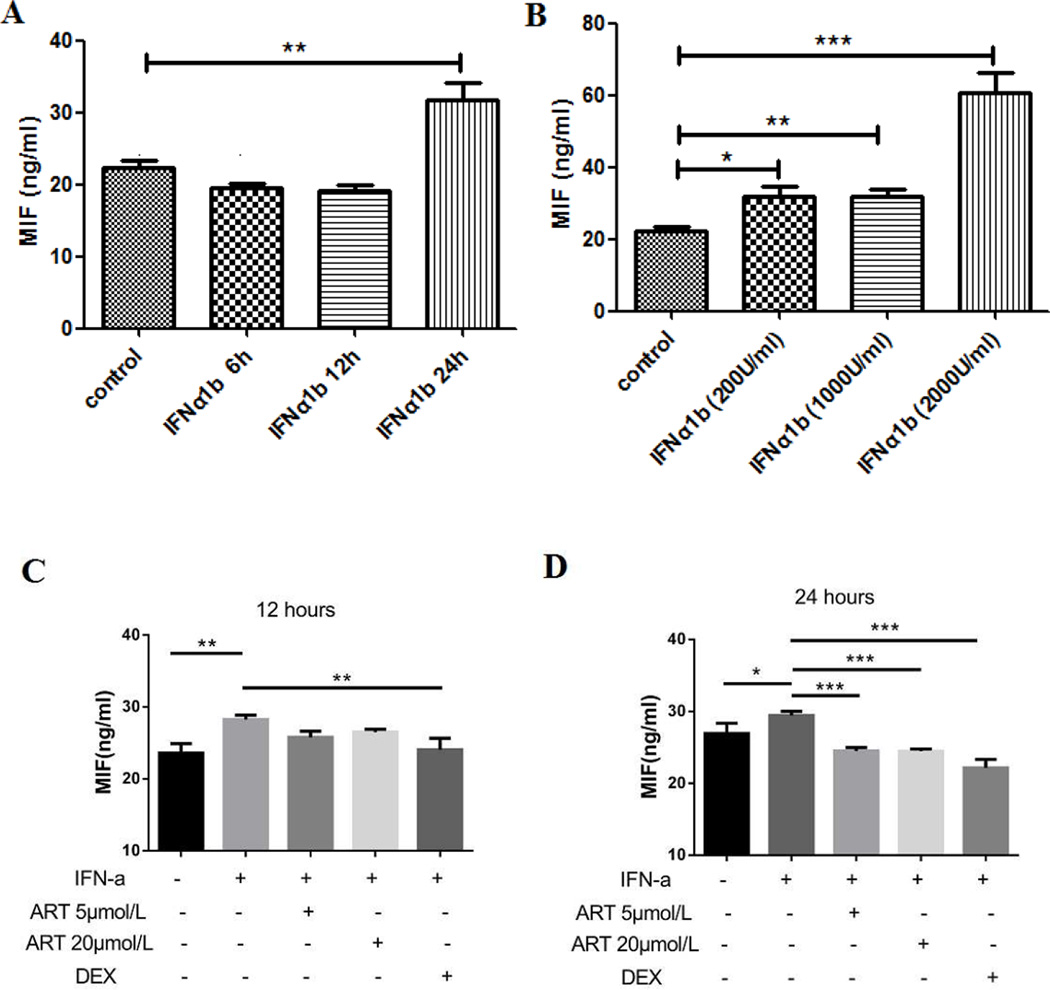
To investigate whether type I IFN did have an effect on MIF regulation, a human endothelial cell line, HUVECs, was cultured with or without the addition of IFNα-1b. As expected, HUVECs expressed higher mRNA levels of type I IFN inducible genes (as represented by LY6E and ISG15) after IFNα stimulation (p < 0.05) (see the control group in Figure 3). MIF levels were significantly elevated at 24 hours (31.8 ± 4.5 ng/ml vs. 22.4 ± 1.8 ng/ml, p < 0.01) but not at 6 hours or 12 hours after IFNα treatment (Figure 4A). After stimulated by different concentrations of IFNα for 24 hours, MIF levels were increased in all three groups compared with that in control group and the effect was most significantly different at the highest dose in the 2000 U/ml group (60.6 ± 11.0 ng/ml vs. 22.4 ± 1.8 ng/ml, p < 0.001) (Figure 4B), indicating that type I IFN is capable of promoting MIF production in a time- and dose-dependent manner.
Figure 3.
Down-regulated IFN inducible gene expression after ART treatment. HUVECs were cultured with or without the presence of IFN-1b for 24 hours, and then treated with ART or dexamethasone (DEX) for 24 hours. Similar to that of DEX, ART at both 5 µM and 20 µM significantly inhibited both natural and IFNα induced LY6E (A, C) and ISG15 (B, D) expressions in HUVEC. Data were present as mean ± SD. *p < 0.05, ***p < 0.001.
Figure 4.
ART counteracted IFNα triggered MIF production in HUVECs. HUVECs were stimulated by 1000 U/ml IFNα-1b for 6 hours, 12 hours and 24 hours (A), and then cultured with different concentrations of IFN-1b (200 U/ml, 1000 U/ml, 2000 U/ml) for 24 hours (B) to detect supernatant MIF levels. Next, HUVECs were cultured with the presence of 1000 U/ml IFN-1b for 24 hours, and treated with ART or dexamethasone (DEX) for another 12 and 24 hours (C, D). Data were present as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
ART down-regulated type I IFN inducible gene expression and MIF production
To explore the role of ART on type I IFN and MIF, HUVECs were exposed to IFNα-1b and then treated by ART (5 and 20 µM) or dexamethasone (as a positive control). As shown in Figure 3, there was a dramatic decline of IFN inducible gene (LY6E and ISG15) expressions after ART and dexamethasone treatment, either with or without prior stimulation of IFNα (1000U/ml), indicating that ART could inhibit the activation of IFN pathway. After culturing with ART for 24 hours, a significant decline of MIF levels was observed (24.5 ± 0.5 ng/ml in ART 5 µM group, 24.4 ± 0.3 ng/ml in ART 20 µM group, all p < 0.001 compared to 29.4 ± 0.6 ng/ml in IFNα control group) (Figure 4D), suggesting that ART could counteract IFNα triggered MIF production.
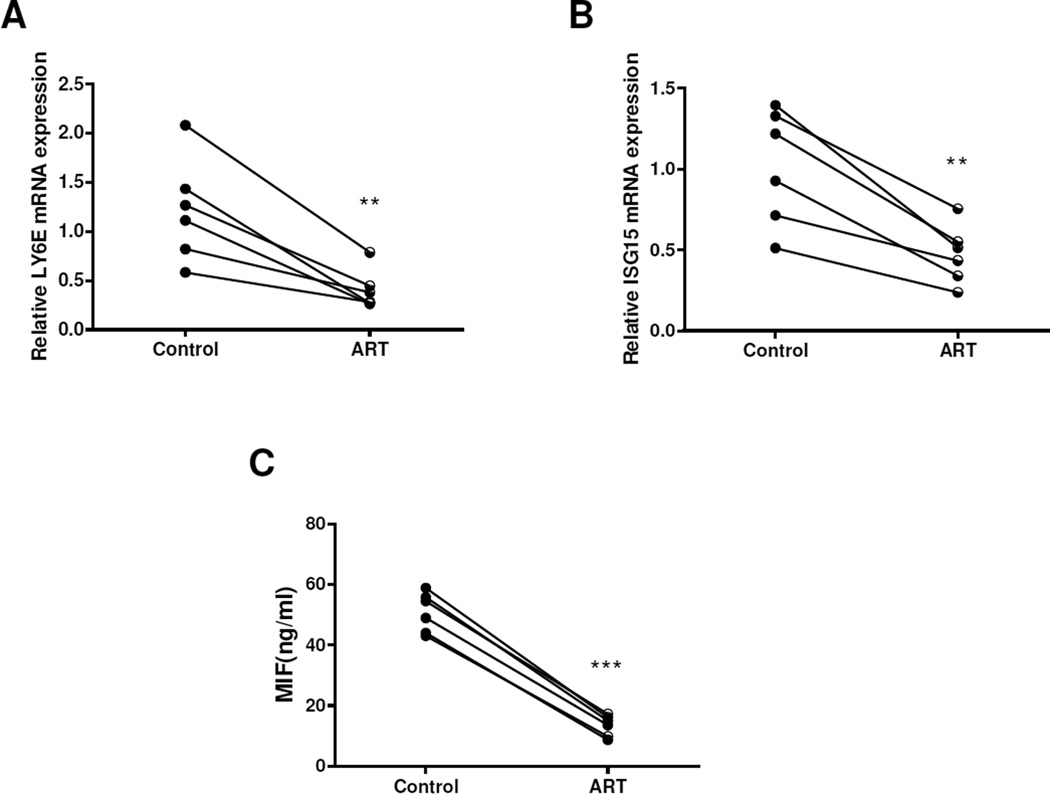
To further confirm the effect of ART, we assessed MIF production in SLE PBMC cultures in the presence or absence of ART at a dose of 20 µM. Similar to that in HUVECs, ART significantly decreased the mRNA expressions of LY6E and ISG15 (both p < 0.01) (Figure 5A–B) and down-regulated MIF levels (13.5 ± 3.5 ng/ml vs. 50.9 ± 6.5 ng/ml, p < 0.001) (Figure 5C). Thus, ART could not only act on endothelial cells, but also act on PBMCs to inhibit MIF production.
Figure 5.
ART inhibited MIF production in PBMC from SLE patients. A-B, compared with that in control group, ART at 20 µM significantly decreased the mRNA expressions of LY6E and ISG15. C, ART treatment significantly down-regulated the production of MIF levels in PBMC from SLE patients. **p < 0.01, ***p < 0.001 vs. the control group.
ART inhibited MIF production through the blockade of STAT1 phosphorylation
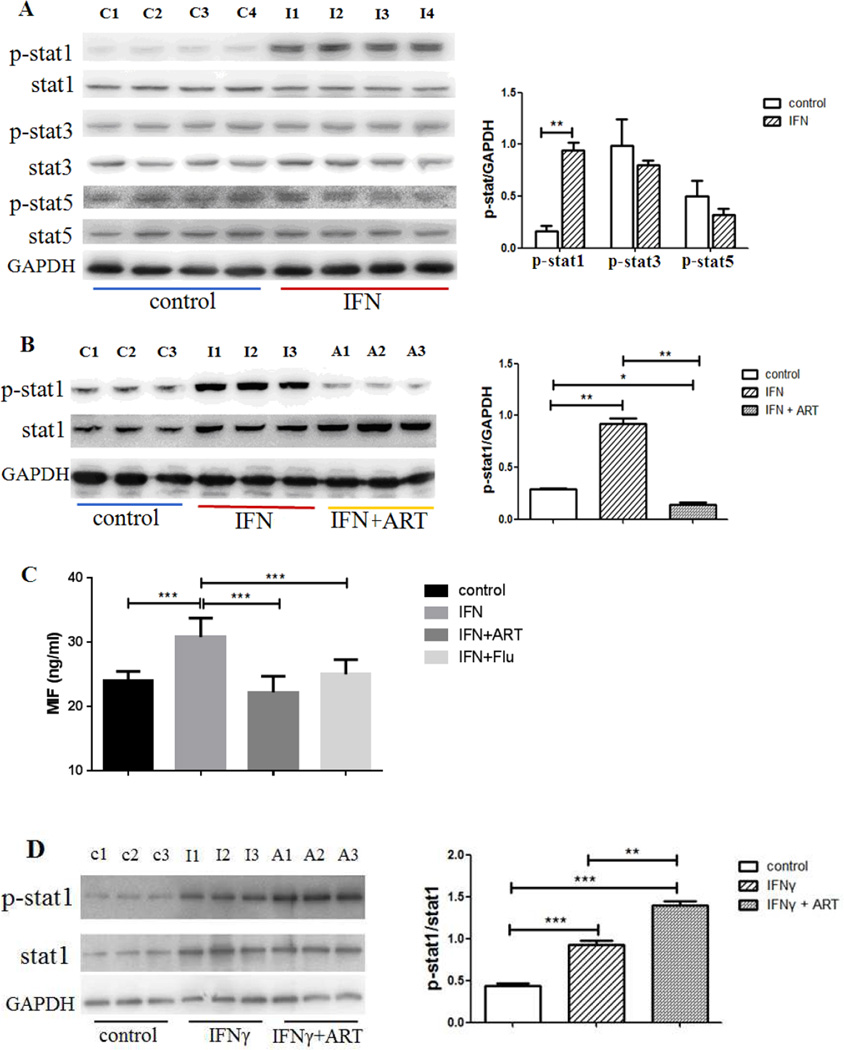
Given that the biological activities of IFNs are triggered by the STAT signaling cascade (20), we studied the activation of STATs after ART treatment. Western blotting demonstrated that IFNα-1b promoted tyrosine phosphorylation of SATA1 but not STAT3 or STAT5 in cultured HUVECs (Figure 6A). When treated by ART at a dose of 20 µM for 24 hours, IFNα-1b induced elevation of p-SATA1 was greatly down-regulated (Figure 6B). Consistently, MIF levels in cultured supernatants were also increased after IFNα-1b stimulation, while ART had a similar effect as that of fludarabine, a specific STAT1 inhibitor, in attenuating IFNα induced MIF production (Figure 6C). To rule out the effect of type II IFN, HUVECs were stimulated with 10ng/ml IFNγ for 24 hours and cultured with ART for another 24 hours. As shown in Figure 6D, ART treatment did not reverse IFNγ-induced STAT1 phosphorylation.
Figure 6.
ART down-regulated MIF production through the inhibition of STAT1 phosphorylation. A, Protein levels of STAT1, 3, 5 and p-STAT1, 3, 5 in HUVECs with or without IFNα stimulation. B, Effect of ART treatment on IFNα induced pSTAT1 production. HUVECs were cultured with or without the presence of 1000U/ml IFNα for 24 hours and then those with IFNα stimulation were treated by 20 µM ART for 24 hours. C, Similar to that of fludarabine, a selective STAT1 inhibitor, ART treatment significantly down-regulated IFNα induced MIF production. D. Effect of ART treatment on IFNγ induced pSTAT1 production. HUVECs were cultured with or without the presence of 10ng/ml IFNγ for 24 hours and then those with IFNγ stimulation were treated by 20 µM ART for 24 hours. Each lane represented an independent sample and data were also shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Individuals with SLE have a striking increase in the incidence of premature atherosclerosis compared to their sex- and age-matched controls, which has been recognized as one of the leading cause of morbidity and mortality for this prototypical autoimmune disease (21). Recently, activation of type I IFN system has been shown to contribute, at least partially, to accelerated atherosclerosis in SLE patients (22), yet the mechanisms remain to be elucidated. In this study, we confirmed that MIF, a chemokine -like inflammatory regulator related to either atherosclerosis or lupus, was highly expressed in SLE patients, especially those with active diseases. Our data demonstrated for the first time that elevated type I IFN could lead to the abnormal production of MIF, while ART, an anti-malarial agent extracted from Chinese herbs, counteracted the effect of type I IFN by blocking STAT1 phosphorylation. Based on these data, we propose that ART may serve as a novel and feasible therapy for both SLE and SLE-associated arthrosclerosis.
Our data also demonstrated that MIF was highly expressed in SLE patients and associated with both disease activity and accumulated organ damage. MIF, first described as a T-cell cytokine important for inhibiting macrophage migration, is now known to be secreted by both immune and nonimmune cell types through the autocrine or paracrine modes of action and to have diverse immunologic functions (23). There is an impressive body of evidence supporting a key role for MIF in the pathogenesis of SLE, as the circulating level and the tissue expression of MIF are elevated in SLE patients, and the MIF high-expression alleles are associated with increased prevalence of SLE (6;24). In addition, lupus-prone mice deficient in MIF have been reported to exhibit prolonged survival and marked reductions in renal and skin lesions despite no effects on T and B cells, while targeting the MIF–MIF receptor interaction by small-molecule antagonism down-regulates MIF-dependent pathways of tissue damage (7;25). MIF may directly accelerate atherosclerosis, since it is highly expressed in different stages of human atherosclerosis (26), and MIF inhibition induces the stabilization and even regression of atherosclerotic plaques in different experimental models (8;27;28). Thus, the elevation of MIF in SLE patients could not only exacerbate lupus diseases but also promote atherosclerosis.
Our next question is why MIF levels fluctuated in SLE. In the past decade, IFNα has been proposed to play an important role in SLE etiology and pathogenesis. There are many studies including ours demonstrating that IFNα levels or type I IFN inducible gene expressions increase during SLE flares and multiple organ involvement (19;29). Recently, a link between type I IFN and premature atherosclerosis in SLE has been established by the data showing that IFNα priming promotes lipid uptake and macrophage-derived foam cell formation (12). In this study, we showed for the first time that MIF levels were related to increased IFN inducible gene expression in SLE patients. In vitro treatment of IFNα confirmed the relationship between type I IFN and MIF, implying that over-expressed type I IFN may promote atherosclerosis in SLE through the modulation of MIF.
Currently, the available therapy for atherosclerosis primarily targets hyperlipidemia and prevention of thrombosis, even it has long been identified as having inflammatory components contributing to its pathogenesis. Unfortunately, routine statin use over 3 years has no significant effect on subclinical atherosclerosis progression in young SLE patients (30). With the exception of mycophenolate mofetil and hydroxychloroquine (31;32), the availability of beneficial treatments to treat SLE-associated atherosclerosis is greatly limited. Since MIF plays an important role in the progression of atherosclerosis, the design of new inhibitors targeting MIF and the study of their effects on the biologic functions of this factor will be extremely valuable and promising for treatment of lupus atherosclerosis (23).
Artemisinins are a family of sesquiterpene trioxane lactone anti-malarial agents originally derived from Artemisia annua L., among which ART is the most studied analog. With its established safety record in millions of malarial patients, recently ART has also been investigated in other diseases such as virus or fungal infections, cancers and autoimmune diseases (33). Consistent with our previous findings (13), this kind of drug significantly ameliorates lupus-like disease in experimental murine models of SLE, with comparable or even superior efficacy to that of GC or immunosuppressant (34;35). The anti-inflammatory effects of ART could be attributed to the inhibition of various cytokines and diverse signaling pathways (36;37). Here we showed that ART directly inhibited the expression of IFN inducible genes and counteracted the effect of IFNα on MIF production, suggesting that ART act through the modulation of type I IFN pathway. Upon IFN-induced activation, the STAT proteins are phosphorylated and translocated into the nucleus to bind specific elements within the promoters of IFN-stimulated genes (20). Treatment of an artemisinin analog has been found to significantly suppress the phosphorylation of STAT-1, STAT-3, and STAT-5 but not STAT-4 and STAT-6 (35). However, only the phosphorylation of STAT-1 was found to be down-regulated by ART in this study, which was compatible to that of fludarabine, a selective STAT1 inhibitor.
In summary, here we showed that the production of MIF was up-regulated by type I IFN in vitro. The IFNα-MIF axis could represent a new pathway for the development of arthrosclerosis in SLE. ART acted through the inhibition of STAT1 phosphorylation to counteract the effect of type I IFN, leading to the decline of MIF level, therefore may be helpful to relieve both lupus and atherosclerosis in SLE patients.
Acknowledgments
We thank all the patients and healthy volunteers participating in this study.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81373198), Jiangsu Provincial Special Program of Medical Science (BE2015602) and Jiangsu Province’s Key Provincial Talents Program. BPT was supported by RO1AR0 43814 and R21AR065626 from the NIAMS NIH.
Footnotes
Conflict of Interest Statement
The authors declare that there are no competing interests.
Reference List
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60(2):221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du BR, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 5.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez E, Gomez LM, Lopez-Nevot MA, Gonzalez-Gay MA, Sabio JM, Ortego-Centeno N, et al. Evidence of association of macrophage migration inhibitory factor gene polymorphisms with systemic lupus erythematosus. Genes Immun. 2006;7(5):433–436. doi: 10.1038/sj.gene.6364310. [DOI] [PubMed] [Google Scholar]
- 7.Leng L, Chen L, Fan J, Greven D, Arjona A, Du X, et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. 2011;186(1):527–538. doi: 10.4049/jimmunol.1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Xia M, Hayford C, Tham e, Semik V, Hurst S, et al. Expression of human tissue factor pathway inhibitor on vascular smooth muscle cells inhibits secretion of macrophage migration inhibitory factor and attenuates atherosclerosis in ApoE−/− mice. Circulation. 2015;131(15):1350–1360. doi: 10.1161/CIRCULATIONAHA.114.013423. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Zhang X, Zhao L, Zhen X, Huang S, Wang S, et al. Attenuation of atherosclerotic lesions in diabetic apolipoprotein E-deficient mice using gene silencing of macrophage migration inhibitory factor. J Cell Mol Med. 2015;19(4):836–849. doi: 10.1111/jcmm.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, et al. Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum. 2011;63(2):492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
- 13.Jin O, Zhang H, Gu Z, Zhao S, Xu T, Zhou K, et al. A pilot study of the therapeutic efficacy and mechanism of artesunate in the MRL/lpr murine model of systemic lupus erythematosus. Cell Mol Immunol. 2009;6(6):461–467. doi: 10.1038/cmi.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Shi L, Yang X, Li S, Guo X, Pan L. Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol. 2010;92(4):587–597. doi: 10.1007/s12185-010-0697-3. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27(2):373–376. [PubMed] [Google Scholar]
- 18.Feng X, Huang J, Liu Y, Xiao L, Wang D, Hua B, et al. Identification of interferon-inducible genes as diagnostic biomarker for systemic lupus erythematosus. Clin Rheumatol. 2015;34(1):71–79. doi: 10.1007/s10067-014-2799-4. [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 20.Brierley MM, Fish EN. Stats: multifaceted regulators of transcription. J Interferon Cytokine Res. 2005;25(12):733–744. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- 21.Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(Suppl 4):S4. doi: 10.1186/ar3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karageorgas TP, Tseronis DD, Mavragani CP. Activation of type I interferon pathway in systemic lupus erythematosus: association with distinct clinical phenotypes. J Biomed Biotechnol. 2011;2011:273907. doi: 10.1155/2011/273907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos LL, Morand EF. Macrophage migration inhibitory factor: a key cytokine in RA, SLE and atherosclerosis. Clin Chim Acta. 2009;399(1–2):1–7. doi: 10.1016/j.cca.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31(2):268–273. [PubMed] [Google Scholar]
- 25.Hoi AY, Hickey MJ, Hall P, Yamana J, O'Sullivan KM, Santos LL, et al. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J Immunol. 2006;177(8):5687–5696. doi: 10.4049/jimmunol.177.8.5687. [DOI] [PubMed] [Google Scholar]
- 26.Burger-Kentischer A, Goebel H, Seiler R, Fraedrich G, Schaefer HE, Dimmeler S, et al. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105(13):1561–1566. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- 27.Noels H, Bernhagen J, Weber C. Macrophage migration inhibitory factor: a noncanonical chemokine important in atherosclerosis. Trends Cardiovasc Med. 2009;19(3):76–86. doi: 10.1016/j.tcm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation. 2004;109(3):380–385. doi: 10.1161/01.CIR.0000109201.72441.09. [DOI] [PubMed] [Google Scholar]
- 29.Niewold TB, Clark DN, Salloum R, Poole BD. Interferon alpha in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:948364. doi: 10.1155/2010/948364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 2012;64(1):285–296. doi: 10.1002/art.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richez C, Richards RJ, Duffau P, Weitzner Z, Andry CD, Rifkin IR, et al. The effect of mycophenolate mofetil on disease development in the gld.apoE (−/−) mouse model of accelerated atherosclerosis and systemic lupus erythematosus. PLoS One. 2013;8(4):e61042. doi: 10.1371/journal.pone.0061042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 33.Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142(1):126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH, et al. SM934 treated lupus-prone NZB × NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One. 2012;7(2):e32424. doi: 10.1371/journal.pone.0032424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63(8):2445–2455. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Wang S, Wang Y, Zhou C, Chen G, Shen W, et al. Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl Res. 2013;161(2):89–98. doi: 10.1016/j.trsl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Cheng C, Ho WE, Goh FY, Guan SP, Kong LR, Lai WQ, et al. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One. 2011;6(6):e20932. doi: 10.1371/journal.pone.0020932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94(1):20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]