Abstract
Drawing on conceptual models of critical periods, major life transitions, and life pathways, we proposed that the life-course features of parenthood are important, but understudied, mechanisms for explaining possibly gendered heart-health outcomes. Using three waves from the Midlife in the U.S. Study (MIDUS), we investigated (a) gender differences in the timing of the transition to parenthood as a pathway linking childhood SES disadvantage to onset of heart problems and (b) life-course factors (which vary by gender) that link the timing of the transition to parenthood to adult heart problems. We found that individuals who were disadvantaged in childhood were more likely to have their first child as teenagers or in early young adulthood. For women only, an early transition to parenthood partially explained the association between childhood disadvantage and onset of heart problems. Furthermore, women who had their first child at younger ages, particularly in their teens, had lower rates of college graduation, more financial difficulties, higher levels of depressive symptoms, and greater risk of smoking and obesity in midlife. These factors partially accounted for the association between early parenthood and onset of heart problems in later life. Our findings underscore the significance of the timing of the transition to parenthood in specifying the associations between childhood disadvantage and adult heart problems. Various factors are involved, including low adult SES, psychological distress, and unhealthy lifestyles.
Keywords: gender, childhood disadvantage, heart disease, parenthood
Introduction
Despite intensive interventions, heart disease remains the number one cause of mortality among U.S. adults, accounting for one in every four deaths (Xu et al., 2016). Biological factors, such as sex and age, are well-known determinants of heart disease; men have higher susceptibility to heart disease with generally higher prevalence than women, although women’s risk of heart disease increases after menopause (Khalil, 2010). The influence of early life socioeconomic status (SES) on adult heart problems has also been well-documented (Galobardes et al., 2006). However, few studies have investigated life-course pathways that explain how men and women from disadvantaged backgrounds develop heart problems.
Early-life circumstances influence the material, psychological and behavioral elements of adult heart disease, such as earnings, distress, smoking, drinking, and exercise (Galobardes et al., 2006; Hamil-Luker & O’Rand, 2007). Yet, men and women are differentially exposed to and vulnerable to these life-course determinants (Denton et al., 2004), indicating that the contribution of these factors to heart problems may vary by gender, perhaps with fewer socioeconomic opportunities for women and more heart-harming behaviors for men. Although these underlying factors largely explain the association between early-life SES and adult heart disease, prior work indicates that the main effect of early-life disadvantage remains significant even after adjustment for these mechanisms (e.g., Hamil-Luker & O’Rand, 2007), suggesting the need to explicate other pathway mediators.
The transition to parenthood, a major life event that determines individuals’ health and wellbeing, may be particularly important (Umberson et al., 2010). Early-life SES is significantly associated with the timing of the transition, with individuals from disadvantaged families being more likely to have a child at younger ages (Pears et al., 2005). Earlier transitions to parenthood, particularly as teenagers, create life-course trajectories of cumulative disadvantage, which can increase the risk of developing heart problems, potentially through negative socioeconomic, psychological, and behavioral pathways (Hardy et al., 2009). Importantly, childhood disadvantage may harm heart health more for women than men through timing of reproduction because women from low SES backgrounds are more likely to experience pregnancy at earlier ages, bear their first child at an earlier age, and sustain postpartum weight gain, all of which are linked to risks of heart problems (Gore et al., 2003; Siega-Riz et al., 2010). The current study introduces timing of transition to parenthood, the age at birth of first child, as an important life-course pathway involving multiple chains of risk from childhood disadvantage to adult heart problems.
Gender, childhood disadvantage, and heart health
Childhood is a critical period of the life course, when environments and experiences strongly predict later-life health outcomes (Ben-Shlomo & Kuh, 2002). Early-life exposure to a variety of stressors, including low SES, poor childhood health, and a harsh family environment contribute to the early development of risk factors for heart disease, including elevated blood pressure, high blood cholesterol level, and systemic arterial stiffness (Klassen et al., 2016; Stein et al., 2010). Childhood SES, typically measured by the educational attainments of parents and the occupational prestige of the head of household, is the most widely studied early-life predictor of heart disease in adulthood (Galobardes et al., 2004), yet gender differences in this relationship are not well understood.
Biological sex is a key determinants of heart problems. Although sex differences in heart disease vary by the type of problem, women generally experience a lower or delayed risk of heart disease than men (Mosca et al., 2011). This gendered pattern suggests that men are biologically more vulnerable than women to heart disease. This difference suggests that when men and women are exposed to structural inequality in early life, men may be more likely than women to develop heart problems. However, evidence regarding gender differences in the link between low SES and heart problems is mixed: some studies find no gender differences (Morton et al., 2014) or greater effects for men (S. E. Taylor et al., 2004) while others indicated that the deleterious effects of having low SES in early life are greater for women (Hamil-Luker & O’Rand, 2007). These disparate findings may be partially attributed to methodological and sampling differences, including the types of heart problems measured and age of respondents. Nonetheless, extant findings point to the need to better understand life-course factors beyond biological vulnerability that may influence the gendered patterns in pathways to adult heart problems.
Gender differences in intervening processes
Life-course pathway models suggest that early-life circumstances influence subsequent socioeconomic, psychological, and behavioral trajectories of individuals which, in turn, determine adult health outcomes (Power & Hertzman, 1997). Early-life disadvantages may make men and women differently vulnerable to adult heart health through distinct exposures and mechanisms. Women are more likely than men to experience social and material difficulties and negative life events in early life, while simultaneously having fewer economic and psychological resources to cope with such challenges (Denton et al., 2004; deVries & Watt, 1996; Turner & Avison, 2003). In addition, women who grow up in disadvantaged families are less likely than men to have opportunities for upward mobility, e.g., social stunting, particularly if they are overweight or obese, a key risk factor for heart disease (Heraclides & Brunner, 2010).
Behavioral factors influencing heart problems may also vary by gender: smoking and alcohol consumption are more important predictors of adult health for men, while body weight and physical inactivity are more important for women (Denton & Walters, 1999). Using structural inequality and life-course pathway models, Hamil-Luker and O’Rand (2007) investigated adult SES, social relationships, lifestyle behaviors, and access to health care as potential mediators that structure the gender-specific health consequences of early-life disadvantage. They found that the impact of early-life disadvantage on women’s risk of heart disease remained significant even after adjusting for these mediators. Similarly, Beebe-Dimmer et al. (2004) reported that the effect of childhood SES on mortality related to cardiovascular disease remained significant among women even after taking into account adult SES and lifestyle risk factors. These findings suggest the need to consider other intervening factors.
Early parenthood as a life-course pathway to explain gender differences
Stressful life transitions, such as having a child, often prompt individuals to substantially adjust their lives (Wheaton, 1990). The age at which individuals have their first child is influenced by early-life environments. Individuals, especially women, who grow up in disadvantaged families are more likely to enter parenthood at earlier ages (Pears et al., 2005) and have more children over the life course with shorter birth spacing (Setty-Venugopal & Upadhyay, 2002). Early transition to parenthood can lead to physiological tolls for women, such as hypertension and excessive weight gain, which may persist for decades after childbirth (Adamo et al., 2012), thus increasing risk for cardiovascular diseases in later life (Kharazmi et al., 2013).
Beyond biological processes, an early transition to parenthood may set up a trajectory of elevated risk of heart disease through lifestyle risk behaviors and limited life opportunities. For both men and women, early parenthood is associated with school disengagement and dropping out of school, which restrict occupational opportunities (Fletcher & Wolfe, 2009). Individuals confronting parenting-related stress may engage in less healthy practices, such as poor or irregular diet and insufficient exercise (Nomaguchi & Bianchi, 2004; Reczek, 2014), both known to be associated with compromised heart health in later life. Teen motherhood, in fact, is associated with welfare dependency and less prestigious occupations in young adulthood as well as poor mental and physical health outcomes in midlife (Hardy et al., 2009; Henretta, 2007). Through socialization of masculinities and femininities, women, particularly those in disadvantaged environments, are more likely than men to have household obligations of cooking and child-rearing, which are linked with gender differences in emotional distress, diet, and physical activity during parenthood (M. A. Martin & Lippert, 2012). These differences may put women at higher risk of developing heart problems than men. Yet, only one study (Gustafsson & Hammarström, 2012) has tested whether the life-course features of parenthood, e.g., having a child by age 21 and number of children by age 43, might mediate the association between childhood SES and risk of metabolic syndrome for middle-aged women.
Aims and hypotheses of the present study
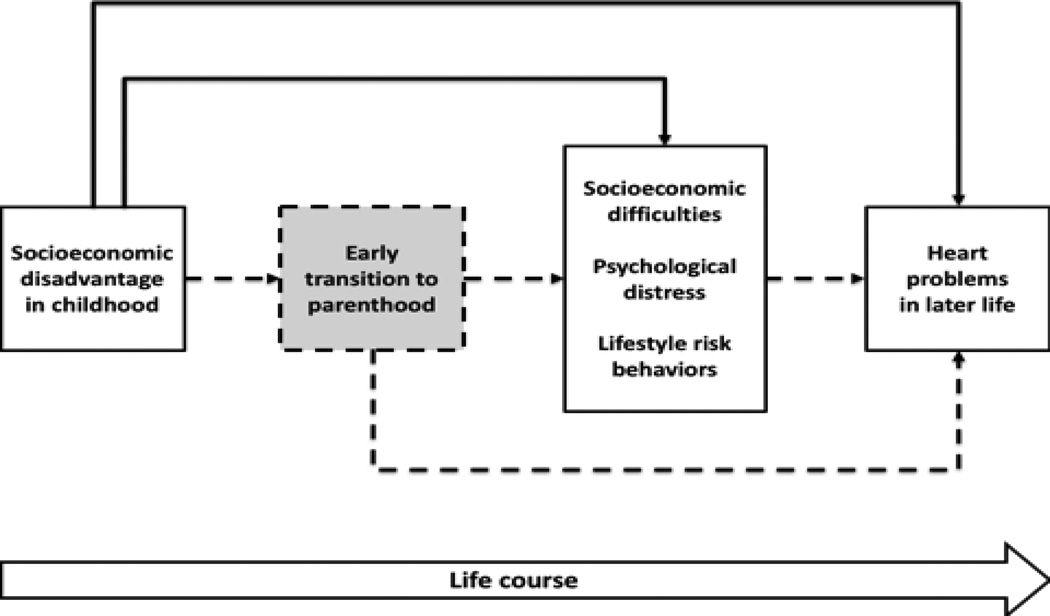
Figure 1 illustrates the conceptual framework guiding the present inquiry. It integrates concepts from research on critical periods, major life transitions, and life-course pathways. The dashed lines point to the mechanisms tested in the present study. The early transition to parenthood contributes to the association between childhood disadvantage and adult heart problems via life-course risk factors. Four key hypotheses emerge from this formulation.
H1: Individuals who were disadvantaged during childhood are likely to have their first child at younger ages.
H2: Particularly for women, the early transition to parenthood is associated with more financial strain and health-related problems in midlife, measured in terms of psychological distress and unhealthy lifestyle.
H3: Particularly for women, early transition to parenthood partially explains the association between childhood disadvantage and heart problems in later life.
H4: Material, psychological, and behavioral factors further explain why early transition to parenthood plays a significant role in accounting for the association between childhood disadvantage and adult heart problems.
Figure 1.
Conceptual model of life-course pathways of parenthood. The dashed pathways are proposed mechanisms that specify transition to parenthood as a mediator in the association between childhood disadvantage and heart problems in midlife and old age.
Data and Methods
The current study includes participants in MIDUS, a nationally representative survey designed to assess the role of social, psychological, and behavioral factors in understanding differences in mental and physical health (n=7,108; 52% women). MIDUS began in 1995/1996 (Wave [W] 1) with non-institutionalized, English-speaking adults aged 25 to 74 in the 48 contiguous states (Brim et al., 2004). MIDUS consists of a two-stage survey: a telephone interview and a self-administered questionnaire. Follow-up interviews with MIDUS respondents were completed every 9 to 10 years: n=4,963 in 2004–2006 (W2) and n=3,294 in 2013–2014 (W3). By W3 (October 2015), 1,299 respondents from W1 had died. Longitudinal retention rates for both W2 and W3 were 75% after adjustment for mortality.
Our analytic sample comprises data from the three MIDUS surveys. Because MIDUS has no information on respondent’s age when heart problems occurred, we focused on the incidence of heart problems between waves (i.e., W1–W2 and W2–W3), thus limiting the sample to respondents who had no heart problems at W1. For the first observational interval (W1–W2), we analyzed 4,342 respondents. For the second interval (W2–W3), we excluded respondents who had already developed heart problems in the first interval (because they are no longer at risk of incident heart problems); thus, we analyzed 2,722 respondents in the second interval. The analytic sample, therefore, included 7,064 observations across waves. There was an average of 1.6 observations per respondent over the two intervals.
Measures
To fully investigate the onset of heart problems between the beginning of each survey interval (t–1) and the end of each survey interval (t), all potential mediators are timing varying (i.e., measured at the beginning of each interval, t–1). Childhood disadvantage and all control variables, except number of children (time varying), are based on measurements at baseline.
Childhood disadvantage
Using four indicators (parental education, parental occupation, welfare status, and financial level growing up), we created a summary score of childhood disadvantage with a possible range of 0 to 8. Using highest levels of parental education reported by respondents, we created parental education, with higher scores indicating more disadvantage (0=some college, 1=high school graduate, 2=less than high school). Respondents reported each parent’s occupation during their adolescent years. Based on Duncan’s Socioeconomic Index (Hauser & Warren, 1997), we created a measure of parental occupational status, where the highest parental level of occupational prestige was coded as 0=top 40%, 1=30–60%, and 2=no job or bottom 30%. Respondents were asked whether, during childhood and adolescence, there was ever a period of six months or longer when their family was on welfare or Aid to Families with Dependent Children. We coded welfare status as 0=never on welfare and 2=ever on welfare. Finally, we created a measure of financial level growing up based on respondents’ recollections of their family’s financial situation when they were growing up, relative to the average family (0=better off than others, 1=about the same as others, 2=worse off than others).
Onset of heart problems
The outcome variable in this analysis is a series of repeated measures across waves of whether respondents had a doctor suspect or confirm that they had any heart problems, e.g., heart attack, angina, valve disease, blocked artery, irregular heartbeat, heart failure, or others. Onset of heart problems is indicated when respondents reported at least one type of heart problem at t, but had not reported any heart problems at t–1. We examine the onset of heart problems to ensure temporal ordering between intervening and outcome variables. Self-reports of cardiovascular events, such as stroke or heart attack, have been previously validated and used to assess the prevalence of heart problems (Okura et al., 2004).
Timing of transition to parenthood
Age at first child was calculated as the difference between the respondent’s age and the age of their oldest biological child. We created a set of five dummy variables to capture timing of transition to parenthood: (1) age 19 or younger, (2) ages 20–23, (3) ages 24+, and (4) nulliparous. Each cutoff value of parental timing represents a social turning point: age 19 is the threshold for teen parenthood, and 23 is the age by which most individuals complete their higher education/certification. Though prior studies have indicated that childbearing after age 35 is associated with increased risk of complications in pregnancy (Jolly et al., 2000), we did not create a separate category for ages 35+ for two reasons: (1) < 5% of respondents in our sample had their first child after age 35 and (2) there is a temporal ordering issue between timing of transition to parenthood and college graduation.
Life-course intervening factors
We included two measures of adult SES. College graduation is a dummy variable coded as “1” if respondents have a 2 year+ college degree or “0” if they did. Respondents were asked about three topics related to their financial strain: (1) level of difficulty paying monthly bills (1=not at all difficult to 4=very difficult), (2) having enough money for needs (1=more money than is needed to 3=not enough money), and (3) current financial situation (0=best to 10=worst). The internal consistency reliabilities of the three items were .83 (W1) and .85 (W2). We created financial strain index by standardizing the three indicators and summing them.
Difficulties in social relationships are a consequence of early parenthood and a significant predictor of heart disease (Hamil-Luker & O’Rand, 2007; J. L. Taylor, 2009). We thus included family strain, which was measured four items: how often family members made too many demands on the respondent, criticized them, let them down, or got on their nerves (response categories ranged from 1=never to 4=often). We created the measure of family strain by calculating the mean of the four items. A depressive symptom scale followed criteria in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R, 1987) and was measured by the short form of the Composite International Diagnostic Interview (CIDI), Version 10 (Kessler et al., 1998). The short-form of CIDI shows satisfactory validity and reliability (Wittchen, 1994). Respondents reported whether, in the past 12 months, they had a period of at least 2 weeks where they had experienced any of following seven items on most days: losing interest in most things, lacking energy, having poor eating or sleep habits, problems with concentration, feeling worthless, having suicidal thoughts or actions. The depression scale ranges from 0 to 7, with 0 indicating no measurable depression and scores between 1 and 7 indicating increasingly severe symptoms of depression.
Early childbearing may affect heart health via maladaptive health behaviors. We used three lifestyle risk measures: smoking, heavy drinking, and obesity. Smoking status is a set of three dummy variables: never smoked, formerly smoked, and currently smoking. Heavy drinking was coded as “1” for respondents who reported that they currently drink more than four drinks per day; “0” for others. Obesity is defined by body mass index (BMI) of 30 or above, calculated as kilograms/meters2 from self-reported weight and height.
Control variables
In addition, we adjusted for demographic covariates: age and race. Family history of heart attack was added to reduce the possibility of the intergenerational/genetic transmission of heart disease (Morton et al., 2014). To adjust for early health-based selection into both timing of transition to parenthood and heart health, we included childhood health (1=poor to 5=excellent), averaging the values of self-rated physical and mental health at age 16 (internal consistency=.70). Number of biological children was included as a control because of the significant link between parity and risk factors of heart disease, e.g., BMI (Wolfe et al., 1997).
Analytic strategy
One or more of the variables of interest were missing for respondents who participated in the two inter-survey periods, i.e., item-specific non-response: W1–W2 (17%) and W2–W3 (23%). To alleviate potential statistical power issues in subsequent analyses, we used Stata’s ICE command for multiple imputation (Royston, 2005). We implemented ten imputations to predict missing variables by generating imputed values, including dependent variables, and then deleting observations with imputed dependent variables (Von Hippel 2007). We confirmed that our overall conclusions were consistent using data before and after multiple imputations, although there was a lack of statistical power when individuals with missing data were removed.
For the descriptive statistics, we used t and χ2 tests to assess gender differences in each indicator. We performed a three-step analysis to test our hypotheses. First, using a multinomial logistic regression model with robust standard error estimation, we investigated whether individuals from lower SES backgrounds are more likely to have their first child at younger ages than higher SES individuals (Hypothesis 1). Second, we investigated the extent to which early transition to parenthood is associated with life-course intervening factors of heart problems (Hypothesis 2). Finally, we tested the role of timing of transition to parenthood as a pathway linking childhood disadvantage and onset of heart problems (Hypotheses 3 and 4). As noted above, the analyses included up to two observations for each respondent (W1–W2 and W2–W3). Thus, for the second and third steps of the analysis, we used random effects regression models (continuous variables) or random effects logit regression models (binary variables) to account for intra-individual correlation in repeated measures of outcome variables across the survey intervals and to reduce the likelihood of having biased estimates due to the omission of random effects. For the ordinal categorical outcome (i.e., smoking), we used a continuation ratio logit model by estimating two random effects logit models: a) the probability of ever having smoked vs. never having smoked and b) the probability, among those who have ever smoked, of being current smokers.
All required pathways meet the prerequisites for Baron and Kenny’s (1986) mediation model. The contributions of potential mediators were evaluated by (a) calculating changes in coefficients (percentage) and (b) comparing the significance levels of the coefficients between models. In the crude models, we estimated the effect of each predictor separately, while in the adjusted models we looked at the effects of predictors combined. To yield comparable estimates, all models included controls and continuous variables are standardized at mean (SD=1). Given that biological risk of heart disease, timing of entering parenthood, and underlying mechanisms vary by gender, we constructed gender-stratified models. Gender differences in the proposed hypotheses were explicitly tested by pooling data from both genders and testing gender interaction terms. We used STATA 14.0 to conduct the analyses (StataCorp, 2015).
Results
Table 1 shows gender differences in each variable. Women had lower risk of heart problems than men, but they were more likely to have their first child at younger ages. They also showed poorer profiles than men on education, financial and family strain, and depressive symptoms. However, women were less likely than men to engage in health risk behaviors, such as smoking and drinking.
Table 1.
Descriptive Statistic by Interval and Gender, Mean, (SD) or Proportion (n)
| Beginning (t–1) – End (t) of survey interval | Wave 1 (t–1) – Wave 2 (t) | Wave 2 (t–1) – Wave 3 (t) | ||||
|---|---|---|---|---|---|---|
| Women (n=2,328) |
Men (n=2,014) |
p-value | Women (n=1,521) |
Men (n=1,201) |
p-value | |
| Childhood disadvantage at baseline (Wave 1) | 3.09(1.95) | 2.91(1.93) | <.01 | 2.99(1.95) | 2.66(1.84) | <.001 |
| Onset of heart problems between t–1 and t | 9%(220) | 13%(266) | <.001 | 10%(153) | 15%(177) | <.001 |
| Timing of transition to parenthood at t–1 | ||||||
| Nulliparous | 21%(487) | 25%(510) | <.01 | 17%(259) | 21%(250) | <.05 |
| Under age 20 | 18%(430) | 5%(106) | <.001 | 17%(253) | 4%(52) | <.001 |
| Ages 20–23 | 27%(633) | 21%(430) | <.001 | 26%(396) | 18%(220) | <.001 |
| Ages 24+ | 33%(778) | 48%(968) | <.001 | 40%(613) | 57%(679) | <.001 |
| Life-course factors at t–1 | ||||||
| College graduation | 38%(881) | 49%(988) | <.001 | 45%(688) | 57%(683) | <.001 |
| Standardized financial strain index | −.04(1.03) | −.06(.96) | <.01 | −.02(1.02) | .14(.93) | <.001 |
| Family strain | 2.16(.61) | 2.05(.58) | <.001 | 2.11(.59) | 2.01(.57) | <.001 |
| Depressive symptoms | .83(1.99) | .52(1.57) | <.001 | .72(1.86) | .30 (1.21) | <.001 |
| Smoking status | ||||||
| Never smoked | 30%(702) | 20%(400) | <.001 | 31%(475) | 22%(263) | <.001 |
| Formerly smoked | 50%(1165) | 60%(1210) | <.001 | 55%(838) | 65%(781) | <.001 |
| Current smoker | 20%(404) | 20%(461) | .832 | 14%(208) | 13%(157) | .647 |
| Heavy drinking | 18%(426) | 41%(834) | < .001 | 20%(303) | 42%(508) | <.001 |
| Obesity | 23%(522) | 24%(475) | .364 | 29%(446) | 27%(321) | .135 |
| Covariates at baseline | ||||||
| Age | 45.96(12.63) | 45.46(11.95) | .182 | 44.95(11.45) | 44.08(10.75) | <.05 |
| Non-white | 8%(193) | 6%(128) | <.05 | 7%(111) | 6%(71) | .151 |
| Family history of heart attack | 36%(847) | 36%(731) | .952 | 36%(543) | 35%(426) | .901 |
| Childhood health | 4.24(.81) | 4.32(.77) | <.001 | 4.23(.81) | 4.31(.78) | <.05 |
| Number of children at t – 1 | 2.02(1.53) | 1.83(1.47) | <.001 | 2.13(1.50) | 1.92(1.39) | <.001 |
Timing of transition to parenthood
In Table 2, we found a modest and statistically significant association between childhood disadvantage and early transition to parenthood. For every SD increase in childhood disadvantage, women were 1.95 times more likely to have their first child before age 20 than at ages 24+. Similarly, women were 1.48 times more likely to have their first child at ages 20–23 (vs. ages 24+). However, childhood disadvantage had no significant effect on women’s chances of being nulliparous vs. having their first child at ages 24+. Similar patterns were observed for men; those who experienced disadvantage in childhood were more likely to have their first child before age 20 or at ages 20–23 (vs. ages 24+). Childhood SES had no significant effect on whether men had their first biological child at ages 24+ or did not have a biological child. Childhood disadvantage was more strongly associated with teen parenthood (vs. ages 24+) more so for women than men, although the gender difference was marginally significant (p=.07).
Table 2.
Multinomial Regression Model Estimates (Relative Risk Ratio) for the Effects of Childhood Disadvantage on the Timing of Transition to Parenthood
| Nulliparous | Ages 24+ (Reference) |
Ages 20–23 |
Under age 20 |
|
|---|---|---|---|---|
| RRR | RRR | RRR | RRR | |
| Panel 1: Women | ||||
| Childhood disadvantage | .94 | 1.00 | 1.48*** | 1.95*** |
| Panel 2: Men | ||||
| Childhood disadvantage | 1.06 | 1.00 | 1.52*** | 1.68*** |
p < .001
Life-course intervening factors
Table 3 shows that early parenthood had significant impacts on most life-course factors even after controlling for childhood disadvantage. Compared to women who had their first child after age 23, those who become mothers as teenagers had lower rates of college graduation (OR=.05). In midlife, they reported more financial difficulties (Beta=.23), and higher levels of depressive symptoms (Beta=.23), and greater risk of obesity (OR=1.75). They were more likely to have ever smoked (OR=2.31) and be smokers over time (OR=9.85). Similarly, but to a lesser degree, having a first child between ages 20 and 23 (vs. ages 24+) was associated with lower rates of having a college degree (OR=.14), higher levels of depressive symptoms (Beta=.12) and being current smokers (OR=3.29). For men, those who had a first child before age 20 (vs. ages 24+) were more likely to be poorly educated (OR=.03), depressed (Beta=.20), ever having smoked (OR=16.40), being continuous smokers (OR=5.83), and heavy drinkers (OR=2.45). A similar pattern, but a lesser degree, was found for men who had a first child between ages 20 and 23, in terms of education, smoking, and drinking. There were greater negative effects of early parenthood on financial strain (p=.08; below 20 vs. ages 24+) and depressive symptoms (p=.09; ages 20–23 vs. ages 24+) for women than men, although this association is only marginally significant. Distinctively, early parenthood has a greater impact on heavy drinking (p<.01; ages 20–23 vs. ages 24+) and (ever) smoking for men than women (p<.01; under 20 vs. ages 24+)
Table 3.
Random Effects Estimates for the Effects of Childhood Disadvantage and Timing of Transition to Parenthood on Life-course Factors
| College graduation (logit) |
Financial strain (OLS) |
Family strain (OLS) |
Depressive symptoms (OLS) |
Never vs. Ever smoked (logit) |
Former vs. Current smoker (logit) |
Heavy drinking (logit) |
Obesity (logit) |
|
|---|---|---|---|---|---|---|---|---|
| OR | Beta | Beta | Beta | OR | OR | OR | OR | |
| Panel 1: Women | ||||||||
| Crude models | ||||||||
| Childhood disadvantage | .23*** | .10*** | .04* | .04 | 1.14 | 1.56*** | 1.29** | 1.40** |
| Adjusted models | ||||||||
| Childhood disadvantage | .28*** | .08*** | .04 | .02 | 1.05 | 1.34* | 1.27** | 1.36** |
| Timing of transition to parenthooda |
||||||||
| Nulliparous | 1 .00 | −.09 | −.01 | .04 | .65 | 2.11* | 1.26 | 1.43 |
| Ages 20–23 | .14*** | .08 | −.01 | .12* | 1.33 | 3.29*** | .82 | 1.13 |
| Under age 20 | .05*** | .23*** | .06 | .23*** | 2.31** | 9.85*** | 1.41 | 1.75* |
| Panel 2: Men | ||||||||
| Crude models | ||||||||
| Childhood disadvantage | .25*** | .13*** | .05* | . 04** | 1.35* | 2.01*** | 1.58*** | 1.56*** |
| Adjusted models | ||||||||
| Childhood disadvantage | .28*** | .12*** | .06** | . 04** | 1.27* | 1.81*** | 1.51*** | 1.54*** |
| Timing of transition to parenthooda |
||||||||
| Nulliparous | 1.10 | −.14** | −.04 | .01 | .73 | 1.02 | 1.43 | .87 |
| Ages 20–23 | .12*** | .03 | −.08 | −.01 | 1.62 | 3.79*** | 1.92** | 1.06 |
| Under age 20 | .03*** | .09 | .00 | .20** | 16.40*** | 5.83*** | 2.45* | 1.53 |
Ages 24+ is the reference group.
OLS=ordinary least squares regression model; Logit=logistic regression model; Beta=standardized coefficient; OR=odds ratio
p < .05,
p < .01,
p < .001
Onset of heart problems in later life
Table 4 presents a series of models to test Hypotheses 3 and 4. By comparing the coefficients of childhood disadvantage in Model 1vs. Model 2a, we assessed the intervening effects of life-course factors. Similarly, we estimated the extent to which timing of parenthood explained the association between childhood disadvantage and onset of heart problems by comparing coefficients in Model 1 vs. Model 2b. Finally, in Model 2c, we included all potential mediators to evaluate (a) intervening effects of all mediators in the association between childhood disadvantage and onset of heart problems (Models 1 vs. 2c) and (b) intervening effects of life-course factors in the association between timing of parenthood and onset of heart problems (Models 2b vs. 2c). The percentage change in the coefficients (β) that were statistically significant in Model 1 was calculated as 100 × (βmodel 2 – βmodel 1)/βmodel 1., where β=log (odds). A summary of the percentage change is included as an Appendix.
Table 4.
Random Effects Logit Regression Estimates (Odds Ratio) for the Effects of Childhood Disadvantage and Life-course Factors on Onset of Heart Problems
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Crude Models |
Adjusted models | Crude Models |
Adjusted models | |||||
| Model 1 | Model 2a | Model 2b | Model 2c | Model 1 | Model 2a | Model 2b | Model 2c | |
| Childhood disadvantage | 1.21** | 1.14* | 1.16* | 1.12 | 1.14* | 1.07 | 1.13 | 1.07 |
|
Timing of transition to parenthood |
||||||||
| Nulliparous | .87 | .86 | .88 | .81 | .81 | .81 | ||
| Ages 20–23 | 1.50** | 1.42* | 1.37* | 1.19 | 1.15 | 1.09 | ||
| Under age 20 | 1.69** | 1.54** | 1.40* | 1.22 | 1.16 | 1.01 | ||
| Life-course factors | ||||||||
| College graduation | .72** | .85 | .90 | .73** | .84 | .85 | ||
| Financial strain | 1.28*** | 1.16* | 1.16* | 1.04 | .97 | .97 | ||
| Family strain | 1.32*** | 1.24*** | 1.24*** | 1.12 | 1.09 | 1.09 | ||
| Depressive symptoms | 1.17** | 1.09 | 1.08 | 1.05 | 1.02 | 1.02 | ||
| Formerly smoked | 1.36* | 1.32* | 1.32* | 1.09 | 1.05 | 1.04 | ||
| Current smoker | 1.59** | 1.40 | 1.34 | 1.44* | 1.35 | 1.33 | ||
| Heavy drinking | 1.03 | .87 | .88 | 1.11 | .95 | .95 | ||
| Obesity | 1.36* | 1.22 | 1.21 | 1.95*** | 1.89*** | 1.89*** | ||
Ages 24+ and never smoked are the reference groups for timing of transition to parenthood and smoking status, respectively.
p < .05,
p < .01,
p < .001
For women in Model 1, for each additional SD increase of childhood disadvantage, the risk of developing heart problems increased by 1.21. After adding life-course factors, the coefficient decreased by 31% from 1.21 to 1.14, indicating that these factors partially explained the association between childhood disadvantage and heart problems. Yet, the coefficient of childhood disadvantage remained significant (p < .05). Introducing timing of parenthood decreased the coefficient of childhood disadvantage from 1.21 to 1.16 (22%, Model 1 vs. Model 2b). The main effects of timing of transition to parenthood remained significant: compared to women who had their first child after age 23, those who had their first child before age 20 (OR=1.42) and between ages 20 and 23 (OR=1.54) had higher risk of developing heart problems. Finally, including all mediators resulted in non-significance in the coefficient of childhood disadvantage. The coefficient of timing of parenthood before age 20, though still significant, was attenuated from 1.54 to 1.40 (22%, Model 2b vs. Model 2c), indicating that these life-course mediators partially explained the association between teen motherhood and onset of heart problems in later life. However, the magnitude of the change in coefficients of having their first child at ages 20–23 was relatively small (10%).
For men, the association between childhood disadvantage and heart problems was largely mediated by life-course factors as shown by a substantial attenuation in the coefficient of childhood disadvantage between Model 1 and Model 2a (from 1.14 to 1.07; 48 %). There was little change in the coefficient of childhood disadvantage between Model 1 and Model 2b (6%), and none of the coefficients of timing of parenthood was statistically significant, indicating that transition to parenthood contributed little to the association between childhood disadvantage and heart problems for men. Overall, Table 4 supported Hypotheses 3 and 4—for women, but not for men, early transition to parenthood explained the association between childhood disadvantage and adult heart problems. Life-course factors partially explained why women who had their first child at younger ages were at greater risk of heart problems.
Discussion
The present study explored why and how childhood disadvantage increases the risk of heart problems in adulthood. The dominant explanations in prior work are that socioeconomic disadvantage persists from childhood to adulthood, contributing to future heart problems through enhanced psychosocial vulnerabilities and the use of health-threatening practices to cope with stress. However, prior studies found that the effects of childhood disadvantage remained significant, especially for women, even after accounting for these mechanisms. We included the timing of parenthood as a key intervening pathway linking childhood disadvantage to onset of heart problems via life-course risk factors. Our study yielded four major findings.
First, we showed that early childbearing plays a critical role in the association between childhood disadvantage and onset of heart problems for women. To our knowledge, only one study (Gustafsson & Hammarström, 2012) has investigated the intervening role of reproductive events in the association between early-life SES and the health (metabolic syndrome) of middle-aged women, but the results were non-significant. This outcome may have reflected the limited temporal measurement of parenthood, e.g., did the respondent have a child by age 21. We operationalized the timing of transition to parenthood using a broader range of cutoff values, including adolescence and later ages, as well as biological childlessness into midlife. Our approach revealed that the timing of the transition to parenthood explained around one fifth of the association between childhood disadvantage and women’s adult heart problems. Consistent with prior work, the effect of childhood disadvantage on heart problems declined substantially for both men and women when life-course factors were included as mediators. However, we added the timing of the transition to parenthood as an additional mediator, which resulted in an additional decline in the effect of childhood disadvantage for women and the effect of childhood disadvantage on heart problems no longer being statistically significant. This finding indicates that timing of parenthood is an important but neglected mediator in prior research.
Our linking age at the birth of first child to women’s health problems replicate prior findings (Hardy et al., 2009; Henretta, 2007; J. L. Taylor, 2009), but we further reveal that early childbearing, especially before age 20, is likely to result in heart problems for women in midlife and old age, several decades after their first delivery. This new knowledge has important implications for women’s health disparities in the U.S., where despite declining for the more than half a century, the teen birth rate remains higher than the rate in most other developed countries (Ventura et al., 2014). Given that structural problems, such as poverty and income inequality, are well-known precursors to teen births (Gold et al., 2002), our findings suggest that intervening along the pathway from childhood disadvantage to delayed parenthood would pay dividends by decreasing the prevalence of heart problems, particularly for women. However, interventions to reduce early childbearing may require deeper understanding of cultural practices and identity accomplishments for women from high-poverty communities, where teen births and kin support of childrearing are common (Geronimus, 2003). Some poor women who had children at earlier ages may view parenthood as a way to find meaning and purpose in life in the midst of limited life opportunities (Edin & Kefalas, 2011).
Third, we found that early transition to parenthood has long-term effects on various life-course risk factors. Having a first child as a teenager or in early young adulthood interferes with higher educational attainment for both genders, but only women, are likely to encounter financial difficulties in midlife as a consequence of teen parenthood. Alternatively, having a first child during teenage years is significantly associated with elevated depressive symptoms and being current smokers in midlife for both genders. Teen parenthood is significantly associated with obesity for women and heavy drinking for men in midlife. Such outcomes are illuminated by the stress process model which suggests that stressors are rooted in one’s social location, which is based on individual characteristics including age, race, and gender (Pearlin, 1989). Major life events, such as having a child, can trigger broader role transitions that increase exposure to chronic stressors over the life course. Women may thus be more likely than men to adopt stress-soothing but self-damaging lifestyle behaviors, such as consuming more unhealthy foods and being inactive, in response to parenting stress (Nomaguchi & Bianchi, 2004; Reczek, 2014), while men are more likely to cope their stress via alcohol use (Esper & Furtado, 2013).
Finally, the attenuation of the association between timing of parenthood and heart problems after adjusting for potential mediators suggests that lifestyle-related factors in midlife partially drive the association. We found that encountering economic difficulties and engaging in health-threatening practices in midlife, such as smoking and being obese, partially explains why women who had their first child as teenagers are at risk of developing heart problems in later life. Obesity and smoking may thus be chronic health-threatening practices from childhood or adolescence. Childhood disadvantage is more strongly tied to obesity for women than men, which may reflect poor family environments that influence obesity-related lifestyle behaviors, such as diets high in fat or sugar and physical inactivity. Girls are known to be more vulnerable than boys to such lifestyles (Crossman et al., 2006; K. S. Martin & Ferris, 2007). Given that teen mothers are more likely to smoke during pregnancy (Cornelius et al., 2007), significant associations between early motherhood and midlife smoking in our study indicate that smoking habits in early life may persist into later life, thus contributing to cumulative risk for adult heart problems. In contrast to findings for women, timing of parenthood had little effect on men’s heart health. We expected that having a first child at younger ages would make demands on men, too, adding to their workload and prompting unhealthy coping mechanisms, such as smoking and heavy drinking, in addition to parenting stress. Yet, we saw no evidence of an association with men’s heart problems in later life.
The limitations of our study should be noted. First, the etiology and latency of individual heart problems could not examined because only 10% of individuals developed any heart problems between waves. It is possible that some heart problems are more strongly related than others to childhood disadvantage and early parenthood. Second, our analysis was limited to those who survived until Wave 2 or Wave 3. Since those who struggled most with early-life disadvantage may thus have been lost due to attrition or death, our results might be underestimates. In addition, to establish temporal ordering between mediators and the outcome variable, we limited our sample to individuals who did not have any heart problems in Wave 1. Thus, individuals who were disadvantaged in early life and developed heart problems at earlier ages were excluded from our analyses. Third, if there was variation in access to medical care by SES, then measuring heart problems as suspected or confirmed by a doctor may have led to underestimation of actual onset of heart problems for low SES individuals. Fourth, the study relied on retrospective recall of childhood SES, which might produce some recall bias. However, the validity of recall of childhood SES is supported in twin studies (Krieger et al., 1998), and if retrospective reports have caused any biases, it is likely to be underestimation (Senese et al., 2009). Finally, it is difficult to rule out the possibility that omitted variables produced the observed association in this study. Some communities may promote teen parenthood (or stigmatize it to a lesser degree) while individuals in these communities may be more likely to engage in heart-harming lifestyles.
Despite these limitations, our study is the first to document the gendered pathway from childhood disadvantage to heart problems for older adults by incorporating a major life transition, namely, the transition to parenthood, into the life-course pathway model. Our findings provide new insights into older women’s health, particularly for those who suffered from early-life disadvantages. While most research on childhood disadvantage and adult health has focused on psychosocial and behavioral risk factors, our work reveals the importance of when individuals become parents for understanding the journey from early-life adversity to adult heart health.
Supplementary Material
Highlights.
The timing of parenthood was examined as a pathway linking childhood disadvantage to adult heart problems.
Individuals from disadvantaged families are more likely to have their first child at younger ages.
For women, early parenthood partially explains why childhood disadvantage increases risk of heart problems.
Psychosocial distress and unhealthy behaviors partially explain why early parenthood harms heart health.
Acknowledgments
Research reported in this publication was supported by grants from the National Institute On Aging of the National Institutes of Health (K99AG052458) and the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (UL1TR000427). The work was also supported by the National Institute on Aging (P01AG020166) to conduct a longitudinal follow-up of the MIDUS investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Support also came from M01RR023942 (Georgetown) and M01RR00865 (UCLA) from the General Clinical Research Centers Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Dana Glei for helpful comments on analytic strategies.
REFERENCES
- Adamo KB, Ferraro ZM, Brett KE. Pregnancy is a critical period for prevention of obesity and cardiometabolic risk. Canadian Journal of Diabetes. 2012;36:133–141. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beebe-Dimmer J, Lynch JW, Turrell G, Lustgarten S, Raghunathan T, Kaplan GA. Childhood and adult socioeconomic conditions and 31-year mortality risk in women. American Journal of Epidemiology. 2004;159:481–490. doi: 10.1093/aje/kwh057. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–293. [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. How healthy are we?: A national study of well-being at midlife. University of Chicago Press; 2004. [Google Scholar]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: Effects on behavioral problems in offspring. Nicotine & Tobacco Research. 2007;9:739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman A, Anne Sullivan D, Benin M. The family environment and American adolescents’ risk of obesity as young adults. Social Science and Medicine. 2006;63:2255–2267. doi: 10.1016/j.socscimed.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Denton M, Prus S, Walters V. Gender differences in health: A Canadian study of the psychosocial, structural and behavioural determinants of health. Social Science and Medicine. 2004;58:2585–2600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Denton M, Walters V. Gender differences in structural and behavioral determinants of health: an analysis of the social production of health. Social Science and Medicine. 1999;48:1221–1235. doi: 10.1016/s0277-9536(98)00421-3. [DOI] [PubMed] [Google Scholar]
- deVries B, Watt D. A lifetime of events: Age and gender variations in the life story. International Journal of Aging and Human Development. 1996;42:81–102. doi: 10.2190/FM22-V5VT-B60Y-6UGC. [DOI] [PubMed] [Google Scholar]
- Edin K, Kefalas M. Promises I can keep: Why poor women put motherhood before marriage. University of California Press; 2011. [Google Scholar]
- Esper LH, Furtado EF. Gender differences and association between psychological stress and alcohol consumption: A systematic review. Journal of Alcoholism and Drug Dependence. 2013;1:116. [Google Scholar]
- Fletcher JM, Wolfe BL. Education and labor market consequences of teenage childbearing evidence using the timing of pregnancy outcomes and community fixed effects. Journal of Human Resources. 2009;44:303–325. [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Geronimus AT. Damned if you do: Culture, identity, privilege, and teenage childbearing in the United States. Social Science and Medicine. 2003;57:881–893. doi: 10.1016/s0277-9536(02)00456-2. [DOI] [PubMed] [Google Scholar]
- Gold R, Kennedy B, Connell F, Kawachi I. Teen births, income inequality, and social capital: Developing an understanding of the causal pathway. Health & Place. 2002;8:77–83. doi: 10.1016/s1353-8292(01)00027-2. [DOI] [PubMed] [Google Scholar]
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: A review of the evidence. Annals of Behavioral Medicine. 2003;26:149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Hammarström A. Socioeconomic disadvantage in adolescent women and metabolic syndrome in mid-adulthood: An examination of pathways of embodiment in the Northern Swedish Cohort. Social Science and Medicine. 2012;74:1630–1638. doi: 10.1016/j.socscimed.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Hamil-Luker J, O’Rand AM. Gender differences in the link between childhood socioeconomic conditions and heart attack risk in adulthood. Demography. 2007;44:137–158. doi: 10.1353/dem.2007.0004. [DOI] [PubMed] [Google Scholar]
- Hardy R, Lawlor DA, Black S, Mishra GD, Kuh D. Age at birth of first child and coronary heart disease risk factors at age 53 years in men and women: British birth cohort study. Journal of Epidemiology and Community Health. 2009;63:99–105. doi: 10.1136/jech.2008.076943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. Sociological Methodology. 1997;27:177–298. [Google Scholar]
- Henretta JC. Early childbearing, marital status, and women’s health and mortality after age 50. Journal of Health and Social Behavior. 2007;48:254–266. doi: 10.1177/002214650704800304. [DOI] [PubMed] [Google Scholar]
- Heraclides A, Brunner E. Social mobility and social accumulation across the life course in relation to adult overweight and obesity: the Whitehall II study. Journal of Epidemiology and Community Health. 2010;64:714–719. doi: 10.1136/jech.2009.087692. [DOI] [PubMed] [Google Scholar]
- Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Human Reproduction. 2000;15:2433–2437. doi: 10.1093/humrep/15.11.2433. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization composite international diagnostic interview short-form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. [Google Scholar]
- Khalil RA. Potential approaches to enhance the effects of estrogen on senescent blood vessels and postmenopausal cardiovascular disease. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2010;8:29–46. doi: 10.2174/187152510790796156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi E, Fallah M, Luoto R. Maternal age at first delivery and risk of cardiovascular disease later in life. ISRN Epidemiology. 2013;2013:1–6. [Google Scholar]
- Klassen SA, Chirico D, O’Leary DD, Cairney J, Wade TJ. Linking systemic arterial stiffness among adolescents to adverse childhood experiences. Child Abuse and Neglect. 2016;56:1–10. doi: 10.1016/j.chiabu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Krieger N, Okamoto A, Selby JV. Adult female twins’ recall of childhood social class and father’s education: A validation study for public health research. American Journal of Epidemiology. 1998;147:704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- Martin KS, Ferris AM. Food insecurity and gender are risk factors for obesity. Journal of Nutrition Education and Behavior. 2007;39:31–36. doi: 10.1016/j.jneb.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Martin MA, Lippert AM. Feeding her children, but risking her health: The intersection of gender, household food insecurity and obesity. Social Science and Medicine. 2012;74:1754–1764. doi: 10.1016/j.socscimed.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton PM, Mustillo SA, Ferraro KF. Does childhood misfortune raise the risk of acute myocardial infarction in adulthood? Social Science and Medicine. 2014;104:133–141. doi: 10.1016/j.socscimed.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Barrett-Connor E, Kass Wenger N. Sex/Gender Differences in Cardiovascular Disease Prevention: What a Difference a Decade Makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomaguchi KM, Bianchi SM. Exercise time: Gender differences in the effects of marriage, parenthood, and employment. Journal of Marriage and Family. 2004;66:413–430. [Google Scholar]
- Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Pearlin LI. The sociological study of stress. Journal of Health and Social Behavior. 1989;30:241–256. [PubMed] [Google Scholar]
- Pears KC, Pierce SL, Kim HK, Capaldi DM, Owen LD. The timing of entry into fatherhood in young, at-risk men. Journal of Marriage and Family. 2005;67:429–447. doi: 10.1111/j.0022-2445.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Hertzman C. Social and biological pathways linking early life and adult disease. British Medical Bulletin. 1997;53:210. doi: 10.1093/oxfordjournals.bmb.a011601. [DOI] [PubMed] [Google Scholar]
- Reczek C, Beth Thomeer M, Lodge AC, Umberson D, Underhill M. Diet and exercise in parenthood: A social control perspective. Journal of Marriage and Family. 2014;76:1047–1062. doi: 10.1111/jomf.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Mutliple imputation of missing values: Update of ice. Stata Journal. 2005;5:527–536. [Google Scholar]
- Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between childhood socioeconomic position and adulthood obesity. Epidemiologic Reviews. 2009;31:21–51. doi: 10.1093/epirev/mxp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty-Venugopal V, Upadhyay UD. Birth spacing: Three to five saves lives. Population Reports. Series L: Issues in World Health. 2002:1–23. [PubMed] [Google Scholar]
- Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity. 2010;18:1996–2003. doi: 10.1038/oby.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- Stein DJ, Scott K, Abad JMH, Aguilar-Gaxiola S, Alonso J, Angermeyer M, et al. Early childhood adversity and later hypertension: Data from the World Mental Health Survey. Annals of Clinical Psychiatry. 2010;22:19–28. [PMC free article] [PubMed] [Google Scholar]
- Taylor JL. Midlife impacts of adolescent parenthood. Journal of family issues. 2009;30:484–510. doi: 10.1177/0192513X08329601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72:1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Avison WR. Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. Journal of Health and Social Behavior. 2003:488–505. [PubMed] [Google Scholar]
- Umberson D, Pudrovska T, Reczek C. Parenthood, childlessness, and well-being: A life course perspective. Journal of Marriage and Family. 2010;72:612–629. doi: 10.1111/j.1741-3737.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura SJ, Hamilton BE, Matthews T. National and state patterns of teen births in the United States, 1940–2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2014;63:1–34. [PubMed] [Google Scholar]
- Wheaton B. Life transitions, role histories, and mental health. American Sociological Review. 1990:209–223. [Google Scholar]
- Wittchen H-U. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Wolfe WS, Sobal J, Olson CM, Frongillo EA. Parity-associated body weight: Modification by sociodemographic and behavioral factors. Obesity Research. 1997;5:131–141. doi: 10.1002/j.1550-8528.1997.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. National Vital Statistics Reports. 2016:64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.