Summary
Sleep inertia, sleep homeostatic, and circadian processes modulate cognition, including reaction time, memory, mood, and alertness. How these processes influence higher-order cognitive functions is not well known. Six participants completed a 73-daylong study that included two 14-daylong 28h forced desynchrony protocols, to examine separate and interacting influences of sleep inertia, sleep homeostasis, and circadian phase on higher-order cognitive functions of inhibitory control and selective visual attention. Cognitive performance for most measures was impaired immediately after scheduled awakening and improved over the first ~2-4h of wakefulness (sleep inertia); worsened thereafter until scheduled bedtime (sleep homeostasis); and was worst at ~60° and best at ~240° (circadian modulation, with worst and best phases corresponding to ~9AM and ~9PM respectively, in individuals with a habitual waketime of 7AM). The relative influences of sleep inertia, sleep homeostasis, and circadian phase depended on the specific higher-order cognitive function task examined. Inhibitory control appeared to be modulated most strongly by circadian phase, whereas selective visual attention for a spatial-configuration search task was modulated most strongly by sleep inertia. These findings demonstrate that some higher-order cognitive processes are differentially sensitive to different sleep-wake regulatory processes. Differential modulation of cognitive functions by different sleep-wake regulatory processes has important implications for understanding mechanisms contributing to performance impairments during adverse circadian phases, sleep deprivation, and/or upon awakening from sleep.
Keywords: Executive function, Visual search, Stroop color word task, Cognitive throughput, Neurobehavioral, Cognitive speed
Introduction
Many daily activities and safety-sensitive tasks require higher-order cognitive functions to guide and control other cognitive processes. Executive and cognitive control functions are critical for situational awareness, adapting to environmental challenges, decision making, impulse control, and goal directed behaviors. Cognition is modulated across the 24-hour day to a large extent by three central nervous system processes referred to as sleep inertia, sleep homeostasis, and the circadian system (Achermann and Borbely, 1994; Akerstedt and Folkard, 1997; Jewett et al., 1999). Upon awakening at habitual waketime, sleep inertia initially impairs cognition (Wertz et al. 2006). Thereafter, the circadian rhythm in brain arousal facilitates relatively stable levels of cognition across the waking day despite increasing homeostatic sleep pressure (Czeisler et al., 1994; Dijk and Czeisler, 1995).
The influence of these central nervous system processes on cognition has been studied mostly using sleep deprivation, sleep restriction, sleep fragmentation, and nap protocols (Herscovitch and Broughton, 1981; Van Dongen and Dinges, 2003; Wertz et al., 2006; Wilkinson, 1969). While informative, such protocols do not permit precise estimation of the individual and interacting contribution of the three processes on cognition. The forced desynchrony protocol permits the mathematical separation of the effect of these processes and quantitatively estimates their separate contributions as well as their interactions on cognitive function. Findings from prior forced desynchrony studies indicate that sleep homeostatic and circadian processes interact to modulate the following cognitive functions: reaction time, sustained visual attention, working and delayed probed recall memory and cognitive speed, subjective happiness, sleepiness, alertness, and motivation (Boivin et al., 1997; Cohen et al., 2010; Czeisler et al., 1994; Dijk et al., 1992; Grady et al., 2010; Hull et al., 2003; Wyatt et al., 1999; Zhou et al., 2011a). Findings from forced desynchrony studies also indicate that performance immediately upon awakening is worst during the biological night in healthy young (Scheer et al., 2008) and older (Silva and Duffy, 2008) adults, with the former study showing a circadian rhythm in the magnitude of sleep inertia. However, the relative magnitude of sleep inertia on performance compared to sleep homeostasis and circadian phase remains unknown. Therefore, we examined the independent and interacting contributions of sleep inertia, sleep homeostatic, and circadian processes on multiple cognitive functions. We especially were interested in the influence of these processes on higher-order cognitive functions of inhibitory control and selective visual attention that are critical to guide and control other cognitive functions. We also included measures of working memory, visual vigilance, and subjective sleepiness previously shown to be influenced by sleep homeostatic and circadian processes in forced desynchrony protocols.
Materials and Methods
Participants and protocol
Six healthy adults (1 female), aged 26.8±5.2y (mean±SD), participated in a 73–day in–laboratory study (Scheer et al., 2007). Written informed consent was obtained and the Partners Human Research Committee approved the protocol and the University of Colorado Boulder IRB approved data analysis. Physical and psychological screenings determined health status and participants were free from medications and drugs as verified by blood and urine toxicology. Participants reported no night work three years prior or travel more than one time zone three months prior to study. Participants maintained regular ~8h sleep–wakefulness schedules based on habitual sleep–waketimes for three weeks as verified via sleep logs, time–stamped voice–recorder of bed–waketimes, and wrist actigraphy (Actiwatch, Minimitter, Bend, OR).
Participants were studied at the Center for Clinical Investigation at the Brigham and Women's Hospital in an environment free of time cues. Protocol events (e.g. meal times and sleep opportunities) were scheduled relative to wake time. The 73–day long study included two–28h forced desynchrony protocol segments (12×28h days each) following circadian entrainment to non–24h day lengths (Scheer et al., 2007). The forced desynchrony protocols used in the current study consisted of 18.67h scheduled wakefulness in dim light (~1.8 lux, angle of gaze) and 9.33h scheduled sleep in darkness (0 lux), maintaining the baseline 2:1 ratio of wakefulness-sleep (see Supporting Information Methods for details).
Core body temperature, cognitive performance and subjective assessments
Core body temperature (CBT) was recorded with rectal thermistors (Yellow Springs Instrument Company, Yellow Spring, OH, USA) every minute. Computerized test batteries began at scheduled waketime and every 2h thereafter until bedtime. Participants were tested in a semi-recumbent 35-45° head up posture seated in a hospital bed immediately upon awakening and at the 18h time point versus a seated position in a chair for other time points. Participants were requested to sit immediately prior to each test, unless they were already seated. The first forced desynchrony assessed, in order: a 20–min visual vigilance task [Psychomotor Vigilance Task (PVT)], and working memory/ cognitive speed tasks [2–min mathematical addition test (ADD), 1.5–min Digit Symbol Substitution Test (DSST)] (Wright, et al., 2002). The second forced desynchrony assessed, in order: Karolinska Sleepiness Scale (KSS), and goal directed behaviors and cognitive control on selective visual attention tasks [Spatial–configuration visual search task (SPATIAL) and Conjunction visual search task (CONJ)] (Horowitz et al., 2003), and cognitive speed and the inhibitory control component of executive function [Stroop Color Word Task (STROOP)](Frey et al., 2011) (descriptions of STROOP and visual search tasks in Supporting Information).
Data analysis
The circadian period of the CBT rhythm was analyzed with nonorthogonal spectral analysis (Czeisler et al., 1999; Scheer et al., 2007; Wright, et al., 2001). Cognitive functions were averaged into circadian bins of 60° (15°=one circadian hour), with the temperature minimum assigned to 0°, and into hours awake bins every 2h beginning at scheduled waketime (Scheer et al., 2008; Wright, et al., 2002). Visual search performance was examined for overall cognitive throughput-the number correct per minute (Thorne, 2006), and for percent of missed targets-errors of omission. Inhibitory control for the STOOP was calculated as the difference between congruent and incongruent median reaction times for correct responses. Cognitive performance data were analyzed with mixed-model ANOVA, with subject as a random factor and hours awake and circadian phase as fixed factors using Statistica (version 10.0, Statsoft, Tulsa, OK). Generalized eta squared (η2G) effect sizes were used to compare magnitudes of sleep inertia, sleep homeostasis, circadian phase and interactions (see Supporting Information for details).
Results
Stroop color word task
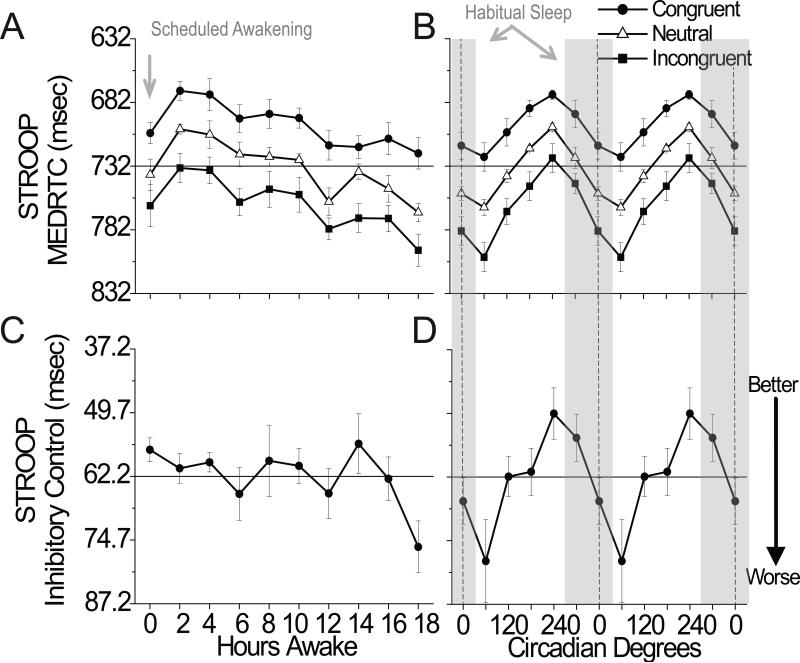
Cognitive speed as assessed by the median reaction time for correct responses on the Stroop color word test, was fastest for congruent, intermediate for neutral, and slowest for incongruent stimuli (main effect of stimulus type: F(2, 10)=12.52, p=0.0019) with significant main effects of hours awake and circadian phase for each (Table 1). Cognitive speed improved over the first 2h after lights on (reflecting the dissipation of sleep inertia) and declined thereafter (reflecting an increase in homeostatic sleep pressure; Fig. 1A). From a circadian perspective, cognitive speed was worst near 60° (corresponding to ~9AM) and best near 240° (~9PM; Fig. 1B). Cognitive speed performance during sleep inertia was similar to being awake for 10–12h and to circadian phase near 120° or 0° (Fig.1A and 1B). Furthermore, cognitive speed was relatively stable across days (Fig. S1), but was modulated to a similar extent by sleep homeostasis and circadian phase for congruent and neutral stimuli and to a greater extent by circadian phase for incongruent stimuli (Fig. 2). Color contour plots for cognitive speed, illustrating the interaction between hours awake and circadian phase are provided in the supplementary information (Fig. S2). Inhibitory control showed a significant main effect of circadian phase only, with worst performance near 60° and best performance near 240° (Table 1; Fig. 1D). Figures 1C, 1D, 2 and 3 also show that inhibitory control was modulated primarily by circadian phase.
Table 1.
Mixed Model
| HA |
CP |
HA × CP |
|
|---|---|---|---|
| Measure | F (9,45) | F (5,25) | F (45,225) |
| STROOP: | |||
| Congruent MEDRTC | 5.27**** | 11.53***** | 1.02 |
| Neutral MEDRTC | 5.52**** | 17.03***** | 1.11 |
| Incongruent MEDRTC | 6.28**** | 30.34***** | 1.37 (0.07) |
| Inhibitory Control | 1.48 | 2.98* | 0.88 |
| SPATIAL: | |||
| Overall CT | 6.88***** | 1.20 | 1.50* |
| Target Present CT | 4.67*** | 1.44 | 1.10 |
| Target Absent CT | 6.12**** | 1.45 | 1.52* |
| Missed Targets (%) | 1.76 | 0.21 | 1.06 |
| CONJ: | |||
| Overall CT | 4.98*** | 14.41***** | 1.75** |
| Target Present CT | 5.58**** | 12.50***** | 1.96*** |
| Target Absent CT | 4.09*** | 12.66***** | 1.37 (0.07) |
| Missed Targets (%) | 3.73** | 4.74** | 1.93*** |
| ADD (# correct) | 4.16*** | 10.94***** | 0.93 |
| DSST (# correct) | 9.15***** | 12.72***** | 2.10*** |
| PVT (1/MEDRT) | 5.28**** | 18.76***** | 2.39**** |
| KSS Score | 10.48***** | 7.67*** | 2.35**** |
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
P < 0.0001.
Values in parentheses show non-significant trends (P>0.05).
Analysis of cognitive measures by hours awake (HA), circadian phase (CP), and the interaction (HA×CP). STROOP, Stroop Color Word Task; CONJ, Conjunction Visual Search Task; SPATIAL, Spatial–Configuration Visual Search Task; ADD, mathematical addition test; DSST, Digit Symbol Substitution Task; PVT, Psychomotor Vigilance Task; KSS, Karolinska Sleepiness Scale; MEDRTC, Median Reaction Time Correct; CT, Cognitive Throughput; MEDRT, Median Reaction Time.
Figure 1. Influence of sleep inertia, homeostatic, and circadian components on Stroop Color Word Task (STROOP).
Left panels depict influence of hours awake (sleep inertia and sleep homeostasis) and right panels depict influence of circadian phase and are double plotted. Data aligned by fitted core body temperature minimum (CBT Min), defined as 0° (dashed line right panels). Gray bars depict when sleep habitually occurred prior to in-laboratory procedures. Data points represent deviations from mean±standard error (DFM±SEM). The grand mean (solid horizontal line) was incorporated into the figures to illustrate magnitude of change. Lower scores represent worse performance. MEDRTC, median reaction time for correct responses in msec for congruent (Cong), neutral (Neu) and incongruent (Inc) stimuli; inhibitory control (INH) score represents the difference between the congruent and incongruent stimuli MEDRTC in msec.
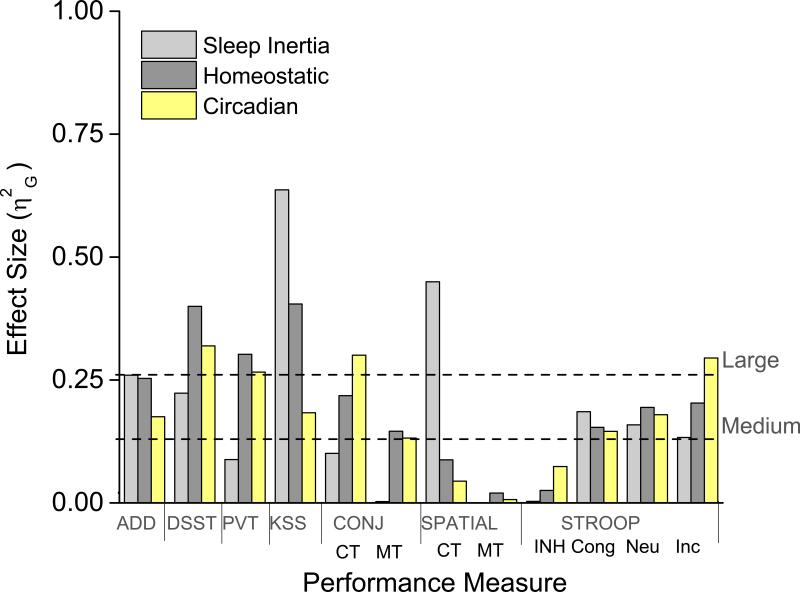
Figure 2. Generalized eta squared (η2G) effect size comparison of sleep inertia, homeostatic, and circadian phase on performance.
Mathematical addition test (ADD) [number correct additions of two, 2-digit numbers in 2 min]; Digit Symbol Substitution Task (DSST) [number correct responses matching digits (0–9) to symbols (circle, X, triangle, etc.) in 1.5 min]; Psychomotor Vigilance Task (PVT) [reciprocal median reaction time in msec, data for the PVT were transformed using the reciprocal transformation to control for violations of homogeneity of variance and normality]; Karolinska Sleepiness Scale (KSS) [a 9 point Likert–type scale with 9 indicating the greatest sleepiness]. Conjunction (CONJ) and Spatial–Configuration (SPATIAL) Visual Search Tasks Cognitive throughput (CT) (Thorne, 2006), represents number of trials per minute for when targets were correctly identified as being present and/or absent and MT represents missed targets. See Fig 1 for STROOP definitions.
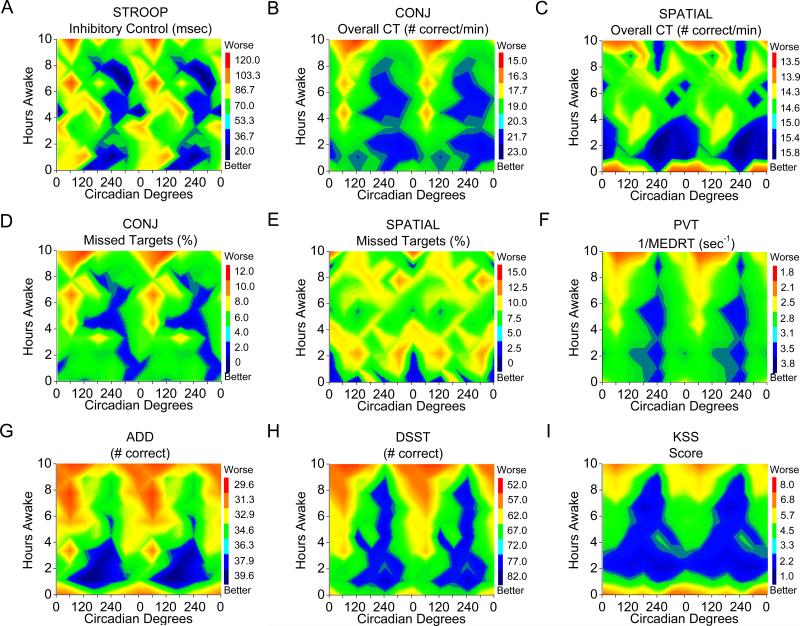
Figure 3. Color contour plots of the interaction between sleep inertia, homeostatic, and circadian influences on STROOP Inhibitory Control, visual search and cognitive performance.
The right y–axis represents deviation from the mean (DFM)±SEM with grand mean incorporated to illustrate magnitude of change. Increasingly red colors (up) indicate worse performance and increasingly blue colors (down) indicate better performance. See Fig 1 for task definitions.
Conjunction and spatial–configuration visual search tasks
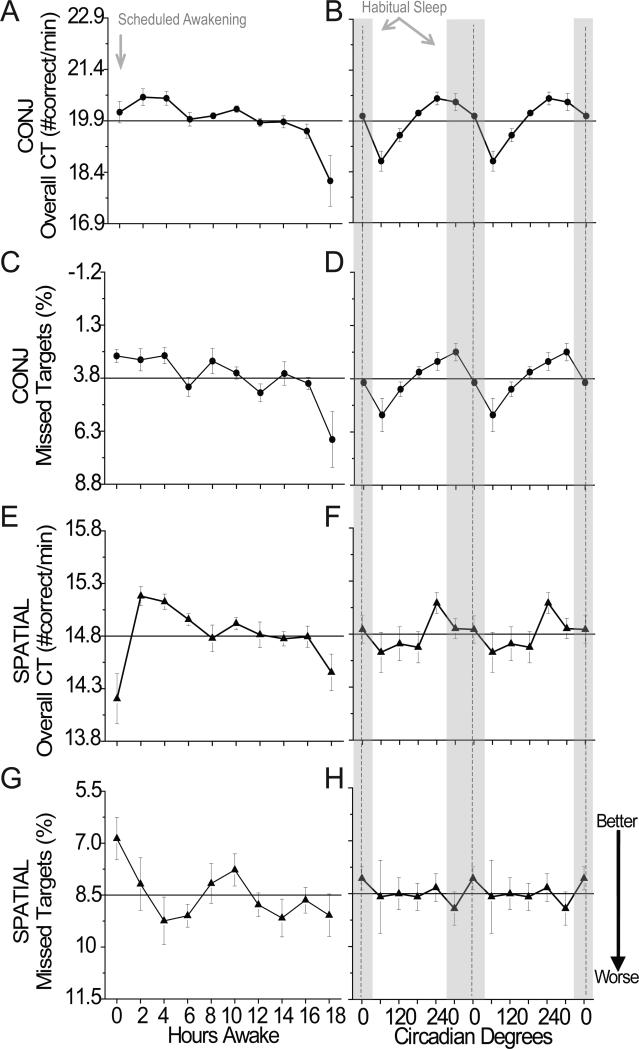
Cognitive throughput was faster for conjunction (a combination of orientation and color) versus spatial (spatial location) visual search (F(1, 5)=64.25, p<0.001). Conjunction cognitive throughput was significantly influenced by hours awake and circadian phase; whereas spatial cognitive throughput was significantly influenced by hours awake, but not circadian phase (Table 1). Both conjunction and spatial visual search were influenced by an interaction between hours awake and circadian phase (Table 1). Figures 4A–4D show the influence of sleep inertia (worse performance at 0 versus 2 and 4 hours awake; p < 0.05) for conjunction cognitive throughput was similar to performance observed near 6–14h awake whereas figures 4E–4F show spatial cognitive throughput was modulated the greatest extent by sleep inertia. Figures 2 and 4A–4D show homeostatic and circadian phase have larger influence than sleep inertia for conjunction cognitive throughput, whereas sleep inertia had larger influence than homeostatic and circadian phase for spatial throughout. Color contour plots show amplitude of the circadian modulation in conjunction cognitive throughput (Fig. 3B) grew with hours awake whereas amplitude of the circadian modulation in spatial cognitive throughput (Fig. 3C) was largest during sleep inertia with a relatively small increase in amplitude with hours awake until near the very end of scheduled wakefulness.
Figure 4. Influence of sleep inertia, homeostatic, and circadian components on Conjunction (CONJ) and Spatial–Configuration (SPATIAL) Visual Search Tasks.
A non-significant trend for more missed targets for spatial than conjunction was observed (F(1, 5)=5.64, p=0.064). Missed targets on conjunction, but not spatial, were significantly influenced by hours awake, circadian phase, and their interaction (Table 1). Conjunction missed targets gradually increased with hours awake (Fig. 4C). From a circadian perspective, conjunction missed targets were most frequent near 60° and least frequent near 300° (Fig. 4D). Furthermore, conjunction missed targets were modulated the greatest extent by sleep homeostasis and circadian phase (Figs. 2, 4C and 4D). The circadian modulation of missed targets for conjunction grew with hours awake (Fig. 3D), whereas there was a relatively small increase in circadian modulation with hours awake in missed targets for spatial (Fig. 3E; Fig. S3).
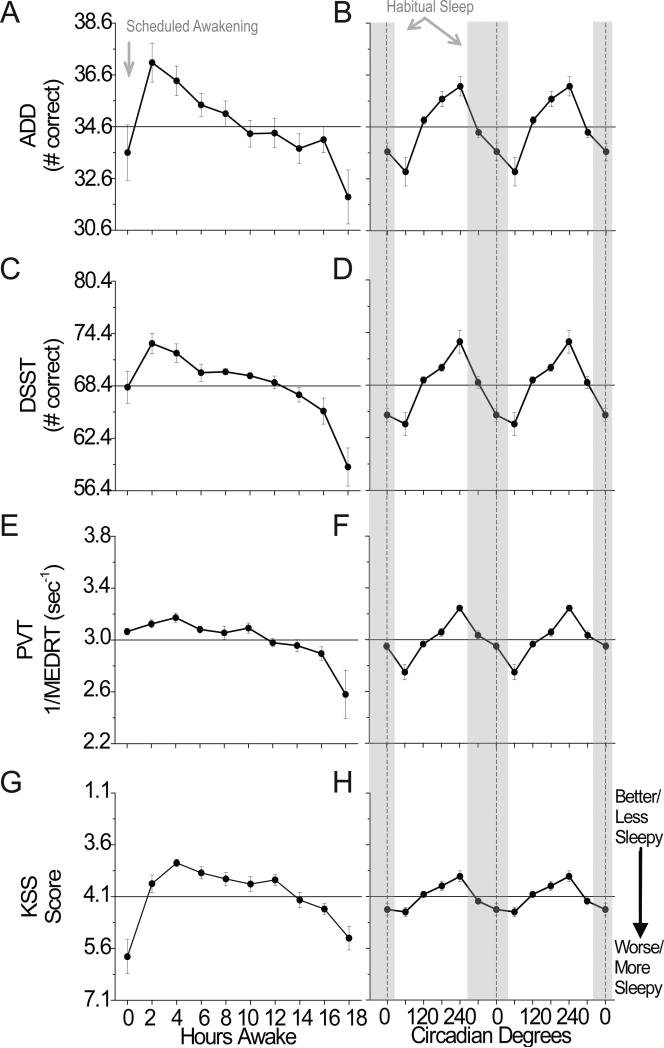
Mathematical addition, digit symbol substitution, psychomotor vigilance task, and subjective sleepiness
Significant effects of hours awake, circadian phase, and their interaction were observed for most of the ADD, DSST, PVT, and KSS measures examined (Table 1). In general, performance and sleepiness improved over the first 0–4h of scheduled wakefulness and thereafter performance declined until 18h awake (Fig. 5A, 5C, 5E, 5G). From a circadian perspective, performance/sleepiness on these tasks was worst near 60° and best near 240° (Fig. 5B, 5D, 5F and 5H). Figures 2 and 5 also show the relative contribution of the three processes. We focus on the relative comparison of sleep inertia to sleep homeostasis and circadian phase as relative comparisons between sleep homeostasis and circadian phase have been reported for these more general tasks (Czeisler et al., 1994; Dijk et al., 1992; Wright, et al., 2002; Wyatt et al., 1999). Sleep inertia (worse performance at 0 versus 2 and 4 hours awake; p < 0.05) influenced ADD and DSST tasks similarly to being awake for ~14–16 and ~12h, respectively and similarly to a circadian phase near 0° or 120°/300°. Response speed on the PVT appeared to be less influenced by sleep inertia, whereas average sleepiness on the KSS appeared to be more influenced by sleep inertia (worse performance at 0 versus 2 and 4 hours awake; p < 0.05). The relative magnitude of sleep inertia for KSS and ADD were similar to sleep homeostasis, whereas for the DDST and PVT was less than sleep homeostasis and circadian components (Fig 2). Following dissipation of sleep inertia, circadian amplitude in performance increased with hours awake for these tasks (Fig. 3F-I).
Figure 5. Influence of sleep inertia, homeostatic, and circadian components on cognitive performance.
See Fig 2 for task details.
Discussion
Findings demonstrate that the inhibitory control component of executive function and components of selective visual attention are differentially modulated by sleep–wakefulness processes. Specifically, inhibitory control was primarily under circadian control while selective visual attention on a spatial–configuration visual search task was most strongly influenced by sleep inertia. The latter findings demonstrate task–dependent changes in cognitive impairment caused by sleep inertia, homeostatic and circadian processes. Findings also demonstrate that sleep inertia, sleep homeostasis, and circadian phase independently and interactively modulate a variety of other cognitive functions.
The findings that inhibitory control as measured by the STROOP is primarily modulated by the circadian system and that the circadian amplitude of inhibitory control changed very little with sleep homeostatic pressure is unique among the cognitive functions previously examined (e.g., Wyatt et al., 1999; Wyatt et al., 2004). Inhibitory control showed a robust circadian variation with worst performance near 60°, corresponding to approximately 1–2h after habitual waketime regardless of the level of sleep inertia or homeostatic sleep pressure. Why this measure of executive function was not significantly impaired by increased time awake like other cognitive tasks examined, especially during the biological night, is unknown, but the finding suggests that the circadian system plays a fundamental role in modulation of inhibitory control. Significant modulation by sleep homeostasis and circadian phase for the non–executive function STROOP cognitive speed measures are consistent with findings for previously examined tasks (Czeisler et al., 1994; Dijk et al., 1992; Wright, et al., 2002; Wyatt et al., 1999). That executive function appears to be most vulnerable to circadian phase may help to understand inconsistent findings of impaired executive function in some but not all sleep deprivation studies (e.g., Drummond et al., 2006; McCarthy and Waters, 1997). Interference on the STROOP task is typically greatest on the first presentation. Although repeated assessments likely changes cognitive processing of the task, we still observed an interference response to incongruent stimuli suggesting that our task version provides some assessment of executive function (see Supporting Information for discussion). Groeger et al. (2011) have also shown working memory executive function to be influenced by sleep inertia from afternoon naps. Additional research is necessary to determine if other executive functions (Alvarez and Emory, 2006), are primarily influenced by circadian phase, and test such executive functions under real world conditions (Horne and Moseley, 2011).
The visual search tasks assessed selective visual attention/goal directed behavior requiring determination of whether or not a target is present among non–target distractor stimuli. We found conjunction cognitive throughput was primarily influenced by sleep homeostasis and circadian phase, whereas spatial cognitive throughput was primarily influenced by sleep inertia. Differential modulation by sleep inertia, sleep homeostatic, and circadian processes and their interaction for different visual search tasks suggests that unshared components of these tasks (Horowitz et al., 2003; Wolfe, 1994) are more vulnerable to sleep inertia versus sleep homeostasis and circadian phase. Missed targets were in general less common for conjunction than spatial; however, missed targets on conjunction were more sensitive to homeostatic sleep pressure and circadian phase. Unexpectedly, missed targets for spatial were fewer during sleep inertia and worsened over the first 4h of wakefulness. As cognitive throughput was slower during sleep inertia on spatial, it is possible that participants missed fewer targets because they were taking more time to search (e.g., speed/accuracy tradeoff).
Findings for other tasks assessed were generally consistent with prior reports (Czeisler et al., 1994; Santhi, et al. 2013; Wright, Jr., et al. 2002; Wyatt et al., 1999; also see Supporting Information). The influence of sleep inertia on the 20–min PVT, a measure of visual vigilance/sustained attention, appeared small, influencing performance only near the core body temperature minimum.
Limitations of the study include sample size and that subjects were tested in a semi-recumbent 35-45 degree head up posture in a hospital bed immediately upon awakening and at the 18h time point versus a seated position in a chair for other time points, which may have an influence on the performance observed. We likely underestimate the magnitude of sleep inertia for tasks that occur later in the performance battery versus those given at scheduled awakening, especially given the fixed order of the tasks, and under conditions where participants had awoken prior to the time of scheduled awakening. Longer duration wake episodes (e.g., 28h in a 42.85 hour forced desynchrony) (Grady et al., 2010) or increasing the wake to sleep ratio (Cohen et al., 2010; Zhou et al., 2011b) could increase the magnitude of the sleep homeostatic/wake dependent component observed. It is possible the inhibitory component of executive function would have been influenced by higher levels of homeostatic sleep pressure associated with such extended wakefulness. We likely underestimate influences on executive function given the repeated testing of the task. It is also possible that the fixed order of tasks within each forced desynchrony could result in systematic influence of prior tasks on task performance.
In summary, findings provide evidence that higher–order cognitive functions involved in goal-directed behaviors and cognitive control are influenced by circadian phase, homeostatic sleep drive, and/or sleep inertia. These tasks are relevant for safety–sensitive performance, daily activities and social behaviors. For example, visual search is critical for selecting the correct medication from a pharmacy shelf, searching for weapons in carry-on luggage, and for finding a car in a full parking lot. Cognitive control components of executive function are critical for modulation of other cognitive processes such as looking the correct direction when crossing the street while visiting a country where people drive on a different side of the road, or inhibition of inappropriate outbursts and behaviors in patients with dementia. Our findings may also have important implications for the millions of individuals working at adverse circadian phases, long work hours, and/or immediately upon awaking (Horne and Moseley, 2011), including health care professionals treating patients, with the acknowledgement that findings from laboratory studies may not directly translate to real world performance. Lastly, our findings highlight the importance of considering how sleep inertia, hours awake and circadian processes may influence clinical assessments of cognitive impairment.
Supplementary Material
Acknowledgements
Thanks to participants and research staff. Supported in part by NIH R01–NS41886. Data collection carried out in the Brigham and Women's Hospital Center for Clinical Investigation (formally supported by NIH M01-RR02635 currently part of Harvard Clinical and Translational Science Center supported by NIH 1UL1 TR001102). KPW and TMB were supported by NIH R01-HL081761, NIH R01-HL085705 and NIH R01 HL109706; CAC, KPW and TMB supported by NASA Cooperation Agreement NCC 9–58 with the National Space Biomedical Research Institute. FAS was supported by NIH P30-HL101299, R01 HL094806.
KPW has grant support from Philips Inc. CAC has received consulting fees from or served as a paid membe r of scientific advisory boards for: Boston Celtics; Boston Red Sox; Citgo Inc.; Cleveland Browns; Merck; Novartis; Purdue Pharma LP; Quest Diagnostics, Inc.; Teva Pharmaceuticals Industries Ltd.; Valero Inc.; Vanda Pharmaceuticals, Inc. CAC currently owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc., and between October 2012 and October 2013, Apple, Inc. and Microsoft, Inc. CAC received royalties from McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics, Inc. and has received grants and research support from Cephalon Inc., National Football League Charities, Philips Respironics, ResMed Foundation, San Francisco Bar Pilots and Sysco. CAC is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, CAC has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms, including matters involving Bombardier, Inc.; Delta Airlines; FedEx; Greyhound; Michael Jackson's mother and children; Purdue Pharma, L.P.; United Parcel Service and the United States of America.
Footnotes
Conflicts of Interest. The authors TMB, FAS, and JMR declare no conflicts of interest.
Author Contributions: Secured funding (CAC, KPW), data collection (FAS, JMR, KPW), data analysis and interpretation (TMB, FAS, JMR, CAC, KPW), manuscript preparation (TMB, FAS, JMR, CAC, KPW).
References
- Achermann P, Borbely AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol. Cybern. 1994;71:115–121. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Folkard S. The three-process model of alertness and its extension to performance, sleep latency, and sleep length. Chronobiol. Int. 1997;14:115–123. doi: 10.3109/07420529709001149. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci. Transl. Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Dijk DJ, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh JR, editors. Sleep Onset: Normal and Abnormal Processes. American Psychological Association; Washington, D.C.: 1994. pp. 89–110. [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP., Jr. Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J. Am. Geriatr. Soc. 2011;59:73–81. doi: 10.1111/j.1532-5415.2010.03229.x. [DOI] [PubMed] [Google Scholar]
- Grady S, Aeschbach D, Wright KP, Jr., Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology. 2010;35:1910–1920. doi: 10.1038/npp.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger JA, Lo JC, Burns CG, Dijk DJ. Effects of sleep inertia after daytime naps vary with executive load and time of day. Behav Neurosci. 2011;125:252–260. doi: 10.1037/a0022692. [DOI] [PubMed] [Google Scholar]
- Herscovitch J, Broughton R. Performance deficits following short-term partial sleep deprivation and subsequent recovery oversleeping. Can. J. Psychol. 1981;35:309–322. doi: 10.1037/h0081197. [DOI] [PubMed] [Google Scholar]
- Horne J, Moseley R. Sudden early-morning awakening impairs immediate tactical planning in a changing ‘emergency’ scenario. J. Sleep Res. 2011;20:275–278. doi: 10.1111/j.1365-2869.2010.00904.x. [DOI] [PubMed] [Google Scholar]
- Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol. Sci. 2003;14:549–557. doi: 10.1046/j.0956-7976.2003.psci_1464.x. [DOI] [PubMed] [Google Scholar]
- Hull JT, Wright KP, Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J. Biol. Rhythms. 2003;18:329–338. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J. Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- McCarthy ME, Waters WF. Decreased attentional responsivity during sleep deprivation: orienting response latency, amplitude, and habituation. Sleep. 1997;20:115–123. doi: 10.1093/sleep/20.2.115. [DOI] [PubMed] [Google Scholar]
- Santhi N, Groeger JA, Archer SN, et al. Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PLoS One. 20138:e79688. doi: 10.1371/journal.pone.0079688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J. Biol. Rhythms. 2008;23:353–361. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Wright KP, Jr., Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PLoS. ONE. 2007;2:e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EJ, Duffy JF. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav. Neurosci. 2008;122:928–935. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne DR. Throughput: a simple performance index with desirable characteristics. Behav. Res. Methods. 2006;38:569–573. doi: 10.3758/bf03193886. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J. Sleep Res. 2003;12:181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- Wertz AT, Ronda JM, Czeisler CA, Wright KP., Jr. Effects of sleep inertia on cognition. JAMA. 2006;295:163–164. doi: 10.1001/jama.295.2.163. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT. Sleep deprivation: Performance tests for partial and selective sleep deprivation. Prog. Clin. Psychol. 1969;8:28–43. [Google Scholar]
- Wolfe JM. Guided Search 2.0 - A Revised Model of Visual-Search. Psychon. Bull. Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr., Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr., Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul. Integr. Comp Physiol. 2002;283:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ferguson SA, Matthews RW, Sargent C, et al. Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep. 2011a;34:57–63. doi: 10.1093/sleep/34.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ferguson SA, Matthews RW, Sargent C, et al. Sleep, Wake and Phase Dependent Changes in Neurobehavioral Function under Forced Desynchrony. Sleep. 2011b;34:931–941. doi: 10.5665/SLEEP.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.