Abstract
Spinal cord injury (SCI) results in both acute and chronic inflammation, as a result of activation of microglia, invasion of macrophages and activation of the NADPH oxidase (NOX) enzyme. The NOX enzyme is a primary source of reactive oxygen species (ROS) and is expressed by microglia and macrophages after SCI. These cells can assume either a pro- (M1) or anti-inflammatory (M2) polarization phenotype and contribute to tissue response to SCI. However, the contribution of NOX expression and ROS production to this polarization and vice versa is currently undefined. We therefore investigated the impact of SCI on NOX expression and microglial/macrophage polarization over time in a mouse model of contusion injury. Adult C57Bl/6 mice were exposed to a moderate T9 contusion SCI and tissue was assessed at acute, sub-acute and chronic time points for NOX isoform expression and co-expression with M1 and M2 microglia/macrophage polarization markers. Two NOX isoforms were increased after injury and were associated with both M1 and M2 markers, with an M1 preference for NOX2 acutely and NOX4 chronically. M2 cells were primarily found at acute time points only; the peak of NOX2 expression was associated with the decline in M2 polarization. In vitro, NOX2 inhibition shifted microglial polarization toward the M2 phenotype. These results now show that microglial/macrophage expression of NOX isoforms is independent of polarization state, but that NOX activity can influence subsequent polarization. These data can contribute to the therapeutic targeting of NOX as a therapy for SCI.
Keywords: Microglia, inflammation, spinal cord injury, oxidative stress, polarization
Introduction
Spinal cord injury (SCI) results in both acute (hours) and chronic (days to months) inflammation. This inflammation includes activation of microglia, invasion of macrophages and up-regulation of the NADPH oxidase (NOX) enzyme. This inflammatory process plays an important role in the secondary tissue damage and cell death that follows the initial mechanical insult. This contribution to the pathological outcome of the injury makes it an important therapeutic target.
In a comprehensive study, a histopathological post-mortem assessment of injured human spinal cords showed an increased expression of NOX2 associated with a transient neutrophil population and activated microglia/macrophages with a peak expression at 3 days after injury (Fleming et al., 2006). SCI also results in acute and chronic up-regulation of NOX components in both rats and mice (Byrnes et al., 2006, Byrnes et al., 2011, Pajoohesh-Ganji et al., 2012). We have previously shown that NOX, particularly the NOX2 and 4 isoforms, are up-regulated after central nervous system (CNS) injury in a number of cells, including microglia and macrophages in a rats (Cooney et al., 2013, Cooney et al., 2014).
The NOX family of proteins is a primary source of reactive oxygen species (ROS) and oxidative stress (Xiong et al., 2007). There are several isoforms of this enzyme, NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2, each with slightly different enzyme components and oxidative products. NOX2, for example, is composed of four cytosolic (gp40PHOX, p47PHOX, p67PHOX and GTP-binding protein p21-Rac1) and two membrane subunits (gp91PHOX and p22PHOX) (Cross and Segal, 2004). Activation of NOX typically involves two steps: up-regulation of expression of the individual protein components and PKC- or MAPK-mediated phosphorylation of cytosolic components (such as p47PHOX in the NOX2 isoform). Once phosphorylated, the cytosolic subunits translocate to the plasma membrane, where they assemble with the membrane subunits to form the active enzyme (Bey et al., 2004, Choi et al., 2005, El Benna et al., 1996, Zhao et al., 2005). Unlike NOX2, NOX4 is constitutively active, although it can be influenced by a number of factors including chemical changes. Active NOX2 produces O2-, which can be converted into other superoxide-derived oxidants such as hydrogen peroxide (H2O2), hydroxyl radicals and peroxynitrite (Patel et al., 2005), while NOX4 produces H2O2 directly (Takac et al., 2011). These ROS can have severe cytotoxic effects (Block et al., 2007, Qin et al., 2004) as well as facilitate pro-inflammatory pathways by activating MAPK and NFκB signaling, ultimately inducing transcription of pro-inflammatory cytokines and other inflammatory mediators such as tumor necrosis factor (TNF)α, inducible nitric oxide synthase (iNOS), and monocyte chemotractant protein (MCP)-1 (Aldskogius and Kozlova, 1998). ROS can also produce a positive feedback loop by inducing transcription and/or activation of NOX components, further increasing NOX activity (Brandes et al., 2001, El Benna et al., 1996, Pawate et al., 2004).
In injured human spinal cords, activated microglia and macrophages were present at 1 day post injury, becoming the predominant inflammatory cells at and beyond 5 days post-injury; this response is sustained weeks to months (Fleming et al., 2006). Despite differences in lesion evolution and cavity formation in rats and mice, similarly to human injury, macrophages from the periphery and activated microglia appear in the spinal cord between 12 and 24 hours post-injury, peaking between 4 and 8 days post-injury (Carlson et al., 1998, Pajoohesh-Ganji et al., 2012, Popovich et al., 1997, Sroga et al., 2003). In a comprehensive study Kigerl et al. demonstrated that this microglial/macrophage response is primarily composed of 2 different cellular phenotypes: M1 and M2 (Kigerl et al., 2009). Classically activated, or M1, microglia/macrophages, express a number of identifiable cell surface markers, such as CD86 and CD16/32, and secrete pro-inflammatory molecules such as TNFα, interleukin (IL) 1β, ROS and nitrogen intermediates (Aloisi, 2001). Alternatively activated, or M2, microglia/macrophages have an anti-inflammatory phenotype and are associated with CD206/mannose receptor and arginase-1 expression and secretion of anti-inflammatory cytokines such as IL4 and transforming growth factor (TGF)-β (Kigerl et al., 2009). Acutely after injury, both M1 and M2 phenotypes are equally present in the damaged spinal cord, while, at later time points (i.e., beyond 2 weeks in a rodent SCI model), the M1 phenotype is dominant and M2 markers are often below levels of detection (Kigerl et al., 2009).
It is currently unclear what factors in the injured spinal cord control the shifts in microglial and macrophage polarization. However, in 2011, Choi et al. (Choi et al., 2011) found that knockout of gp91PHOX or p47PHOX or administration of the non-specific NOX inhibitor apocynin could increase M2 marker expression in microglia in an in vivo inflammation model. Further, our previous work demonstrated that acute inhibition of the NOX2 enzyme with the specific inhibitor gp91ds-tat resulted in an elevation in the number of cells expressing the M2 marker CD206 (Khayrullina et al., 2015). The goal of this work was therefore to further explore this phenomenon and identify the NOX enzyme isoform expression profile after SCI and determine if there was a correlation between this expression profile and microglial/macrophage polarization. We now demonstrate that the expression of NOX2 and NOX4 enzymes is both temporally and polarization related. Further, we show that NOX enzyme inhibition in microglia can alter polarization status, although polarization does not alter NOX expression.
Material and Methods
Animal Handling and Surgical Methods
Adult male C57Bl6 mice (20-25g, Taconic Farms, Derwood, MD) were used for all experiments. Mice were group housed and received food and water ad libitum with a 12:12 hour light cycle. A total of 72 male mice were used for this study; 3 injured mice and 1 sham injured mouse were removed from the study due to post-surgical complications or inadequate injury. All experiments complied fully with the principles set forth in the “Guide for the Care and Use of Laboratory Animals” and were approved by the Uniformed Services University IACUC.
Moderate spinal cord contusion injury was performed using the Infinite Horizons Impactor (50 kdyne; Precision Systems and Instrumentation, Fairfax Station, VA) positioned over the exposed spinal cord at vertebral level T-9 while animals were anesthetized with isoflurane (4% induction, 2% maintenance). Sham injured mice underwent the same experimental procedures, but received a laminectomy only. Naïve mice underwent no surgery. Animals were allowed to recover on heating pads, after which they were returned to home cages (group housing, 4 mice/cage with cell-sorb bedding) and received acetaminophen (200mg/kg) in drinking water for 72 hours post-injury. Manual bladder expression was performed daily until normal bladder expression returned.
At 1 (n = 5 injured, 5 sham), 4 (n = 4 injured, 4 sham), 7 (n = 5 injured, 5 sham), 28 (n = 3 injured, 4 sham), or 60 (n = 5 injured, 5 sham) days after surgery or no surgery (naïve; n = 4), mice were anesthetized (Euthasol, 0.22ml/kg, I.P.) and intracardially perfused with 50 ml of 0.9% saline followed by 100 ml of 10% buffered formalin. In injured or sham/naïve mice, a 5mm section of the spinal cord centered at the lesion epicenter was dissected, post-fixed in 10% buffered formalin overnight and cryoprotected in 30% sucrose for 48 hours. Tissue was cut into serial 20 μm thick transverse sections and collected at every 60μm (Supplementary Figure 1). At the above-mentioned time points, an additional 18 mice (naïve n = 4; 1 day n = 3; 4 days n = 4; 7 days n = 3; 28 days n = 4) were euthanized and tissue sample protein was obtained from a 5 mm section of the spinal cord centered at the lesion epicenter was dissected and immediately frozen on dry ice for western blotting.
Immunochemistry
Standard single or double fluorescent immunohistochemistry (IHC) and immunocytochemistry (ICC) was performed as described previously (Cooney et al., 2013). Primary antibodies were used as described in the Table 1. Appropriate secondary antibodies linked to AlexaFluor dyes (1:2000; Invitrogen, Carlsbad, CA) were incubated with tissue sections (serial 20μm thick transverse sections 60μm apart, supplementary Fig. 1) or fixed cells for 1 hour at room temperature. Slides were coverslipped using mounting media containing DAPI to counterstain for nuclei (Vector Labs, Burlingame, CA). Round coverslips were mounted onto slides using mounting media containing DAPI (Vector Labs).
Table 1.
Immunochemistry and western blot antibodies.
| Antibody | Host | Company | Concentration | Application |
|---|---|---|---|---|
| 3-Nitrotyrosine | Mouse | Abcam | 5 μg/ml, 2 μg/ml | IHC, WB |
| 8OHdG | Rabbit | Abcam | 1 μg/ml | IHC |
| CD206 (Mannose receptor) | Rat | Serotec | 20 μg/ml | ICC |
| CD206 (Mannose receptor) | Rabbit | Abcam | 15 μg/ml, 1 μg/ml | IHC, WB |
| CD206 (Mannose receptor) | Mouse | Abcam | 15 μg/ml | IHC |
| CD32 | Goat | R&D systems | 5 μg/ml | IHC |
| CD86 | Rabbit | Abcam | 5 μg/ml | IHC |
| CD86 | Rat | Abcam | 5 μg/ml | IHC |
| iNOS | Rabbit | Abcam | 20 μl/ml, 2 μg/ml | ICC, WB |
| Liver Arginase | Goat | Abcam | 5 μg/ml | IHC |
| NOX2/gp91PHOX | Mouse | BD Transduction Laboratories | 0.5 μg/ml, 0.5 μg/ml, 1 ug/ml | ICH, ICC, WB |
| NOX4 | Rabbit | Thermo Fisher Scientific | 1 μg/ml, 1 μg/ml | ICC, IHC |
| Phospho-p47PHOX | Rabbit | Sigma-Aldrich | 20 ug/ml | WB |
Legend. IHC: Immunohistochemistry; ICC: Immunocytochemistry; WB: Western blot
To ensure accurate and specific staining, negative controls were used in which the primary antibody was not applied, and only staining that labeled cells that were labeled with nuclei and had expected labeling patterns (i.e., classic microglia morphology) was confirmed as positive labeling. It should be noted that NOX specific antibodies from a number of sources were tested, as NOX antibodies have a history of lacking specificity and fidelity. Each antibody was tested in conjunction with cell specific antibodies and with western blotting to confirm that each resulted in appropriate, expected staining and/or expected band sizes in western blots (NOX2 - supplementary figure 2; NOX4 data not shown, but consistent with the testing in our previous work (Cooney et al., 2013)).
Imaging and quantification
ICC fluorescence was detected and photographed in at least 5 randomly chosen regions (20X) using an Olympus DP72 microscope with Olympus cellSens microscopy software (Olympus). IHC fluorescence was detected and photographed using an Olympus DP72 microscope with Olympus cellSens microscopy software (Olympus, Center Valley, PA) or NanoZoomer Digital Pathology system (Hamamatsu Photonics, K.K., Japan). For 8OHdG, fluorescence was quantified as previously described using pixel density measurement in Scion Image (Donnelly et al., 2009). For all other immunohistochemistry, semi-quantification was performed manually on 3 to 5 equally spaced sections per animal spanning a 1.7mm section encompassing the lesion center (Supplementary Figure 1) focused on the dorsal columns and surrounding gray matter at a 20X magnification, as this was the area most affected by the dorsal contusion. Cells were evaluated through the presence of a labeled nucleus and expected cellular morphology by two investigators blinded to group. The score system was used as follows: 0=no labeled cells observed, 1=<5, 2=5-10, 3=10-50, 4=>50 labeled cells observed (Supplementary Figure 3). Score for each section was then averaged with all other sections for each animal, and mean per group represents average for each animal in that group.
This scoring system was chosen as standard cell counting was found to underestimate cell number due to overlapped cells (data not shown). In addition, given the injured nature of the tissue and common artifactual fluorescence, pixel density analysis was unable to specifically identify microglial/macrophage labeling or to detect double-labeling of cells. In all analyses, no significant difference was noted between sham and naïve data. Therefore, in all data presentation and analysis, sham and naïve data are combined and, in graphs, indicated as day 0.
Western blot Analysis
Protein from tissue samples was isolated in RIPA Buffer (Pierce, Rockford, IL) containing protease inhibitor (1%, Halt Protease Inhibitor Single-Use Cocktail, Thermo Scientific). The in-vitro sample protein was obtained 24 hours after treatment with LPS, gp91ds-tat or scrambled-tat, cells were scraped and lysed in Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) containing protease inhibitor (1%, Halt Protease Inhibitor Single-Use Cocktail, Thermo Scientific). Twenty-five μg of sample protein was run in a Mini-PROTEAN® TGX™ Precast Gel (Bio-Rad, Hercules, CA) and transferred to a Trans-Blot® Turbo™ (Bio-Rad) nitrocellulose membrane. Primary antibodies were probed overnight at 4°C as described in the table 1. Immune complexes were detected with appropriate secondary antibodies and chemiluminescence reagents (Pierce, Rockford, IL). GAPDH (0.5μg/ml; Millipore, Temecula, CA) or β-actin (0.5 ug/ml; Abcam) was used as a control for gel loading and protein transfer. NIH Image J was used to assess pixel density of resultant blots for quantitation.
In Vitro Analysis
The BV2 microglial cell line (a generous gift from Dr. Carol Colton) was cultured and replated to wells at passage 14 - 19. Cells were incubated at 37°C with 5% CO2 in Dulbecco's modified Eagle media (Gibco, Carlsbad, CA) with 10% fetal calf serum (Hyclone, Logan, UT), 1% L-glutamine (Gibco), 1% sodium pyruvate (Gibco), and 1% Pen/Strep (Fisher, Pittsburgh, PA). Cells were used 24 hours after plating for experimentation.
BV2 cells were treated with the toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS; 100ng/ml, Sigma, St. Louis, MO) or vehicle media for 24 hours. The NOX2 inhibitor gp91ds-tat peptide (50 μM, AnaSpec, Fremont, CA), scrambled ds-tat (50μM, AnaSpec) or recombinant rat IL-4 (7.5 μM, R&D systems) was applied one hour after LPS or control stimulation. All drugs were prepared and stored according to the manufacturer's guidelines. At 24 hours after treatment cells were fixed with 4% paraformaldehyde on round coverslips or protein was isolated. NOX isoforms, microglial polarization markers and oxidative stress markers were examined.
Nitric Oxide Assay
BV2 microglia were exposed to LPS for 1 hour prior to addition of gp91ds-tat (50μM) or scrambled ds-tat and incubated at 37°C and 5% CO2 for 24 hours. NO• production was assayed using the Griess Reagent Assay kit (Invitrogen) and absorption assessed at 540nm, according to the manufacturer's instructions.
Statistics
Power analysis was completed based on our previously published studies (Cooney et al., 2013, Cooney et al., 2014) and pilot work indicated that an n of 4/group for immunohistochemical analysis and an n of 3/group for western blotting would be sufficient to demonstrate a significant difference between groups with 80% power. Post-hoc analysis demonstrated that an n of 4 was sufficient to obtain this power in all studies; however, due to post-injury mortality in the 28 day injured group, the final n was 3. Analysis of this group did show that, while underpowered, no significant difference would have been observed without an extraordinarily large n (n = 10 – 15/group) for immunohistochemical analysis, and the result would likely be biologically insignificant. Therefore, 28 day data remains in the manuscript. All assays were carried out by investigators blinded to subject group. Quantitative data are presented as mean +/− standard error of the mean. Semi-quantitative score data were analyzed using the Kruskal-Wallis non-parametric test followed by Dunn's post-test. Quantitative in vivo and in vitro data were analyzed using one-way ANOVA with Dunnett's Multiple Comparison's post-test. All statistical tests were performed using the GraphPad Prism Program, Version 6.03 for Windows (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
SCI leads to a significant acute and chronic increase in oxidative stress
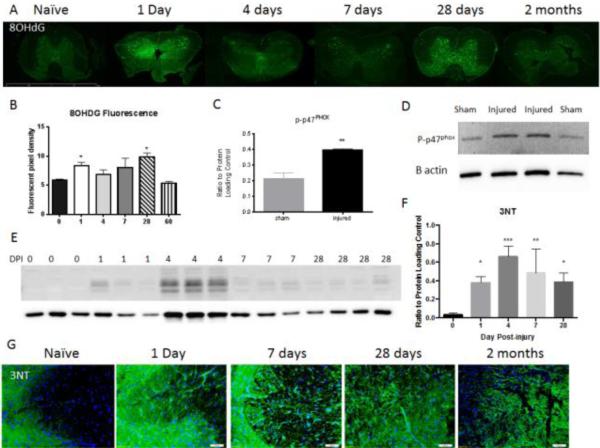
The release of ROS can lead to oxidative damage to DNA, which can be detected using the 8OHdG antibody, and production of further oxidative compounds, such as peroxynitrate. In turn, peroxynitrate can lead to the nitrosylation of proteins, which can be detected using the 3NT antibody. We therefore investigated oxidative stress in the spinal cord at acute and chronic time points after SCI or in naïve tissue using immunohistochemistry and western blotting for 8OHdG and 3NT (Fig. 1A, B). A marked increase in nuclear staining of 8OHdG, particularly in large motor neurons within the gray matter, was observed in injured tissue in comparison to naïve tissue. This expression was most prevalent at 1 and 28 days post-injury. Immunolabeling was quantified as pixel density using stain intensity as a threshold; quantification confirmed a significant increase in immunofluorescence over naïve tissue at 24 hours and 28 days post-injury (*p<0.05). Elevated 3NT was also observed in injured tissue from 24 hours to 60 days post-injury (Fig. 1E-G). Quantification of nitrosylated proteins in a western blot showed that this was significantly elevated above naïve levels by 1 day through 28 days peaking at 4 days post-injury (p<0.001).
Figure 1. Oxidative stress markers significantly increase after injury.
8OHdG immunohistochemistry was used as a marker of oxidative stress in naïve and injured tissue (A). Size bar = 2mm. Expression was measured by pixel density of immunolabeling using stain intensity as threshold. The staining showed a significant increase in expression over naïve tissue (n = 4) at 24 hours (n =5) and 28 days (n = 3) post-injury, with no significant difference at 4 days (n = 4), 7 days (n = 5) or 60 days (n = 5) post-injury (B). Phosphorylated p47PHOX was assessed by western blot in sham and injured tissue 4 days post-injury (n = 4/group). Quantification shows a significant increase in injured tissue over naïve (C). Representative blot is shown in D. Protein nitrosylation (3NT) was assessed by western blot in naïve (n = 3) and injured tissue at 24 hours (n = 3), 4 days (n = 3), 7 days (n = 3) and 28 days (n = 4) post-injury (E). Quantification shows a significant increase of 3NT in injured tissue over naïve at all time points, peaking at 4 days (F). 3NT was also assessed through immunohistochemistry and showed an elevated immunoreactivity in injured tissue from day 1 to day 60, particularly in grey matter (G). Size bar = 50μm. Bars represent mean +/- SEM. *p<0.05, **p<0.01, ***p<0.001, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
A number of enzymes are known to contribute to post-injury oxidative stress. In order to begin to understand whether NOX may play a role, we investigated if activation of NOX was observed at the same time point of elevated oxidative stress. Immunoblotting for the phosphorylated p47PHOX subunit showed a significant increase at 4 days post-injury in comparison to sham/naïve tissue (Fig. 1C-D).
Moderate SCI results in changes in NOX isoform expression and microglia/macrophage polarization
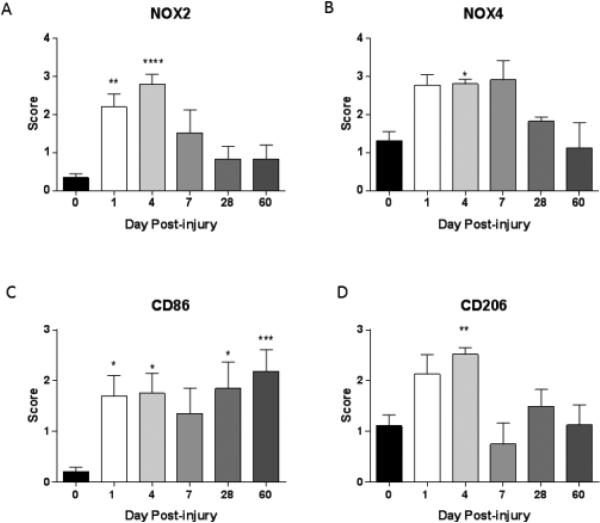
We next aimed to determine if NOX isotype expression was also altered following SCI. Immunolabeled NOX isotypes were semi-quantified using a 5 point scale based on the number of positive cells and show clear changes in NOX2 and NOX4 isotype expression after injury (Fig. 2). NOX2 expression showed a significant increase at 1 and 4 days, returning to levels that trended toward higher but were not significantly different than sham/naïve levels by 7 days after injury, with no further change. NOX4 also showed an initial increase, significantly elevating above sham by 4 days, but returned to sham levels by 28 days after SCI. A trend toward significance was observed at 1 and 7 days post-injury, but did not reach significance with the non-parametric Kruskal Wallis test. To further support the immunohistochemistry results, expression of NOX2 was confirmed at 4 days post-injury using immunoblotting techniques (supplementary Fig. 2).
Figure 2. Moderate SCI changes the expression of NOX isoforms and polarized microglia markers in the spinal cord.
NOX isoforms and M1 (CD86) and M2 (CD206) markers were assessed on a 5 point scale in naïve/sham tissue (n = 11) or at 1 (n = 5), 4 (n = 4), 7 (n = 5), 28 (n = 3) or 60 (n = 5) days after a moderate contusion injury. NOX2+ cells showed a significant increase at 1 and 4 days post-injury, after which expression declined, but remained elevated through 60 days post-injury over sham-injured tissue (A). The NOX4+ cell population showed a similar pattern, although significance was only observed at 4 days post-injury (B). CD86+ cells in the spinal cord increased significantly acutely and continued rising at chronic time points (C). CD206+ cells were increased at 4 days post-injury and decreased by 7 days but remained elevated over sham/injured levels at chronic time points (D). Bars represent mean score +/− SEM. *p<0.05, **p<0.01, ***p<0.001, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
Using the same 5 point scale, microglia/macrophage polarization markers were evaluated and semi-quantified as well (Fig. 2). CD86 was used as a marker of M1 polarized macrophage/microglia. Its expression increased significantly after injury, with a slight reduction in expression at 7 days. Expression elevated again at 28 days and continued increasing during the rest of the study. CD206 was used as a marker of M2 polarized macrophage/microglia. This marker showed an increase in expression acutely but at 7 days post-injury expression levels returned to sham/naïve levels where it remained for the remainder of the study. Additional M1 and M2 markers were examined using immunohistochemistry and immunoblotting techniques to confirm these polarization trends (supplementary Fig. 2).
Acute NOX2 expression is associated with M1 and M2 microglia/macrophages
In order to assess any temporal or phenotypic dependent expression of NOX isoforms in macrophages/microglia, double immunostaning of polarization markers and NOX isoforms was performed. Single and double-labeled cells were manually semi-quantified spanning a representative area of the tissue always including the white and grey matter within the lesion epicenter and perilesional region (supplementary Fig. 1). In addition to double labeling, temporal changes in expression of each cell population were analyzed. Immunoreactive cells for each antibody were taken as a population and the proportion of this population expressing each marker at all different time points is reported. The overall pattern of staining suggests that at acute (24 hours to 4 days) time points, both M1 and M2 polarized cells were associated with NOX2, which was confirmed with confocal microscopy at higher magnifications (supplementary Fig. 4).
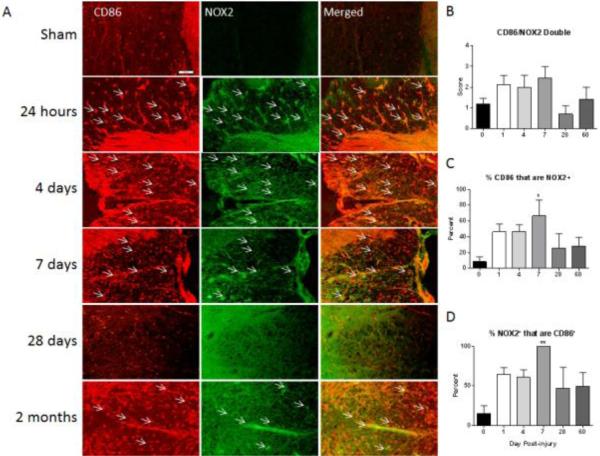
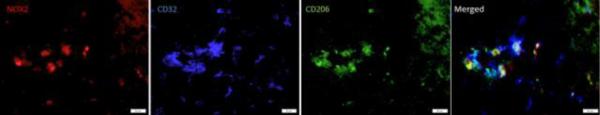
NOX2 co-labeling with the M1 marker CD86 was slightly elevated over sham by 24 hours post-injury through 7 days post-injury, although this did not reach statistical significance (Fig. 3). At this 7 day time point, all of the cells expressing NOX2 were CD86+, but only about 75% of the CD86+ cell population expressed NOX2. Interestingly, at these acute time points, while 100% of the NOX2 positive cells were CD86+, 10 – 20% were also positive for the M2 marker CD206 (Fig. 4). Investigation of triple labeling for NOX2 and the M1 and M2 markers showed that at this time point, a subset of cells were positive for both M1 and M2 markers, suggesting an intermediate polarization state (Fig. 5). At 28 days post-injury, co-labeling decreased to resemble sham/naïve but returned to increased levels by 60 days post-injury. At chronic time points, about 30% of the M1 polarized cells expressed NOX2.
Figure 3. M1 polarized macrophages/microglia express NOX2 after SCI.
Macrophages/Microglia were labeled with CD86 (red) and immunostained with an antibody against NOX2 (green) in sham-injured tissue and at all examined time points post-injury (A). Co-labeled cells are indicated with arrows. CD86+/NOX2+ cells increased by 24 hours post-injury through 7 days post-injury. At 28 days post-injury staining resembled sham but returned to increased levels at 60 days post-injury (A). Bar = 50 μm. Double-immunolabeling was then semi-quantitated using a 5 point scale and is presented as total amount of double label (B), percent of CD86 positive cells that are also NOX2 positive (C) and percent of NOX2 positive cells that are also CD86 positive (D). NOX2 expression by M1 polarized cells increased acutely post-injury, and at 7 days after injury, 100% of the cells expressing NOX2 were CD86+. Of the CD86+ cell population, 75% expressed NOX2. Bars represent mean score or percent +/- SEM of naïve/sham tissue (n = 11) or 1 (n = 5), 4 (n = 4), 7 (n = 5), 28 (n = 3) or 60 (n = 5) days post-injury. *p<0.05, **p<0.01, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
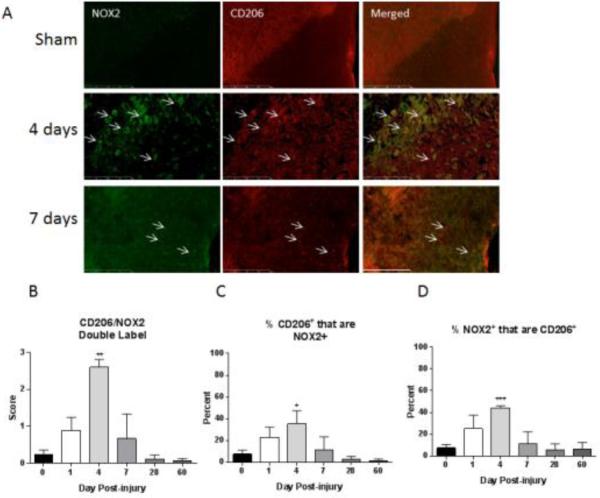
Figure 4. NOX isoforms are associated with M2 polarized microglia/macrophage at acute time points.
M2 polarized macrophages/microglia were labeled with CD206 (red) and an antibody against NOX2 (green) in sham-injured tissue and at all examined time points post-injury. Four and 7 day post-injury tissue is shown. Co-labeled cells are indicated with arrows. CD206+ cells co-expression of NOX2 increases acutely after injury but returned to sham/naïve beyond 7 days post injury (A). Size Bar = 200 μm. Double-immunolabeling was then semi-quantitated using a 5 point scale and is presented as total amount of double label (B), percent of CD206 positive cells that are also NOX2 positive (C) and percent of NOX2 positive cells that are also CD206 positive (D). M2 polarized cells positive for NOX2 showed a peak at 4 days after SCI. At this point, the percent of CD206+ cells expressing NOX2 had shifted from less than 10% to 30-50%. Of the NOX2+ cells, 40-50% co-labeled with CD206 antibody. Bars represent mean score or percent +/− SEM of naïve/sham tissue (n = 11) or 1 (n = 5), 4 (n = 4), 7 (n = 5), 28 (n = 3) or 60 (n = 5) days post-injury. *p<0.05, **p<0.01, ***p<0.001, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
Figure 5. Evidence of an intermediate polarization state.
Triple labeling for NOX2, CD32 and CD206 shows that NOX2 is present in cells in an intermediate state of polarization at 7 days post injury. Bar = 20 μm.
CD206 was used as an M2 polarized macrophage/microglia marker. Standard double immunostaining was performed with CD206 and an antibody against NOX2 in tissue from sham-injured and all examined time points post-injury (Fig. 5). Although CD206 positive cells can be observed in tissue from sham/naïve and all the examined time points, co-labeling with NOX2 was most prominent at 4 and 7 days after injury with a peak at 4 days. Semi-quantification indicated that double-labeled population increased significantly from 1 day to 4 days post-injury; by 7 days the double labeled cell population diminished to sham injured levels. Up to 4 days post injury, the percent of CD206 positive cells expressing NOX2 had shifted from less than 10% to 25-35% while of all the NOX2+ cells, 40-50% were M2 polarized cells. This involved an increase from less than 10% at 1 day post injury to 40 – 50% by day 4. Both of these proportions returned to sham injured levels by 7 days, the time point at which M2 polarization decreased in the spinal cord.
NOX4 isoform is associated with the M1 and M2 polarized phenotype
Standard double immunostaining was also performed with an antibody against NOX4 and the M1 marker CD86 (Fig. 6). As with NOX2, single and double-labeled cells were manually semi-quantified spanning a representative area of the tissue always including the white and grey matter within the lesion epicenter and perilesional region. The overall pattern of staining suggests that NOX4 was observed in M2 polarized cells acutely after injury and in M1 polarized cells at more chronic time points.
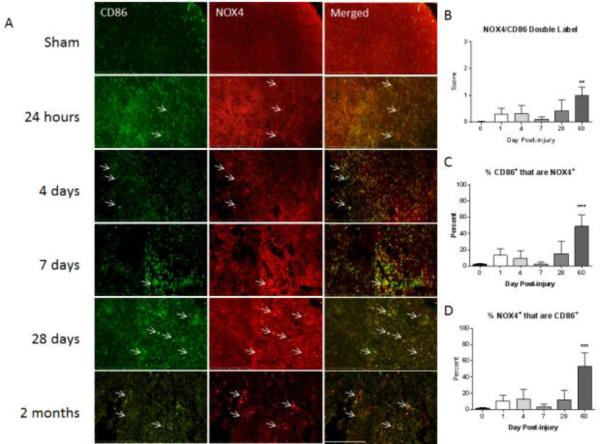
Figure 6. M1 polarized macrophages/microglia express NOX4 post injury.
Macrophages/Microglia were labeled with CD86 (green) and immunostained with an antibody against NOX4 (red) in sham-injured tissue and at all examined time points post-injury. Co-labeled cells are indicated with arrows. CD86+/NOX4+ cells increased steadily from 1 to 60 days post-injury at all time points except at 7 days. At 7days post-injury staining resembled sham but returned to increased levels at 28 days post-injury (A). Bar = 200 μm. Double-immunolabeling was then semi-quantitated using a 5 point scale and is presented as total amount of double label (B), percent of CD86 positive cells that are also NOX4 positive (C) and percent of NOX4 positive cells that are also CD86 positive (D). NOX4/CD86 double labeled cell population increased gradually following the first day post-injury, reaching significance at 60 days. The percent of CD86+ cells that were also NOX4+ shifted from approximately 10% at 1 day post-injury to approximately 60% at 60 days post-injury. Of the NOX4+ cell population around 60% was also CD86+ at 60 days post-injury gradually increasing from approximately 10% at 1 day post-injury. Bars represent mean score or percent +/- SEM of naïve/sham tissue (n = 11) or 1 (n = 5), 4 (n = 4), 7 (n = 5), 28 (n = 3) or 60 (n = 5) days post-injury. . **p<0.01, ***p<0.001, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
The number of M1 and NOX4 double labeled cells was increased slightly over sham, although without reaching statistical significance, during the study time frame from 1 to 28 days post-injury (Fig. 6), with a slight dip to sham levels observed at 7 days. However, by 60 days, the amount of M1 and NOX4 double labeled cells was significantly elevated over sham. The percent of M1 polarized cells expressing NOX4 changed from approximately 10% at 1 day post-injury to approximately 60% at 60 days post-injury. In a similar manner, of all the cells found to be NOX4+ in the tissue at the examined time points, approximately 10% at 1 day post-injury were M1 polarized cells. This proportion increased to 60% by the end of the study time frame.
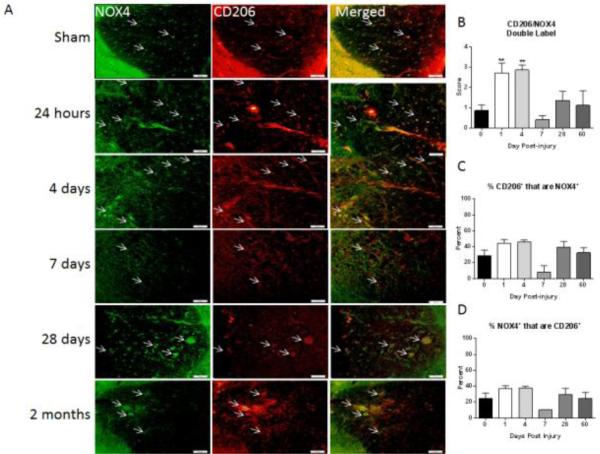
NOX4 and CD206 double staining was also evaluated (Fig. 7). At 1 and 4 days post injury, a noticeable increase in co-labeling was observed and found to be significant in the semi-quantification at both time points. During these acute time points, 40-50% of cells expressing NOX4 were M2 polarized cells. Of the CD206+ cells, approximately 50% expressed NOX4+ at the same time points. By 7 days, the double-labeled cell population declined to sham injured levels but climbed again at chronic time points and remained above sham/injured levels although not reaching significance as at acute time points. During these chronic time points the proportion of M2 cells expressing NOX4 was between 30 and 40% while of the NOX4+ cells present in the tissue at these time points, 20-25% were M2 polarized cells.
Figure 7. M2 polarized macrophages/microglia express NOX4.
Macrophages/microglia were labeled with CD206 (red) and immunostained with an antibody against NOX4 (green) in sham-injured tissue and at all examined time points post-injury. Co-labeled cells are indicated with arrows. CD206+ cells co-expression of NOX4 increases acutely after injury but returned to sham/naïve at 7 days post injury (A). Bar = 50 μm. Double-immunolabeling was then semi-quantitated using a 5 point scale and is presented as total amount of double label (B), percent of CD206 positive cells that are also NOX4 positive (C) and percent of NOX4 positive cells that are also CD206 positive (D). CD206/NOX4 double labeled population increased significantly at 1 and 4 days post-injury with 40-50% of NOX4+ cells co-labeling with CD206 antibody. Of the CD206+ cells approximately 50% were NOX4+ at the same time points . The M2/NOX4 double labeled population declined by 7 days but returned at chronic time points and remained above sham/injured levels. Bars represent mean score or percent +/− SEM of naïve/sham tissue (n = 11) or 1 (n = 5), 4 (n = 4), 7 (n = 5), 28 (n = 3) or 60 (n = 5) days post-injury. **p<0.01, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
In vitro, NOX2 activity is associated with changes in polarization and NOX2 inhibition alters polarization marker expression
In order to understand if the observed alterations in NOX isoform expression and polarization changes are related, we moved to a cell culture model. First, the BV2 microglial cell line was cultured for 1 – 24 hours to the well characterized M1 polarizing agent LPS (Orihuela et al., 2016), and the levels of M1 and M2 markers were assessed. Phosphorylation of p47PHOX was found to be significantly elevated by 6 hours post-injury, indicating activation of the enzyme at that time point (Fig. 8A, B). At the same time, NO production, a marker for M1 polarization, was significantly elevated and continued to rise throughout the remaining time points (Fig. 8C). Conversely, the M2 marker CD206 was significantly decreased in LPS treated BV2 cultures by 16 hours, following the elevation of phosphorylated-p47PHOX (Fig. 8D).
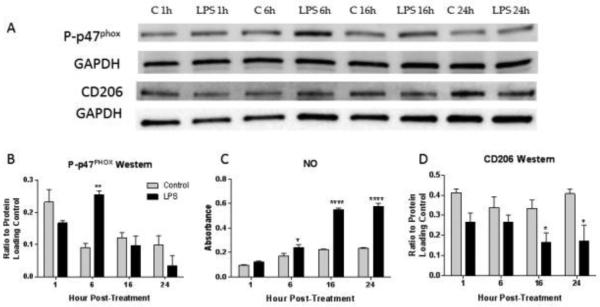
Figure 8. Induction of NOX2 activity is associated with induction of the M1 marker NO production, and reduction of the M2 marker CD206.
BV2 microglia were collected at 1, 6, 16 and 24 hours after stimulation with LPS or control media. CD206, p47PHOX phosphorylation and nitric oxide production were assessed using western blotting (A) or Griess Assay. Phosphorylation of p47PHOX peaked at 6 hours post-treatment (B). At the same time, NO production began to increase (C), followed by a peak at 16 hours and continued elevation by 24 hours. In contrast, CD206 was significantly decreased by 16 hours (D), following the peak in p47PHOX phosphorylation. Bars represent mean +/- SEM. All experiments completed in triplicate.
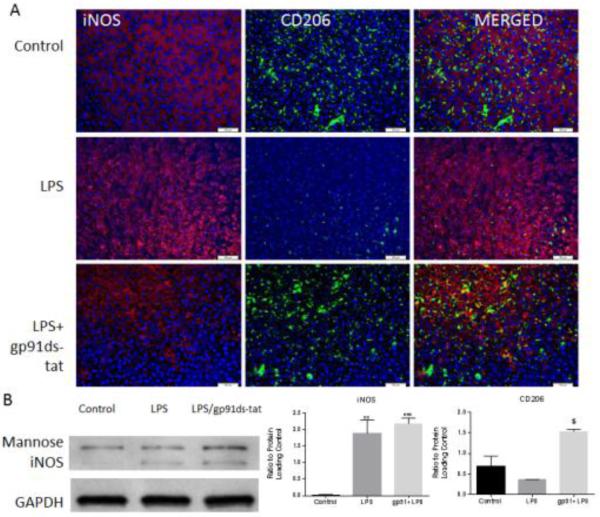
Next, to test the hypothesis that NOX2 contributes to polarization, BV2 cells were cultured following exposure to known polarizing agents (LPS, IL4) and to a NOX2 antagonist, gp91ds-tat and polarization markers were evaluated. NOX2 and NOX4 expression were not altered by LPS or IL4 administration nor by gp91ds-tat peptide administration (data not shown), suggesting that NOX isoform expression is not altered by microglial polarization. However, polarization markers were altered by administration of the NOX2 inhibitor. Resting microglia were found to be iNOS (M1) negative, but showed a moderate amount of baseline M2 marker (CD206) staining (Fig. 9). Treatment with LPS increased iNOS expression (Fig. 9A), although it did not lead to a significant reduction in CD206 protein expression as measured by western blotting. However, administration of the gp91ds-tat peptide 1 hour after LPS administration significantly elevated CD206 expression above LPS levels. iNOS expression remained elevated, as evidenced by both immunocytochemistry and western blotting.
Figure 9. NOX2 inhibition increases CD206 expression in LPS stimulated microglia.
BV2 microglial cells were stimulated with LPS and treated with the NOX2 inhibitor gp91ds-tat. BV2 microglia were labeled with antibodies against iNOS and CD206 (A). Bar = 100 μm. Microglia show initial CD206 but no iNOS expression. CD206 immunolabeling was diminished after LPS stimulation while iNOS staining increased. NOX2 inhibition with gp91ds-tat increased CD206 expression compared to untreated cells, with evidence of double labeling. Polarization markers expression was also assessed by western blotting (B). Quantification showed a basal M2 marker expression with no significant change in protein expression after LPS stimulation. CD206expression was significantly elevated above LPS levels by gp91ds-tat treatment. This treatment did not significantly alter iNOS expression. Bars represent mean +/− SEM. All experiments repeated in triplicate. **p<0.01, ***p<0.001 vs. control; $p<0.05 vs. LPS, using One-Way ANOVA with Dunnett's Multiple Comparison post-test.
Discussion
A number of studies have found evidence of oxidative damage in the spinal cord that can be detected within hours of injury, peaks at around 7 days, and remains present for weeks to months after (for review, see (Jia et al., 2012)). We have also previously shown that components of the NOX enzyme are elevated at both acute and chronic time points after SCI (Byrnes et al., 2006, Cooney et al., 2014). We now expand upon those findings and demonstrate that there is evidence of oxidative stress in the injured spinal cord from 1 to 28 days post-injury, and that two NOX isoforms, NOX2 and NOX4, are elevated in expression after SCI and are expressed in both M1 and M2 polarized microglia/macrophages. The overall pattern of staining demonstrates that at acute time points, M1polarized cells are associated with NOX2 whereas at later time points, M1 polarized cells seem most associated with NOX4. On the other hand, the transient M2 population is associated with both NOX isoforms. This association appears to change primarily due to the shift in the M1/M2 ratio over time, although our in vitro work suggests that activity of the NOX2 isoform may bear an influence on these observations. This sheds light into our previous in vivo work where acute NOX2 inhibition appears to limit the shift towards M1 polarization after SCI.
NOX expression and activity has been noted to be elevated as early as 24 hours post-injury (Byrnes et al., 2006) and remains upregulated for months after injury (Byrnes et al., 2011). NOX has previously been shown to contribute to oxidative stress following injury, and we now show that 2 different markers of oxidative stress, DNA oxidation (8OHDG immunohistochemistry) and protein nitrosylation (3NT western blot and immunohistochemistry) are elevated following SCI. Both DNA oxidation and protein nitrosylation were found to be significantly elevated at 1 day post-injury. While DNA oxidation was found to be significantly elevated at 1 and 28 days, 3NT showed significant elevation at all time points after injury, peaking at 4 days and remaining significantly elevated through 28 days. Interestingly, 3NT peak elevation was found at the same point as peak in NOX2 expression and observation of activation of the NOX2 enzyme, as measured by phosphorylation of p47PHOX, suggesting that we are observing not just an increase in protein expression, but activity that is accompanied by an elevation in downstream oxidative events.
The NOX2 isoform is expressed in microglia, macrophages, neurons and astrocytes (Cooney et al., 2013, Cooney et al., 2014), and this work now shows that the NOX2 expression is not restricted to a specific polarization phenotype, with up to 70% of M1 macrophages/microglia and up to 40% of M2 macrophages/microglia expressing NOX2. Following SCI in a rat model, we have shown that NOX2 peaks at 7 days post-injury, but is elevated above baseline at both 24 hours and 28 days (Cooney et al., 2014). Our current work is in agreement with this, although at a slightly earlier time frame (1 day and 4 day peaks) in the mouse model. It is interesting to note that by 7 days post-injury, 100% of the NOX2 positive cells were CD86 positive, despite the finding that NOX2 is expressed by a number of cells in the CNS.
The NOX4 isoform, on the other hand, has been found to peak in microglia at approximately 24 hours in the rat model, decreasing in expression through 28 days (Cooney et al., 2014); in contrast, the mouse model demonstrates an elongated NOX4 expression, which remained elevated through 7 days before dropping at 28 days. NOX4 is also expressed in microglia, macrophages, neurons and astrocytes (Cooney et al., 2013, Cooney et al., 2014). This enzyme produces H2O2 and has been associated with pain after peripheral nerve injury (Kallenborn-Gerhardt et al., 2012) and glutamate release from microglia (Harrigan et al., 2008). It is considered to have the widest cellular distribution of the NOX's in the body (Altenhofer et al., 2012). Like NOX2, NOX4 expression is not restricted to a specific polarization phenotype, showing expression in up to 70% of M1 macrophages/microglia and up to 50% of M2 macrophages/microglia. Interestingly, this elevated expression in M1 macrophages/microglia was only observed at the final time point (60 days post-injury). Prior to that, NOX4 expression was markedly low in both macrophages and microglia, reaching at most 40-50%. This suggests that NOX4 is primarily expressed by other cells in the CNS, but may play a role in chronic inflammation.
It has been shown that M2 polarized microglia and macrophages decrease in lesion areas in the CNS over time. In the spinal cord, Kigerl et al. performed a comprehensive investigation of M1/M2 polarization markers over time after injury, and showed that M2 markers are elevated during the first 2 weeks after injury and swiftly decline after that (Kigerl et al., 2009). Similar to our work, they found equivalent M1 and M2 staining within the first few days after SCI, often within the same cells. After a moderate traumatic brain injury in rats and mice, M2 markers also peaked at 5 days and were reduced dramatically thereafter (Turtzo et al., 2014, Wang et al., 2013), while M1 markers remained consistent throughout the post-injury time period (Bedi et al., 2013, Turtzo et al., 2014), which is similar to our findings in the injured mouse spinal cord. We now provide evidence that this M1/M2 staining may be present in the same cell (Fig. 4), demonstrating that microglia and macrophages exist in intermediate states that express both M1 and M2 markers along with NOX2 in the injured mouse spinal cord. In agreement with our findings, Kumar et al. (Kumar et al., 2015) also found a robust increase of NOX2 colocalization with Iba1-positive macrophage/microglia at 7 days post-injury in a moderate controlled cortical impact injury model in mice. Furthermore, this gp91PHOX expression was significantly co-localized with microglia expressing only M1 or both M1 and M2 markers but negligibly expressed in M2-like microglia. The significance of this intermediate polarization state is currently unclear.
Our current in vitro data suggests that NOX activity influences polarization, as inhibition of NOX2 led to a significant increase in CD206 expression. In vivo we have previously observed that acute NOX2 inhibition limits the decline of CD206+ population at 7 days after SCI (Khayrullina et al., 2015). Our current in vivo data also seems to support this influence, demonstrating that the peak of NOX2 expression was followed by the reduction in CD206 expressing cells at 7 days post-injury. It is important to note that, despite the reduction in NOX2 expression at 7 days, CD206/M2 marker expression did not increase, as our in vitro data would suggest. In fact, this is the situation in the periphery, with tissue wounds or peripheral nerve injury, in which M2 markers are elevated in later stages of healing (Chen et al., 2015, Ferrante and Leibovich, 2012). While speculative at this point, we now propose that microglial polarization reflects the redox state of the lesion microenviroment. It is possible that despite low expression, NOX2 may still be active and contributing to oxidative stress and suppression of the M2 phenotype, or the NOX4 enzyme may contribute to this suppression as well. The observations of elevated oxidative stress markers at chronic time points support this proposal, but future research is needed to further explore this phenomenon.
In addition, our in vitro p47PHOX phosphorylation data also provides a chronological insight into the interaction between NOX2 and M2 polarization, demonstrating that the peak of phosphorylation of p47PHOX was associated with the down-regulation of M2 expressing cells. Interestingly, our in vitro data did not show a sustained phosphorylation of p47PHOX, despite sustained changes in microglial polarization. This is likely due to a loss of stimulatory action of LPS due to degradation over time, unlike what will happen in the injured spinal cord with continual pro-inflammatory signals. However, despite the loss of obvious NOX2 activity, sustained changes in polarization were observed, suggesting that either NOX2 activity initiates a series of downstream events leading to sustained protein changes. Alternatively, additional signal transduction pathways may be at work to contribute to these sustained changes. For example, Kumar et al suggest that NOX2 activity acts like a switch driving M2 to an M1 polarization state after traumatic brain injury and proposes an upregulation of IL-4Rα expression as a mechanism for this phenomenon, although this upregulation was only found on infiltrating macrophages (Kumar et al.).
Further, previous work has shown that NOX inhibition has a number of anti-inflammatory effects, which may be related to this shift in polarization. For example, in vitro, reduction of NOX activity can ameliorate microglial pro-inflammatory activity (Byrnes et al., 2006, Cheret et al., 2008, Peng et al., 2009) and reduce neuronal cell death (Gao et al., 2002). Reduction or knockout of the p47PHOX component also impairs cytokine and nitric oxide (NO) production in astrocytes and microglia stimulated with LPS and/or interferon (IFN) γ (Pawate et al., 2004). More specifically, diphenyleneiodonium (DPI), a non-specific NOX inhibitor, blocks NFκB activation in microglia, with subsequent reductions in iNOS and pro-inflammatory cytokine production (Min et al., 2004). Similarly, NFκB activation, pro-inflammatory cytokine production and inflammation were all reduced after SCI in mice following apocynin administration (Impellizzeri et al., 2011). Finally, in an in vivo model of inflammation utilizing LPS or Aβ injection, Choi et al. found that knockout of gp91PHOX or p47PHOX or administration of apocynin could increase M2 marker expression in microglia (Choi et al., 2011). In our hands the administration of the NOX2 inhibitor gp91ds-tat limits the decline of CD206+ after SCI (Khayrullina et al., 2015). These data are in agreement with our current in vitro and in vivo findings and suggest that, in the SCI model, the activity of NOX2 or 4 may be influencing the shift from an equal M1/M2 ratio towards a higher M1 expression rather than polarized microglia associated with any of the NOX isotypes.
Conclusion
In summary, our current data therefore provide a descriptive analysis of NOX isoform expression in combination with the post-injury M1/M2 ratio in the injured spinal cord. These data demonstrate that NOX expression, particularly NOX2 and NOX4, is increased after mouse SCI. Further, while both M1 and M2 microglia/macrophages express NOX isoforms, there is an influence of NOX on polarization. As polarization plays a significant role in outcome (Yao et al., 2014), these data support the idea that modulation of NOX activity after SCI may improve recovery.
Supplementary Material
Highlights.
We examined NADPH oxidase expression and microglia polarization after injury
Spinal cord injury leads to a significant increase in NOX2 and NOX4 expression
M1 cells preferentially express NOX2 acutely and NOX4 chronically after injury
The reduction in M2 polarization is associated with a peak in NOX2 expression
Inhibition of NOX2 activity in vitro increases M2 polarization of microglia
Acknowledgements
The authors thank Fiona Brabazon for editorial contributions. This work was funded by the NINDS/NIH (Grant number 1R01NS073667-01A1).
Abbreviations
- NOX
NADPH oxidase
- LPS
lipopolysaccharide
- DPI
diphenyleneiodinium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–43. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi SS, Smith P, Hetz RA, Xue H, Cox CS. Immunomagnetic enrichment and flow cytometric characterization of mouse microglia. J Neurosci Methods. 2013;219:176–82. doi: 10.1016/j.jneumeth.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, et al. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–8. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Viedt C, Nguyen K, Beer S, Kreuzer J, Busse R, et al. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost. 2001;85:1104–10. [PubMed] [Google Scholar]
- Byrnes KR, Garay J, Di Giovanni S, De Biase A, Knoblach SM, Hoffman EP, et al. Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia. 2006;53:420–33. doi: 10.1002/glia.20295. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Washington PM, Knoblach SM, Hoffman E, Faden AI. Delayed inflammatory mRNA and protein expression after spinal cord injury. J Neuroinflammation. 2011;8:130. doi: 10.1186/1742-2094-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, et al. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–51. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ifuku M, Noda M, Guilarte TR. Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia. 2011;59:219–30. doi: 10.1002/glia.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee da Y, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013;10:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney SJ, Zhao Y, Byrnes KR. Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic Res. 2014;48:929–39. doi: 10.3109/10715762.2014.927578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Segal AW. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochimica et biophysica acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Advances in wound care. 2012;1:10–6. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–69. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd'heuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106:2449–62. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri D, Mazzon E, Esposito E, Paterniti I, Bramanti P, Cuzzocrea S. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic Res. 2011;45:221–36. doi: 10.3109/10715762.2010.526604. [DOI] [PubMed] [Google Scholar]
- Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50:264–74. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- Kallenborn-Gerhardt W, Schroder K, Del Turco D, Lu R, Kynast K, Kosowski J, et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci. 2012;32:10136–45. doi: 10.1523/JNEUROSCI.6227-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayrullina G, Bermudez S, Byrnes KR. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. Journal of Neuroinflammation. 2015;12:172. doi: 10.1186/s12974-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2015 doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Barrett JP, Alvarez-Croda D-M, Stoica BA, Faden AI, Loane DJ. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain, Behavior, and Immunity. doi: 10.1016/j.bbi.2016.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–65. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Knoblach SM, Faden AI, Byrnes KR. Characterization of inflammatory gene expression and galectin-3 function after spinal cord injury in mice. Brain Res. 2012;1475:96–105. doi: 10.1016/j.brainres.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Li QY, Chang LY, Crapo J, Liang LP. Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J Neurochem. 2005;92:123–31. doi: 10.1111/j.1471-4159.2004.02838.x. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–51. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Oo ML, Andersen JK. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic Biol Med. 2009;46:312–20. doi: 10.1016/j.freeradbiomed.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. The Journal of comparative neurology. 1997;377:443–64. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–21. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. The Journal of comparative neurology. 2003;462:223–40. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–74. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–49. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- Yao A, Liu F, Chen K, Tang L, Liu L, Zhang K, et al. Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics. 2014;11:636–50. doi: 10.1007/s13311-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, et al. Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. Journal of leukocyte biology. 2005;77:414–20. doi: 10.1189/jlb.0504284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.