To the Editor
Urinary lipid bilayer–bound extracellular vesicles (EVs) are released from all nephron segments under both physiologic and pathologic conditions1,2 and carry cytoplasmic protein, lipids, and microRNAs (miRs). Accumulating evidence implicates miRs in kidney injury and BP control,3–6 but whether miR levels are altered in kidneys exposed to hypertension of different causes remains unclear. We tested the hypothesis that urinary EVs show different expression of selected miRs in essential (EH) and renovascular hypertension (RVH) and in healthy volunteers (HVs).
This HIPAA-compliant study was institutional review board– approved, and written informed consent was obtained. We prospectively recruited 30 hypertensive adults diagnosed with EH (n = 15) or RVH (n = 15). Data and samples were obtained from the Mayo Biobank for age- and BMI-matched HVs without diabetes, hypertension, or drug treatment. miR-21, -92a, -93, -126, -148, -192, -200b, -377, and -433, known for regulation of renal fibrosis and angiogenesis,3,6–9 were quantified from total RNA extracted from EVs isolated from urine from 24-hour collections in hypertensive patients and spot samples in HVs (detailed methods in Item S1). We used analysis of variance, χ2 test, or Fisher exact test to compare groups. Analysis of covariance (to adjust miR levels by clinical parameters) and partial correlation analysis (for comparison between miRs and clinical values) were performed with JMP 10.0. Pairwise comparisons were only performed for overall P ≤ 0.05.
Table 1 shows group characteristics. EH miR-21, -93, and -200b levels were lower than in RVH and HVs; EH miR-92a, -192, and -433 levels were only lower than in HVs. After adjustments for eGFR, systolic BP, triglycerides, HDL, and albuminuria, EH miR-21, -93, and -200b remained lower than in HVs and RVH (Fig 1). Levels of the other tested miRs were not different among groups (fig a of Item S1).
Table 1.
Demographic, Clinical, and Laboratory Data
| HV (n = 15) | EH (n = 15) | RVH (n = 15) | P | |
|---|---|---|---|---|
| Age, y | 71.9 ± 5.7 | 67.0 ± 7.4 | 69.2 ± 7.4 | 0.1 for trend |
| Men | 5 (33) | 9 (60) | 10 (67) | 0.1 for trend |
| BMI, kg/m2 | 26.2 ± 6.2 | 28.5 ± 3.6 | 27.8 ± 4.2 | 0.4 for trend |
| Systolic BP, mm Hg | 117.3 ± 5.9 | 132.0 ± 17.8 | 140.7 ± 18.0 | <0.001 for trend; 0.2 (RVH vs EH); <0.001 (RVH vs HV), 0.02 (EH vs HV) |
| Diastolic BP, mm Hg | 71.9 ± 5.9 | 71.6 ± 13.7 | 69.0 ± 9.4 | 0.7 for trend |
| No. of anti-HTN drugs | — | 2.8 ± 1.0 | 3.0 ± 1.2 | 0.6 (RVH vs EH) |
| ARB or ACEi | 15 (100) | 15 (100) | ||
| CCB | 5 (33) | 7 (47) | 0.5 (RVH vs EH) | |
| β-Blocker | 7 (47) | 11 (73) | 0.1 (RVH vs EH) | |
| Diuretics | 13 (87) | 11 (73) | 0.4 (RVH vs EH) | |
| Statins | 8 (53) | 10 (67) | 0.5 (RVH vs EH) | |
| Total cholesterol, mg/dL | 202.4 ± 31.9 | 179.3 ± 34.0 | 180.0 ± 31.1 | 0.1 for trend |
| Triglycerides, mg/dL | 100.4 ± 38.5 | 141.4 ± 65.5 | 158.0 ± 74 | 0.05 for trend; 0.2 (RVH vs EH); 0.04 (RVH vs HV); 0.8 (EH vs HV) |
| HDL, mg/dL | 70.8 ± 28.7 | 48.4 ± 12.6 | 51.8 ± 23.0 | 0.02 for trend; 0.9 (RVH vs EH); 0.08 (RVH vs HV); 0.02 (EH vs HV) |
| LDL, mg/dL | 110.9 ± 23.9 | 102.6 ± 24.0 | 96.6 ± 21.6 | 0.3 for trend |
| eGFR, mL/min/1.73 m2 | 72.0 ± 15.9 | 73.3 ± 22.0 | 50.3 ± 17.3 | 0.002 for trend; 0.004 (RVH vs EH); 0.007 (RVH vs HV); 0.9 (EH vs HV) |
| Albuminuria, μg/mL | 4.1 [2.2–5.8] | 5.0 [4.8–7.8] | 5.3 [4.9–9.5] | 0.4 for trend |
Note: Values for normally distributed continuous variables given as mean ± SD, non-normally distributed, as median [interquartile range]; categorical variables, as no. (%).
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein cholesterol; HTN, hypertension; LDL, low-density lipoprotein cholesterol.
Figure 1.
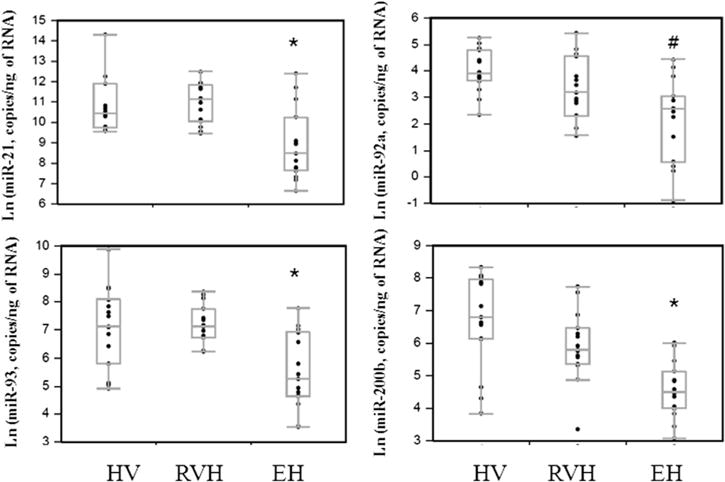
miR levels adjusted for eGFR, systolic BP, triglycerides, HDL, and albuminuria in urinary EVs of the HV, RVH, and EH groups. Each point represents the relative miR level after normalization using Cel-miR-39. miR-21, -93, and -200b levels were lower in EH vs both HVs and RVH, whereas miR-92a level was lower vs HVs alone. *P < 0.05 vs HVs and RVH; #P < 0.05 vs HVs.
Therefore, this study shows lower levels of several miRs in urinary EVs obtained from patients with EH versus RVH or HVs, suggesting miR-21, -93, and -200b in urinary EVs as useful markers for discriminating RVH from EH. Reduced levels of miRs postulated to modulate fibrosis and angiogenesis may reflect different pathogenesis or target-organ injury imposed by primary versus secondary hypertension. Because eGFRs in patients with EH did not differ from HVs, their kidneys were likely relatively preserved. However, modulation of fibrosis-related renal miRs might precede a decrease in eGFR in EH. All hypertensive patients were treated with antihypertensive agents, which may also modulate miRs, yet miR levels in patients with RVH did not differ from HVs. Although advanced RVH may have fewer functional cells to release EVs, eGFR was only slightly decreased in our patients. Moreover, release of urinary EVs from both stenotic and nonstenotic kidneys might obscure changes in urinary EVs. Notably, patients with EH and RVH were studied under well-controlled conditions, whereas HVs gave random urine samples. Hydration status or medications might also affect EV content in urine samples, but were similar in patients with EH and RVH.
Urinary EV miRs likely reflect their expression along the nephron because circulating miRs are too large to undergo glomerular filtration.10 Furthermore, salt sensitivity has been linked to decreased urinary EV miR levels in hypertensive patients.10 However, whether altered miR expression in urinary EVs is a primary or secondary event in EH is incompletely understood.
Importantly, we did not find correlation with clinical parameters (table a of Item S1), possibly because kidney function was relatively preserved and our patients were treated with RAAS blockers. Further studies are needed to define how miRs influence long-term outcomes in hypertension.
Limitations of our study include a small number of patients, lack of renal pathology, and different urine specimen sources in hypertensive patients and HVs.
In conclusion, our hypothesis-generating study implies that miRs may be linked to hypertensive kidney injury or define a unique renal phenotype in EH. Larger studies are needed to elucidate the role of miRs in hypertension, which may provide novel biomarkers for diagnosis, predicting outcomes, or designing therapeutic strategies for hypertension.
Supplementary Material
Item S1: Detailed methods, table a, and figure a.
Acknowledgments
Support: Partly supported by NIH grants DK100081, DK73608, DK102325, and HL123160; the NIH had no input into study design or conduct, data analyses or management, or the decision to submit the work for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: SHK, LOL; data acquisition: SHK, HT, AS, JRW; data analysis/interpretation: SHK, AL, LOL; statistical analysis: SHK, LOL; supervision/mentorship: AL, SCT, LOL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring the questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. LOL takes responsibility that this study has been reported honestly, accurately, and transparently; no important aspect of the study have been omitted; and any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by an external peer reviewer, a Statistical Editor, a Co-Editor, and the Editor-in-Chief.
Supplementary Material
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2016.01.027) is available at www.ajkd.org
References
- 1.Hara M, Yanagihara T, Kihara I, et al. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J Am Soc Nephrol. 2005;16(2):408–416. doi: 10.1681/ASN.2004070564. [DOI] [PubMed] [Google Scholar]
- 2.Ramezani A, Devaney JM, Cohen S, et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest. 2015;45(4):394–404. doi: 10.1111/eci.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Wang Y, Minto AW, et al. MicroRNA-377 is upregulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22(12):4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Kwan BC, Lai FM, et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens. 2010;23(1):78–84. doi: 10.1038/ajh.2009.208. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Kwan BC, Lai FM, et al. Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest. 2010;90(1):98–103. doi: 10.1038/labinvest.2009.118. [DOI] [PubMed] [Google Scholar]
- 6.Putta S, Lanting L, Sun G, et al. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23(3):458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Chung AC, Dong Y, et al. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-beta/Smad3-Azin1 pathway. Kidney Int. 2013;84(6):1129–1144. doi: 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 8.Chung AC, Dong Y, Yang W, et al. Smad7 suppresses renal fibrosis via altering expression of TGF-beta/Smad3-regulated microRNAs. Mol Ther. 2013;21(2):388–398. doi: 10.1038/mt.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Li Q, Xu Q, Liu L, Jiang B. MiR-148a inhibits angio-genesis by targeting ERBB3. J Biomed Res. 2011;25(3):170–177. doi: 10.1016/S1674-8301(11)60022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem. 2013;46(12):1131–1134. doi: 10.1016/j.clinbiochem.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Detailed methods, table a, and figure a.


