Abstract
Pharmacological activation of the heptahelical G protein-coupled receptor GPER by selective ligands counteracts multiple aspects of cardiovascular disease. We thus expected that genetic deletion or pharmacological inhibition of GPER would further aggravate such disease states, particularly with age. To the contrary, we found that genetic ablation of Gper in mice prevented cardiovascular pathologies associated with aging by reducing superoxide (.O2−) formation by NADPH oxidase (Nox) and reduced expression the Nox isoform Nox1. Blocking GPER activity pharmacologically with G36, a synthetic, small molecule, GPER-selective blocker (GRB), decreased Nox1 abundance and .O2− production to basal amounts in cells exposed to angiotensin II and in mice chronically infused with angiotensin II. Thus, this study revealed a role for GPER activity in regulating Nox1 abundance and associated .O2−-mediated structural and functional damage that contributes to disease pathology. Our results indicated that GRBs represent a new class of drugs that can indirectly reduce Nox activity and could be used for the treatment of chronic disease processes involving excessive .O2− formation, including arterial hypertension and diastolic heart failure.
Introduction
G protein-coupled receptors (GPCRs) exert both rapid and chronic effects (1). The G protein-coupled estrogen receptor GPER is a heptahelical receptor, originally designated GPR30, with amino acid homology to GPCRs for angiotensin II (Ang II) and chemokines, that is found in multiple cell types including vascular cells (2-4). GPER is localized predominantly to the endoplasmic reticulum and Golgi apparatus (5) and mediates cellular responses to estrogens, selective estrogen receptor modulators (SERMs), and xenoestrogens (6) through non-genomic as well as genomic mechanisms (5, 7-9). GPER activation results in the rapid mobilization of intracellular calcium (5, 10, 11) and activation of nitric oxide (NO) synthase (12), Akt (11, 13-16) and ERK (7, 11, 16), among other pathways (6). In addition, GPER, like many other GPCRs (1, 17), may also exhibit basal or intrinsic activities that contribute to the chronic regulation of genomic pathways. Such genomic effects have been suggested to be responsible for the increased vasoconstrictor tone observed in Gper-deficient mice (18). The beneficial cardiovascular effects of GPER-selective synthetic ligands in multiple disease models (4, 12, 19) have led to the currently prevailing concept that activation of GPER conveys organ protection (6, 11, 20-23).
Reactive oxygen species (ROS) are short-lived intermediates of oxidative metabolism that are essential for cardiovascular homeostasis (24). Excessive ROS production, however, occurs in many chronic disease processes and aggravates vasoconstriction and cell growth, thereby contributing to increased vascular tone, myocardial hypertrophy, fibrosis, heart failure, and aging (25, 26). The NADPH oxidase (Nox) family represents the principal physiological source of ROS in the cardiovascular system and is composed of 7 catalytic subunits termed Nox1-5 and Duox1-2 (27-29). Of these, Nox1, Nox2 and Nox4 have been implicated in both experimental and human hypertension and heart failure, yet their role(s) in cardiovascular aging are less understood (27, 28).
Given that studies utilizing the GPER-selective agonist G-1 have demonstrated that GPER activation conveys partial protection from vascular and myocardial disease (4, 13, 30) and given the central role of ROS in these chronic disease processes (24, 25, 27), we expected that pathologies characterized by increased bioavailability of ROS, particularly those associated with aging, would be exacerbated in the absence of functional GPER, resulting in even greater ROS production. We therefore set out to determine the functional and structural effects of genetic deletion (20) as well as pharmacologic inhibition (31, 32) of GPER on ROS-dependent pathologies affecting the cardiovascular system.
Results
GPER increases Nox-dependent vascular .O2− and vascular tone in aged arteries
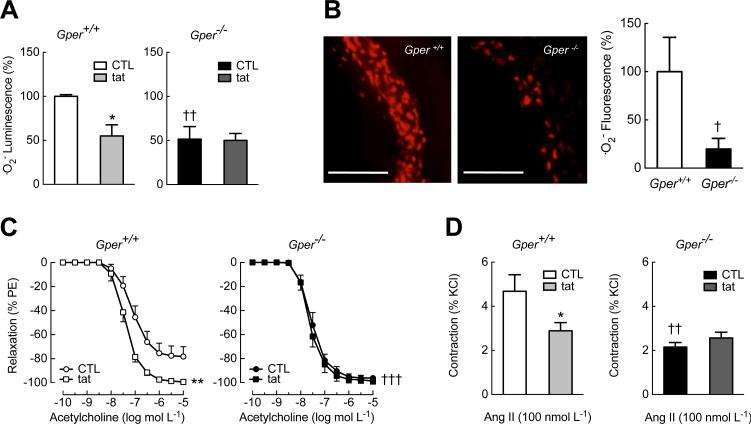
We first examined the effects of Gper deletion on vascular oxidative stress by measuring the production of the unstable free radical superoxide (.O2−) in the aorta of aged mice. To determine whether Nox enzymes are involved in the generation of .O2−, we used a peptide termed gp91dstat (33), which is derived from a gp91phox (now named Nox2) sequence in the region that interacts with the organizer protein p47phox, thus disrupting p47phox binding to and activation of associated catalytic Nox subunits, particularly Nox2 (34), but also Nox1 in vascular smooth muscle cells (VSMCs) (29, 35-37). We found that in aged wild-type mice, ~50% of .O2− formation was Nox-dependent as it was blocked by gp91ds-tat (Fig. 1A, left panel). In contrast to our expectation of exacerbated .O2− production, .O2− formation in aged Gper−/− mice was instead blunted by ~50-80% compared to wild-type mice (Fig. 1, A and B) and was unaffected by gp91ds-tat treatment (Fig. 1A), suggesting an inactive or absent Nox-mediated .O2−-producing pathway.
Fig. 1.
Genetic deletion of Gper abrogates Nox activity and prevents enhanced vasoconstriction in vascular aging. Intact arteries of aged (24 month-old) wild-type (Gper+/+) and Gper−/− mice were analyzed. (A), (B) Nox activity was determined by measuring vascular superoxide (.O2−) production using chemiluminescence (A) or DHE fluorescence (B, scale bar, 200 μm). To quantify the amount of .O2− generated by Nox, subsets of arteries were treated with the Nox inhibitor gp91ds-tat (tat). (C), (D) Endothelium-dependent, NO-mediated vasodilation in response to acetylcholine (C) and contractions to angiotensin II (Ang II, D) in intact arteries in the presence or absence of gp91ds-tat (tat). Data are mean±sem; n = 3–4 mice per group in (A), n = 5–10 mice per group in (B), n = 4–5 mice per group in (C), (D). *P < 0.05, **P < 0.01 compared to control (CTL); †P < 0.05, ††P < 0.01, †††P < 0.001 compared to wild-type mice (ANOVA with Bonferroni post-hoc tests in (A), (D); repeated measures ANOVA with Bonferroni post-hoc tests in (C); Student's t-test in (B)).
We next determined the effects of aging on vascular tone, which is characterized by increased .O2− formation that inactivates endothelium-derived vasodilatory NO (38). Impaired endothelium-dependent vasodilation represents an important predictor of mortality in patients with heart failure and hypertension (38-41). As expected, NO-mediated endothelium-dependent relaxation induced by acetylcholine (42) was reduced in aged (Fig. 1C) compared to young wild-type mice (Fig. S1A). This impairment was completely reversed by incubating arteries with gp91ds-tat, restoring vasodilation to an extent similar to that observed in young mice (Fig. 1C and fig. S1A). Further supporting the notion that impaired NO bioactivity was a result of oxidative stress in aged wild-type mice, the smooth muscle sensitivity to NO alone, generated by an exogenous NO donor, was not affected by aging (Fig. S2). In contrast, aged Gper−/− mice were completely protected from the impairment in endothelium-dependent vasodilation observed in aged wild-type mice; in fact, the vasodilatory capacity was preserved and identical to that of young mice (Fig. 1C, fig. S1A and fig. S2).
In agreement with these observations, we found that in aged wild-type mice, vascular contractions in response to Ang II (a vasoactive peptide that stimulates Nox (43, 44)) were partially (~50%) blocked by gp91ds-tat (Fig. 1D), whereas gp91ds-tat had no effect on Ang II-mediated contractions of arteries from aged Gper−/− mice. In line with the reduced .O2− formation in Gper−/− mice (Fig. 1, A and B, and fig. S1B), contractions in response to Ang II were attenuated in aged (Fig. 1D) as well as in young Gper−/− mice (fig. S1C). These findings, which contrast with the protective vascular role of Gper expression and/or GPER stimulation reported in previous studies (4, 12, 13, 30, 45), indicate instead that constitutive Gper expression is essential for increased vascular Nox bioactivity as well as Nox-mediated vasoconstriction and impaired endothelial cell function, particularly in the context of vascular aging.
GPER deletion prevents structural and functional cardiac aging and myocardial dysfunction
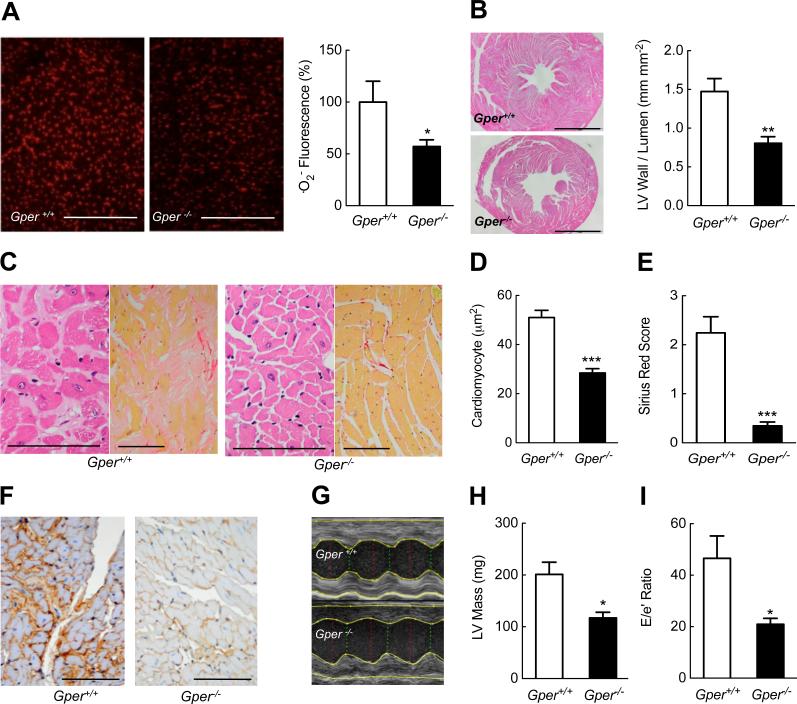
To determine whether Gper-dependent regulation of oxidative stress also plays a role in age-dependent structural and functional cardiac abnormalities, we next assessed .O2− production in the aging heart. Compared to wild-type mice, myocardial .O2− amounts were markedly lower in aged Gper−/− mice (Fig. 2A). Given that oxidative stress is centrally involved in the structural changes that occur with cardiac aging (25, 26), we next examined myocardial histopathology. Whereas aging increased the left ventricular (LV) wall-to-lumen ratio by ~60% in wild-type mice, Gper−/− mice were completely protected from age-dependent myocardial hypertrophy (Fig. 2B and fig. S3A). In addition, histological analyses of the myocardium of Gper−/− mice revealed an absence of cardiomyocyte hypertrophy (Fig. 2, C and D). Organ failure resulting from fibrosis accounts for at least one third of deaths worldwide (46), with myocardial fibrosis being a key feature of cardiac aging (25, 26). Aging in wild-type mice was associated with prominent and diffuse interstitial myocardial fibrosis and collagen IV accumulation, which again was generally absent in aged Gper−/− mice (Fig. 2, C, E and F). The cardioprotective effects of Gper deletion on myocardial fibrosis and hypertrophy were already detectable at 12 months of age (although the differences were less prominent due to the reduced disease pathology in the wild-type mice), resulting in a lower LV wall-to-lumen ratio (fig. S3A), reduced cardiomyocyte hypertrophy (fig. S3B) and reduced myocardial fibrosis, as assessed by Sirius Red (fig. S3C) and collagen IV (fig. S3D) staining, although the reduction in the former did not reach significance at this age.
Fig. 2.
Obligatory role for GPER in aging cardiomyopathy and diastolic dysfunction. Hearts of aged (24 month-old) wild-type and Gper−/− mice were analyzed. (A) Myocardial superoxide (.O2−) production determined by DHE fluorescence. Scale bar, 500 μm. (B) Myocardial hypertrophy as measured by left ventricular wall-to-lumen ratio in cardiac cross sections. Scale bar, 2 mm. (C) Representative histologic myocardial sections stained with hematoxylin-eosin (left, scale bar, 100 μm) and Sirius Red (right, scale bar, 200 μm). Cardiomyocyte hypertrophy analyzed by cross-sectional area (D), quantitation of interstitial fibrosis (E) and representative histologic sections stained for type IV collagen (F, scale bar, 100 μm). (G-I) Dimensions and function of the left ventricle (LV) determined by echocardiography. Representative parasternal M-mode images (G), calculated LV mass (H) and measures of LV filling pressures and diastolic dysfunction (E/e’ ratio, I) are shown. Data are mean±sem; n = 4–5 mice per group in (A), n = 6– 7 mice per group in (B), n = 6–8 mice per group in (C-F), n = 3 mice per group in (G-I). *P < 0.05, **P < 0.01, ***P < 0.001 compared to wild-type mice (Student's t-test).
Given that Gper deletion prevented the structural cardiac abnormalities observed with aging, we next determined whether this translated into improved myocardial function in vivo. Echocardiography confirmed the marked increase in LV relative wall thickness and mass in wild-type mice compared to Gper−/− mice (Fig. 2G and 2H and table S1). Consistent with the reduced ventricular fibrosis and stiffness, analysis of LV filling and diastolic mitral valve annulus velocities (47) revealed improved diastolic function and lower LV filling pressures in aged Gper−/− mice (Fig. 2I and table S1). Together, the overall absence of myocardial fibrosis and hypertrophy in aged Gper−/− mice translated into increased ventricular elasticity, as indicated by improved LV diastolic filling. These differences were independent of changes in systolic LV function or systemic hemodynamics (Table S1).
GPER is essential for .O2− production in murine and human VSMCs through Nox1
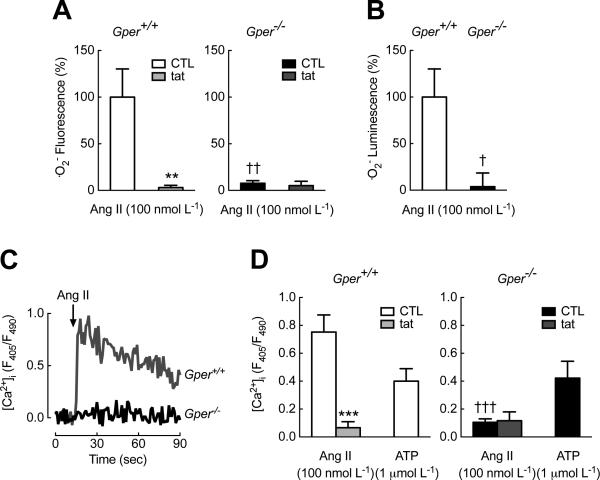
Cardiac fibrosis involves an age-dependent localized activation of the renin-angiotensin system (RAS) (41, 46, 48), with its primary vasoactive peptide Ang II also promoting premature senescence through the induction of Nox (49, 50). Moreover, Ang II-induced ROS promote redox-sensitive cell functions such as intracellular calcium mobilization and contraction (51). Having established that Gper deficiency abrogates Nox-generated .O2− production in aged mice, we next examined the underlying mechanisms of the molecular regulation in vascular smooth muscle cells (VSMCs) isolated from wild-type and Gper−/− mice (fig. S4, A and B). Consistent with the activation of Nox in intact arteries of wild-type mice (Fig. 1), Ang II-stimulated .O2− production in wild-type VSMCs was inhibited by gp91ds-tat (Fig. 3A, left panel). In cells lacking Gper, the Ang II-stimulating effect on .O2− generation was completely absent (Fig. 3, A and B), which was confirmed by electron paramagnetic resonance (EPR) spectroscopy using BMPO as a spin trap for .O2− (52-54) (fig. S5). Similarly, Ang II-induced, Nox-dependent mobilization of intracellular calcium (51) was absent in Gper-deficient VSMCs (Fig. 3, C and D). By contrast, intracellular calcium mobilization responses to the purinergic receptor agonist ATP (a Nox-independent stimulus (55)) were comparable in VSMCs from wild-type and Gper−/− mice, thus excluding inherent defects in calcium signaling in VSMCs lacking Gper (Fig. 3D). In addition, absence of Gper did not affect the expression of the genes encoding the Ang II AT1A and AT1B receptors (fig. S4C).
Fig. 3.
GPER is required for Ang II-induced superoxide (.O2−) production and mobilization of intracellular calcium in VSMCs. Nox activity stimulated by Ang II was inhibited using gp91dstat (tat). (A), (B) Ang II-induced .O2− production detected by DHE fluorescence (A) and chemiluminescence (B). (C), (D) Mobilization of intracellular calcium ([Ca2+]i) determined utilizing the Ca2+ sensor dye indo1-AM in response to Ang II (representative tracing in C, cumulative data in D) and ATP (D). F405/F490, ratiometric detection of emitted light at 405 nm and 490 nm. **P < 0.01, ***P < 0.001 compared to control (CTL); †P < 0.05, ††P < 0.01, †††P < 0.001 compared to VSMCs isolated from wild-type (Gper+/+) mice. Data are mean±sem; n = 5–7 independent experiments per group in (A), n = 5 independent experiment per group in (B), 3–5 independent experiments per group in (D), all with VSMCs from 2 independent isolations. ANOVA with Bonferroni post-hoc tests in (A), (D); Student's t-test in (B).
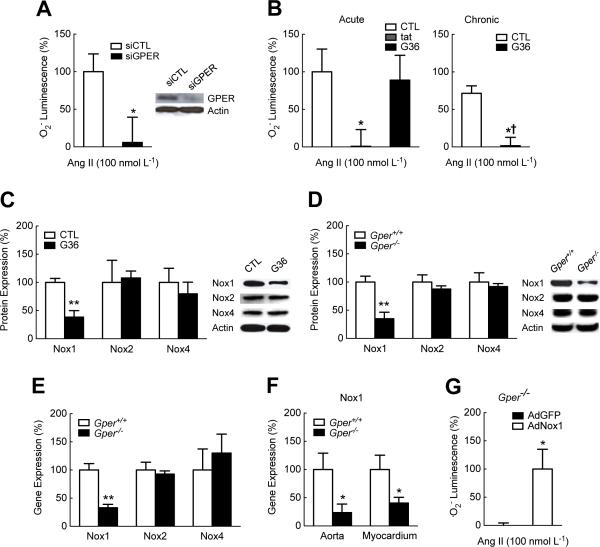
We next sought to determine whether the effects of GPER on .O2− production observed in murine VSMCs extended to human VSMCs. Knockdown of GPER with siRNA abolished the ability of primary human VSMCs to generate .O2− in response to Ang II (Fig. 4A). To determine whether the effects of GPER were mediated through rapid non-genomic signaling alone or involved long-term genomic effects, we treated human VSMCs with the GPER-selective antagonist G36, a synthetic, small molecule GPER blocker (GRB) (32). Acute treatment (30 min) with gp91ds-tat, but not G36, abolished Ang II-stimulated .O2− production (Fig. 4B). In contrast, prolonged treatment with G36 (for 72 h) completely abrogated Ang II-induced .O2− formation (Fig. 4B), suggestive of mechanisms regulating gene transcription. Consistent with the lack of acute effects, G36 did not display direct antioxidant activity (fig. S6).
Fig. 4.
GPER increases Nox1 abundance and activity. (A) Ang II-induced superoxide (.O2−) production detected by chemiluminescence in human VSMCs with GPER-targeted gene silencing (siGPER). *P < 0.05 compared to control siRNA (siCTL). siGPER treatment decreased GPER protein abundance by 89+/-5% compared to siCTL treatment (representative data shown in inset). (B), (C) Ang II-induced .O2− production (detected by chemiluminescence, B) and protein abundance of Nox1, Nox2 and Nox4 (C) in human VSMCs treated with the GPER-selective antagonist G36 (1 μmol L−1) for 30 min (acute, B) or 72 h (chronic, B, C). Nox activity was inhibited using gp91ds-tat (tat). *P < 0.05, **P < 0.01 compared to control (CTL, DMSO 0.01%); †P < 0.05 compared to acute treatment. (D), (E) Protein (D) and mRNA (E) abundance of Nox1, Nox2 and Nox4 in VSMCs isolated from wild-type (Gper+/+) and Gper−/− mice. **P < 0.01 compared to wild-type mice. (F) Nox1 mRNA abundance in aorta and myocardium of aged (24 month-old) Gper+/+ and Gper−/− mice. *P < 0.05 compared to Gper+/+. (G) Ang II-induced .O2 − production detected by chemiluminescence in VSMCs isolated from Gper−/− mice and infected with Nox1-expressing adenovirus (AdNox1). *P < 0.05 compared to vector control (AdGFP). Data are mean±sem; n = 3 independent transfections per group in (A), n = 4–8 independent experiments per group in (B), n = 3–4 independent experiments and Western blots per group in (C) and (D), n = 3-4 VSMC preparations in (E), n = 4–10 mice per group in (F), n = 3 independent infections per group in (G). ANOVA with Bonferroni post-hoc tests in (B); Student's t-test in (A), (C-G).
Given that Nox inhibition by gp91ds-tat reduced vascular .O2− production in mice as well as in murine and human VSMCs only in the presence of GPER, we next determined whether the vascular abundance of Nox1, Nox2 or Nox4 catalytic subunits, which have been implicated in both experimental and human hypertension (27-29), was affected by intrinsic GPER activity. Although gp91ds-tat is traditionally thought only to disrupt Nox2 activity (33), studies have demonstrated that gp91ds-tat also blocks .O2−-mediated effects mediated by Nox1 in VSMCs, likely through its interaction with p47phox (56, 57). In fact, p47phox in VSMCs facilitates activation of Nox1 (58), the closest homologue of Nox2, but not that of Nox4 (59), and also mediates Ang II-induced, redox-dependent signaling (37, 60). Feed-forward mechanisms have also been observed in which ROS production by one Nox subtype or other sources results in the activation of additional Nox subtype(s), suggesting that inhibition of any intermediate could block downstream events (61, 62). We found that in human VSMCs treated with G36 for 72 h, the protein abundance of Nox1 was reduced by ~70% compared to solvent, whereas that of Nox2 and Nox4 was unaffected (Fig. 4C). Similarly, only the protein abundance of Nox1, but not that of Nox2 or Nox4, was substantially lower in murine VSMCs from Gper−/− mice as compared to wild-type mice (Fig. 4D). The reduced Nox1 protein abundance was commensurate with a similar reduction in the mRNA abundance in VSMCs isolated from Gper−/− mice, with gene expression of Nox2 and Nox4 again being unaffected (Fig. 4E). Gper deficiency also reduced Nox1 mRNA abundance in the aorta and myocardium of aged Gper−/− mice (Fig. 4F), both of which displayed markedly reduced .O2− bioactivity compared to wild-type mice (Fig. 1 and 2). To verify that the decreased Nox1 abundance indeed accounted for the inability of Gper-deficient VSMCs to generate .O2− in response to Ang II, we restored Nox1 abundance in these cells using a Nox1-expressing adenovirus. Reintroduction of Nox1 into Gper-deficient VSMC restored their capacity to generate .O2− in response to Ang II (Fig. 4G), further suggesting an obligatory role for GPER in the increase in Nox1 abundance and associated ROS-dependent cellular functions.
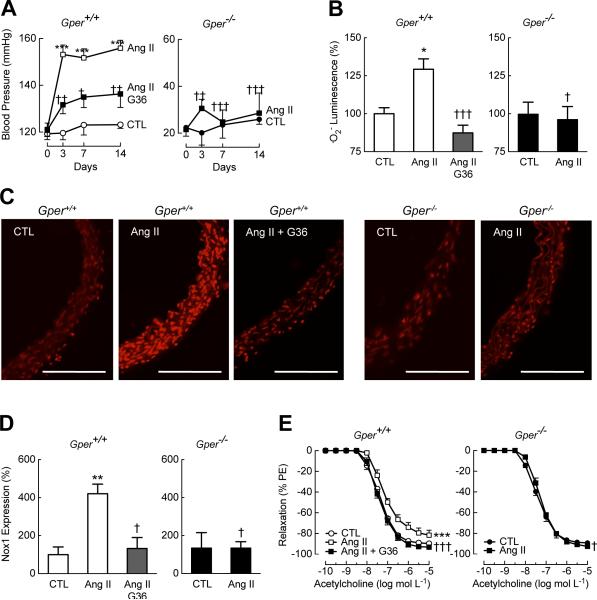
Genetic ablation or pharmacologic inhibition of GPER prevents arterial hypertension
To explore whether the protective effects of Gper deletion extended to cardiovascular disease conditions other than those associated with aging, we increased Nox1 abundance and activity in vivo by infusing mice with Ang II, a critical mediator of Nox1-depdendent .O2− production, vascular dysfunction and increased vascular tone (44). Animals lacking Gper were resistant to the Ang II-induced increase in blood pressure observed in wild-type mice (Fig. 5A). Furthermore, vascular .O2− generation and the increase in vascular Nox1 abundance in mice in response to Ang II infusion required the presence of Gper (Fig. 5, B to D). As previously reported in wild-type mice (44), .O2− generated in response to Ang II impaired endothelial cell function as evident from the blunted NO-dependent vasodilation in response to acetylcholine. By contrast, the attenuation of the vasodilator response was completely absent in Gper−/− mice infused with Ang II (Fig. 5E). In line with .O2−-mediated impairment of NO bioactivity, the inherent vascular smooth muscle sensitivity to NO, as determined with an exogenous NO donor, was not affected by either Ang II infusion or by GPER deficiency or inhibition (fig. S7). These data further confirm that Gper is required to increase Nox1 abundance and the resulting .O2− production, vascular dysfunction and increases in vascular tone.
Fig. 5.
Genetic or pharmacologic ablation of GPER prevents Ang II-induced hypertension, oxidative stress, and increases in Nox1 abundance. Wild-type (Gper+/+) and Gper−/− mice were infused with Ang II (0.7 mg kg−1 per day) or vehicle (control, CTL) for 14 days. A subset of wild-type mice was also treated with the GRB G36. (A) Systolic arterial blood pressure in conscious animals. (B), (C) Vascular .O2− generation as measured by chemiluminescence (B) and DHE staining (C, scale bars, 300 μm). (D) Vascular Nox1 protein abundance detected by immunofluorescence. (E) Endothelium-dependent, NO-mediated vasodilation in response to acetylcholine. Data are mean±sem; n = 4–5 mice in (A), n = 3–5 mice in (B), n = 3–4 mice in (C), n = 3 mice in (D), n = 5–9 mice in (E). *P < 0.05, **P < 0.01, ***P < 0.001 compared to genotype-matched CTL; †P < 0.05, ††P < 0.01, †††P < 0.001 compared to Ang II-treated wild-type mice (repeated measures ANOVA with Bonferroni post-hoc tests in (A), (E); ANOVA with Bonferroni post-hoc tests in (B), (D)). PE, phenylephrine.
The Nox pathway has been recognized as a therapeutic target for ROS-dependent pathologies in humans (46, 63, 64). To determine in vivo whether decreasing Nox1 protein by pharmacological GPER inhibition can be achieved, we again utilized the GRB G36 (32). Not only did G36 treatment prevent the Ang II-mediated increase in Nox1 protein abundance (Fig. 5D), it also markedly reduced vascular .O2− production (Fig. 5, B and C) and restored the vasodilatory response (Fig. 5E). These effects of G36 resulted in a substantial inhibition of the Ang II-mediated increase in blood pressure (Fig. 5A). Given that GPER mediated increases in Nox1 protein abundance, these results identify GRBs as a member of a new class of drugs that act as Nox down-regulators.
Discussion
The results presented in the current study demonstrate that inhibiting GPER activity conveys protection from myocardial and vascular diseases associated with increased Nox1-derived oxidative stress, including cardiovascular aging and arterial hypertension. These data may seem counterintuitive at first when compared to the current body of evidence suggesting the protective role of GPER-selective agonists (GRAs) in the cardiovascular system (4). GRAs, such as G-1 (10), rapidly activate Nox-independent pathways thought to mediate salutary vascular effects, such as Akt and ERK (4). In particular, G-1, unlike the GRB G36, induces eNOS phosphorylation and the subsequent generation of NO, which mediates indirect antioxidant effects through inactivation of .O2− (12). Thus, both GRBs and GRAs improve disease outcome by reducing ROS activity through the same receptor, albeit through entirely distinct mechanisms. These findings place GPER at the center of the balance between the beneficial L-arginine-NO synthase pathway and harmful excessive ROS generation. Such dichotomous effects also exist for other hormones (such as insulin) with the ultimate (patho)physiogical effect dependent upon the stage of the disease process (65).
Our results have identified new and important chronic functions of intrinsic GPER activity that determine Nox1 abundance. Indeed, regulation of gene expression through constitutive signaling is prevalent among G protein-coupled receptors (17). The GPER-dependent increase in Nox1 abundance and Nox1-dependent ROS formation was likely a key factor in the pathogenesis of increased vasoconstriction associated with hypertension or aging, as well as age-dependent cardiac remodeling. Indeed, arterial hypertension, left-ventricular hypertrophy and the associated diastolic dysfunction in humans are important predictors of the development of heart failure (26), the prevalence of which increases with age and has reached epidemic proportions (66).
The present study has certain limitations. The results were obtained in experimental models of vascular and myocardial aging, heart failure and arterial hypertension. Investigating the association between oxidative excess and cardiovascular injury in humans is complicated because most of these patients are on ROS-inhibiting treatments (such as angiotensin converting enzyme inhibitors and statins), which makes it difficult to unmask mechanisms as described in the present study (67). Moreover, whether human Nox enzymes such as Nox5, which is not encoded in the rodent genome (27, 63), are involved in these disease processes requires further study. Given the results from the in vitro studies with primary vascular human cells, it remains to be shown whether G36 inhibits Nox1 activity in humans, which would also require monitoring the safety of GRB treatment. Because ROS are implicated in the progression of many chronic non-communicable diseases, GRBs may find application in a broader array of indications.
In summary, this study has identified an obligatory role for the intrinsic activity of GPER as an activator of Nox1 expression that mediates structural and functional injury in vascular and myocardial diseases. Although the role of ROS in the aging process and chronic diseases is well recognized, simple scavenging of ROS with antioxidants has been largely unsuccessful therapeutically (68), likely due to the localized production and ensuing effects of ROS. Inhibition of Nox activity through the use of small molecules, some with limited selectivity (69), is currently being evaluated in clinical trials. The present study however introduces a new class of drugs, Nox down-regulators, which, as shown for G36, reduce Nox protein abundance, thus directly limiting .O2− production by one of its main sources. Therapeutically reducing the expression of Nox could provide an effective approach to target chronic disease conditions involving excessive Nox-mediated .O2− formation. The therapeutic application of GRBs may include prevention of organ injury due to Nox-dependent chronic diseases such as heart failure and arterial hypertension, while also treating or delaying pathologies associated with aging and rare diseases such as progeria syndromes (40).
Materials and Methods
Study Design
The aim of this study was to explore whether and through which mechanisms GPER promotes cardiovascular oxidative stress induced by aging or Ang II. For this purpose, we used wild-type and Gper-deficient mice as a model of aging, as well as mice chronically infused with Ang II. In addition, the GRB G36 was employed to test whether pharmacological targeting of GPER reduced oxidative stress. Detailed mechanistic studies were carried out in primary VSMCs isolated from wild-type and Gper−/− mice, as well as in human VSMCs. Mice were randomly and equally assigned to different treatment groups. Animal studies were conducted in a controlled and non-blinded manner. Study designs included: (i) two-way factorial designs comparing Gper+/+ and Gper−/− mice, in the presence or absence of inhibitors; (ii) similar two-way factorial designs with repeated measures (concentrations, time); (iii) one-way factorial repeated measure designs within Gper+/+ cohort, controlling for baseline differences, over time and (iv) two-way factorial designs with repeated measures designs between the Gper+/+ and Gper−/− groups, controlling for baseline differences, over time.
Transgenic mice and aging model
Male Gper−/− mice (provided by Jan S. Rosenbaum, Proctor & Gamble Co.) were generated and backcrossed 10 generations onto the C57BL/6 background (Harlan Laboratories) as described (12). Wild-type C57BL/6 and Gper−/− littermates were housed at the Animal Resource Facility of the University of New Mexico Health Sciences Center under controlled temperature of 22–23 °C on a 12 h light-dark cycle with unrestricted access to standard chow and water. Mice aged 24 months, which show functional and structural changes resembling human cardiovascular aging (25), were used as a model of aging. Animals were euthanized at the age of 4, 12 or 24 months by intraperitoneal injection of sodium pentobarbital (2.2 mg g−1 body weight). All procedures were approved by and carried out in accordance with institutional policies and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic Ang II infusion model
Micro-osmotic pumps (Alzet model 1002, Durect) were implanted subcutaneously in the midscapular region of wild-type and Gper−/− mice under isoflurane (3%) anesthesia. Pumps continuously delivered PBS or Ang II (MP Biomedicals) at a rate of 0.7 mg kg−1 per day for 14 days (43, 44). Three days prior to pump implantation, pellets continuously releasing the GRB G36 (32) (33 μg per day, Innovative Research of America) or placebo were implanted subcutaneously into the right hindlimb of a subset of wild-type mice.
Vascular smooth muscle cells
Primary aortic VSMCs from wild-type and Gper−/− mice (n = 13 per genotype) were isolated and cultured as described (12). Human aortic VSMCs (Lonza) were cultured according to the provider's recommendations. Experiments were performed with cells derived from passages 2 to 5 for murine and 2 to 8 for human VSMCs. For functional assays, cells at sub-confluence were rendered quiescent by overnight serum starvation.
Measurement of superoxide (.O2−) by lucigenin-enhanced chemiluminescence
Following euthanization, the aorta was immediately excised, carefully cleaned from perivascular adipose and connective tissue, opened longitudinally, and cut into segments of identical size (3 mm) in cold (4 °C) physiological saline solution (PSS, composition in mmol L−1: 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 0.43 NaH2PO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose; pH 7.4). Tissues were transferred and equilibrated in HEPES-buffered PSS (composition in mmol L−1: 134 NaCl, 6 KCl, 1 MgCl, 10 HEPES, 2 CaCl2, 0.026 EDTA, and 10 glucose; pH 7.4) in a humidified incubator at 37 °C for 60 min. In addition to intact isolated arteries, vascular smooth muscle cells (VSMCs) and a cell free .O2− generating system by adding the substrate xanthine (100 μmol L−1, Calbiochem) to xanthine oxidase (0.05 mU, Calbiochem) were employed. Chemiluminescence was measured in dark-adapted HEPES-PSS containing 5 μmol L−1 lucigenin (Enzo Life Sciences) at 37 °C (70). After equilibrating for 15 min, .O2− production was induced by Ang II (100 nmol L−1) (70). Where indicated, tissues or cells were pretreated with the Nox-selective inhibitor gp91ds-tat (Anaspec, 3 μmol L−1) (28, 33), the GRB G36 (32) (10 nmol L−1, 100 nmol L−1, or 1 μmol L−1), the .O2− dismutase mimetic tempol (100 μmol L−1, Tocris Bioscience) (71), or vehicle (DMSO 0.01%). Luminescence was measured 10-times in 20 sec intervals using a Synergy H1 multi-mode microplate reader (BioTek), and readings were averaged to reduce variability (70). A background reading was subtracted, and .O2− production normalized to surface area of vascular segments (72) or to VSMC number (70), respectively.
In-situ detection of .O2− by dihydroethidium (DHE)
The thoracic aorta was equilibrated in HEPES-PSS in a humidified incubator at 37 °C for 60 min, and treated with the Nox-selective inhibitor gp91ds-tat (3 μmol L−1) (28, 33) for 30 min when indicated. Tissues were frozen in optimum cutting temperature (O.C.T.) compound (Sakura Finetek), cut on a cryostat into 10 μm thick sections, and stored on glass slides at –80 °C. For staining, sections were incubated with DHE (5 μmol L−1, Invitrogen) in HEPES-PSS for 15 min at room temperature in the dark (73). In separate experiments, VSMCs were grown on poly-L lysine coated coverslips, which were incubated with DHE (5 μmol L−1) in HEPES-PSS for 30 min at 37 °C in the dark (73). Where indicated, VSMCs were pretreated with Nox-selective inhibitor gp91ds-tat (3 μmol L−1) (28, 33) for 30 min, and .O2− production was stimulated by Ang II (100 nmol L−1) for 20 min prior to imaging. Slides with VSMCs or aortic sections were carefully washed, mounted in HEPES-PSS with coverslips, and immediately imaged by epifluorescence microscopy (Axiovert 200M, Zeiss) using a rhodamine filter with exposure intensity adjusted to background fluorescence (73). Signal intensity was quantified using ImageJTM software (National Institutes of Health).
.O2− detection by spin trapping combined with electron paramagnetic resonance (EPR) spectroscopy
The nitrone 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO, Enzo Life Sciences) was used as the spin trap for .O2− generated from VSMC, which was monitored using EPR spectroscopy as described (52-54). Briefly, serum-starved VSMCs were suspended in serum-free medium supplemented with BMPO (50 mmol L−1) and diethylenetriaminepentaacetic acid (100 μmol L−1), and .O2− production was stimulated by Ang II (100 nmol L−1, 30 min at 37°C). Supernatant containing spin-trapped .O2− was snap-frozen in liquid nitrogen and stored at −80 °C for less than one week. After thawing, supernatant was immediately transferred to custom-made gas-permeable Teflon tubing (Zeus Industries), folded four times, and inserted into a quartz EPR tube open at each end. The quartz EPR tube was inserted into the cavity of an EPR spectrometer (EleXsys 540 X-band, Bruker) operating at 9.8 GHz and 100-kHz field modulation, and the spectra of BMPO-OOH, spin-trapped .O2−, was recorded after spectrometer tuning at room temperature. The EPR spectrum was acquired with a scan time of 40 s, and 20 scans were obtained and averaged to produce significant signal-to-noise ratio. Instrument settings were as follows: magnetic field, 3,509 G; scan range, 70 G; microwave power, 21 mW; modulation frequency, 100 kHz; modulation amplitude, 1.0 G; time constant, 20 ms. The EPR spectra were collected, stored, and manipulated using Xepr software (Bruker).
Vascular reactivity studies
The aorta was immediately excised after euthanization, carefully cleaned from perivascular adipose and connective tissue and cut into 2 mm long rings in cold (4 °C) PSS. Aortic rings were mounted in myograph chambers (multi-channel myograph system 620M, Danish Myo Technology) onto 200 μm pins (18, 74). A PowerLab 8/35 data acquisition system and LabChart Pro software (AD Instruments) were used for recording of isometric tension. Experiments to determine vascular reactivity of aortic rings were performed as described (18, 74). Briefly, rings were equilibrated in PSS (37 °C; pH 7.4; oxygenated with 21% O2, 5% CO2, and balanced N2) for 30 min and stretched step-wise to the optimal amount of passive tension for force generation. Functional integrity of vascular smooth muscle was confirmed by repeated exposure to KCl (PSS with substitution of 60 mmol/L potassium for sodium), with resulting contractions demonstrating no differences between groups. Selected arteries were pretreated with the Nox-selective inhibitor gp91ds-tat (3 μmol L−1) (28, 33) for 30 min. Contractions to Ang II (100 nmol L−1) were studied in the abdominal aorta in the presence of the NO synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME, 300 μmol L−1, incubation for 30 min, Cayman Chemical) (75) to exclude Ang II-mediated release of NO (76). Ang II-induced contractions exhibit rapid desensitization in the mouse vasculature with a nearly complete loss of tension after about 2 min, thus preventing the recording of responses to increasing concentrations (75, 77). To study endothelium-dependent, NO-mediated relaxations, rings from the thoracic aorta were precontracted with phenylephrine (Sigma-Aldrich) to 80% of KCl-induced contractions, and responses to acetylcholine (0.1 nmol L−1 – 10 μmol L−1, Sigma-Aldrich) were recorded. Similarly, endothelium-independent, NO-mediated relaxations to sodium nitroprusside (SNP, 1 – 10 μmol L−1, MP Biomedicals) were determined. Precontraction did not differ between groups. To exclude any GPER-dependent effects on vasoconstrictor prostanoids (18), responses were obtained in the presence of the cyclooxygenase-inhibitor meclofenamate (1 μmol L−1, incubation for 30 min, Cayman Chemical). Contractions were calculated as the percentage of contraction to KCl, and relaxation was expressed as the percentage of phenylephrine-induced precontraction.
Cardiac histopathology
After sacrifice, hearts were excised, fixed in 4% paraformaldehyde and embedded into paraffin blocks. Histological sections (2 μm) were stained with hematoxylin-eosin, Sirius Red or by immunohistochemistry using a polyclonal goat antibody recognizing collagen IV (Southern Biotech) as described (78). Morphometric analysis of free left ventricle wall thickness and ventricular lumen area was performed using light microscopy at 400-fold magnification and cellSensTM software (Olympus), with left ventricular wall thickness based on analysis of 10 randomly selected measure points. Cardiomyocyte cross-sectional area was determined by analysis of 15 anterolaterally located cardiomyocytes using cellSens software. Myocardial fibrosis on Sirius Red or collagen type IV stained paraffin sections was graded using a semi-quantitative fibrosis score (0 = no staining; 1 = less than 25%; 2 = 26–50 %; 3 = 51–75%; 4 = more than 75% of cardiac tissue with positive staining). For each heart, the mean score evaluated on 10 power fields at 200-fold magnification was calculated.
High-resolution, high-frequency echocardiography
Mice were lightly sedated using inhaled isoflurane anesthesia, placed on a heat-pad to maintain body temperature, and echocardiography was performed using a Vevo® LAZR photoacoustic imaging system (VisualSonics) using high-resolution, high-frequency ultrasound at 40 mHz (47, 79). Conventional B-mode, M-mode, pulsed wave- and tissue-doppler images were acquired by an experienced, blinded operator to ensure a standardized, consistent technique, and LV dimensions were quantified as described (47, 79). LV ejection fraction was determined by speckle-tracking based wall motion analysis (47, 79, 80) using VevoStrain software (VisualSonics). Analysis of diastolic function included transmitral flow velocity waveforms obtained from pulsed-wave Doppler to calculate the ratio of early (E)-to-late (atrial, A) LV filling velocities (E/A ratio), and the mitral annulus diastolic velocity (e’ waves) obtained from pulsed-wave tissue Doppler imaging as well as the calculated E/e’ ratio as measures of diastolic function and LV filling pressures.
Measurement of arterial blood pressure
Systolic blood pressure was measured in conscious mice using a volume-pressure recording noninvasive monitoring system (CODA-6, Kent Scientific) as described (12), which produces blood pressure readings with similar sensitivity and specificity as invasive measurements (12). This blood pressure measurement technique has successfully been applied in the chronic Ang II infusion model (43, 44).
Intracellular calcium mobilization
VSMCs were loaded with 5 μmol L−1 indo1-AM (Invitrogen) and 0.05% pluronic F-127 (Invitrogen) in Hanks’ buffered salt solution (HBSS) supplemented with NaCl (150 mmol L−1), CaCl2 (2 mmol L−1), and HEPES (20 mmol L−1; pH 7.4) for 30 min at room temperature in the dark. Cells were washed and resuspended in HBSS (106 cells per mL), and calcium mobilization in response to Ang II (100 nmol L−1) and adenosine triphosphate (ATP, 1 μmol L−1, Sigma-Aldrich) was determined ratiometrically using λex 340 nm and λem of 405 and 490 nm at 37 °C in a QM-2000-2 spectrofluorometer (Photon Technology International).
VSMC transduction with Nox1 adenovirus or transfection with GPER-targeted siRNA
VSMCs from Gper−/− mice were plated at ~600,000 cells per T25 flask, washed in PBS, and infected with Nox1GFP and GFP control adenovirus constructs (59) at 400 MOI overnight in low serum (1% FBS) DMEM. Cells were allowed to recover for 48 h prior to experiments. Transduction efficiency was determined by GFP expression. For siRNA, human aortic VSMCs were transfected with siGPER (Dharmacon ON-Targetplus J-005563-08) or control (siCTL) siRNA (Dharmacon ON-Targetplus D-001810-02) using Lipofectamine 2000 (Invitrogen) for 6–8 h in serum-free medium, washed, and returned to normal medium as described by the manufacturer. Subsequent experiments were performed 72 h after transfection.
Quantification of gene and protein expression
RNA was extracted, reverse-transcribed and analyzed using SYBR Green-based detection of amplified gene-specific cDNA fragments by qPCR performed in triplicate as described (18). The following primer pairs have been used: 5′-CAT CCA GTC TCC AAA CAT GAC A-3′ (forward) and 5′-GCT ACA GTG GCA ATC ACT CCA G-3′ (reverse) for amplification of a specific cDNA fragment encoding mouse Nox1 (GenBank ID: NM_172203.1); 5′-ACT CCT TGG GTC AGC ACT GG-3′ (forward) and 5′-GTT CCT GTC CAG TTG TCT TCG-3′ (reverse) for amplification of a specific cDNA fragment encoding mouse Nox2 (GenBank ID: NM_007807.4); 5′-TGA ACT ACA GTG AAG ATT TCC TTG AAC-3′ (forward) and 5′-GAC ACC CGT CAG ACC AGG AA-3′ (reverse) for amplification of a specific cDNA fragment encoding mouse Nox4 (GenBank ID: NM_015760.4); 5′-GCG GTC TCC TTT TGA TTT CC-3′ (forward) and 5′-CAA AGG GCT CCT GAA ACT TG-3′ (reverse) for amplification of a specific cDNA fragment encoding mouse AT1A receptor (GenBank ID: NM_177322.3); 5′- TAT TTT CCC CAG AGC AAA GC-3′ (forward) and 5′-TGT TGC TTC CTT GTC CCT TG-3′ (reverse) for amplification of a specific cDNA fragment encoding mouse AT1B receptor (GenBank ID: NM_175086.3); and 5’-TTC ACC ACC ATG GAG AAG GC-3’ (forward) and 5’-GGC ATG GAC TGT GGT CAT GA-3’ (reverse) for amplification of a specific cDNA fragment encoding mouse GAPDH (GenBank ID: NM_008084.2), which served as the housekeeping control.
For determination of protein abundance by Western blot, VSMCs were lysed in NP-40 buffer supplemented with protease inhibitor (1 μg/mL), 10% SDS, 0.5% sodium fluoride, and 0.5% sodium orthovanadate. 20 or 40 μg of lysate were loaded on 10% SDS-PAGE gel (Thermo Scientific), blotted onto polyvinylidene fluoride membrane (Millipore), and blocked with 3% newborn calf serum in Tris-buffered saline with Tween-20 (0.1%). Blots were incubated overnight at 4 °C with primary antibodies recognizing Nox1 (Sigma-Aldrich), Nox2 (Boster), or Nox4 (Boster), washed, incubated with secondary HRP-conjugated antibodies (1:5000) for 1 h at room temperature, and developed with Super Signal West Pico Chemiluminescent substrate (Thermo Scientific). Blots performed in duplicate were imaged and quantified using ImageJ densitometry analysis software.
Immunofluorescence of Nox1 and GPER
Aortic sections frozen in O.C.T. compound were fixed in 4% paraformaldehyde, blocked and permeabilized in PBS containing normal goat serum (3%) and TritonX-100 (0.01%, EM Science). Sections were incubated with rabbit antibody recognizing murine Nox1 (1:100, Sigma-Aldrich) or negative control IgG (1:100, Sigma-Aldrich) overnight at 4 °C, washed, incubated with goat antibody recognizing rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen) for one hour, washed, mounted in Vectashield (Vector Laboratories), and imaged utilizing a Leica SP5 confocal microscope. Signal intensity was quantified using ImageJ software. VSMCs were stained with a rabbit antiserum recognizing murine GPER as described (12).
Statistical analysis
Statistical analysis for in vitro and in vivo experiments was performed using GraphPad Prism™ version 5.0 for Macintosh (GraphPad Software). When comparing two groups, the two-tailed, unpaired Student's t-test was performed. When comparing multiple groups, data were analyzed by two-way analysis of variance (ANOVA), with repeated measures as appropriate, followed by Bonferroni's post-hoc test to correct for multiple comparisons. Values are expressed as mean±sem; n equals the number of independent animals or cell preparations used. Statistical significance was accepted at a P value < 0.05.
Supplementary Material
Acknowledgments
We thank C. Hu, D. Cimino, M. Reutelshöfer, K. Schmitt and S. Söllner for expert technical assistance, J. Weaver for assistance with EPR spectroscopy, and B. Deeley (FUJIFILM VisualSonics Inc., Toronto, ON, Canada) for support with the echocardiography studies. We gratefully acknowledge K. K. Griendling and B. Lassègue (Emory University School of Medicine, Atlanta, GA, USA) for providing the Nox1/GFP adenovirus. We thank J. S. Rosenbaum (Proctor and Gamble Co.) for providing the male Gper−/− mice. Funding: This study was supported by the National Institutes of Health (NIH R01 CA127731 & CA163890 to E.R.P.), Dedicated Health Research Funds from the University of New Mexico School of Medicine allocated to the Signature Program in Cardiovascular and Metabolic Diseases (to E.R.P.), the Swiss National Science Foundation (grants 135874 & 141501 to M.R.M. and grants 108258 & 122504 to M.B.), and the Interdisciplinary Centre for Clinical Research (IZKF) Erlangen, project F1 (to K.A.). E.R.P. was also supported by the UNM Comprehensive Cancer Center (NIH grant P30 CA118100). N.C.F. was supported by NIH training grant HL07736. The EPR core facility of the University of New Mexico Biomedical Research and Integrative Neuroimaging Center is supported by NIH grant P30 GM103400, and the University of New Mexico & UNM Comprehensive Cancer Center Fluorescence Microscopy Shared Resource is supported by NIH grant P30 CA118100 as detailed: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml. Biostatistics support was provided by the UNM Clinical and Translational Science Center supported by NIH grant UL1 TR001449.
Footnotes
This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Author contributions: M.R.M., N.C.F., C.D. and G.S. performed experiments; J.B.A. synthesized G36; M.R.M., N.C.F., C.D., G.S., M.B., and E.R.P. analyzed data; M.R.M., N.C.F., K.A., M.B., and E.R.P. interpreted results of experiments; M.R.M., M.B. and E.R.P. prepared figures and wrote the manuscript; all authors approved the final version of manuscript; M.R.M., M.B., and E.R.P. were involved in conception and/or design of research.
Competing interests: M.R.M., G.S., M.B., and E.R.P. are inventors on a U.S. patent application for the therapeutic use of compounds targeting GPER. E.R.P. and J.B.A. are inventors on U.S. patent Nos. 7,875,721 and 8,487,100 for GPER-selective ligands and imaging agents. The other authors declare that they have no competing interests.
References and Notes
- 1.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt's lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 3.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 4.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 6.Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 8.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 9.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, Prossnitz ER. G protein-coupled estrogen receptor protects from atherosclerosis. Sci Reports. 2014;4:7564. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zekas E, Prossnitz ER. Estrogen-mediated inactivation of FOXO3a by the G protein- coupled estrogen receptor GPER. BMC Cancer. 2015;15:702. doi: 10.1186/s12885-015-1699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin AL, Steurer MA, Aronstam RS. Constitutive Activity among Orphan Class- A G Protein Coupled Receptors. PLoS One. 2015;10:e0138463. doi: 10.1371/journal.pone.0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton M, Prossnitz ER. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab. 2015;26:185–192. doi: 10.1016/j.tem.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prossnitz ER, Hathaway HJ. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol. 2015;153:114–126. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma G, Prossnitz ER. GPER/GPR30 Knockout Mice: Effects of GPER on Metabolism. Methods Mol Biol. 2016;1366:489–502. doi: 10.1007/978-1-4939-3127-9_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154:4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 25.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16:1492–1526. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 27.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimenez M, Schickling BM, Lopes LR, Miller FJ., Jr. Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin Sci (Lond) 2016;130:151–165. doi: 10.1042/CS20150404. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi SI, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 34.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 38.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 39.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 40.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO, 3rd, Gahl WA, Introne WJ. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minamino T, Komuro I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med. 2008;5:637–648. doi: 10.1038/ncpcardio1324. [DOI] [PubMed] [Google Scholar]
- 42.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 43.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 44.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 45.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 47.Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:H1765–1780. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsusaka T, Katori H, Homma T, Ichikawa I. Mechanism of cardiac fibrosis by angiotensin. New insight revealed by genetic engineering. Trends Cardiovasc Med. 1999;9:180–184. doi: 10.1016/s1050-1738(00)00018-9. [DOI] [PubMed] [Google Scholar]
- 49.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 50.Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76:506–516. doi: 10.1016/j.cardiores.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM, Angiotensin II NADPH. oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai P, Ichikawa K, Mailer C, Pou S, Halpern HJ, Robinson BH, Nielsen R, Rosen GM. Esters of 5-carboxyl-5-methyl-1-pyrroline N-oxide: a family of spin traps for superoxide. J Org Chem. 2003;68:7811–7817. doi: 10.1021/jo0350413. [DOI] [PubMed] [Google Scholar]
- 53.Shi H, Timmins G, Monske M, Burdick A, Kalyanaraman B, Liu Y, Clement JL, Burchiel S, Liu KJ. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch Biochem Biophys. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Ramiro-Diaz JM, Giermakowska W, Weaver JM, Jernigan NL, Gonzalez Bosc LV. Mechanisms of NFATc3 activation by increased superoxide and reduced hydrogen peroxide in pulmonary arterial smooth muscle. Am J Physiol Cell Physiol. 2014;307:C928–938. doi: 10.1152/ajpcell.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schreiner CE, Kumerz M, Gesslbauer J, Schachner D, Joa H, Erker T, Atanasov AG, Heiss EH, Dirsch VM. Resveratrol blocks Akt activation in angiotensin II-or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc Res. 2011;90:140–147. doi: 10.1093/cvr/cvq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 58.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 59.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 60.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox- mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 61.Al Ghouleh I, Frazziano G, Rodriguez AI, Csanyi G, Maniar S, St Croix CM, Kelley EE, Egana LA, Song GJ, Bisello A, Lee YJ, Pagano PJ. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res. 2013;97:134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li WG, Miller FJ, Jr., Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol. 2012;56:216–231. doi: 10.1016/j.vph.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 66.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr., Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montezano AC, Touyz RM. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28:288–295. doi: 10.1016/j.cjca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 68.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. P. A. Nutrition Committee of the American Heart Association Council on Nutrition, Metabolism, Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 69.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective nox inhibitors and therapeutics: challenges, triumphs and pitfalls. Antioxid Redox Signal. 2014;20:2741–2754. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guzik TJ, Channon KM. Measurement of vascular reactive oxygen species production by chemiluminescence. Methods Mol Med. 2005;108:73–89. doi: 10.1385/1-59259-850-1:073. [DOI] [PubMed] [Google Scholar]
- 71.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zanetti M, d'Uscio LV, Peterson TE, Katusic ZS, O'Brien T. Analysis of superoxide anion production in tissue. Methods Mol Med. 2005;108:65–72. doi: 10.1385/1-59259-850-1:065. [DOI] [PubMed] [Google Scholar]
- 74.Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013;8:e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kretz M, Mundy AL, Widmer CC, Barton M. Early aging and anatomic heterogeneity determine cyclooxygenase-mediated vasoconstriction to angiotensin II in mice. J Cardiovasc Pharmacol. 2006;48:30–33. doi: 10.1097/01.fjc.0000242061.18981.d3. [DOI] [PubMed] [Google Scholar]
- 76.Schena M, Mulatero P, Schiavone D, Mengozzi G, Tesio L, Chiandussi L, Veglio F. Vasoactive hormones induce nitric oxide synthase mRNA expression and nitric oxide production in human endothelial cells and monocytes. Am J Hypertens. 1999;12:388–397. [PubMed] [Google Scholar]
- 77.Zhou Y, Dirksen WP, Babu GJ, Periasamy M. Differential vasoconstrictions induced by angiotensin II: role of AT1 and AT2 receptors in isolated C57BL/6J mouse blood vessels. Am J Physiol Heart Circ Physiol. 2003;285:H2797–2803. doi: 10.1152/ajpheart.00466.2003. [DOI] [PubMed] [Google Scholar]
- 78.Schlote J, Schroder A, Dahlmann A, Karpe B, Cordasic N, Daniel C, Hilgers KF, Titze J, Amann K, Benz K. Cardiovascular and renal effects of high salt diet in GDNF+/− mice with low nephron number. Kidney Blood Press Res. 2013;37:379–391. doi: 10.1159/000355716. [DOI] [PubMed] [Google Scholar]
- 79.Foster FS, Hossack J, Adamson SL. Micro-ultrasound for preclinical imaging. Interface Focus. 2011;1:576–601. doi: 10.1098/rsfs.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Derumeaux G, Ichinose F, Raher MJ, Morgan JG, Coman T, Lee C, Cuesta JM, Thibault H, Bloch KD, Picard MH, Scherrer-Crosbie M. Myocardial alterations in senescent mice and effect of exercise training: a strain rate imaging study. Circ Cardiovasc Imaging. 2008;1:227–234. doi: 10.1161/CIRCIMAGING.107.745919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.