Abstract
Cannabinoid receptors contribute to learning and synaptic plasticity mechanisms. The cerebellum contains a high density of cannabinoid receptors and manipulations of cannabinoid receptors affect synaptic plasticity within the cerebellar cortex. In vivo studies have found that cannabinoid agonists impair learning of cerebellum-dependent eyeblink conditioning in rodents and humans. However, the role of cannabinoid receptors or endocannabinoids in memory consolidation within the cerebellum has not been examined. In the current study, we examined the role of cannabinoid receptors and endocannabinoids during learning and consolidation of eyeblink conditioning in rats. Administration of the cannabinoid receptor agonist WIN55,212-2 or drugs that increase/decrease endocannabinoid levels directly into the cerebellar cortex before each training session resulted in marked learning impairments. When administered 1 hr after each training session, during memory consolidation, the cannabinoid inverse agonist SR141716A or the endocannabinoid suppressor THL impaired memory. In contrast, increasing endocannabinoid levels with JZL-184 or infusion of WIN55,212-2 within the cerebellar cortex facilitated memory consolidation 1 hr post-training. Intracerebellar manipulations of cannabinoid receptors or endocannabinoid levels had no effect on memory consolidation when administered 3 or 6 hr after each training session. The results demonstrate that cannabinoids impair cerebellar learning, but facilitate memory consolidation mechanisms within the cerebellar cortex 1– 3 hr after training.
Keywords: cannabinoid receptors, 2-AG, cerebellum, eyeblink conditioning, consolidation
1. INTRODUCTION
Cannabis sativa is the most widely used psychoactive drug in the United States with rates continuing to increase over the past decade (Substance Abuse Mental Health Services Administration, 2014). The major active ingredient in cannabis, Δ9-tetrahydrocannabinol, binds to G-protein coupled cannabinoid receptors CB1R and CB2R (Gaoni and Mechoulam, 1964; Devane et al., 1988). CB1Rs are the most abundant G-protein coupled receptor in the mammalian brain with the highest density of receptors within the cerebellar cortex (Herkenham et al., 1990; 1991). Two endogenous cannabinoids (endocannabinoids) have been discovered, 2-Arachidonoylglycerol (2-AG) and Anandamide. Cannabinoid receptors have many diverse roles throughout the brain including synaptic plasticity during learning and memory. CB1Rs within the cerebellar cortex have been demonstrated to be important for the induction of long-term depression (LTD) in parallel fiber synapses with Purkinje cells in vitro (Lévénés, et al., 1998; Safo and Regher, 2005; van Beugen, Nagaraja, and Hansel, 2006). In addition, systemic or genetic CB1R manipulations, which impair CB1R function throughout the brain, result in decrements in the rate of eyeblink conditioning, a type of associative learning that depends on the cerebellar cortical plasticity (Kishimoto and Kano, 2006; Skosnik et al., 2008; Edwards et al., 2008; Steinmetz and Freeman, 2010; 2011; 2013; Steinmetz et al., 2012). The hypothesis drawn from these studies is that the decrement in acquisition of eyeblink conditioning is the result of impaired synaptic plasticity mechanisms within Purkinje cells.
Less is known about the role of cerebellar cannabinoid receptors in memory consolidation. Studies examining the role of CB1Rs and endocannabinoids during consolidation have been conducted primarily in the hippocampus, amygdala, and prefrontal cortex (Wise, Thorpe, and Lichtman, 2009; Tan et al., 2011). Each of these areas plays an important role in memory consolidation for different tasks, but contains moderate levels of CB1Rs as compared to the cerebellar cortex. To date, there have been no studies examining the role of CB1Rs during memory consolidation within the cerebellum. As mentioned above, the cerebellar cortex contains the highest density of CB1Rs and also is an important site of synaptic plasticity underlying motor learning (Safo and Regehr, 2005). Manipulations of cannabinoid receptors or endocannabinoid levels should therefore affect memory consolidation within the cerebellum.
The current study employed cerebellum-dependent delay eyeblink conditioning to examine the role of CB1Rs and endocannabinoids during learning and memory consolidation. Eyeblink conditioning involves the presentation of a conditioned stimulus (CS) that does not elicit eyelid closure prior to learning (e.g., a tone), followed by an unconditioned stimulus (US) that elicits eyelid closure before learning (e.g., periorbital stimulation). After repeated CS-US pairings an adaptively timed eyelid closure conditioned response (CR) develops. The cerebellum, specifically the cerebellar cortex and anterior interpositus nucleus, is essential for acquisition and retention of the CR (McCormick and Thompson, 1984; Freeman and Steinmetz, 2011). In rats, the cerebellar cortical area at the base of the primary fissure is necessary for the acquisition of eyeblink conditioning (the eyeblink conditioning microzone, Steinmetz & Freeman, 2014). In the current study, rats received 5 daily sessions of eyeblink conditioning in which a CB1R agonist (WIN55,212-2), CB1R inverse agonist (SR141716A), monoacylglycerol lipase inhibitor (JZL-184; increases endocannabinoid levels), or diacylglycerol lipase inhibitor (THL; decreases endocannabinoid levels) was infused into the eyeblink conditioning microzone before (learning manipulation) or after (consolidation manipulation) each training session. Activating CB1Rs or increasing endocannabinoid levels within the eyeblink conditioning microzone of the cortex were predicted to impair memory consolidation since CB1R agonists impair learning when given before training sessions.
2. MATERIALS AND METHODS
2.1 Subjects
The subjects were 113 male Long-Evans rats (250–300 g). The rats were housed in the animal colony in Spence Laboratories of Psychology at the University of Iowa (Iowa City, IA). All rats were maintained on a 12 h light/dark cycle and given ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
2.2 Surgery
One week before training, rats were anesthetized with isoflurane. After the onset of anesthesia, the rats were fitted with differential electromyography (EMG) electrodes implanted into the upper left orbicularis oculi muscle. The reference electrode was a silver wire attached to a skull screw. The EMG electrode leads terminated in gold pins inserted into a plastic connector. A bipolar stimulating electrode for delivering the periorbital stimulation US was implanted subdermally, caudal to the left eye. A 23 gauge guide cannula was implanted 1 mm dorsal to the base of the primary fissure of the cerebellar cortex. A 30 gauge stylet was inserted into the guide cannula. The stereotaxic coordinates taken from bregma for the cannula was 11.4 mm posterior, 3.0 mm lateral, and 3.2 mm ventral. The plastic connector housing the EMG electrode leads, bipolar stimulating electrode, the guide cannula, and skull screws were secured to the skull with bone cement.
2.3 Microinjections
Before each infusion, the stylet was replaced with a 30 gauge infusion cannula that extended 1.0 mm beyond the guide cannula. The infusion cannula was connected to polyethylene tubing (PE 10), which was connected to a 10 µl syringe. The syringe was placed into an infusion pump and 0.5 µl of each drug was infused over 5 min at a rate of 6.0 µl/h. After the infusion, the cannula was left in place for 3 min in order to allow diffusion of the drug.
2.4 Drugs
The CB1R/CB2R agonist WIN55,212-2 ([R]-(+)-[2,3-Dihydro-5-methyl-3- (4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone), CB1R inverse agonist SR141716A [5-(4-Chlorophenyl)-1- (2,4-dichloro-phenyl)- 4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide], the monoacylglycerol lipase inhibitor JZL-184 [4-nitrophenyl-4-[bis(1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate] or the diacylglycerol lipase inhibitor THL [(S)-((S)-1-((2S,3S)-3-Hexyl-4-oxooxetan-2-yl)tridecan-2-yl) 2-formamido-4-methylpentanoate] were administered intracranially to rats. WIN55,212-2 and THL were dissolved in a vehicle of 1:1:18 solution of ethanol, cremaphor, saline and SR141716A and JZL-184 were dissolved in a vehicle of 1:1:18 solution of ethanol, tween 80, saline. WIN55,212-2, JZL-184 and THL were purchased from Sigma/RBI. SR141716A was a generous gift from NIDA Drug Supply Program (Rockville, MD).
2.5 Apparatus
A detailed description of the apparatus has been published (Nicholson and Freeman, 2000). Briefly, conditioning occurred within small-animal operant chambers (BRS/LVE). The electrode leads from the rat's head stage was connected to peripheral equipment by lightweight cables that allowed the rat to move during conditioning. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC).
2.6 Conditioning Procedure
The rats were allowed to adapt to the training environment for 5 min before each training session. Paired training sessions occurred in which 5 blocks of 9 paired CS–US presentations and 1 CS-alone probe trial were presented. Fewer trials were administered than previous studies from our laboratory in order to minimize within-session memory consolidation. The CS was a 400-ms tone (2 kHz; 85 dB) and terminated with a 25-ms shock US. The US intensity was set such that a rat would elicit eyelid closure and slight head movement (2.5 mA). CRs were defined as EMG activity that exceeded a threshold of 0.4 units (amplified and integrated units) above the baseline mean during the CS period after 80 ms. EMG responses that exceeded the threshold during the first 80 ms of the CS period were defined as startle responses. On CS-alone probe trials, the duration for scoring CRs was extended beyond the CS to the end of the trial period (1.0 s). Measurements of CR amplitude, onset latency, and peak latency were taken from CS-alone probe trials. URs were defined as responses that crossed the threshold after the onset of the US.
2.7 Experimental Design
The rats received either vehicle, WIN55,212-2 (10 µg/µL), SR141716A (1.0 µg/µL), JZL-184 (0.5 ng/µL), or THL (1 ng/µL) infusions into the cerebellar cortex. Additionally, rats given WIN55,212-2 or SR141716A were randomly assigned to receive the infusions at different time points. These time points were immediately before the session (pre), immediately following the session (0 hr), 1 hour following the session (1 hr), 3 hours following the session (3 hr), or 6 hr following the session (6 hr). JZL-184 and THL administration was limited to pre, 1 hr, and 3 hr because of the initial findings with WIN55,212-2 and SR141716A.
2.8 Histology
After training, the rats were given sodium pentobarbital (150 mg/kg) and then transcardially perfused with saline followed by 10% buffered formalin. After perfusion, the brains were cryoprotected in 30% sucrose/formalin and subsequently sectioned at 50 µm. Sections were then stained with thionin. The location of the cannula placements was verified using a light microscope.
3. RESULTS
Cannula placements were verified with light microscopy. An example of a cannula placement is given in Figure 1. Four animals were removed from the analysis due to cannula placements that were outside of the targeted area of the cerebellar cortex (outside the circle on Figure 1). Missed cannula were either lateral (n=2) or ventral (n=2) to the defined EBC microzone (Steinmetz and Freeman 2014).
Figure 1.
Example of a cannula placement included in the study. Injection cannula (IC) were kept if they lied within the Pfb (black circle). GC: Guide Cannula, AV: Anterior Cortex, HVI: Lobule HVI.
3.1 Experiment 1. Intracerebellar WIN55,212-2 Impairs Learning but Enhances Memory Consolidation
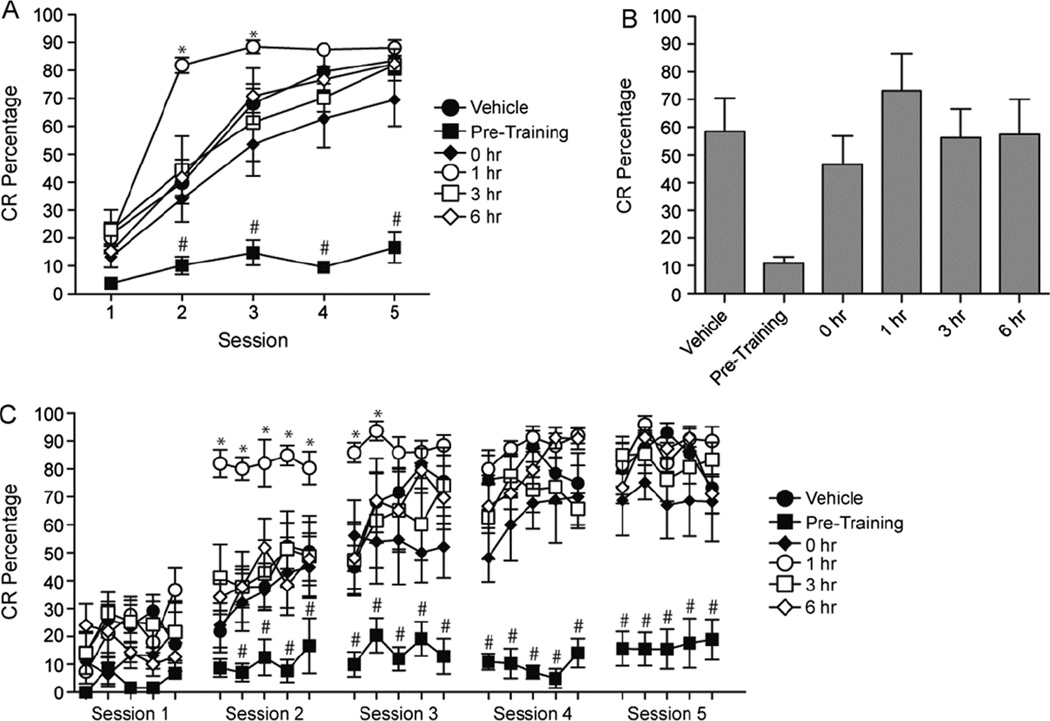
It has been previously reported by our laboratory that systemic WIN55,212-2 administration prior to each acquisition session impairs acquisition of eyeblink conditioning (Steinmetz and Freeman, 2010); however, post-training administration of WIN55,212-2 during the memory consolidation phase has not been examined. To examine memory consolidation rats underwent 5 sessions of CS-US paired training with administration of WIN55,212-2 into the cerebellar cortex either immediately before training or at different intervals after training. The post-training intervals at which WIN55,212-2 was administered included immediately following training (0 hr), 1 hr, 3 hr, or 6 hr post-training. In order to examine the effects of the administration of WIN55,212-2 on learning at the different time points a 6 (Group) × 5 (Session) repeated measures ANOVA was conducted for the CR percentage data (Figure 2A,B). A significant Group × Session interaction was found, F(4,32)=4.520, p <.001 and post-hoc tests (Tukey-Kramer) indicated that WIN55,212-2 administered prior to each training session resulted in a significant decrease in the percentage of CRs on Sessions 2–5 as compared to the vehicle group (p<.01). However, there was a significant increase in CRs during Sessions 2 and 3 when WIN55,212-2 was administered 1 hr following training as compared to the vehicle group. There were no significant differences relative to the vehicle control group when WIN55,212-2 was given immediately following the session or at 3 or 6 hr following the session. In order to further examine the effects, each session of 50 trials was divided into 5 blocks of 10 trials (Figure 1C). A 6 (Group) × 25 (Block) repeated measures ANOVA was conducted and found a significant interaction, F(24,120)= 2.466, p <.001. Post-hoc tests indicated that administration prior to each session resulted in decreased CRs during blocks 7–25. The enhancement in learning from administration 1 hr following the session was found on blocks 6 – 12.
Figure 2.
The effects of CB1R agonist (WIN55,212-2) infusions before and following training. Infusions were made either before, immediately (0 hr), 1, 3, or 6 hours following training. A: CR percentages for 5 daily sessions of conditioning. B: Average of the CR percentages across the 5 sessions of training. C: Block data (5 blocks per session) for each day of conditioning. * significant increase (p<0.05), and # significant decrease (p<0.05)
Additional measures of CR performance, including CR amplitude and timing (onset latencies and peak latency), were examined to further understand the role of cannabinoid receptor activation during and following training. A significant Group × Session interaction was found, F(5,35)=2.528, p = .047 for the CR amplitude data. Post-hoc tests indicated that WIN55,212-2 administered before sessions decreased the amplitude of CRs during sessions 3–5. Similar to results for CR percentage, administration of WIN55,212-2 1 hr following training increased CR amplitude during session 2. No other significant differences were found for the CR amplitude data. CR timing was not altered for any of the groups, but main effects of sessions were found for both CR onset latency, F(4,20)=7.528, p <.001, and CR peak latency, F(4,20)=3.957, p =.004.
Finally, we examined whether pre- or post-session administration of WIN55,212-2 changed sensory or motor function. This potential confound is primarily a concern for the pre-session infusions, but not for the post-session infusions, since WIN55,212-2’s effects will have ended by the next day. Drug-induced changes to sensory processing could be found by measuring the frequency and amplitude of startle responses (SR) to the onset of the tone CS. There were no significant drug effects on either measure when comparing the drug group, time of administration, or an interaction of the two variables. Additionally, changes to motor responses could be found by measuring the amplitude and peak latency of the UR. There were no significant differences in UR measures between groups. The findings of the startle and UR analyses suggest that administration of WIN55,212-2 did not alter sensory or motor function.
3.2 Experiment 2. Intracerebellar SR141716A Impairs Memory Consolidation
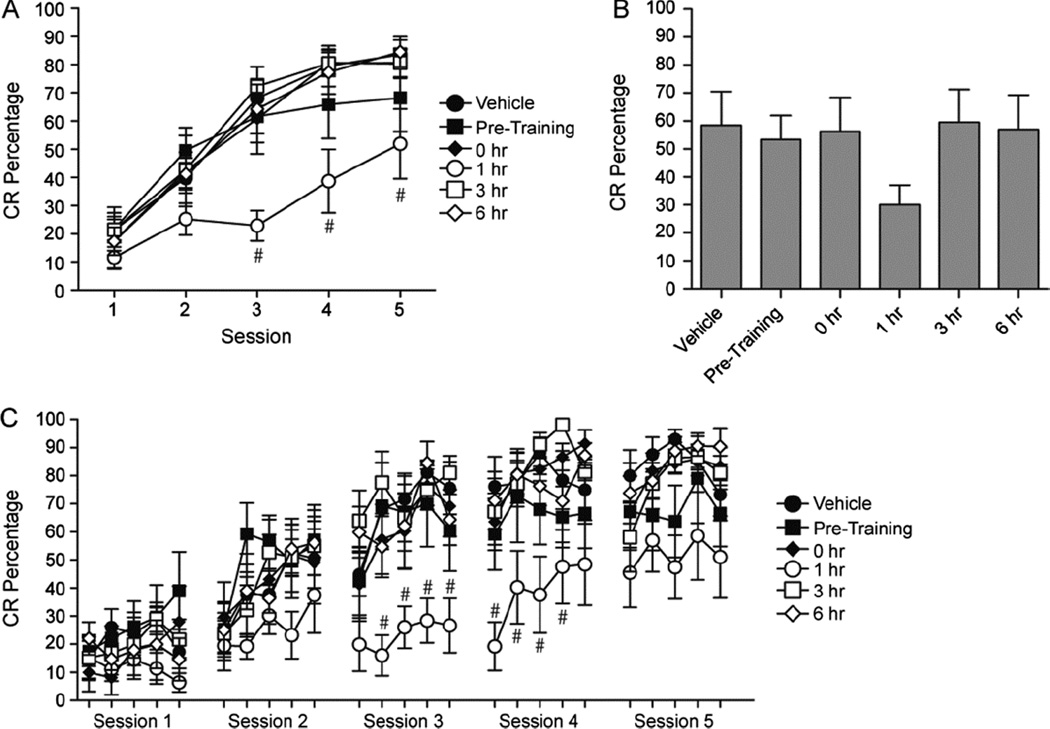
Systemic administration of SR141716A has been reported to impair cerebellar learning, although the magnitude of the impairment was lower than with WIN55,212-2 (Kishimoto and Kano, 2005; Steinmetz and Freeman, 2010). Animals underwent 5 sessions of paired training with administration of SR141716A given either before or after each training session. The post-training intervals at which the drug was administered were the same as with the WIN55,212-2 experiment. A 6 (Group) × 5 (Session) repeated measures ANOVA was conducted for the CR percentage data (Figure 3A,B) and found a significant interaction, F(4,31)=1.822, p =.041. Post-hoc tests indicated that CR percentage was impaired relative to the vehicle group on sessions 3–5 when SR141716A was administered 1 hr following training (p<.01). There were no other significant differences when SR141716A was given immediately before or after 3 or 6 hr following the session. Block data (5 blocks of 10 trials) were also examined (Figure 3C). A 6 (Group) × 25 (Block) repeated measures ANOVA was conducted and found a significant interaction, F(24,120)= 1.416, p = .048. Post-hoc tests revealed that administration of SR141716A 1 hr following the session caused a deficit during blocks 12–19 as compared to the vehicle group.
Figure 3.
The effects of CB1R inverse agonist (SR141716A) infusions before and following training. Infusions were made either before, immediately (0 hr), 1, 3, or 6 hours following training. A: CR percentages for 5 daily sessions of conditioning. B: Average of the CR percentages across the 5 sessions of training. C: Block data (5 blocks per session) for each day of conditioning. # significant decrease (p<0.05)
To further examine the role of SR141716A during acquisition and consolidation CR amplitude and timing were examined. There were no significant interactions or main effects involving the group factor. However, there were main effects of Session for CR amplitude, F(4,20)=11.600, p <.001, onset latency, F(4,20)=2.748, p = .046, and peak latency, F(4,20)=17.167, p <.001, which indicate that all groups showed changes across training sessions.
As in Experiment 1, we examined whether SR141716A had effects on SRs or URs over the time points. We did not find any significant drug effects on SR percentage, SR amplitude, UR peak latency, or UR amplitude. Thus, SR141716A did not alter sensory or motor responses.
3.3 Experiment 3. Intracerebellar JZL-184 Impairs Learning but Enhances Memory
Endocannabinoids have been shown to be important in the induction of synaptic plasticity in Purkinje cells in vitro (Safo and Regehr, 2005). However, it is not known if endocannabinoids are necessary for cerebellum-dependent learning in vivo. To examine the role of endocannabinoids in acquisition and consolidation of cerebellum-dependent learning the monoacylglycerol lipase (MAGL) inhibitor JZL-184 was infused into the cerebellar cortex for 5 consecutive sessions of eyeblink conditioning either prior to the session or following the sessions, at 1 hr or 3 hr post sessions. MAGL is the primary enzyme necessary for the degradation of the endocannabinoid 2-arachidonoylglycerol (2-AG), the primary endocannabinoid in the cerebellum. Thus, inhibition of MAGL results in an increase in 2-AG levels within the cerebellar cortex. A 4 (Group) × 5 (Session) repeated measures ANOVA was conducted for the CR percentage data and found a significant interaction, F(4,21)=4.219, p <.001. Post-hoc tests revealed that when JZL-184 was administered prior to training there were significantly fewer CRs during Sessions 2–5 as compared to the vehicle group (p<.01)(Figure 4A,B). In contrast, there was a significant increase in CR percentage during Session 2 when JZL-184 was administered 1 hr following training (Figure 4A,B). There were no significant group differences when JZL-184 was given 3 hr following the session. Block data were examined to further understand how endocannabinoids are involved in acquisition and consolidation (Figure 4C). Each session was broken down into 5 blocks containing 10 trials resulting in 25 blocks. A 6 (Group) × 25 (Block) repeated measures ANOVA was conducted and found a significant interaction, F(24,72)= 3.192, p < .001. Post-hoc tests revealed that JZL-184 administration prior to the session caused a deficit during blocks 9–25, whereas when administered 1 hr following training an enhancement occurred during blocks 6–10 as compared to the vehicle group.
Figure 4.
The effects of monoacylglycerol lipase inhibitor (JZL-184) infusions before and following training. Infusions were made either before, 1, or 3 hours following training. A: CR percentages for 5 daily sessions of conditioning. B: Average of the CR percentages across the 5 sessions of training. C: Block data (5 blocks per session) for each day of conditioning. * significant increase (p<0.05), and # significant decrease (p<0.05)
CR amplitude and latency (onset and peak) were examined across the 5 sessions. A significant Group × Session interaction was found for the CR amplitude data, F(4,12)=2.069, p =.026. Post-hoc tests revealed a decrease in CR amplitude during sessions 4 and 5 when JZL-184 was administered prior to each session and an increase in CR amplitude during sessions 2 and 3 when administered 1 hr following each session. There was a significant main effect of session for CR peak latency, F(4,12)=6.181, p <.001, which reflected a change across sessions for all groups. No significant effects were found for CR onset latency. There were no significant effects of JZL-184 on SR percentage, SR amplitude, UR latency, or UR amplitude.
3.4 Experiment 4. Intracerebellar THL Impairs Learning and Memory Consolidation
In contrast to JZL-184, administration of THL results in a decrease in 2-AG levels. THL inhibits 2-AG formation via blocking diacylglycerol lipase (DAGL). In order to test the effects of THL during acquisition and consolidation infusions occurred either before or after (1 hr or 3 hr) 5 consecutive CS-US paired training sessions. A 6 (Group) × 5 (Session) repeated measures ANOVA was conducted for the CR percentage data and found a significant interaction, F(4,21)=2.638, p =.007 (Figure 5A,B). Post-hoc tests indicated that THL administration prior to training produced a reduction in CR percentage on Sessions 2–5 as compared to the vehicle group (p<.01). Additionally, there was a significant decrease in CR percentage during sessions 2–5 when THL was administered 1 hr following training. There were no significant group differences when THL was given immediately following the session or 3 hr following the session. In order to further examine the effects of THL each session of 50 trials was divided into 5 blocks of 10 trials (Figure 5C). A 4 (Group) × 25 (Block) repeated measures ANOVA was conducted and found a significant interaction, F(24,72)= 2.855, p < .001. Post-hoc tests indicated that THL administration prior to each session and 1 hr following each session resulted in a deficit in CR percentage during blocks 9–25 and 12–25 respectively.
Figure 5.
The effects of diacylglycerol lipase inhibitor (THL) infusions before and following training. Infusions were made either before, 1, or 3 hours following training. A: CR percentages for 5 daily sessions of conditioning. B: Average of the CR percentages across the 5 sessions of training. C: Block data (5 blocks per session) for each day of conditioning. # significant decrease (p<0.05)
Additional measures of CR performance, amplitude and latency, were examined for the 5 CS-US training sessions. For CR amplitude, a significant Group × Session interaction was found, F(4,12)=2.093, p =.024. Lower amplitude CRs were evident in Sessions 4 and 5 in the group given THL prior to each session and in the group given THL 1 hr following each session. An ANOVA conducted for CR peak latency revealed a main effect of Session, F(4,12)=2.805, p =.030, which was due to a change in latency across sessions. There were no significant differences for the CR onset latency data. There were no significant effects of THL on SR percentage, SR amplitude, UR latency, or UR amplitude.
4. DISCUSSION
This is the first study to demonstrate effects of cannabinoid manipulations on cerebellar memory consolidation. CB1R agonist administration within the EBC microzone of the cerebellar cortex before each training session impaired acquisition of eyeblink conditioning. We extended these findings to show that changing endocannabinoid levels within the cerebellar cortex prior to training also resulted in impaired eyeblink conditioning. However, an enhancement in eyeblink conditioning was found with CB1R activation or increasing endocannabinoid levels 1 hr following each training session. The reverse, impaired eyeblink conditioning, occurred if CB1Rs were blocked or endocannabinoids were decreased 1 hr after each training session. The time-course of these consolidation effects occurred within a relatively small window. Manipulations made either immediately following the session or 3–6 hr after the session did not affect memory consolidation. Taken together, these results indicate a dynamic role for cannabinoid receptors and endocannabinoids in consolidation within the cerebellum.
The drug-related changes in eyeblink conditioning in the current study are attributed primarily to receptor and endocannabinoid effects within the cerebellar cortex. It is possible, however, that the drugs may have spread from the cortical injection site to the cerebellar nuclei. This concern is mitigated by a previous study which demonstrated that intracerebellar infusions of cannabinoid drugs within the cortex produced a learning deficit, whereas infusions into the deep nuclei or neighboring cerebellar cortical areas produced no effect on learning (unpublished results). Thus, it is most likely that the memory consolidation effects reported in this study are attributable to drug effects on cerebellar cortical function.
Manipulations of cannabinoid receptors before training sessions, via agonist/inverse agonist action or removal of the receptors in knockout mice, result in impaired cerebellar learning (Kishimoto and Kano 2006; Steinmetz and Freeman, 2010; 2011; 2013). The current set of experiments show that manipulating cannabinoid activity within the cerebellar cortex by either a CB1R agonist (WIN55,212-2), increasing endocannabinoid levels (JZL), or decreasing endocannabinoid levels (THL) resulted in impaired learning if administered prior to each session. These results indicate that maintaining normal cannabinoid receptor function and endocannabinoid levels during training trials is important for establishing cerebellar learning. Both cannabinoid receptors and endocannabinoids have been reported to be essential for the induction of LTD in Purkinje cells in vitro (Levenes, et al., 1998; Safo and Regehr, 2005). Induction of LTD in the cerebellar cortex is hypothesized as an important early step in the acquisition of eyeblink conditioning (Mauk & Donegan, 1997). Manipulations of cannabinoid receptors and endocannabinoids starting before training sessions may therefore impair cerebellum-dependent learning by impairing LTD induction or induction of other plasticity mechanisms (Johansson et al., 2015). resulting in impaired eyeblink conditioning across the five training sessions in the current study.
The role of cannabinoid receptors during consolidation of memories in other brain areas such as the hippocampus, amygdala, and prefrontal cortex has also been studied (Blum et al., 2006; Campolongo et al. 2009; Marsicano et al. 2002). These previous studies reported both enhancements and impairments of consolidation, depending on the paradigm and brain area (Marsicano et al., 2002; Yim et al., 2008). In the current study, we manipulated cannabinoid receptor activity and endocannabinoid levels within the cerebellar cortex during memory consolidation with post-training infusions. Activating CB1Rs or enhancing endocannabinoid levels in the cerebellar cortex after training sessions resulted in enhanced consolidation, whereas blocking CB1Rs or decreasing endocannabinoid levels after training sessions impaired consolidation. The enhancement in consolidation with WIN55,212-2 and JZL-184 was only evident transiently, since CR acquisition quickly reached asymptote in these groups (Figures 2 and 4, Session 2). In contrast, the consolidation deficit with SR141716A and THL given 1 hr after training sessions was persistent across the five training session, presumably because the drugs continued to impair consolidation. The post-training effects of the manipulations used in this study are hypothesized to affect the mechanisms underlying consolidation of plasticity mechanisms in the cerebellar cortex. Consolidation of plasticity mechanisms might be affected by these manipulations by altering spontaneous post-training Purkinje cell activity. Increasing CB1R binding, either through CB1R agonist administration or increasing endocannabinoid levels, decreases the release of glutamate from granule cells and GABA from interneurons, resulting in increased spontaneous Purkinje cell activity (de Solanges et al. 2008). In contrast, CB1R blockade or decreasing endocannabinoid levels result in increases of glutamate and GABA release, which decrease Purkinje cell activity. The results from the current study suggest that increasing Purkinje cell activity might increase consolidation and decreasing activity might impair consolidation. This activity-based hypothesis is supported by results showing that inactivation of the cerebellar cortex following training sessions impairs eyeblink conditioning (Attwell, Cooke, and Yeo, 2002; Kellett et al., 2010). It is also supported by studies showing that increasing or decreasing amygdala activity post-training results in increased and decreased changes to consolidation, respectfully (Vafaei, et al., 2007; Huff et al., 2013).
The molecular mechanisms underlying cannabinoid-mediated modulation of cerebellar memory consolidation have not been examined, but studies of cannabinoid effects in other brain areas may be informative. Cannabinoid receptor activation has also been reported to cause AMPA receptor endocytosis within the ventral tegmental area (VTA) which resulted in increased LTD expression (Liu et al., 2010). Thus, CB1R activation during the consolidation phase, 1–3 hr after training, could increase AMPA receptor endocytosis in Purkinje cells, which in turn, would increase LTD formation. Cannabinoids might also affect consolidation by increasing protein synthesis through mTOR pathway activation (Puighermanal et al., 2009; 2013) or through activation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Maroso et al., 2016). The HCN channels mediate the h-current (Ih), which plays a role in dendritic excitability. The CB1Rs that are involved in the modulation of HCN channels are post-synaptic and activation of this pathway impairs memory consolidation mechanisms within the hippocampus when given immediately after training (Maroso et al., 2016). It is possible that the post-training effects of cannabinoids in the current study affected consolidation through postsynaptic CB1RHCN mechanisms. The mechanisms underlying cannabinoid effects on memory consolidation within the cerebellum were not revealed in the current study, but it is clear that whatever the mechanisms are, they are regulated during a relatively narrow period between 1 and 3 hours after training.
5. CONCLUSIONS
The current study demonstrates that post-training manipulations of cannabinoid receptors or endocannabinoid levels within the cerebellar cortex alter memory consolidation. Memory underlying eyeblink conditioning was facilitated by enhancement of cannabinoid action and impaired by reducing cannabinoid action when given 1 hour after training. The time window for these effects was relatively narrow, with cannabinoid effects at 1 hour after training, but not after 0, 3, or 6 hours. Furthermore, this is the first study to show that cannabinoid activation impairs and enhances memory within the same brain area, depending only upon the timing of the infusion. Thus, cannabinoid and endocannabinoid manipulations within the cerebellar cortex made at discrete time points resulted in either impairment (before training) or enhancement (following training) in learning. Additional studies will examine the molecular mechanisms underlying these pharmacological effects.
Highlights.
Cannabinoid agonists or alteration of endocannabinoids impair cerebellar learning
Blocking CB1Rs or suppressing 2-AG 1 hr post-training impairs memory
CB1R agonist infusion or increasing 2-AG 1 hr post-training facilitates memory
No effect of cannabinoid/endocannabinoid alterations 3 or 6 hr post-training
Cannabinoids impair cerebellar learning, but facilitate memory consolidation
Acknowledgments
This research was supported by the National Institutes of Health (MH080005) to JHF and an American Psychological Association Dissertation Research Award to ABS. SR141716A was a gift from the National Institute for Drug Abuse Supply Program (Rockville, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests in relation to the work described.
REFERENCES
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proceedings of the National Academy of Sciences. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Léna C. High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron. 2008;58:775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz F, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron–Purkinje cell recordings. The Journal of neuroscience. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, Vollmer JM, O’Donnell BF, Hetrick WP. Assessment of forebrain-dependent trace eyeblink conditioning in chronic cannabis users. Neuroscience letters. 2008;439:264–268. doi: 10.1016/j.neulet.2008.04.102. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning & Memory. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American chemical society. 1964;86:1646–1647. [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the national Academy of sciences. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Carlsson HAE, Rasmussen A, Yeo CH, Hesslow G. Activation of a temporal memory in purkinje cells by the mGluR7 receptor. Cell Reports. 2015;13:1–6. doi: 10.1016/j.celrep.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Fukunaga I, Chen-Kubota E, Dean P, Yeo CH. Memory consolidation in the cerebellar cortex. PLoS One. 2010;5:e11737–e11737. doi: 10.1371/journal.pone.0011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. The Journal of neuroscience. 2006;26:8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévénès C, Daniel H, Soubrié P, Crépel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. The Journal of Physiology. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Han J, Jia L, Maillet JC, Bai G, Xu L, Zhang X. Synaptic neurotransmission depression in ventral tegmental dopamine neurons and cannabinoid-associated addictive learning. Plos One. 2010:e15634. doi: 10.1371/journal.pone.0015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH. Developmental changes in eye-blink conditioning and neuronal activity in the inferior olive. The Journal of Neuroscience. 2000;20:8218–8226. doi: 10.1523/JNEUROSCI.20-21-08218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature Neuroscience. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, Marsicano G, Maldonado R, Ozaita A. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology. 2013;38:1334–1343. doi: 10.1038/npp.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O'Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33:1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Freeman JH. Central cannabinoid receptors modulate acquisition of eyeblink conditioning. Learning & Memory. 2010;17:571–576. doi: 10.1101/lm.1954710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Freeman JH. Retention and extinction of delay eyeblink conditioning are modulated by central cannabinoids. Learning & Memory. 2011;18:634–638. doi: 10.1101/lm.2254111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Edwards CR, Vollmer JM, Erickson MA, O’Donnell BF, Hetrick WP, Skosnik PD. Examining the effects of former cannabis use on cerebellum-dependent eyeblink conditioning in humans. Psychopharmacology. 2012;221:133–141. doi: 10.1007/s00213-011-2556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Freeman JH. Differential effects of the cannabinoid agonist WIN55, 212–2 on delay and trace eyeblink conditioning. Behavioral neuroscience. 2013;127:694. doi: 10.1037/a0034210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Freeman JH. Localization of the cerebellar cortical zone mediating acquisition of eyeblink conditioning in rats. Neurobiology of learning and memory. 2014;114:148–154. doi: 10.1016/j.nlm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48. HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. The Journal of Neuroscience. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beugen BJ, Nagaraja RY, Hansel C. Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. The Journal of neuroscience. 2006;26:8289–8294. doi: 10.1523/JNEUROSCI.0805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Fuchs JR, Green JT, Morielli AD. Cellular mechanisms and behavioral consequences of Kv1. 2 regulation in the rat cerebellum. The Journal of Neuroscience. 2012;32:9228–9237. doi: 10.1523/JNEUROSCI.6504-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB1 receptors mediate the memory impairing effects of Δ9-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim TT, Hong NS, Ejaredar M, McKenna JE, McDonald RJ. Post-training CB1 cannabinoid receptor agonist activation disrupts long-term consolidation of spatial memories in the hippocampus. Neuroscience. 2008;151:929–936. doi: 10.1016/j.neuroscience.2007.08.037. [DOI] [PubMed] [Google Scholar]