Abstract
Background
Vulvodynia is a chronic vulvar pain disorder and fibromyalgia is a chronic widespread musculoskeletal pain disorder; both of unknown etiology. Association of these conditions is well documented. Intra-vaginal algometer measurement of tenderness to pressure applied to the pelvic floor muscles helps to define vulvodynia associated with musculoskeletal factors. Women with both vulvodynia and fibromyalgia might have increased pelvic muscle pain compared to women with vulvodynia alone, defining the possible link of these two conditions.
Objective
1) correlate pain intensity during the non-genital tender point tenderness examination to pain intensity with the vaginal algometer in women with provoked vestibulodynia, 2) determine whether subjects with provoked vestibulodynia and fibromyalgia had higher pain intensity scores with the vaginal algometer than those without fibromyalgia.
Study Design
Ninety-two subjects referred for vulvar pain were confirmed to have provoked vestibulodynia using the cotton swab test. A diagnosis of fibromyalgia was made if pain was present (Numerical rating scale> 1) in at least 11 sites of the 18-point non-genital tender point tenderness exam. Vaginal pain sensitivity was measured using an intra-vaginal pressure algometer, where 0.1, 0.3, and 0.5 kg/cm2 forces were applied digitally in random assignment by force and location to the right and left iliococcygeus muscle regions and the posterior vaginal wall. Both tender point tenderness and algometer pain intensity were reported on a 0 (no pain) to 10 (worse pain) numeric rating scale. Correlations were computed between the composite pain intensity (total of rating scale from each pressure threshold at specified site) of non-genital and those of iliococcygeus regions and the posterior vaginal wall. Independent t-tests were used to determine differences in iliococcygeus regions and the posterior vaginal algometer pain ratings and presence or absence of fibromyalgia. The significance level was at P < .05. The data were expressed as mean ± standard deviation.
Results
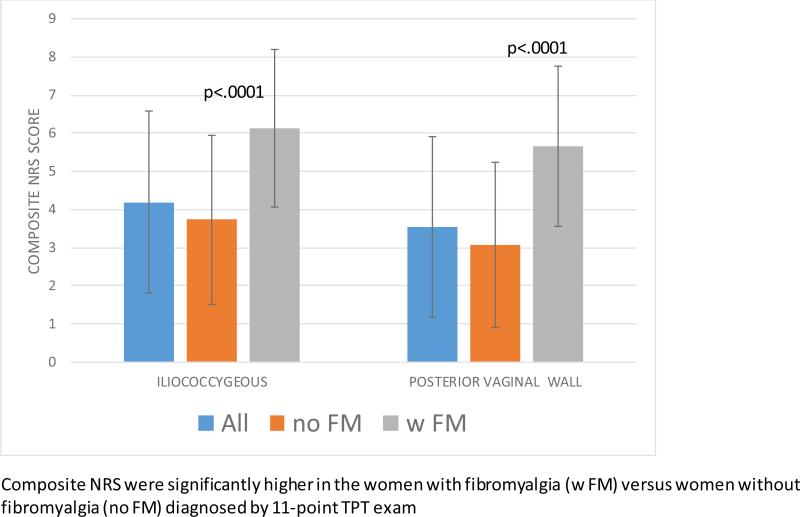
A significant correlation was found between numerical rating scale pain ratings on the non-genital tender point tenderness exam and algometer testing on the iliococcygeus region (r = 0.44, p <.0001), and the posterior vaginal wall (r = 0.45, p < .0001). Subjects with fibromyalgia by tender point tenderness, had significantly higher iliococcygeal pain (6.14 ± 2.07 vs. 3.74 ± 2.22, p = 0.0001), and posterior vaginal wall pain (5.67 ± 2.10 vs. 3.07 ± 2.16, p < 0.0001) than women without fibromyalgia by tender point tenderness.
Conclusion
Women with provoked vestibulodynia who experience more severe pain with nongenital tender point palpation also experience more deep vaginal pain on pelvic exam. Those who fulfill the diagnosis of fibromyalgia show significantly more intense deep vaginal pain to palpation of iliococcygeus muscles and posterior vaginal wall. Further research using a more precise definition of fibromyalgia is necessary to confirm this relationship, but findings suggest that women with provoked vestibulodynia coexisting with fibromyalgia have greater risk of superimposed vaginal muscle pain and may be candidates for early adjunctive pelvic floor physical therapy. These findings need to be explored in women with generalized, non-provoked vulvodynia.
Keywords: Vestibulodynia, fibromyalgia, pain intensity ratings
Introduction
Vulvodynia is a chronic pain disorder of the vulva, occurring in the absence of relevant findings or a specific clinically identified neurologic disorder. (1) Vulvodynia is estimated to affect 8 – 15% of women (2,3,4) and has been described as the leading cause of dyspareunia in women under the age of fifty. (5) Symptoms of vulvodynia may include stinging, burning, irritation or itching. The etiology of vulvodynia is unknown, and likely multifactorial.
In 2015, a consensus vulvar pain terminology committee re-defined vulvodynia as vulvar pain of at least 3 months’ duration, without clear identifiable cause, which has potential associated factors. (6) (Table 1) A key objective of the new terminology introduced in 2015 was to identify potential factors associated with vulvodynia, such as genetic or hormonal factors, inflammation, musculoskeletal or neurological mechanisms, neuroproliferation, psychosocial or structural issues. (1). The recognition of these factors in a given patient could be used to assist in developing individualized treatment plans. For example, those women with a perceived neuropathic etiology might benefit from tricyclic antidepressants or gabapentin, whereas those with musculoskeletal factors might respond better to pelvic floor physical therapy.
Table 1.
2015 Consensus Terminology and Classification of Persistent Vulvar Pain
| Vulvodynia |
| Definition: vulvar pain of at least 3 months’ duration, without clear identifiable cause, which may have potential associated factors. |
| Descriptors: |
| • Localized, generalized or mixed |
| • Provoked, spontaneous or mixed |
| • Onset (primary or secondary) |
| • Temporal pattern (intermittent, persistent, constant, immediate, delayed) |
| Potential Factors Associated with Vulvodynia |
| • Co-morbidities and other pain syndromes |
| • Genetics |
| • Hormonal factors |
| • Inflammation |
| • Musculoskeletal |
| • Neurologic mechanisms |
| ○ Central |
| ○ Peripheral |
| • Neuroproliferation |
| • Psychosocial factors |
| • Structural defects |
Adapted from: Bornstein J, Goldstein A, Coady D. 2015 Consensus terminology and classification of persistent vulvar pain.
Provoked vestibulodynia (PVD), the most common subset of vulvodynia, is localized to the vaginal vestibule (entry to the vagina, including the introitus, and extending to the hymenal ring)) and present only with contact, such as from tampon insertion or intercourse. Diagnosis of PVD is based on history, physical exam (which excludes other pathology) and a positive cotton swab test, whereby pain is elicited when the vestibule is gently touched with a cotton swab at the 2-, 4-, 6-, 8-, and 10-o'clock positions. (7) Complete evaluation should also include palpation of the levator muscles immediately inside the vagina. Pelvic floor dysfunction, with hypertonicity and pain has been demonstrated in PVD patients versus controls. (8,9)
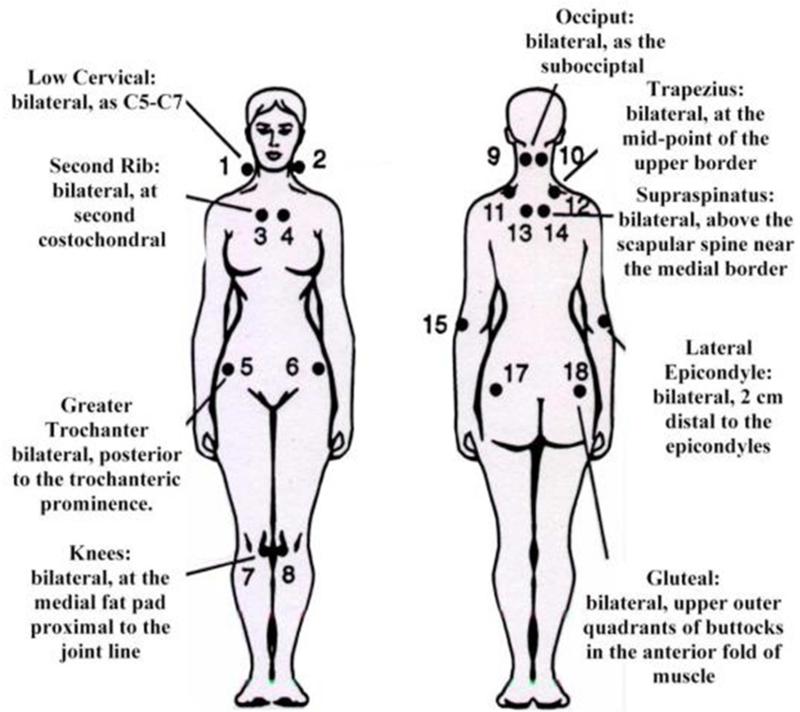
Association of vulvodynia with fibromyalgia (FMS) is well documented. (10,11) Fibromyalgia is a syndrome of unknown etiology, associated with chronic widespread musculoskeletal pain. Fibromyalgia is diagnosed by history, absence of other identified pathology and the presence of pain in 11 of 18 specified tender points on physical exam (tenderpoint tenderness exam (TPT)). (Diagram 1) In 2010, an alternative diagnostic criterion for fibromyalgia, which did not require TPT examination was developed (12), and subsequently validated (13). Investigators and clinicians still disagree on the utility of the TPT exam.
The vaginal algometer is a device developed by researchers to assess pain deeper in the vagina than could be evaluated with the cotton swab test. The device consists of a probe containing a pressor sensor that is inserted into the vagina and a measurement circuit that converts the signal from the probe into a force measurement, with a resultant threshold value. (14) The vaginal algometer was originally intended to measure pressure pain threshold (PPT) over the lateral walls of the vagina, over the area of the pudendal nerve, but has been subsequently validated in women without pain in vaginal sites including the pubococcygeous and iliococcygeous muscles, and the anterior and posterior vaginal wall. (15) The intra-vaginal algometer has also been shown to assess muscle pressure pain threshold independently of mucosal pain in women with vulvodynia. (9)
The vaginal algometer may be considered a TPT examination of the vagina. In this regard, the objective of this study was to correlate ratings of pain intensity during the non-genital tender point tenderness (TPT) examination to ratings of pain intensity with the intra-vaginal algometer in women with provoked vestibulodynia (PVD), and additionally, to determine whether subjects with PVD and non-genital TPT scores consistent with the diagnosis of fibromyalgia (FMS) had higher pain intensity scores with the vaginal algometer compared to those without FMS.
Materials and Methods
Women included were enrolled in a multi-site, NIH-funded, randomized, double-blind, placebo-controlled crossover study of extended release gabapentin in the treatment of PVD. Institutional Review Board approval was obtained at all three study sites, the University of Tennessee Health Science Center, Rutgers-Robert Wood Johnson Medical School, and the University of Rochester Medical Center. Women were enrolled in the study if they were over the age of 18 years old, had greater than 3 continuous months of insertional dyspareunia, pain to touch or pain with tampon insertion (modified Freidrich's Criteria for vulvodynia (16)), and demonstrated an average of “4” or greater on the 11-point tampon test (17) (0=no pain at all; 10 = worse pain ever) during the 2-week screening period.
Exclusion criteria included: (1) other vulvar conditions or active infections identified clinically or by a positive Affirm test (VPIII microbial identification test), (2) a prior vestibulectomy, (3) pregnant or at risk for pregnancy and not using a reliable contraceptive for 3 months prior to and during the study (4) a major medical or psychiatric condition, including substance abuse, (5) a score of greater than or equal to ≥ 12 on the hospital anxiety and depression scale (HADS), indicating a major depressive episode (18), (6)use of centrally-acting medications, excluding selective-serotonin receptor inhibitors, and (7) use of topical lidocaine.
At the screening visit, an interviewer-administered questionnaire collected detailed subject demographic data and a complete medical and gynecological history, including current medications. A visual inspection of the vulva, speculum exam and bimanual pelvic exam was performed. A cotton swab test was used to clinically confirm PVD, an Affirm test collected, and a Rakoff stain evaluated to determine vaginal estrogen status. If the Affirm test was positive for yeast, gardnerella or trichomonas, the patient was treated and re-screened. If the Rakoff showed greater than 10% parabasal cells, patients were treated with local estrogen, if appropriate, and rescreened after 6 weeks. Non-genital TPT and intra-vaginal algometry was performed at a separate visit, only after screening criteria were met.
TPT was performed by using the thumb pad of the dominant hand to apply 4 kg of pressure in a perpendicular direction for 4 seconds to 18 tender points. Vaginal pain sensitivity was measured using a prototype intra-vaginal pressure algometer, where 0.1, 0.3, and 0.5 kg/cm2 forces were applied digitally in random assignment by force and location to the right and left iliococcygeus muscle regions and the posterior vaginal wall, for a total of nine data points. Both TPT and algometer pain intensity were reported on a 0 (no pain) to 10 (worse pain) numeric rating scale (NRS). A diagnosis of FMS was made if pain was present (NRS > 1) in at least 11 of the tender point sites.
Correlations were computed between the composite pain intensity of TPT and those of iliococcygeus regions and the posterior vaginal wall. Composite scores were calculated by totaling the NRS at individual non-genital TPT, and for intra-vaginal algometry by totaling the NRS from the right and left iliococcygeous muscle. Independent t-tests were used to determine differences in iliococcygeus regions and the posterior vaginal algometer pain intensity ratings and presence or absence of FMS. The significance level was at P < .05 and the data were expressed as mean ± standard deviation.
Results
Ninety-two women were successfully screened and admitted into the study. These women underwent TPT examination and vaginal algometer testing by trained examiners adhering to protocol methodology. Two women were on SSRI's and were included in the analysis. Two women had incomplete data sets and were excluded from analysis. Of the remaining 90 women, 17 met TPT criteria for FMS.
For all women, the mean number of positive tender points was 4.87 ± 5.72. The mean ± SD algometer pain intensity following 0.1, 0.3, and 0.5 kg/cm2 force on the iliococcygeus region was 2.74 ± 2.48, 4.32 ± 2.78, and 5.52 ± 2.64, and that on the posterior vaginal wall was 1.73 ± 2.443.81 ± 2.88, 5.13 ± 3.13, respectively by NRS. The composite NRS score was 4.19 ± 2.36 on the iliococcygeus region and 3.56± 2.37 on the posterior vaginal wall. A significant correlation was found between composite NRS pain ratings on the TPT exam and algometer testing on the iliococcygeus region (r = 0.44, p <.0001), and the posterior vaginal wall (r = 0.45, p < .0001). The result was also significant at each of the three levels of force applied to each region independently. (see table 2)
Table 2.
Correlation TPT and Vaginal Algometer Pain Intensity, All subjects
| Iliococcygeus | P-value | Posterior vaginal wall | P-value | |
|---|---|---|---|---|
| 0.1kg/cm2 | 0.40 | <.0001 | 0.34 | 0.0012 |
| 0.3kg/cm2 | 0.39 | 0.0001 | 0.36 | 0.0005 |
| 0.5kg/cm2 | 0.40 | <.0001 | 0.43 | <.0001 |
| Total | 0.44 | <.0001 | 0.45 | <.0001 |
Pearson Correlation Coefficients, N=90
The 73 subjects who did not meet the criteria for FMS had a mean number of positive TP of 3.85 ±2.70. The mean ±SD algometer pain intensity following 0.1, 0.3, and 0.5 kg/cm2 force on the iliococcygeus region was 2.30±2.28, 3.85±2.70, and 5.07±2.58, and that on the posterior vaginal wall was 1.26±2.06, 3.37±2.80, and 4.58±3.08, respectively by NRS. The composite result was 3.74±2.22 at the iliococcygeus and 3.07±2.16 at the posterior vaginal wall.
Subjects with 11 or more painful TPs, fulfilling criteria for FMS, had a mean number of positive tender points of 14.65±2.55. The mean ±SD algometer pain intensity following 0.1, 0.3, and 0.5 kg/cm2 force on the iliococcygeus region was 4.65±2.50, 6.32±2.20, and 7.44±2.01, and that on the posterior vaginal wall was 3.76±2.92, 5.71±2.47, and 7.53±2.03, respectively by NRS. The women with FMS had significantly higher iliococcygeal pain by NRS (6.14 ± 2.07 vs. 3.74 ± 2.22, p = 0.0001), and significantly higher posterior vaginal wall pain (5.67 ± 2.10 vs. 3.07 ± 2.16, p < 0.0001) than women without FMS. (Diagram 2)
Discussion
Vulvodynia continues to present challenges to women and health care providers alike. For the women, this includes delay to diagnosis and impact on overall quality of life, and for the provider lack of proven strategies and evidence based guidelines for treatment. (19,20) These challenges exist, in part, because vulvodynia likely represents a spectrum of disorders, each with distinct contributors, associated factors and treatment requirements. The 2015 Consensus guidelines took a clinical step forward in recognizing the diversity of the associated factors of vulvodynia, including the presence of co-morbid conditions. (6)
Fibromyalgia has long been recognized as a comorbidity of vulvodynia, but this paper is the first to show an elevated algometer score in this population. A 2015 cross sectional study of 40 women with dyspareunia and 30 healthy controls showed lower non-genital pressure pain thresholds and a higher number of tender points in the women with dyspareunia. (22) However, no vaginal examinations were performed in this study, limiting its application to the vulvodynia population. Our study shows not only a higher than expected incidence of fibromyalgia by nongenital TPT score in patients with PVD versus the general population (18 versus 2.2 – 6 (23)), but also a significantly increased intravaginal muscle pain score in women with PVD with FM versus those without.
Our findings have several potential clinical implications. First, they underscore that women with PVD should be screened for co-morbid conditions. If co-morbid conditions are identified, their potential impact on PVD should be evaluated. Second, they suggest that women with PVD and FM may benefit more from early initiation of pelvic floor physical therapy (PFPT) in their treatment plan than women without FM. PFPT has been shown to improve outcomes in all women with vulvodynia and increased muscle tone (20). Third, they help us recognize the need for more research. For example, does treatment of the comorbid condition, for example pregabalin in fibromyalgia, improve vulvodynia symptoms?
Strengths of this study include the number of enrolled patients and the rigorous screening process to eliminate patients with any vulvar or vaginal disorder in addition to PVD. Intra-vaginal algometer examinations were standardized by training investigators at all sites on obtaining algometer measurements, used identical equipment, and had site visits to calibrate machines. All data was analyzed centrally.
Weaknesses of the study include the use of TPT as a diagnostic tool for FMS. The American Academy of Rheumatology 2010 diagnostic criteria for FMS does not require a TPT examination, citing concerns over not only the training of non-specialists in performing the exam, but also the frequency with which the exam was not performed, limiting its accuracy for diagnosis. (12,24) However, all investigators for this study did undergo training on TPT examination, including training on calipers to simulate 4 kg of pressure. The new criteria rely more on history and a scale for measurement of number and severity of symptoms characteristic of FMS, and future studies should incorporate these criteria as well.
Another potential weakness of the study was lack of a control population for measurements of algometer NRS. The goal of the study was to determine if the NRS of the algometer correlated with TPT and as such the control group was the 73 women with a TPT score less than 11. A future study could evaluate the difference of vaginal algometer NRS score between women with and without FMS in the absence of PVD. Additionally, there is no direct algometer comparison of women with PVD and women without PVD. However, prior studies which have looked at intra-vaginal algometry in women without dyspareunia, showed pain with a pressure threshold of 1.52 ± 0.62 kg/cm2for vaginal muscle and 1.65 ± 0.64 kg/cm2 for non-muscle areas (anterior and posterior vaginal wall), values well below out thresholds of 0.1, 0.3 and 0.5 kg/cm2 which elicited pain in our population. (15)
A third weakness of this study is the inclusion of only women with PVD. Women with generalized, non-provoked vestibulodynia would not have a positive CST and therefore would not have qualified for the study. Our findings therefore may not be consistent in this population.
Conclusion
Women with PVD who experience more severe pain with tender point palpation also experience more vaginal pain on pelvic exam as measured by intra-vaginal muscle algometry. Those who fulfill the diagnosis of FMS by use of non-genital TPT evaluation show significantly more intense vaginal pain by NRS to palpation of iliococcygeus muscles and posterior vaginal wall. Further research using a more precise definition of FMS is necessary to confirm this relationship, but findings suggest that women with PVD coexisting with FMS have greater risk of superimposed vaginal muscle pain. Clinical evaluation for vaginal muscle pain remains an important in the evaluation of vulvodynia, and intra-vaginal algometry may have a clinical role in this evaluation, especially in women with comorbid musculoskeletal disorders, like fibromyalgia. This relationship needs to be explored in women with generalized, non-provoked vestibulodynia. Treatment aimed at pelvic floor muscles such as pelvic floor physical therapy may be warranted early in the course of management in this population and studies to look at medications used for treatment of FMS in this population should be considered.
Figure 1.
Tenderpoint Tenderness Diagrammatic Scheme
Figure 2.
COMPOSITE INTRA-VAGINAL ALGOMETER NUMERICAL RATING SCORES (NRS)
Acknowledgments
Financial Support : NIH Grant # 1R01HDO65740-0181
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure Statement
Nancy Phillips reports no conflict of interest.
Candace Brown reports no conflict of interest.
Gloria Bachmann reports no conflict of interest.
Jim Wan reports no conflict of interest.
Ronald Wood reports no conflict of interest.
Dangy Ulrich reports no conflict of interest.
Candi Bachour reports no conflict of interest.
David Foster reports no conflict of interest.
Disclaimers: none
Prior presentation of findings:
Brown C, Bonham A, Bachmann G, Wan J, Wood R, Foster D. Relationship between tender point tenderness and vaginal algometer pain intensity ratings in women with vestibulodynia: Implications for treatment. International Pelvic Pain Society (IPPS) Annual Meeting, Chicago, Oct 2014.
Clinical Trial number: clinicaltrial.gov NCT01301001
REFERENCES
- 1.Haefner HK. Report of the International Society for the Study of Vulvovaginal Disease terminology and classification of vulvodynia. J Low Gen Tract Dis. 2007;11:48–49. doi: 10.1097/01.lgt.0000225898.37090.04. [DOI] [PubMed] [Google Scholar]
- 2.Goetsch MF. Vulvar vestibulitis: [prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164:1609–14. doi: 10.1016/0002-9378(91)91444-2. [DOI] [PubMed] [Google Scholar]
- 3.Harlow BL, Stewart E. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia. J Am Women Assoc. 2003;58:82. [PubMed] [Google Scholar]
- 4.Reed BD, Haefner HK, Harlow SD, Gorenflo DW, Sen A. Reliability and Validity of Self-reported symptoms for predicting vulvodynia. Obstet Gynecol. 2006;108:906–13. doi: 10.1097/01.AOG.0000237102.70485.5d. [DOI] [PubMed] [Google Scholar]
- 5.Meana M, Binik YM, Khalife S, Cohen DR. Biopsychosocial profile of women with dyspareunia. Obstet Gynecol. 1997;90:583. doi: 10.1016/s0029-7844(98)80136-1. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein Jacob, Goldstein Andrew, Coady Deborah, for the consensus vulvar pain terminology committee Consensus terminology and classification of persistent vulvar pain from the International Society for the Study of Vulvovaginal Disease (ISSVD), the International Society for the Study of Women's Sexual Health (ISSWSH), and the International Pelvic Pain Society (IPPS) 2015 [Google Scholar]
- 7.Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43:689–700. doi: 10.1097/00003081-200009000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Reissing ED, Brown C, Lord MJ, Binik YM, Khalife S. Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. J Psychosom Obstet Gynaecol. 2005;26(2):107–13. doi: 10.1080/01443610400023106. [DOI] [PubMed] [Google Scholar]
- 9.Zolnoun D, Bair E, Essicks G, Gracely R, Goyal V, Maixner W. Reliability and Reproducibility of Novel Methodology for Assessment of Pressure Pain Sensitivity in Pelvis. J Pain. 2012 Sep;13(9):910–920. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner H. Relationship between vulvodynia and chronic comorbid pain conditions. Obstet Gynecol. 2012;120(1):145–151. doi: 10.1097/AOG.0b013e31825957cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biasi G, Di Sabatino V, Ghizzani A, Galeazzi M. Chronic pelvic pain: comorbidity between chronic musculoskeletal pain and vulvodynia. Reumatismo. 2014;66(1):87–91. doi: 10.4081/reumatismo.2014.768. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 13.Bennett RM, Friend R, Marcus D, Bernstein C, Han BK, Yachoui R, Deodhaur A, Kaell A, Bonafede P, Chino A, Jones KD. Criterial for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American college of rheumatology criterial and the development of alternative criteria. Arth Care Res. 2014;66(9):1364–1373. doi: 10.1002/acr.22301. [DOI] [PubMed] [Google Scholar]
- 14.Baguley SDK, Curnow JSH, Morrison GD, Barron LF. Vaginal algometer: development and application of a device to monitor vaginal wall pressure pain threshold Physiol. Meas. 2003;24:833–836. doi: 10.1088/0967-3334/24/4/302. [DOI] [PubMed] [Google Scholar]
- 15.Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Vaginal pain pressure thresholds: initial validation and reliability assessment in healthy women. Clin J Pain. 2008;24(1):45–50. doi: 10.1097/AJP.0b013e318156db13. [DOI] [PubMed] [Google Scholar]
- 16.Freidrich EG. Vulvar vestibulitis syndrome. J Reprod Med. 1987;32:110–114. [PubMed] [Google Scholar]
- 17.Foster DC, Kotok MB, Huang LS, Watts BS, Oakes D, Howard FM, Stodgell CJ, Dworkin RH. The tampon test for vulvodynia research: reliability, construct validity, responsiveness. Obstet Gynecol. 2009;113:825–32. doi: 10.1097/AOG.0b013e31819bda7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoffstetter S, Shah M. Vulvodynia. Clin Obstet Gyne. 2015;58(3):536–545. doi: 10.1097/GRF.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 20.Shah M, Hoffstetter S. Vulvodynia. Obstetrics & Gynecology Clinics of North America. 2014 Sep;41(3):453–64. doi: 10.1016/j.ogc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Latremoliere A1, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terzi H, Terzi R, Kale A. The relationship between fibromyalgia and pressure pain threshold in patients with dyspareunia. Pain Res Manag. 2015;20(3):137–140. doi: 10.1155/2015/302404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldenberg DL. Clinical manifestations and diagnosis of fibromyalgia in adults. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-offibromyalgia-in-adults/abstract/44.
- 24.Shleyfer E, Jotkowitz A, Karmon A, Nevzorov R, Cohen H, Buskila D. Accuracy of the diagnosis of fibromyalgia by family physicians: is the pendulum shifting? J Rheumatol. 2009;36(1):170–173. doi: 10.3899/jrheum.080468. [DOI] [PubMed] [Google Scholar]