Abstract
Background
The complex process by which lactation is initiated upon neonate delivery remains incompletely understood. Microvesicles (MVs) can transmit microRNAs (miRNAs) into recipient cells to influence cell function, and recent studies have identified miRNAs essential for mammary gland development and lactation. This study aimed to investigate the expression of lactation-related miRNAs in MVs isolated from human umbilical cord blood immediately after delivery.
Material/Methods
Umbilical cord blood samples were collected from 70 healthy pregnant women, and MVs were isolated through differential centrifugation and characterized by transmission electron microscopy, Western blotting, and nanoparticle tracking analysis. Lactation-related miRNAs were screened using bioinformatics tools for miRNA target prediction, gene ontology, and signaling pathway analyses. miRNA PCR arrays were used for miRNA expression analysis, and the results were validated by real-time PCR. Upon exposure of HBL-100 human mammary epithelial cells to MVs, MV uptake was examined by fluorescence confocal microscopy and β-casein secretion was detected by ELISA.
Results
Spherical MVs extracted from umbilical cord blood expressed CD63 and had an average diameter of 167.0±77.1 nm. We profiled 337 miRNAs in human umbilical cord blood MVs and found that 85 were related to lactation by bioinformatics analysis. The 25 most differentially expressed lactation-related miRNAs were validated by real-time PCR. MV uptake by HBL-100 cells was after 4 h in culture, and significantly increased secretion of β-casein was observed after 96 h from cells exposed to MVs (P<0.05).
Conclusions
Umbilical cord blood MVs contain many lactation-related miRNAs and can induce β-casein production by HBL-100 cells in vitro. Thus, umbilical cord blood MVs may mediate secretion of β-casein through miRNAs, thereby playing an important role in fetal-maternal crosstalk.
MeSH Keywords: Lactation, MicroRNAs, Microvesicles
Background
Microvesicles (MVs), also known as shedding vesicles, are nanovesicles (100–1000 nm in diameter) that contain miRNAs, mRNA, and proteins [1]. miRNAs, which are short (20–22 nucleotides in length), non-coding RNAs that control the translation of proteins from many genes, can be delivered via MVs into recipient cells, where they regulate target gene expression and cell function [2]. Mincheva et al. found that placenta-derived microvesicles function as immune regulators in fetal-maternal crosstalk, improving maternal adaptation to the ongoing pregnancy and promoting fetal allograft survival [3]. It has been demonstrated that circulating syncytiotrophoblast MVs from umbilical cord blood can support normal pregnancy by binding to monocytes and B cells and inducing the release of specific cytokines (e.g., tumor necrosis factor alpha [TNF-α] and interleukin 1 alpha [IL-1α]) [4]. Using pathway analysis, a recent study revealed that umbilical cord blood exosomes collected from pregnant sheep contain miRNAs targeting cellular and organismal development [5]. These early studies indicated that miRNAs in umbilical cord blood plays key roles in the regulation of pregnancy-related processes.
The complex initiation of lactation has been studied extensively at the genetic, physiological, and morphological levels due to its importance to the health of the neonate. Several miRNAs have been shown to be indispensable for mammary development and lactation [6]. Ucar et al. showed that miR-212 and miR-132 are essential for mouse mammary gland development, particularly for the regulation of epithelial duct outgrowth [7]. Li et al. showed that miR-15a regulates growth hormone receptor expression, influences mammary epithelial cell viability, and alters the secretion of β-casein [8]. Thus, multiple miRNAs are known to be involved in the regulation of milk protein synthesis and the development of mammary glands in cows and other mammals [9]. However, little is known about the miRNAs in MVs that may be involved in the regulation of milk protein synthesis in humans. In the present study, we investigated whether umbilical cord blood MVs contained miRNAs that could regulate lactation. We isolated MVs from human umbilical cord blood samples, identified lactation-related miRNAs within these MVs, and considered the potential roles of the miRNAs in inducing the secretion of milk proteins.
Material and Methods
Sample collection and MV isolation
Umbilical cord blood samples (100 ml) were collected by midwives from 70 healthy pregnant women (age 29.26±3.61 years, body mass index 23.63±1.89 kg/m2, gestational age 38.51±1.67 weeks) into umbilical cord blood collection bags. Fresh umbilical cord blood samples were processed within 6 h and briefly mixed with an equal volume of phosphate-buffered saline (PBS, pH 7.4). All donors delivered in the Department of Obstetrics and Gynaecology of the First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China, between February 2015 and October 2015. The study was approved by the Ethics Review Committee on Human Research of the Chongqing Medical University (Reference Number: 2015004), and informed consent was obtained from all donors. MVs were isolated from the umbilical cord blood by continuous differential centrifugation, as previously described [10,11]. The cells and debris were removed by centrifugation at 80×g for 20 min, 2000×g for 15 min, and 5000×g for 15 min, sequentially. Then the MVs were pelleted by further centrifugation at 12 000×g for 30 min at 4°C. The resulting precipitant was collected, suspended in 1 ml PBS, and then centrifuged at 12 000×g for 70 min.
Transmission electron microscopy and Western blot analysis
MVs isolated from the umbilical cord blood were suspended in PBS containing 0.1% bovine serum albumin (BSA). The resuspended MVs were fixed in 1% glutaraldehyde at 4°C overnight and stained with 1% uranyl acetate for 10 min. Excess fluid was removed with a piece of Whatman filter paper. All transmission electron micrographs were obtained using an H7500 transmission electron microscope (Hitachi, Japan) at 120 kV. Extracted total proteins were incubated with cold radioimmunoprecipitation (RIPA) lysis buffer and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins were transferred to 0.22-μm polyvinylidene difluoride (PVDF) membranes (Bio-Rad) and reacted with anti-CD63 (1: 1000, Cat #ab59479, Abcam) and anti-cytochrome c (1;1000, Cat #ab50050, Abcam) primary antibodies at 4°C overnight. After washing with Tris-buffered saline containing Tween 20 (TBST), the membranes were incubated with peroxidase-conjugated secondary antibodies (1: 1000, 0.01M PBST). Protein expression was normalized to β-actin expression. Antibodies bound on the membrane were detected using an enhanced chemiluminescence detection system (Millipore) according to the manufacturer’s instructions.
Nanoparticle tracking analysis
The nanoparticle tracking analysis of the MVs was performed using a NanoSight NS300 instrument (Malvern instruments Ltd., UK) calibrated with 100-nm polystyrene beads (Polysciences, Warrington, PA). Particle suspensions were diluted in PBS to a concentration of 1–8×108 particles/ml for the nanoparticle tracking analysis. The Stokes-Einstein equation was employed to determine the size distribution and number of particles (concentration) within the sample.
Bioinformatics analysis
The miRNA expression profile was obtained by literature mining and database searching. We used BLAST + 2.2.31 to compare sequences of miRNAs found to be expressed during the lactation period of other mammals with human sequences listed in the miRbase database. The 11 miRNA target genes included DIANA-microT, MicroInspector, miRanda, MirTarget2, miTarget, NBmiRTar, PicTar, PITA, RNA22, RNAhybrid, and TargetScan/TargertScanS. These were used to predict the expression of human homolog miRNA target genes. A gene was considered the corresponding miRNA target gene if it was predicted as a target gene by 5 or more tools. ClueGO determines the distribution of genes on the target gene list across the gene ontology (GO) terms and pathways. We analyzed all of the differentially expressed genes using GO and KEGG pathway analyses. The map of interactions among these target genes was constructed using Cytoscape 3.2.1.
Polymerase chain reaction (PCR) array
The miRNA expression profile in umbilical cord blood MVs was detected by a 384-well miRNA qPCR array. Quantitative real-time PCR reactions were performed on the ViiA™ 7 high-throughput real-time PCR system (Thermo Fisher Scientific).
The 25 most differentially expressed miRNAs were further validated by real-time PCR. The total RNA was isolated using a total RNA extraction kit (Quanto Bio, cat #0960601). TaqMan-based quantitative PCR was performed using the 7900HT fast real-time PCR system (Applied Biosystems). EC4 and EC5 were used as the external control for miRNA in the real-time qPCR analyses. The gene expression amount was calculated as a cycle threshold (CT value). The real-time reverse transcription PCR primers used are listed in Table 1.
Table 1.
Primers used in real-time PCR.
| Primer | Sequence |
|---|---|
| Down-regulated miRNA | |
| miR-181d | (F)5′-AACAUUCAUUGUUGUCGGUGGGU-3′ |
| miR-107 | (F)5′-AGCAGCAUUGUACAGGGCUAUCA-3′ |
| let-7c | (F)5′-UGAGGUAGUAGGUUGUAUGGUU-3′ |
| miR-10a | (F)5′-UACCCUGUAGAUCCGAAUUUGUG-3′ |
| miR-100 | (F)5′-AACCCGUAGAUCCGAACUUGUG-3′ |
| miR-199a-3p | (F)5′-ACAGUAGUCUGCACAUUGGUUA-3′ |
| miR-221 | (F)5′-AGCUACAUUGUCUGCUGGGUUUC-3′ |
| miR-146b | (F)5′-UGAGAACUGAAUUCCAUAGGCU-3′ |
| miR-199b-5p | (F)5′-CCCAGUGUUUAGACUAUCUGUUC-3′ |
| miR-26a | (F)5′-UUCAAGUAAUCCAGGAUAGGCU-3′ |
| miR-181c | (F)5′-AACAUUCAACCUGUCGGUGAGU-3′ |
| miR-23a | (F)5′-AUCACAUUGCCAGGGAUUUCC-3′ |
| miR-27a | (F)5′-UUCACAGUGGCUAAGUUCCGC-3′ |
|
| |
| Up-regulated miRNA | |
| miR-181a | (F)5′-AACAUUCAACGCUGUCGGUGAGU-3′ |
| miR-106a | (F)5′-AAAAGUGCUUACAGUGCAGGUAG-3′ |
| miR-200c | (F)5′-UAAUACUGCCGGGUAAUGAUGGA-3′ |
| miR-20a | (F)5′-ACUGCAUUAUGAGCACUUAAAG-3′ |
| miR-181b | (F)5′-AACAUUCAUUGCUGUCGGUGGGU-3′ |
| miR-29b | (F)5′-UAGCACCAUUUGAAAUCAGUGUU-3′ |
| miR-103 | (F)5′-AGCAGCAUUGUACAGGGCUAUGA-3′ |
| miR-10a | (F)5′-UACCCUGUAGAUCCGAAUUUGUG-3′ |
| miR-145-5p | (F)5′-GUCCAGUUUUCCCAGGAAUCCCU-3′ |
| miR-155 | (F)5′-UUAAUGCUAAUCGUGAUAGGGGU-3′ |
| miR-142-3p | (F)5′-UGUAGUGUUUCCUACUUUAUGGA-3′ |
| miR-18a | (F)5′-UAAGGUGCAUCUAGUGCAGAUAG-3′ |
Cell culture
HBL-100 cells were a kind gift from Dr. Tingxiu Xiang (Laboratory of Molecular Oncology and Epigenetics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China). The cells were routinely maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) plus 10% fetal bovine serum (FBS; Hyclone), 50 U/ml penicillin (Gibco), 50 mg/ml streptomycin (Gibco), and a lactogenic hormone mix of 5 mg/ml insulin (Pepro Tech), 10 ng/ml epidermal growth factor (EGF; Pepro Tech), 1 mM dexamethasone (Aladdin), and 5 mg/ml prolactin (Sigma-Aldrich). The MVs were added directly to HBL-100 cells cultured in a 12-well plate or chamber slide.
Enzyme-linked immunosorbent assay (ELISA)
Mammary epithelial cells (1×105 cells/well) were incubated in 450 μl culture medium per well in a 12-well plate for 1 day at 37°C in a 5% CO2 incubator. Aliquots of MVs (30 μl) were collected and incubated with mammary epithelial cells. The culture supernatant was collected after 24, 48, 72, and 96 h. All experiments were performed in triplicate. The secretion of β-casein was confirmed using a human CSN2 ELISA kit (Shanghai Hushang Biological Technology Co., Ltd., China).
MV labeling and delivery analysis
For antibody labeling, MVs were incubated with anti-CD63 at 4°C overnight. Then, MVs were then exposed to Alexa 488-labeled goat anti-rabbit IgG secondary antibodies at room temperature for 2 h, washed with PBS, incubated with 4,6-diamidino-2-phenylindole (DAPI) for nuclear staining, and then mounted with ProLong® Gold antifade reagent. HBL-100 cells exposed to MVs on coverslips were fixed in 4% paraformaldehyde (PFA), and immunofluorescence labeling was performed following a standard procedure. Images were obtained using a confocal laser scanning microscope (Nikon Microsystems). Digital images were recorded and analyzed using NIS 4.2 Viewer and Image Pro Plus software.
Statistical analysis
In the bioinformatics analysis, the P-value was calculated using right-sided hypergeometric tests. Benjamini step-down adjustments were used for multiple test corrections. The results were considered significant when P<0.05. Thus, the corresponding GO terms and pathways were enriched in the target genes. The statistical analyses were performed with SPSS 13.0 software. Data from at least 3 separate experiments are expressed as mean ± standard deviation (SD) values. Difference in the data were analyzed using t tests between 2 groups. A value of P<0.05 was considered statistically significant.
Results
MVs in umbilical cord blood
MVs were isolated from the umbilical cord blood of healthy pregnant women using differential centrifugation at 12 000×g. Transmission electron microscopy showed that the MVs were approximately 100–500 nm in diameter and had a round shape (Figure 1A). Western blot analysis showed that MVs expressed cytochrome C and CD63, whereas lymphocytes did not express CD63 (Figure 1B). The nanoparticle tracking analysis showed that the MVs had an average diameter of 167±77.1 nm, and the MV size distribution showed 3 main peaks at 114, 141, and 383 nm (Figure 1C).
Figure 1.
Evidence of microvesicles (MVs) in umbilical cord blood. (A) Transmission electron microscopy image of MVs isolated from umbilical cord blood. (B) Western blot showing expression of surface marker CD63 on MVs, with β-actin expression as a control. (C) Results of nanoparticle tracking analysis of MVs. Scale bar, 0.2 μm.
Bioinformatics-based identification of lactation-related miRNAs
Our searches of the literature and databases revealed 112 miRNAs that were differentially expressed during lactation in mammals (mice, rats, cows, goats, and sheep), and we found that down-regulated and up-regulated miRNAs were highly homologous among the species studied previously (Supplementary Tables 1, 2). BLAST analysis identified 69 miRNAs that were highly homologous with human miRNAs. GO and KEGG pathway analyses suggested that these miRNAs may regulate genes associated with macromolecule biosynthetic processes and important lactation pathways, such as the MAPK, mTOR, and PI3K-Akt signaling pathways. Overall, we found that the following 18 of the 69 miRNAs were down-regulated: miR-181d-5p, miR-574-3p, miR-107, let-7c-5p, miR-10a-5p, miR-100-5p, miR-199a-3p, miR-221-3p, miR-205-5p, miR-146b-5p, miR-221-5p, miR-199b-5p, miR-203a-3p, miR-26a-5p, miR-181c-5p, miR-134-5p, miR-23a-5p, and miR-27a-5p. The other 51 miRNAs were up-regulated.
We analyzed all of the differentially expressed genes regulated by these miRNAs using ClueGO for GO and KEGG pathway analyses (Supplementary Tables 3, 4). In the GO analysis, the down-regulated miRNAs were associated with 15 biological processes (P<1.83E-11). As shown in Supplementary Table 3A, the 5 most significantly enriched biological processes were: positive regulation of macromolecule metabolism, positive regulation of cellular metabolism, nervous system development, positive regulation of macromolecule biosynthesis, and positive regulation of cellular biosynthesis. In the KEGG analysis, as shown in Supplementary Table 4A, the down-regulated miRNAs were mainly associated with the following pathways: dorso-ventral axis formation, MAPK signaling pathway, miRNAs in cancer, and the mechanistic target of rapamycin (mTOR) signaling pathway.
As shown in Supplementary Table 3B, the 5 most significantly enriched biological processes associated with the up-regulated miRNAs were: multicellular organismal development, regulation of macromolecule metabolism, negative regulation of biological processes, positive regulation of macromolecule metabolism, and regulation of metabolism. These biological processes, while not unexpected, are all closely connected with macromolecule biosynthesis and metabolism. As shown in Supplementary Table 4B, the up-regulated miRNAs were mainly involved in the following pathways: thyroid hormone signaling, miRNAs in cancer, MAPK signaling, and PI3K-Akt signaling. Interestingly, the significantly enriched pathways were associated with milk protein synthesis, which indicates that miRNA signaling may be an important molecular event during lactation.
Validation of miRNA expression in MVs
We identified 337 miRNAs in umbilical cord blood MVs using a 384-miRNA qPCR array (Supplementary Table 5). As shown in Figure 2A, 55 miRNAs in the MVs were lactation-related, and these represented 79.71% of the total lactation-related miRNAs (55/69). Another 30 miRNAs in MVs were lactation-related miRNAs with −3p/−5p or * forms, and they had the same function. Finally, 85 lactation-related miRNAs in MVs were found and accounted for 25.22% of all miRNAs of the MVs (85/337). The 25 most differentially expressed lactation-related miRNAs according to the bioinformatics analysis were selected for further validation. As shown in Figure 2B, real-time PCR was then performed to assess the miRNA expression in 9 samples, and the averages of the Ct values were between 15 and 35.
Figure 2.
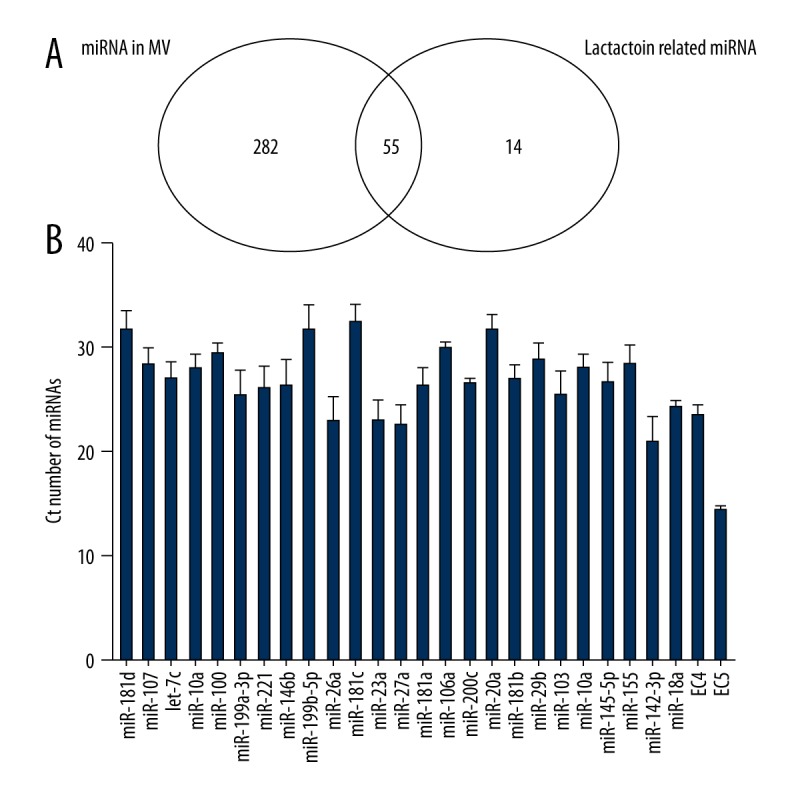
Venn diagram comparing miRNA expression in MVs and lactation-related miRNAs. (A) The number of shared and specific miRNAs. (B) Real-time PCR results for miRNAs in MVs. EC 4 and EC5 were used as external controls for qPCR.
Interaction between HBL-100 mammary epithelial cells and MVs and consequent changes in β-casein secretion
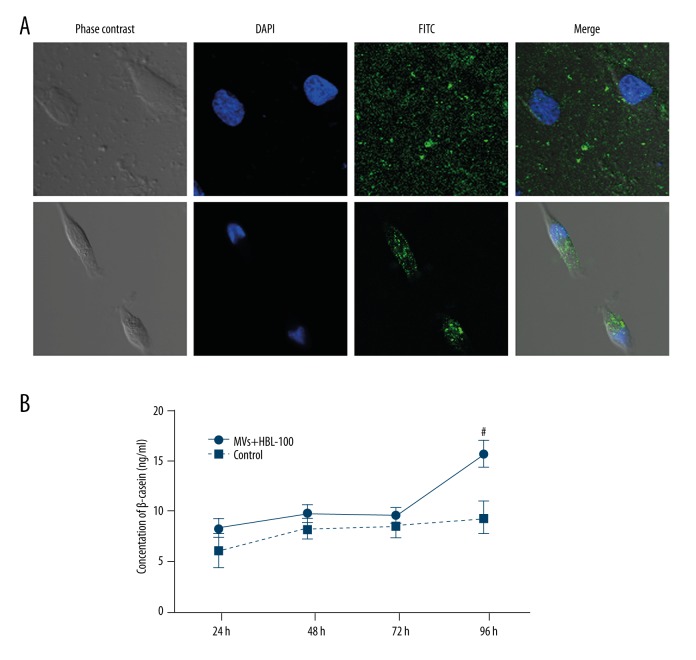
To evaluate the interaction between MVs and cultured HBL-100 cells, MVs were labeled with fluorescein isothiocyanate (FITC) and incubated with HBL-100 cells prior to observation by confocal microscopy. The nuclei of HBL-100 cells were labeled with DAPI. Confocal microscopy images of the treated MVs revealed green spots around cells (Figure 3A, upper panel), whereas after 4 h, MVs were found inside the HBL-100 cells (Figure 3A, lower panel).
Figure 3.
MV uptake by HBL-100 mammary epithelial cells and consequent change in β-casein secretion. (A) Confocal microscopy images of HBL-100 cells (nuclei stained blue by DAPI) exposed to MVs labeled with rat anti-human CD63 monoclonal antibody as a primary antibody and rabbit anti-rat FITC as a secondary antibody (green fluorescence). Initially, the labeled MVs were observed as green spots around the cells (upper panel), whereas MVs were found inside the HBL-100 cells after 4 hours (lower panel). # P<0.05: MVs+HBL-100 group vs. control group. (B) β-casein concentration in the HBL-100 supernatant after 24, 48, 72, and 96 hours of exposure to umbilical cord blood-derived MVs.
Aliquots of MVs (30 μl) were collected and incubated with human mammary epithelial cells. After 24, 48, 72, and 96 h, β-casein production had increased. Specifically, at 96 h, the concentration of β-casein in the supernatant was significantly greater at 16 ng/ml compared to that in the control group (7.5 ng/ml; P<0.05, Figure 3B). These results suggest that β-casein secretion was increased after the addition of MVs from umbilical cord blood.
Discussion
MVs are shed from the plasma membrane into the extracellular environment to facilitate communication between cells. Despite their small size (100–1000 nm), MVs are enriched in bioactive molecules and contain nucleic acids and/or proteins. MVs are known to play roles in growth, differentiation, and cancer progression. Valadi et al. first demonstrated that MVs contain both mRNA and miRNA [12]. Later research showed that MVs have pleiotropic effects on both fetal and maternal environments through the transmission of miRNA or/and proteins during pregnancy [13–15]. For example, MVs from uterine fluid were found to directly transfer miRNAs, such as miR200c, miR17, and miR106a, thereby contributing to the endometrial-embryo crosstalk required for embryo implantation [16]. In addition, MVs from the amniotic fluid were found to support fetal survival via their capture by human monocytic THP-1 cells and subsequent stimulation of cytokine release and nuclear factor kappa B (NF-κB)/STAT3 activation in a Toll-like receptor-dependent manner [17]. Moreover, circulating MVs shed by trophoblasts and isolated from plasma of pregnant women are able to downregulate T-cell activity, suggesting a possible role for these MVs in maintaining pregnancy [18]. Certainly, many questions regarding the role of MVs during pregnancy, and even delivery, remain to be answered. In the present study, we isolated MVs from umbilical cord blood and found that they contained an abundance of miRNAs, with bioinformatics analysis and real-time PCR revealing the presence of 337 miRNAs, 85 of which are lactation-related miRNAs, in these MVs.
Lactation is controlled by a complex interplay of endocrine hormones and proteins that act together locally. The most important genes in the networks of milk protein synthesis are believed to be PRLR, Jak2, Stat5, mTOR, insulin, AMPK, and MAPK [19–21]. A recent study indicated that miRNAs play a key role in mammary gland development; therefore, hormones and proteins are not the only factors influencing this process. Through extensive sequencing analyses, Li et al. explored differentially expressed miRNAs in lactating cow mammary glands and identified 226 differentially expressed miRNAs in the lactation period versus the non-lactation period. They then found that 16 key lactation genes mainly associated with milk synthesis and composition regulation were maintained by 37 differentially expressed miRNAs in lactating cow mammary glands [22]. Another study reported that there are 44 genes involved in milk protein synthesis and regulation in cows [23]; therefore, Li et al. connected 16 of the 44 genes to miRNAs. In the present study, we identified 69 miRNAs previously reported in lactating mammals that were highly homologous with human miRNAs by BLAST. Our GO and KEGG pathway analyses linked these miRNAs to genes associated with macromolecule biosynthesis and important lactation pathways. Overall, we found that 18 genes involved in milk synthesis were regulated by 29 miRNAs isolated from human umbilical cord MVs. These findings suggest that MVs in umbilical cord blood play a role in the regulation of milk protein synthesis.
Previous studies have demonstrated miRNA activities related to lactation via specific effects in mammary gland cells. For example, miR-101a was shown to influence mammary gland development by regulating the expression of cyclooxygenase-2 [24], and miR-126-3p targeting of progesterone receptors can affect the viability of mammary epithelial cells and secretion of β-casein [25]. Our in vitro investigation of the effects of umbilical cord blood MVs on HBL-100 mammary epithelial cells showed that uptake of MVs by HBL-100 cells occurred within 4 h, and consequently, the secretion of β-casein was significantly increased at 96 h. Thus, our in vitro experiments confirmed the lactation-related activity of MVs isolated from umbilical cord blood.
The increased production of milk protein after uptake of MVs could be related to the function of miRNAs independently or the function of miRNAs and other included components (e.g., protein) acting synergistically, and further studies are needed to determine the exact role of the miRNAs. For example, the specific genes associated with milk protein synthesis that are regulated by the miRNAs remain to be identified.
Conclusions
Our study revealed that umbilical cord blood MVs contain a large number of lactation-related miRNAs. Moreover, β-casein production was increased in HBL-100 cells treated with the cord blood-derived MVs. Together, our results suggest that miRNAs in umbilical cord blood MVs likely play a biological role in regulating lactation. Therefore, umbilical cord blood MVs represent a new vehicle of fetal-maternal crosstalk. We will explore the signaling mechanisms underlying the effects of umbilical cord blood MVs on lactation in our future research.
Supplementary Tables
Supplementary Table 1.
Down-regulated lactation-related miRNAs (E-value <6.00E-04).
| Original species | miRNA | Human genome homologous | Conservation | E-value | Number of target genes |
|---|---|---|---|---|---|
| Cow | mir-181d-5p | hsa-miR-181d-5p | 1 | 2.00E-04 | 222 |
| Cow | mir-574-3p | hsa-miR-574-3p | 1 | 6.00E-04 | 6 |
| Cow | mir-107-3p | hsa-miR-107 | 1 | 6.00E-04 | 53 |
| Cow | bta-let-7c | hsa-let-7c-5p | 1 | 6.00E-04 | 64 |
| Cow | mir-10a-5p | hsa-miR-10a-5p | 1 | 6.00E-04 | 19 |
| Cow | mir-100-5p | hsa-miR-100-5p | 1 | 6.00E-04 | 4 |
| Cow | mir-199a-3p | hsa-miR-199a-3p | 1 | 6.00E-04 | 39 |
| Cow | mir-221-3p | hsa-miR-221-3p | 1 | 6.00E-04 | 36 |
| Cow | mir-205-5p | hsa-miR-205-5p | 1 | 6.00E-04 | 43 |
| Cow | mir-146b-5p | hsa-miR-146b-5p | 1 | 6.00E-04 | 13 |
| Cow | mir-327-5p | hsa-miR-221-5p | 1 | 6.00E-04 | 36 |
| Cow | mir-199b-5p | hsa-miR-199b-5p | 1 | 6.00E-04 | 39 |
| Cow | mir-203-3p | hsa-miR-203a-3p | 1 | 6.00E-04 | 94 |
| Cow | mir-26-1-5p | hsa-miR-26a-5p | 0.98 | 6.00E-04 | 118 |
| Cow | mir-181c-5p | hsa-miR-181c-5p | 0.99 | 6.00E-04 | 151 |
| Rat | rno-miR-134-5p | hsa-miR-134-5p | 1 | 6.00E-04 | 8 |
| Rat | rno-miR-23a-5p | hsa-miR-23a-5p | 1 | 6.00E-04 | 103 |
| Rat | rno-miR-27a-5p | hsa-miR-27a-5p | 1 | 6.00E-04 | 118 |
Supplementary Table 2.
Up-regulated lactation-related miRNAs (E-value<6.00E-04).
| Original species | miRNA | Human genome homologous | Conservation | E-value | Number of target genes |
|---|---|---|---|---|---|
| Cow | bta-mir-181a | hsa-miR-181a-5p | 0.98 | 2.00E-04 | 157 |
| Cow | mir-106-5p | hsa-miR-106a-5p | 1 | 2.00E-04 | 237 |
| Cow | mir-200c-3p | hsa-miR-200c-3p | 1 | 2.00E-04 | 157 |
| Cow | mir-20a-5p | hsa-miR-20a-5p | 1 | 2.00E-04 | 205 |
| Mouse | mir-181b-5p | hsa-miR-181b-5p | 1 | 2.00E-04 | 159 |
| Mouse | mir-181a-5p | hsa-miR-181a-5p | 1 | 2.00E-04 | 157 |
| Mouse | mir-29b-3p | hsa-miR-29b-3p | 1 | 2.00E-04 | 95 |
| Mouse | mir-200c-3p | hsa-miR-200c-3p | 1 | 2.00E-04 | 157 |
| Mouse | mmu-miR-103-3p | hsa-miR-103a-3p | 1 | 2.00E-04 | 61 |
| Cow | bta-miR-10a | hsa-miR-10a-5p | 1 | 2.00E-04 | 19 |
| Cow | bta-miR-145 | hsa-miR-145-5p | 1 | 2.00E-04 | 55 |
| Cow | bta-miR-155 | hsa-miR-155-5p | 1 | 2.00E-04 | 46 |
| Rat | rno-mir-7a-5p | hsa-miR-7-5p | 1 | 2.00E-04 | 39 |
| Rat | rno-miR-142-3p | hsa-miR-142-3p | 1 | 2.00E-04 | 32 |
| Rat | rno-miR-342-3p | hsa-miR-342-3p | 1 | 2.00E-04 | 13 |
| Rat | rno-miR-18a-5p | hsa-miR-18a-5p | 1 | 2.00E-04 | 28 |
| Cow | bta-mir-135a | hsa-miR-135a-5p | 1 | 2.00E-04 | 61 |
| Cow | bta-miR-21-5p | hsa-miR-21-5p | 0.9 | 6.00E-04 | 34 |
| Cow | bta-miR-146b | hsa-miR-146b-5p | 0.96 | 6.00E-04 | 13 |
| Cow | mir-210-3p | hsa-miR-210-3p | 1 | 6.00E-04 | 2 |
| Cow | mir-362-3p | hsa-miR-362-3p | 1 | 6.00E-04 | 262 |
| Cow | mir-148a-3p | hsa-miR-148a-3p | 1 | 6.00E-04 | 76 |
| Cow | mir-1-3p | hsa-miR-1-3p | 1 | 6.00E-04 | 76 |
| Cow | mir-375-3p | hsa-miR-375 | 1 | 6.00E-04 | 2 |
| Cow | mir-141-3p | hsa-miR-141-3p | 1 | 6.00E-04 | 84 |
| Cow | mir-92a-3p | hsa-miR-92a-3p | 1 | 6.00E-04 | 80 |
| Mouse | mir-451a-5p | hsa-mir-451a | 1 | 6.00E-04 | 3 |
| Mouse | mir-146b-5p | hsa-miR-146b-5p | 1 | 6.00E-04 | 13 |
| Mouse | mir-200a-3p | hsa-mir-200a-3p | 1 | 6.00E-04 | 131 |
| Mouse | mir-200b-3p | hsa-miR-200b-3p | 1 | 6.00E-04 | 166 |
| Mouse | mir-148a-3p | hsa-miR-148a-3p | 1 | 6.00E-04 | 76 |
| Mouse | mmu-miR-141-3p | hsa-miR-141-3p | 1 | 6.00E-04 | 84 |
| Mouse | mmu-miR-30a-5p | hsa-miR-30a-5p | 1 | 6.00E-04 | 184 |
| Mouse | miR-29a-3p | hsa-miR-29a-3p | 1 | 6.00E-04 | 97 |
| Mouse | miR-26a-5p | hsa-miR-26a-5p | 1 | 6.00E-04 | 118 |
| Mouse | miR-24-3p | hsa-miR-24-3p | 1 | 6.00E-04 | 30 |
| Mouse | miR-22-3p | hsa-miR-22-3p | 1 | 6.00E-04 | 40 |
| Mouse | miR-21-5p | hsa-miR-21-5p | 1 | 6.00E-04 | 34 |
| Mouse | miR-16-5p | hsa-miR-16-5p | 1 | 6.00E-04 | 151 |
| Mouse | let-7i-5p | hsa-let-7i-5p | 1 | 6.00E-04 | 49 |
| Mouse | let-7g-5p | hsa-let-7g-5p | 1 | 6.00E-04 | 48 |
| Mouse | let-7f-5p | hsa-let-7f-5p | 1 | 6.00E-04 | 47 |
| Mouse | let-7c-5p | hsa-let-7c-5p | 1 | 6.00E-04 | 64 |
| Mouse | let-7b-5p | hsa-let-7b-5p | 1 | 6.00E-04 | 68 |
| Mouse | let-7a-5p | hsa-let-7a-5p | 1 | 6.00E-04 | 65 |
| Cow | bta-miR-15b | hsa-miR-15b-5p | 1 | 6.00E-04 | 159 |
| Cow | bta-miR-205 | hsa-miR-205-5p | 1 | 6.00E-04 | 43 |
| Cow | bta-mir-221 | hsa-miR-221-3p | 1 | 6.00E-04 | 36 |
| Cow | bta-mir-223 | hsa-miR-223-3p | 1 | 6.00E-04 | 34 |
| Rat | rno-miR-206-3p | hsa-miR-206 | 1 | 6.00E-04 | 78 |
| Cow | bta-mir-29c | hsa-miR-29c-3p | 1 | 6.00E-04 | 113 |
Supplementary Table 3.
GO analysis based on highly expressed miRNA targets. Note that the biological process category is for lactation-related miRNA targets.
| A Biological process of down-regulated miRNA. | |||
|---|---|---|---|
| GO term | No. genes | Term P Value Corrected with Bonferroni step down | log2P |
| Positive regulation of macromolecule metabolic process | 182 | 8.32E-17 | 16.07996775 |
| Positive regulation of cellular metabolic process | 185 | 8.43E-15 | 14.07396225 |
| Nervous system development | 155 | 8.56E-15 | 14.06754556 |
| Positive regulation of macromolecule biosynthetic process | 122 | 2.44E-14 | 13.61261041 |
| Positive regulation of cellular biosynthetic process | 127 | 1.83E-13 | 12.7382415 |
| Positive regulation of biosynthetic process | 128 | 2.52E-13 | 12.59793599 |
| Positive regulation of nucleobase-containing compound metabolic process | 121 | 9.15E-13 | 12.03834877 |
| Positive regulation of gene expression | 121 | 1.27E-12 | 11.89715016 |
| Positive regulation of transcription, DNA-templated | 107 | 1.30E-12 | 11.88737081 |
| Positive regulation of nucleic acid-templated transcription | 107 | 1.30E-12 | 11.88737081 |
| Positive regulation of nitrogen compound metabolic process | 122 | 1.60E-12 | 11.79704508 |
| Positive regulation of RNA metabolic process | 109 | 4.62E-12 | 11.33535686 |
| Positive regulation of RNA biosynthetic process | 107 | 5.30E-12 | 11.27612632 |
| Regulation of macromolecule biosynthetic process | 227 | 1.07E-11 | 10.97066187 |
| Regulation of nucleobase-containing compound metabolic process | 227 | 1.83E-11 | 10.73639878 |
| B Biological process of up-regulated miRNA. | |||
|---|---|---|---|
| GO Term | No. genes | Term P Value Corrected with Bonferroni step down | log2P |
| Multicellular organismal development | 353 | 5.03E-20 | 19.29808555 |
| Regulation of macromolecule metabolic process | 396 | 6.22E-20 | 19.20595298 |
| Negative regulation of biological process | 334 | 6.74E-20 | 19.17123925 |
| Positive regulation of macromolecule metabolic process | 229 | 4.55E-19 | 18.34163176 |
| Regulation of metabolic process | 446 | 1.54E-18 | 17.81317793 |
| Negative regulation of cellular process | 310 | 1.59E-18 | 17.79753238 |
| System development | 315 | 1.02E-17 | 16.99352731 |
| Regulation of cellular metabolic process | 402 | 1.69E-17 | 16.7718758 |
| Regulation of macromolecule biosynthetic process | 302 | 2.86E-17 | 16.54383494 |
| Regulation of primary metabolic process | 388 | 3.66E-17 | 16.43665168 |
| Regulation of cellular macromolecule biosynthetic process | 294 | 1.13E-16 | 15.94714185 |
| Positive regulation of metabolic process | 271 | 1.37E-16 | 15.86279658 |
| Positive regulation of macromolecule biosynthetic process | 153 | 1.60E-16 | 15.79720478 |
| Regulation of biosynthetic process | 312 | 2.16E-16 | 15.66486045 |
| Regulation of cellular biosynthetic process | 309 | 4.09E-16 | 15.38825063 |
Supplementary Table 4.
KEGG pathway analysis based on highly expressed miRNA targets. Note that KEGG pathway enrichment is for lactation-related miRNA targets.
| A Pathway of down-regulated miRNA target. | |||
|---|---|---|---|
| GOID | GO term | No. genes | Term P Value Corrected with Bonferroni step down |
| KEGG: 04320 | Dorso-ventral axis formation | 10 | 2.15E-06 |
| KEGG: 04010 | MAPK signaling pathway | 26 | 2.28E-04 |
| KEGG: 05206 | MicroRNAs in cancer | 28 | 3.62E-04 |
| KEGG: 05214 | Glioma | 11 | 0.002349762 |
| KEGG: 05223 | Non-small cell lung cancer | 10 | 0.003465807 |
| KEGG: 04022 | cGMP-PKG signaling pathway | 18 | 0.0039885 |
| KEGG: 05205 | Proteoglycans in cancer | 20 | 0.005731077 |
| KEGG: 04012 | ErbB signaling pathway | 12 | 0.007906834 |
| KEGG: 05231 | Choline metabolism in cancer | 13 | 0.008142061 |
| KEGG: 05161 | Hepatitis B | 16 | 0.008760234 |
| KEGG: 04919 | Thyroid hormone signaling pathway | 14 | 0.011458475 |
| KEGG: 05230 | Central carbon metabolism in cancer | 10 | 0.016058162 |
| KEGG: 05200 | Pathways in cancer | 29 | 0.027849671 |
| KEGG: 05202 | Transcriptional misregulation in cancer | 17 | 0.029870001 |
| KEGG: 04150 | mTOR signaling pathway | 9 | 0.03240035 |
| KEGG: 05220 | Chronic myeloid leukemia | 10 | 0.032404467 |
| KEGG: 04930 | Type II diabetes mellitus | 8 | 0.033460878 |
| KEGG: 04961 | Endocrine and other factor-regulated calcium reabsorption | 8 | 0.033460878 |
| B Pathway of up-regulated miRNA target. | |||
|---|---|---|---|
| GOID | GO term | No. genes | Term P Value Corrected with Benjamini-Hochberg |
| KEGG: 04919 | Thyroid hormone signaling pathway | 19 | 5.93E-04 |
| KEGG: 05206 | MicroRNAs in cancer | 33 | 6.34E-04 |
| KEGG: 05205 | Proteoglycans in cancer | 26 | 6.38E-04 |
| KEGG: 04510 | Focal adhesion | 27 | 8.36E-04 |
| KEGG: 04360 | Axon guidance | 18 | 0.002137231 |
| KEGG: 04010 | MAPK signaling pathway | 28 | 0.002293612 |
| KEGG: 05202 | Transcriptional misregulation in cancer | 22 | 0.002612816 |
| KEGG: 05218 | Melanoma | 12 | 0.004135083 |
| KEGG: 04550 | Signaling pathways regulating pluripotency of stem cells | 18 | 0.004544666 |
| KEGG: 05211 | Renal cell carcinoma | 11 | 0.004565614 |
| KEGG: 05214 | Glioma | 11 | 0.004595295 |
| KEGG: 04012 | ErbB signaling pathway | 13 | 0.004598649 |
| KEGG: 05222 | Small cell lung cancer | 13 | 0.004778807 |
| KEGG: 05220 | Chronic myeloid leukemia | 12 | 0.004814658 |
| KEGG: 04151 | PI3K-Akt signaling pathway | 33 | 0.004894532 |
| KEGG: 04152 | AMPK signaling pathway | 16 | 0.005007425 |
| KEGG: 04810 | Regulation of actin cytoskeleton | 23 | 0.005099651 |
| KEGG: 04350 | TGF-beta signaling pathway | 12 | 0.005719938 |
| KEGG: 04710 | Circadian rhythm | 7 | 0.006234951 |
| KEGG: 04068 | FoxO signaling pathway | 16 | 0.009418922 |
Supplementary Table 5.
miRNA expression profile of umbilical cord blood microvesicles.
| miRNAs in microvesicles | |||||
|---|---|---|---|---|---|
| miR-369-3p | miR-29a* | miR-499-5p | miR-325 | miR-149 | miR-202 |
| miR-454 | miR-122* | miR-126-5p | miR-455-5p | miR-421 | miR-497 |
| miR-2861 | miR-455-3p | let-7g | miR-499-3p | miR-196a | miR-497* |
| miR-376c | miR-124-3p | miR-122 | miR-20a* | miR-141 | miR-410 |
| miR-202* | miR-98 | miR-28-5p | miR-32 | miR-181d | miR-26a-2* |
| miR-376a | miR-218 | miR-409-3p | miR-335-3p | miR-200a | let-7i |
| miR-212-3p | miR-219-5p | miR-29b | miR-340-5p | miR-30e | miR-132 |
| miR-135b | miR-493* | miR-135b* | miR-380-3p | miR-494 | miR-206 |
| miR-16 | miR-411 | miR-23b | miR-146b | miR-30c | miR-26a-1* |
| miR-181b | miR-425 | miR-26b* | miR-15a | miR-495 | miR-186 |
| miR-199a-3p | miR-145-5p | miR-27b | miR-16-1* | miR-141* | miR-339-5p |
| miR-20a | let-7c | miR-30a* | miR-200b | miR-148a | miR-483-3p |
| miR-297a | miR-143 | miR-34c | miR-204 | miR-195 | let-7f |
| miR-337-3p | miR-194 | miR-451 | miR-21 | miR-200c | miR-106a |
| miR-369-5p | miR-22 | miR-96 | miR-29c | miR-29a | miR-106b |
| miR-379 | miR-23a | miR-126-3p | miR-30b | miR-148b* | miR-10a |
| miR-331-3p | miR-195* | miR-191 | miR-671-3p | miR-150 | miR-150* |
| miR-381 | miR-129-3p | miR-9* | miR-23b* | miR-18a* | miR-296-5p |
| let-7e | miR-449a | miR-15b* | miR-24 | miR-324-3p | miR-214 |
| miR-20b | miR-187 | miR-100 | miR-346 | miR-674 | miR-34c* |
| miR-92a | miR-199a-5p | miR-19a | miR-326 | miR-877 | miR-93* |
| miR-99b | miR-200b* | miR-30a | miR-210 | miR-99b* | miR-27a* |
| miR-504 | miR-34a | miR-375 | let-7i* | miR-674* | miR-324-5p |
| miR-129-5p | miR-501-5p | miR-377 | miR-501-3p | miR-106b* | miR-92b |
| miR-520g | miR-625 | miR-99a | miR-584 | miR-658 | miR-508-3p |
| miR-376b | miR-422a | miR-27a | miR-875-3p | miR-659 | miR-33b* |
| miR-586 | miR-432 | miR-27b* | miR-155 | miR-769-3p | miR-505-3p |
| miR-147 | miR-18b | miR-29b-1* | miR-523 | miR-527 | miR-139-5p |
| miR-130a | miR-142-5p | miR-338-5p | miR-25 | miR-301a | miR-302a |
| miR-152 | miR-138 | miR-363-3p | miR-196b | miR-30d | miR-30e* |
| has-RNU24 | miR-450a-5p | miR-378 | miR-208b | miR-31* | miR-302d |
| miR-183 | miR-875-5p | miR-382 | miR-223 | miR-335-5p | miR-338-3p |
| miR-328 | miR-151-5p | miR-103 | miR-26b | miR-365 | miR-331-5p |
| miR-21* | miR-412 | miR-493 | miR-31 | miR-496 | let-7a |
| miR-508-5p | miR-199a/b-3p | miR-1281 | miR-340-3p | miR-181a | let-7a-1* |
| miR-15a* | miR-107 | miR-628-5p | miR-9 | miR-181c | miR-144 |
| miR-193b | miR-140 | miR-616-5p | let-7b | miR-17 | miR-10b |
| has-24 | miR-15b | miR-33b | miR-1197 | miR-19b | miR-130b |
| miR-191* | miR-24-2* | miR-616-3p | miR-148a* | miR-200a* | miR-148b |
| miR-572 | miR-26a | miR-628-3p | miR-154 | miR-222 | miR-93 |
| miR-146b* | miR-342-3p | miR-520b | miR-183* | miR-29c* | miR-142-3p |
| miR-371-3p | miR-380-5p | miR-422b | miR-18a | miR-342-5p | let-7d |
| miR-519a* | miR-146a | miR-95 | miR-221 | miR-409-5p | miR-125a-5p |
| miR-711 | miR-429 | miR-602 | miR-24-1* | miR-425* | miR-125b-5p |
| miR-518f | miR-769-5p | miR-387 | has-miR-298 | miR-671-5p | miR-125b-3p |
| miR-28-3p | miR-219-3p | miR-200c* | has-miR-762 | miR-139-3p | miR-139-5p |
| miR-623 | miR-483-5p | let-7g* | has-miR-675-5p | miR-423-5p | has-miR-453 |
| miR-106a* | miR-583 | miR-185 | miR-770-5p | has-miR-663 | miR-615-3p |
| miR-450b-5p | miR-758 | miR-214* | miR-557 | miR-423-3p | miR-370 |
| miR-452-5p | miR-301b | miR-22* | miR-372 | has-miR-718 | miR-296-3p |
| miR-449b | miR-16-2* | miR-433 | miR-512-5p | miR-323-5p | miR-23a* |
| miR-498 | miR-524-5p | miR-182 | miR-337-5p | miR-373* | miR-125a-3p |
| miR-612 | miR-199b-5p | miR-151-3p | miR-524-3p | miR-30b* | miR-675b |
| miR-1469 | miR-424* | miR-489 | miR-526a | miR-329 | miR-198 |
| miR-503 | miR-339-3p | miR-345-5p | miR-17* | miR-638 | miR-373 |
| miR-23*/miR-23 | miR-112 | miR-20b* | miR-25 | miR-176 | miR-235 |
Lactation-related miRNAs are noted in bold.
Acknowledgements
We thank Da-xue Zhou and Ling-yun Zou for assistance with miRNA detection and analysis.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethics standards. The study was approved by the Ethics Review Committee on Human Research of the Chongqing Medical University (Reference Number: 2015004).
Source of support: This study was supported by the National Natural Science Fund of the Chinese National Science Foundation (No. 31571453, 81127901, 11574039, and 11274404) and the National Basic Research Program of China (No. 2011CB707900)
References
- 1.Ochiya T, Lotvall J. Exosome as a novel shuttle for innovation. Preface. Adv Drug Deliv Rev. 2013;65(3):v. doi: 10.1016/S0169-409X(13)00041-0. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–33. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 4.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One. 2011;6:e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleys ER, Halleran JL, McWhorter E, et al. Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep. Mol Reprod Dev. 2014;81:983–93. doi: 10.1002/mrd.22420. [DOI] [PubMed] [Google Scholar]
- 6.Gigli I, Maizon DO. microRNAs and the mammary gland: A new understanding of gene expression. Genet Mol Biol. 2013;36:465–74. doi: 10.1590/S1415-47572013005000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ucar A, Vafaizadeh V, Jarry H, et al. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet. 2010;42:1101–8. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 8.Li HM, Wang CM, Li QZ, Gao XJ. MiR-15a decreases bovine mammary epithelial cell viability and lactation and regulates growth hormone receptor expression. Molecules. 2012;17:12037–48. doi: 10.3390/molecules171012037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JA, Richer JK, Goodall GJ. microRNAs and EMT in mammary cells and breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:213–23. doi: 10.1007/s10911-010-9183-z. [DOI] [PubMed] [Google Scholar]
- 10.Nazarenko I, Rupp AK, Altevogt P. Exosomes as a potential tool for a specific delivery of functional molecules. Methods Mol Biol. 2013;1049:495–511. doi: 10.1007/978-1-62703-547-7_37. [DOI] [PubMed] [Google Scholar]
- 11.Thery C, Amigorena A, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 12.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–59. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Desrochers LM, Bordeleau F, Reinhart-King CA, et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958. doi: 10.1038/ncomms11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong M, Chamley LW. Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb Perspect Med. 2015;5:a023028. doi: 10.1101/cshperspect.a023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns G, Brooks K, Wildung M, et al. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 2014;9:e90913. doi: 10.1371/journal.pone.0090913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng YH, Rome S, Jalabert A, et al. Salamonsen, endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013;8:e58502. doi: 10.1371/journal.pone.0058502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191:5515–23. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- 19.Burgos SA, Cant JP. IGF-1 stimulates protein synthesis by enhanced signaling through mTORC1 in bovine mammary epithelial cells. Domest Anim Endocrinol. 2010;38:211–21. doi: 10.1016/j.domaniend.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Burgos SA, Dai M, Cant JP. Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J Dairy Sci. 2010;93:153–61. doi: 10.3168/jds.2009-2444. [DOI] [PubMed] [Google Scholar]
- 21.Toerien CA, Cant JP. Abundance and phosphorylation state of translation initiation factors in mammary glands of lactating and nonlactating dairy cows. J Dairy Sci. 2007;90:2726–34. doi: 10.3168/jds.2006-778. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Liu H, Jin X, et al. Expression profiles of microRNAs from lactating and non-lactating bovine mammary glands and identification of miRNA related to lactation. BMC Genomics. 2012;13:731. doi: 10.1186/1471-2164-13-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bionaz M, Loor JJ. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform Biol Insights. 2011;5:83–98. doi: 10.4137/BBI.S7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Haneda S, Imakawa K, et al. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation. 2009;77:181–87. doi: 10.1016/j.diff.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Cui W, Li Q, Feng L, Ding W. MiR-126-3p regulates progesterone receptors and involves development and lactation of mouse mammary gland. Mol Cell Biochem. 2011;355:17–25. doi: 10.1007/s11010-011-0834-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Down-regulated lactation-related miRNAs (E-value <6.00E-04).
| Original species | miRNA | Human genome homologous | Conservation | E-value | Number of target genes |
|---|---|---|---|---|---|
| Cow | mir-181d-5p | hsa-miR-181d-5p | 1 | 2.00E-04 | 222 |
| Cow | mir-574-3p | hsa-miR-574-3p | 1 | 6.00E-04 | 6 |
| Cow | mir-107-3p | hsa-miR-107 | 1 | 6.00E-04 | 53 |
| Cow | bta-let-7c | hsa-let-7c-5p | 1 | 6.00E-04 | 64 |
| Cow | mir-10a-5p | hsa-miR-10a-5p | 1 | 6.00E-04 | 19 |
| Cow | mir-100-5p | hsa-miR-100-5p | 1 | 6.00E-04 | 4 |
| Cow | mir-199a-3p | hsa-miR-199a-3p | 1 | 6.00E-04 | 39 |
| Cow | mir-221-3p | hsa-miR-221-3p | 1 | 6.00E-04 | 36 |
| Cow | mir-205-5p | hsa-miR-205-5p | 1 | 6.00E-04 | 43 |
| Cow | mir-146b-5p | hsa-miR-146b-5p | 1 | 6.00E-04 | 13 |
| Cow | mir-327-5p | hsa-miR-221-5p | 1 | 6.00E-04 | 36 |
| Cow | mir-199b-5p | hsa-miR-199b-5p | 1 | 6.00E-04 | 39 |
| Cow | mir-203-3p | hsa-miR-203a-3p | 1 | 6.00E-04 | 94 |
| Cow | mir-26-1-5p | hsa-miR-26a-5p | 0.98 | 6.00E-04 | 118 |
| Cow | mir-181c-5p | hsa-miR-181c-5p | 0.99 | 6.00E-04 | 151 |
| Rat | rno-miR-134-5p | hsa-miR-134-5p | 1 | 6.00E-04 | 8 |
| Rat | rno-miR-23a-5p | hsa-miR-23a-5p | 1 | 6.00E-04 | 103 |
| Rat | rno-miR-27a-5p | hsa-miR-27a-5p | 1 | 6.00E-04 | 118 |
Supplementary Table 2.
Up-regulated lactation-related miRNAs (E-value<6.00E-04).
| Original species | miRNA | Human genome homologous | Conservation | E-value | Number of target genes |
|---|---|---|---|---|---|
| Cow | bta-mir-181a | hsa-miR-181a-5p | 0.98 | 2.00E-04 | 157 |
| Cow | mir-106-5p | hsa-miR-106a-5p | 1 | 2.00E-04 | 237 |
| Cow | mir-200c-3p | hsa-miR-200c-3p | 1 | 2.00E-04 | 157 |
| Cow | mir-20a-5p | hsa-miR-20a-5p | 1 | 2.00E-04 | 205 |
| Mouse | mir-181b-5p | hsa-miR-181b-5p | 1 | 2.00E-04 | 159 |
| Mouse | mir-181a-5p | hsa-miR-181a-5p | 1 | 2.00E-04 | 157 |
| Mouse | mir-29b-3p | hsa-miR-29b-3p | 1 | 2.00E-04 | 95 |
| Mouse | mir-200c-3p | hsa-miR-200c-3p | 1 | 2.00E-04 | 157 |
| Mouse | mmu-miR-103-3p | hsa-miR-103a-3p | 1 | 2.00E-04 | 61 |
| Cow | bta-miR-10a | hsa-miR-10a-5p | 1 | 2.00E-04 | 19 |
| Cow | bta-miR-145 | hsa-miR-145-5p | 1 | 2.00E-04 | 55 |
| Cow | bta-miR-155 | hsa-miR-155-5p | 1 | 2.00E-04 | 46 |
| Rat | rno-mir-7a-5p | hsa-miR-7-5p | 1 | 2.00E-04 | 39 |
| Rat | rno-miR-142-3p | hsa-miR-142-3p | 1 | 2.00E-04 | 32 |
| Rat | rno-miR-342-3p | hsa-miR-342-3p | 1 | 2.00E-04 | 13 |
| Rat | rno-miR-18a-5p | hsa-miR-18a-5p | 1 | 2.00E-04 | 28 |
| Cow | bta-mir-135a | hsa-miR-135a-5p | 1 | 2.00E-04 | 61 |
| Cow | bta-miR-21-5p | hsa-miR-21-5p | 0.9 | 6.00E-04 | 34 |
| Cow | bta-miR-146b | hsa-miR-146b-5p | 0.96 | 6.00E-04 | 13 |
| Cow | mir-210-3p | hsa-miR-210-3p | 1 | 6.00E-04 | 2 |
| Cow | mir-362-3p | hsa-miR-362-3p | 1 | 6.00E-04 | 262 |
| Cow | mir-148a-3p | hsa-miR-148a-3p | 1 | 6.00E-04 | 76 |
| Cow | mir-1-3p | hsa-miR-1-3p | 1 | 6.00E-04 | 76 |
| Cow | mir-375-3p | hsa-miR-375 | 1 | 6.00E-04 | 2 |
| Cow | mir-141-3p | hsa-miR-141-3p | 1 | 6.00E-04 | 84 |
| Cow | mir-92a-3p | hsa-miR-92a-3p | 1 | 6.00E-04 | 80 |
| Mouse | mir-451a-5p | hsa-mir-451a | 1 | 6.00E-04 | 3 |
| Mouse | mir-146b-5p | hsa-miR-146b-5p | 1 | 6.00E-04 | 13 |
| Mouse | mir-200a-3p | hsa-mir-200a-3p | 1 | 6.00E-04 | 131 |
| Mouse | mir-200b-3p | hsa-miR-200b-3p | 1 | 6.00E-04 | 166 |
| Mouse | mir-148a-3p | hsa-miR-148a-3p | 1 | 6.00E-04 | 76 |
| Mouse | mmu-miR-141-3p | hsa-miR-141-3p | 1 | 6.00E-04 | 84 |
| Mouse | mmu-miR-30a-5p | hsa-miR-30a-5p | 1 | 6.00E-04 | 184 |
| Mouse | miR-29a-3p | hsa-miR-29a-3p | 1 | 6.00E-04 | 97 |
| Mouse | miR-26a-5p | hsa-miR-26a-5p | 1 | 6.00E-04 | 118 |
| Mouse | miR-24-3p | hsa-miR-24-3p | 1 | 6.00E-04 | 30 |
| Mouse | miR-22-3p | hsa-miR-22-3p | 1 | 6.00E-04 | 40 |
| Mouse | miR-21-5p | hsa-miR-21-5p | 1 | 6.00E-04 | 34 |
| Mouse | miR-16-5p | hsa-miR-16-5p | 1 | 6.00E-04 | 151 |
| Mouse | let-7i-5p | hsa-let-7i-5p | 1 | 6.00E-04 | 49 |
| Mouse | let-7g-5p | hsa-let-7g-5p | 1 | 6.00E-04 | 48 |
| Mouse | let-7f-5p | hsa-let-7f-5p | 1 | 6.00E-04 | 47 |
| Mouse | let-7c-5p | hsa-let-7c-5p | 1 | 6.00E-04 | 64 |
| Mouse | let-7b-5p | hsa-let-7b-5p | 1 | 6.00E-04 | 68 |
| Mouse | let-7a-5p | hsa-let-7a-5p | 1 | 6.00E-04 | 65 |
| Cow | bta-miR-15b | hsa-miR-15b-5p | 1 | 6.00E-04 | 159 |
| Cow | bta-miR-205 | hsa-miR-205-5p | 1 | 6.00E-04 | 43 |
| Cow | bta-mir-221 | hsa-miR-221-3p | 1 | 6.00E-04 | 36 |
| Cow | bta-mir-223 | hsa-miR-223-3p | 1 | 6.00E-04 | 34 |
| Rat | rno-miR-206-3p | hsa-miR-206 | 1 | 6.00E-04 | 78 |
| Cow | bta-mir-29c | hsa-miR-29c-3p | 1 | 6.00E-04 | 113 |
Supplementary Table 3.
GO analysis based on highly expressed miRNA targets. Note that the biological process category is for lactation-related miRNA targets.
| A Biological process of down-regulated miRNA. | |||
|---|---|---|---|
| GO term | No. genes | Term P Value Corrected with Bonferroni step down | log2P |
| Positive regulation of macromolecule metabolic process | 182 | 8.32E-17 | 16.07996775 |
| Positive regulation of cellular metabolic process | 185 | 8.43E-15 | 14.07396225 |
| Nervous system development | 155 | 8.56E-15 | 14.06754556 |
| Positive regulation of macromolecule biosynthetic process | 122 | 2.44E-14 | 13.61261041 |
| Positive regulation of cellular biosynthetic process | 127 | 1.83E-13 | 12.7382415 |
| Positive regulation of biosynthetic process | 128 | 2.52E-13 | 12.59793599 |
| Positive regulation of nucleobase-containing compound metabolic process | 121 | 9.15E-13 | 12.03834877 |
| Positive regulation of gene expression | 121 | 1.27E-12 | 11.89715016 |
| Positive regulation of transcription, DNA-templated | 107 | 1.30E-12 | 11.88737081 |
| Positive regulation of nucleic acid-templated transcription | 107 | 1.30E-12 | 11.88737081 |
| Positive regulation of nitrogen compound metabolic process | 122 | 1.60E-12 | 11.79704508 |
| Positive regulation of RNA metabolic process | 109 | 4.62E-12 | 11.33535686 |
| Positive regulation of RNA biosynthetic process | 107 | 5.30E-12 | 11.27612632 |
| Regulation of macromolecule biosynthetic process | 227 | 1.07E-11 | 10.97066187 |
| Regulation of nucleobase-containing compound metabolic process | 227 | 1.83E-11 | 10.73639878 |
| B Biological process of up-regulated miRNA. | |||
|---|---|---|---|
| GO Term | No. genes | Term P Value Corrected with Bonferroni step down | log2P |
| Multicellular organismal development | 353 | 5.03E-20 | 19.29808555 |
| Regulation of macromolecule metabolic process | 396 | 6.22E-20 | 19.20595298 |
| Negative regulation of biological process | 334 | 6.74E-20 | 19.17123925 |
| Positive regulation of macromolecule metabolic process | 229 | 4.55E-19 | 18.34163176 |
| Regulation of metabolic process | 446 | 1.54E-18 | 17.81317793 |
| Negative regulation of cellular process | 310 | 1.59E-18 | 17.79753238 |
| System development | 315 | 1.02E-17 | 16.99352731 |
| Regulation of cellular metabolic process | 402 | 1.69E-17 | 16.7718758 |
| Regulation of macromolecule biosynthetic process | 302 | 2.86E-17 | 16.54383494 |
| Regulation of primary metabolic process | 388 | 3.66E-17 | 16.43665168 |
| Regulation of cellular macromolecule biosynthetic process | 294 | 1.13E-16 | 15.94714185 |
| Positive regulation of metabolic process | 271 | 1.37E-16 | 15.86279658 |
| Positive regulation of macromolecule biosynthetic process | 153 | 1.60E-16 | 15.79720478 |
| Regulation of biosynthetic process | 312 | 2.16E-16 | 15.66486045 |
| Regulation of cellular biosynthetic process | 309 | 4.09E-16 | 15.38825063 |
Supplementary Table 4.
KEGG pathway analysis based on highly expressed miRNA targets. Note that KEGG pathway enrichment is for lactation-related miRNA targets.
| A Pathway of down-regulated miRNA target. | |||
|---|---|---|---|
| GOID | GO term | No. genes | Term P Value Corrected with Bonferroni step down |
| KEGG: 04320 | Dorso-ventral axis formation | 10 | 2.15E-06 |
| KEGG: 04010 | MAPK signaling pathway | 26 | 2.28E-04 |
| KEGG: 05206 | MicroRNAs in cancer | 28 | 3.62E-04 |
| KEGG: 05214 | Glioma | 11 | 0.002349762 |
| KEGG: 05223 | Non-small cell lung cancer | 10 | 0.003465807 |
| KEGG: 04022 | cGMP-PKG signaling pathway | 18 | 0.0039885 |
| KEGG: 05205 | Proteoglycans in cancer | 20 | 0.005731077 |
| KEGG: 04012 | ErbB signaling pathway | 12 | 0.007906834 |
| KEGG: 05231 | Choline metabolism in cancer | 13 | 0.008142061 |
| KEGG: 05161 | Hepatitis B | 16 | 0.008760234 |
| KEGG: 04919 | Thyroid hormone signaling pathway | 14 | 0.011458475 |
| KEGG: 05230 | Central carbon metabolism in cancer | 10 | 0.016058162 |
| KEGG: 05200 | Pathways in cancer | 29 | 0.027849671 |
| KEGG: 05202 | Transcriptional misregulation in cancer | 17 | 0.029870001 |
| KEGG: 04150 | mTOR signaling pathway | 9 | 0.03240035 |
| KEGG: 05220 | Chronic myeloid leukemia | 10 | 0.032404467 |
| KEGG: 04930 | Type II diabetes mellitus | 8 | 0.033460878 |
| KEGG: 04961 | Endocrine and other factor-regulated calcium reabsorption | 8 | 0.033460878 |
| B Pathway of up-regulated miRNA target. | |||
|---|---|---|---|
| GOID | GO term | No. genes | Term P Value Corrected with Benjamini-Hochberg |
| KEGG: 04919 | Thyroid hormone signaling pathway | 19 | 5.93E-04 |
| KEGG: 05206 | MicroRNAs in cancer | 33 | 6.34E-04 |
| KEGG: 05205 | Proteoglycans in cancer | 26 | 6.38E-04 |
| KEGG: 04510 | Focal adhesion | 27 | 8.36E-04 |
| KEGG: 04360 | Axon guidance | 18 | 0.002137231 |
| KEGG: 04010 | MAPK signaling pathway | 28 | 0.002293612 |
| KEGG: 05202 | Transcriptional misregulation in cancer | 22 | 0.002612816 |
| KEGG: 05218 | Melanoma | 12 | 0.004135083 |
| KEGG: 04550 | Signaling pathways regulating pluripotency of stem cells | 18 | 0.004544666 |
| KEGG: 05211 | Renal cell carcinoma | 11 | 0.004565614 |
| KEGG: 05214 | Glioma | 11 | 0.004595295 |
| KEGG: 04012 | ErbB signaling pathway | 13 | 0.004598649 |
| KEGG: 05222 | Small cell lung cancer | 13 | 0.004778807 |
| KEGG: 05220 | Chronic myeloid leukemia | 12 | 0.004814658 |
| KEGG: 04151 | PI3K-Akt signaling pathway | 33 | 0.004894532 |
| KEGG: 04152 | AMPK signaling pathway | 16 | 0.005007425 |
| KEGG: 04810 | Regulation of actin cytoskeleton | 23 | 0.005099651 |
| KEGG: 04350 | TGF-beta signaling pathway | 12 | 0.005719938 |
| KEGG: 04710 | Circadian rhythm | 7 | 0.006234951 |
| KEGG: 04068 | FoxO signaling pathway | 16 | 0.009418922 |
Supplementary Table 5.
miRNA expression profile of umbilical cord blood microvesicles.
| miRNAs in microvesicles | |||||
|---|---|---|---|---|---|
| miR-369-3p | miR-29a* | miR-499-5p | miR-325 | miR-149 | miR-202 |
| miR-454 | miR-122* | miR-126-5p | miR-455-5p | miR-421 | miR-497 |
| miR-2861 | miR-455-3p | let-7g | miR-499-3p | miR-196a | miR-497* |
| miR-376c | miR-124-3p | miR-122 | miR-20a* | miR-141 | miR-410 |
| miR-202* | miR-98 | miR-28-5p | miR-32 | miR-181d | miR-26a-2* |
| miR-376a | miR-218 | miR-409-3p | miR-335-3p | miR-200a | let-7i |
| miR-212-3p | miR-219-5p | miR-29b | miR-340-5p | miR-30e | miR-132 |
| miR-135b | miR-493* | miR-135b* | miR-380-3p | miR-494 | miR-206 |
| miR-16 | miR-411 | miR-23b | miR-146b | miR-30c | miR-26a-1* |
| miR-181b | miR-425 | miR-26b* | miR-15a | miR-495 | miR-186 |
| miR-199a-3p | miR-145-5p | miR-27b | miR-16-1* | miR-141* | miR-339-5p |
| miR-20a | let-7c | miR-30a* | miR-200b | miR-148a | miR-483-3p |
| miR-297a | miR-143 | miR-34c | miR-204 | miR-195 | let-7f |
| miR-337-3p | miR-194 | miR-451 | miR-21 | miR-200c | miR-106a |
| miR-369-5p | miR-22 | miR-96 | miR-29c | miR-29a | miR-106b |
| miR-379 | miR-23a | miR-126-3p | miR-30b | miR-148b* | miR-10a |
| miR-331-3p | miR-195* | miR-191 | miR-671-3p | miR-150 | miR-150* |
| miR-381 | miR-129-3p | miR-9* | miR-23b* | miR-18a* | miR-296-5p |
| let-7e | miR-449a | miR-15b* | miR-24 | miR-324-3p | miR-214 |
| miR-20b | miR-187 | miR-100 | miR-346 | miR-674 | miR-34c* |
| miR-92a | miR-199a-5p | miR-19a | miR-326 | miR-877 | miR-93* |
| miR-99b | miR-200b* | miR-30a | miR-210 | miR-99b* | miR-27a* |
| miR-504 | miR-34a | miR-375 | let-7i* | miR-674* | miR-324-5p |
| miR-129-5p | miR-501-5p | miR-377 | miR-501-3p | miR-106b* | miR-92b |
| miR-520g | miR-625 | miR-99a | miR-584 | miR-658 | miR-508-3p |
| miR-376b | miR-422a | miR-27a | miR-875-3p | miR-659 | miR-33b* |
| miR-586 | miR-432 | miR-27b* | miR-155 | miR-769-3p | miR-505-3p |
| miR-147 | miR-18b | miR-29b-1* | miR-523 | miR-527 | miR-139-5p |
| miR-130a | miR-142-5p | miR-338-5p | miR-25 | miR-301a | miR-302a |
| miR-152 | miR-138 | miR-363-3p | miR-196b | miR-30d | miR-30e* |
| has-RNU24 | miR-450a-5p | miR-378 | miR-208b | miR-31* | miR-302d |
| miR-183 | miR-875-5p | miR-382 | miR-223 | miR-335-5p | miR-338-3p |
| miR-328 | miR-151-5p | miR-103 | miR-26b | miR-365 | miR-331-5p |
| miR-21* | miR-412 | miR-493 | miR-31 | miR-496 | let-7a |
| miR-508-5p | miR-199a/b-3p | miR-1281 | miR-340-3p | miR-181a | let-7a-1* |
| miR-15a* | miR-107 | miR-628-5p | miR-9 | miR-181c | miR-144 |
| miR-193b | miR-140 | miR-616-5p | let-7b | miR-17 | miR-10b |
| has-24 | miR-15b | miR-33b | miR-1197 | miR-19b | miR-130b |
| miR-191* | miR-24-2* | miR-616-3p | miR-148a* | miR-200a* | miR-148b |
| miR-572 | miR-26a | miR-628-3p | miR-154 | miR-222 | miR-93 |
| miR-146b* | miR-342-3p | miR-520b | miR-183* | miR-29c* | miR-142-3p |
| miR-371-3p | miR-380-5p | miR-422b | miR-18a | miR-342-5p | let-7d |
| miR-519a* | miR-146a | miR-95 | miR-221 | miR-409-5p | miR-125a-5p |
| miR-711 | miR-429 | miR-602 | miR-24-1* | miR-425* | miR-125b-5p |
| miR-518f | miR-769-5p | miR-387 | has-miR-298 | miR-671-5p | miR-125b-3p |
| miR-28-3p | miR-219-3p | miR-200c* | has-miR-762 | miR-139-3p | miR-139-5p |
| miR-623 | miR-483-5p | let-7g* | has-miR-675-5p | miR-423-5p | has-miR-453 |
| miR-106a* | miR-583 | miR-185 | miR-770-5p | has-miR-663 | miR-615-3p |
| miR-450b-5p | miR-758 | miR-214* | miR-557 | miR-423-3p | miR-370 |
| miR-452-5p | miR-301b | miR-22* | miR-372 | has-miR-718 | miR-296-3p |
| miR-449b | miR-16-2* | miR-433 | miR-512-5p | miR-323-5p | miR-23a* |
| miR-498 | miR-524-5p | miR-182 | miR-337-5p | miR-373* | miR-125a-3p |
| miR-612 | miR-199b-5p | miR-151-3p | miR-524-3p | miR-30b* | miR-675b |
| miR-1469 | miR-424* | miR-489 | miR-526a | miR-329 | miR-198 |
| miR-503 | miR-339-3p | miR-345-5p | miR-17* | miR-638 | miR-373 |
| miR-23*/miR-23 | miR-112 | miR-20b* | miR-25 | miR-176 | miR-235 |
Lactation-related miRNAs are noted in bold.