Abstract
Of the two major subdivisions of the habenula, the medial and lateral nuclei, the medial habenula is the least understood in terms of synaptic transmission, intrinsic properties and plasticity. The medial habenula (MHb) is composed of glutamatergic neurons which receive the majority of their inputs from the septal region and project predominantly to the interpeduncular nucleus (IPN). To understand the synaptic transmission, we studied both glutamatergic and GABAergic synaptic transmission in the dorsal region of the medial habenula (dMHb). While glutamatergic transmission dominates during early development, an attenuation of glutamatergic transmission and an enhancement of GABAergic transmission occur during development leading into adulthood. Furthermore, as reported previously, GABAA receptor-mediated transmission is excitatory in the adult dMHb, which is consistent with the reduced expression of the K-Cl co-transporter KCC2. Given the potential role of the dMHb in aversive behaviors, we examined whether fear conditioning or exposure to foot shock affects excitability in dMHb neurons. We observed a suppression of the excitability of dMHb neurons in mice that either underwent fear conditioning or were exposed to foot shock. Furthermore, we observed a suppression of GABAergic but not glutamatergic transmission in the dMHb neurons following fear conditioning. These results suggest that aversive experience produces a suppression of the dMHb neuronal activity. Given that the medial habenula is upstream of the median raphe nucleus which is believed to be involved in the negative regulation of aversive memory, the suppression of dMHb neurons following an aversive experience might play a role in strengthening of aversive memories.
Keywords: Medial habenula, GABA, Synaptic plasticity
INTRODUCTION
The habenula is a paired epithalamic structure that includes the medial habenula (MHb) and the lateral habenula (LHb) (Aizawa, 2013). While both the MHb and LHb have been implicated in addictive and neuropsychiatric disorders, they exhibit distinct gene expression profiles, cellular organizations and connectivities (Andres et al., 1999; Qin and Luo, 2009; Viswanath et al., 2013). The MHb is subdivided into the dorsomedial habenula (dMHb) and the ventromedial habenula (vMHb), and this subdivision is facilitated by the exclusive expression of substance P and choline acetyltransferase (ChAT) in the dMHb and vMHb, respectively (Holmes et al., 2003; Kobayashi et al., 2013; Yamaguchi et al., 2013). Also, inputs from distinct regions of the posterior septum, the bed nucleus of the anterior commissure and triangular septum, innervate the dMHb and vMHb, respectively (Contestabile and Fonnum, 1983; Qin and Luo, 2009; Yamaguchi et al., 2013). In addition, the MHb receives GABAergic inputs from the medial septum (Qin and Luo, 2009).
The MHb is primarily composed of glutamatergic neurons which project to the interpeduncular nucleus (IPN) through the fasciculus retroflexus (Herkenham and Nauta, 1979; Qin and Luo, 2009). The substance P-positive dMHb neurons project to the lateral part of the IPN, while the ChAT-positive vMHb neurons terminate in the medial part of the IPN (Quina et al., 2009; Yamaguchi et al., 2013). Apart from a strong IPN output to the median raphe nucleus (MRN), recent data suggests that the MRN receives direct input from the MHb (Herkenham and Nauta, 1979; Ogawa et al., 2014). Therefore, the MHb could play a key role in an MRN-mediated modulation of other brain areas, particularly the hippocampus and the medial prefrontal cortex (Szonyi et al., 2014; Varga et al., 2009).
Among the several neurotransmitter and neuromodulator inputs, there has been some interest in the role of GABAergic transmission in the MHb (Lecourtier and Kelly, 2007). Fast GABA-mediated responses in the MHb neurons suggested the presence of GABAA receptors (Mulle et al., 1992). Also, GABA transmission exerts excitatory and inhibitory effects in the vMHb, by activating GABAA and GABAB receptors, respectively (Kim and Chung, 2007). Unlike most other brain areas, the MHb does not express any detectable levels of the K-Cl co-transporter KCC2, which is necessary for the chloride efflux that enables GABAA mediated hyperpolarization (Kim and Chung, 2007). On the other hand, the MHb is one of the brain areas that express the highest level of GABAB receptors (Jones et al., 1998). A recent study reported that GABAB receptors on the MHb axon terminals play an excitatory role by promoting neurotransmitter release as they are coupled to presynaptic Ca2+-channels (Zhang et al., 2016). These studies suggest a unique role for GABA receptors in MHb function.
Although the MHb has been implicated in cognition, attention, regulation of hedonic state, emotion and aversive behaviors (Agetsuma et al., 2010; Hsu et al., 2014; Lecourtier and Kelly, 2007; Soria-Gomez et al., 2015; Viswanath et al., 2013; Yamaguchi et al., 2013), little is known about the synaptic transmission, intrinsic properties and plasticity of the MHb neurons. Our studies revealed a developmental switch from glutamate-predominant synaptic transmission to GABA-predominant transmission in dMHb neurons. Furthermore, fear conditioning (FC) caused a weakening of excitability and GABAergic transmission in the dMHb neurons without affecting glutamatergic transmission. These results suggest that aversive experience can suppress the dMHb activity, which may alter the downstream MRN and hence facilitate the consolidation of aversive memories.
MATERIALS AND METHODS
Animals
Wild-type C57BL/6J mice and heterozygous VGAT-ChR2-EYFP mice (stock #014548) with C57BL/6J background were purchased from Jackson Laboratory and maintained on a 12:12 light-dark cycle at 23°C with ad libitum access to food and water (Zhao et al., 2011). Mice were handled for 3 days prior to the behavioral experiments. All procedures were approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine.
Electrophysiology
After pentobarbital anesthesia (120mg/kg, intraperitoneal), mice were intracardially perfused with artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl (118), glucose (10), KCl (2.5), NaH2PO4 (1), CaCl2 (1) and MgSO4 (1.5) (325 mOsm, pH 7.4) for 60 sec. Following the isolation of the brain, coronal brain slices containing the MHb were cut on a vibratome and allowed to recover for at least I hr in a brain slice keeper at room temperature. Brain slices were transferred to a recording chamber maintained at 32°C under an upright microscope fitted with a 40x long working distance water-immersion objective. Brain slices were perfused with the aforementioned ACSF containing 2.5mM CaCl2. Patch electrodes (4–6 MΩ) were pulled from borosilicate capillary glass and filled with an electrode solution containing the following (in mM): potassium gluconate (130), KCl (10), MgCl2 (5), MgATP (5), GTP (0.2), EGTA (0.5), HEPES (5), pH adjusted to 7.4 with KOH. A Multiclamp 700B amplifier connected to a Digidata 1550A (Molecular Devices, CA, USA) was used to perform electrophysiological measurements. Signals were sampled at 20–100 kHz and filtered at 2 kHz. Spontaneous glutamatergic currents were recorded in the presence of bicuculline (20μM) at −70mV. Spontaneous GABAergic currents were recorded in the presence of NBQX (10μM) and D-2-Amino-5-phosphonovaleric acid (APV, 50 μM) at −60mV. For studying the effect of 1-Naphthyl acetyl spermine trihydrochloride (NASPM, 100 μM), brain slices were incubated in the drug solution for 60–90 min before recording the currents. Parallel vehicle control experiments were carried out in brain slices from the same animals under identical conditions. To evoke GABAergic inputs to dMHb neurons, blue light (470 nm) was emitted from a Lumen 1600-LED (Prior) for 0.2 to 2.0 ms onto brain slices from VGAT-ChR2-EYFP mice.
Passive and active membrane properties were measured as described previously (Koppensteiner et al., 2014). Except for resting membrane potential measurements, the membrane potential was hyperpolarized close to −60 mV to avoid spontaneous action potentials (APs). Cell attached recordings were performed using the same potassium gluconate pipette solution used for whole-cell recordings.
Fear conditioning-induced plasticity in the dMHb neurons
To study fear conditioning-induced plasticity, mice were fear conditioned in a shock-chamber placed in a sound-attenuated box (Coulbourn Instruments). On day 1, following a 2 min acclimation period and two 30 s habituation tones at an interval of 30 s, mice received three tone-shock pairings, each one comprised of 30 s tones (5 kHz, 50 dB) co-terminating with a 1 s foot shock (0.7 mA) with an inter-trial interval of 30 s. Mice remained in the conditioning chamber for 1 min after the last tone-shock pairing. Freezing was tested 24 hr later (day 2) on a non-shock floor by 3 presentations of 30 s tones at an interval of 30 s. The control naïve group stayed in the home cage on day 1 and was tested on day 2. The tone-alone (TA) group received five tone presentations on day 1 on the shock floor and three tone presentations on day 2 on the non-shock floor. The shock-alone (SA) group was placed into the conditioning chamber and immediately received three foot shocks at 5 s interval without any tone presentations, followed by an immediate return to the home cage. On day 2, the SA group was tested on the non-shock floor without any tone presentations. Brain slices for electrophysiology experiments were prepared 1–2 hrs after the fear memory test on day 2. In another set of experiments (Figure 5A), brain slices were prepared 2 hrs after fear conditioning on day 1 and the control group consisted of naïve mice from the home cages.
Figure 5.
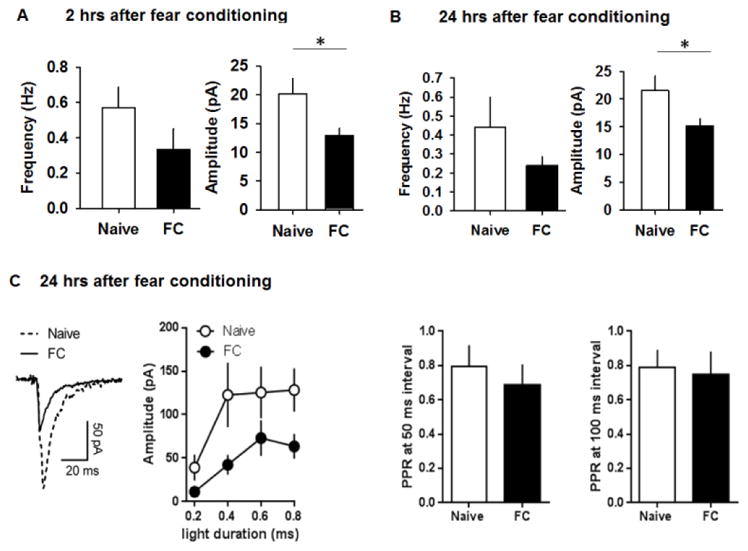
Fear conditioning suppresses GABAergic transmission in dMHb neurons. A&B) Frequency and amplitude of spontaneous GABAergic currents in dMHb neurons of naïve [(13 cells/6 mice) and (11 cells/4 mice), respectively, for 2 and 24 hrs experiments] and FC [(9 cells/4 mice) and (13 cells/4 mice), respectively, for 2 and 24 hrs experiments] groups 2 and 24 hrs after fear conditioning. C) Light-evoked GABAergic currents in naïve and FC groups. PPR remained unaffected by the treatment, at both 50 and 100 ms intervals. Naïve: 14 cells/4 mice; FC: 14 cells/4 mice. Asterisk denotes statistical significance.
Immunohistochemistry
After ketamine anesthesia, mice were intracardially perfused with 40 ml of PBS, followed by 40 ml of freshly prepared 4% paraformaldehyde in PBS. The tissue was dehydrated in 30% sucrose in PBS overnight, embedded in Tissue-Tek (Leica) and frozen over dry ice. Cryosections (20um) were prepared at −20 °C and collected on coated slides (Super-Frost Plus). Sections were air dried for 20 minutes and washed with PBS. Sections were subsequently incubated for 2 days at 4°C in a moist chamber with NKCC1 (Santa Cruz; 1:200 stock diluted in PBS) and KCC2 (Millipore; 1:300 stock diluted in PBS) primary antibodies. After incubation, the sections were washed with PBS (3 x 10 minutes at room temperature) and incubated for 2 hours at room temperature with anti-goat Alexa 488 (Abcam; 1:500) and anti-mouse Alexa 555 (Molecular Probes; 1:500) secondary antibodies. The sections were then washed with PBS (3 x 10 minutes at room temperature) to remove unbound secondary antibody and incubated for 10 minutes at room temperature with Hoechst solution (1:10,000 stock diluted in PBS) for nuclear staining. After a final rinse, the slides were coverslipped using fluoromount G (Southern Biotechnology Associates). Slices were examined and imaged using a Carl Zeiss LSM 700 confocal microscope with three solid-state lasers (405/444, 488, 555 nm) and appropriate filter sets.
Data analysis
Membrane and synaptic properties were analyzed using Clampfit 10.5 (Molecular Devices) and Mini Analysis Programs (Synaptosoft). Results are presented as mean±SEM. One way ANOVA followed by Tukey’s test was used for comparison of more than two groups. Two-tailed t-test was used for the comparison of two groups. Current/AP frequency relations and cell-attached spontaneous spike data were analyzed with two-way ANOVA with Bonferroni post hoc test. p < 0.05 was considered statistically significant.
RESULTS
Passive and active electrophysiological properties of dMHb neurons
To characterize changes in electrophysiological properties of dMHb neurons during development, we measured passive and active membrane properties in the neurons of pre-juvenile (P14–15) and adult (P60–P120) mice (Figure 1A). P14–15 dMHb neurons exhibited more depolarized membrane potentials compared with adult neurons (t(20) = 2.93, p = 0.008). However, passive membrane properties, including input resistance (Rin), membrane time constant tau and membrane capacitance (Cm) were not significantly affected by age (Figure 1B,C). In addition, active membrane properties remained unaffected, with the exception of significantly broader APs in pre-juvenile neurons (t(20) = 2.53, p = 0.02) (Figure 1D,E). These results suggest that dMHb neurons remain highly excitable over the course of postnatal development. In addition, we compared passive and active membrane properties of adult dMHb neurons to those of adult vMHb neurons (Table 1). vMHb neurons had larger (amplitude, t(24) = 3.61, p = 0.001) and broader (FWHM, t(24) = 3.05, p = 0.006) APs and a trend towards lower Rin values (t(24) = 2.06, p = 0.051) compared to dMHb neurons. Other electrophysiological properties were not different between neurons of the dorsal and ventral MHb regions (Table 1).
Figure 1.
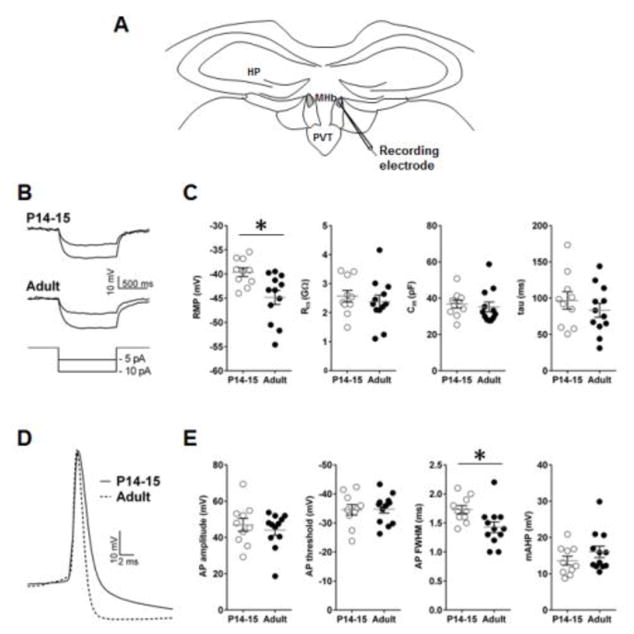
Membrane properties of dMHb neurons in P14–15 (10 cells/3 mice) and adult mice (12 cells/4 mice). A) Schematic presentation of the position of recording electrode (shown in grey) in the MHb slice preparation. B) Example traces of hyperpolarizing current injections in P14–15 and adult dMHb neurons. C) Passive membrane properties of dMHb neurons. D) Magnified AP trace of P14–15 and adult dMHb neurons. E) Active membrane properties of dMHb neurons. Asterisk denotes statistical significance. AHP – after-hyperpolarization, AP – action potential, Cm – membrane capacitance, FWHM – full-width at half maximum, HP – hippocampus, PVT – paraventricular thalamic nucleus, Rin – input resistance, RMP – resting membrane potential.
Table 1.
Passive and active membrane properties of ventral and dorsal MHb neurons. We compared the electrophysiological properties of adult dMHb neurons (n=12 cells/4 mice, same as in Figure 1) and vMHb neurons (n=14 cells/3 mice).
| RMP (mV) | Rin (GΩ) | tau (ms) | Cm (pF) | AP threshold (mV) | AP amplitude (mV) | AP FWHM (ms) | AP AHP (mV) | |
|---|---|---|---|---|---|---|---|---|
| vMHb | −49.2 ± 2.2 | 1.8 ± 0.2 | 72.0 ± 9.0 | 40.1 ± 2.8 | −38.2 ± 1.1 | 55.5 ± 1.7 | 1.9 ± 0.1 | 16.24 ± 1.3 |
| dMHb | −44.8 ± 1.4 | 2.4 ± 0.2 | 83.3 ± 9.6 | 35.2 ± 2.7 | −34.8 ± 1.4 | 44.1 ± 2.8 | 1.4 ± 0.1 | 16.1 ± 1.6 |
| P value | 0.12 | 0.051 | 0.4 | 0.2 | 0.07 | 0.001 | 0.006 | 0.9 |
P values were determined in two-tailed unpaired t-tests. AHP – after-hyperpolarization, AP – action potential, Cm – membrane capacitance, FWHM – full-width at half maximum, Rin – input resistance, RMP – resting membrane potential;
Developmental downregulation of glutamatergic transmission in the dMHb
To study glutamatergic transmission in the dMHb, we recorded spontaneous glutamatergic currents in dMHb neurons from P14–P15 and adult mice. The frequency [p = 0.007, one-way ANOVA (F(3,40) = 7.34, p = 0.001)] and amplitude [p = 0.001, one-way ANOVA (F(3,40) = 7.5, p = 0.001)] of the spontaneous glutamatergic currents were significantly higher in the P14–P15 group compared to the adult group (Figure 2B,C). Calcium permeable AMPA receptors (CPARs) are elevated in several brain areas during early postnatal development (Kumar et al., 2002). To test the potential contribution of CPARs in the increased glutamatergic transmission in P14–P15 mice, we recorded spontaneous glutamatergic currents in P14–15 and adult dMHb neurons following a 1 hr incubation with NASPM (100 μM), a CPAR antagonist. NASPM reduced the frequency (p = 0.003) and amplitude (p = 0.004) of spontaneous glutamatergic currents in P14–15 group but not adult neurons. Although the adult group showed a higher rise time [p = 0.039, one-way ANOVA (F(3,36) = 2.9, p = 0.04)], NASPM affected neither rise nor decay time (Figure 2D,E). These results suggest an elevated CPAR-mediated glutamatergic transmission in pre-juvenile mice and its downregulation during the maturation to adulthood.
Figure 2.
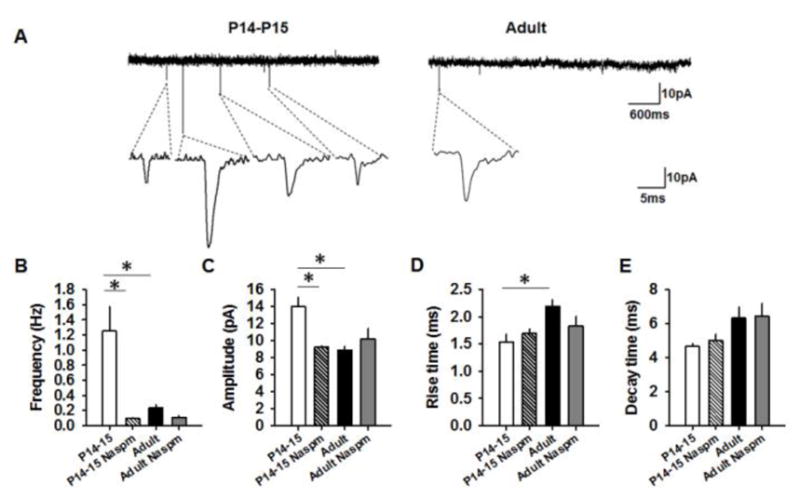
Glutamatergic transmission in dMHb neurons of P14–15 and adult mice. A) Examples of spontaneous glutamatergic currents in the dMHb neurons. B&C) Average frequency and amplitude of spontaneous glutamatergic currents in vehicle-treated P14–15 (15 cells/7mice), NASPM-treated P14–15 (9 cells/4 mice), vehicle-treated adult (11 cells/5 mice) and NASPM-treated adult groups (9 cells/4 mice). D&E) Average rise time and decay time of spontaneous glutamatergic currents in vehicle-treated P14–15 (10 cells/4mice), NASPM-treated P14–15 (9 cells/4 mice), vehicle-treated adult (12 cells/5 mice) and NASPM-treated adult groups (9 cells/4 mice). Asterisk denotes statistical significance.
Developmental upregulation of GABAergic transmission in the dMHb
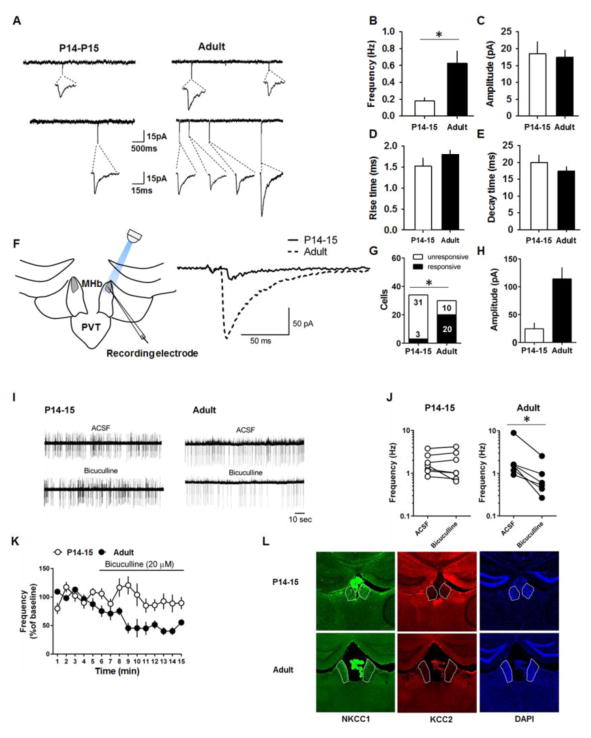
Given the downregulation of glutamatergic transmission during development, we examined whether GABAergic transmission is also developmentally regulated. We recorded spontaneous GABAergic currents in the dMHb neurons from P14–P15 and adult mice. We observed a higher frequency of spontaneous GABAergic currents in the adult mice compared to the P14–15 mice (t(17) = −2.79, p = 0.01) without changes in amplitude, rise time or decay time (Figure 3B–E). The selective increase in frequency suggests an augmentation of GABAergic inputs in the dMHb during development. Next, we used optogenetic stimulation of GABAergic terminals to compare evoked GABAergic currents in dMHb neurons of P14–15 and adult VGAT-ChR2-EYFP mice (Figure 3F). Fewer P14–15 dMHb neurons responded to light-induced stimulation compared to adult dMHb neurons (odds ratio = 0.048; 95% confidence interval 0.01–0.20; p < 0.0001, Fisher’s exact test) (Figure 3G). Although the amplitude of the evoked GABAergic currents was smaller in P14–15 neurons compared to the adult neurons, it did not reach statistical significance (t(21) = 1.64, p = 0.12) (Figure 3H). These results suggest fewer GABAergic inputs in the dMHb neurons of pre-juvenile mice.
Figure 3.
GABAergic transmission in dMHb neurons of P14–15 and adult mice. A) Examples of spontaneous GABAergic currents in the dMHb neurons. B–E) Average frequency, amplitude, rise time and decay time of spontaneous GABAergic currents in P14–15 (10 cells/4mice) and adult mice (10 cells/4 mice). F) Schematic presentation and example traces of light-evoked GABAergic currents in dMHb neurons of VGAT-ChR2 mice. G) The number of dMHb neurons that showed GABAergic currents in response to light stimulation in P14–15 and adult groups. H) Average amplitudes of evoked currents in P14–15 neurons (3 cells/3 mice) and adult neurons (20 cells/4 mice). I) Example traces of spontaneous spikes before and after the application of bicuculline. J&K) Effect of bicuculline on frequency of spontaneous spikes in P14–15 (7 cells/3 mice) and adult neurons (6 cells/3 mice). L) Coronal brain slices showing the expression of KCC2 and NKCC1 in the MHb. Asterisk denotes statistical significance.
Based on the predominant GABAergic transmission in adults and an earlier report that GABA acts as an excitatory neurotransmitter via the activation of GABAA receptors in the MHb (Kim and Chung, 2007), we asked whether inhibition of GABAA receptors affects spontaneous spiking in dMHb neurons. Perfusion of bicuculline produced a significant decrease in the frequency of spontaneous spikes in adult dMHb neurons (t(5) = 5.38, p = 0.003 in a ratio paired t-test) but not P14–15 dMHb neurons (t(6) = 0.94, p = 0.4 in a ratio paired t-test) (Figures 3I,J). Consistently, comparison of the time courses revealed a significant effect of bicuculline in the adult group compared to P14–15 group (main effect of age: F(1,153) = 43.7, p < 0.0001; main effect of time: F(14,153) = 3.3, p = 0.0001; interaction F(14,109) = 2.5, p = 0.002) (Figure 3K). These results suggest that GABAergic transmission plays a role in the excitability of adult but not P14–15 dMHb neurons, in accordance with the diminished GABAergic transmission during the pre-juvenile period.
The strength and polarity of the GABA-mediated effects are largely determined by the intracellular chloride concentration, which is primarily regulated by the Na-K-2Cl cotransporter-1 (NKCC1) and K-Cl cotransporter-2 (KCC2) (Kaila et al., 2014). Mature neurons in most brain areas maintain an active chloride extrusion process through KCC2 (Kaila et al., 2014). However, an earlier study showed that the MHb does not express KCC2 (Kim and Chung, 2007). Consistently, we observed lack of expression of KCC2 in the MHb supporting the notion that GABA acts as an excitatory neurotransmitter via the activation of GABAA receptors in the MHb (Figure 3L) (Kim and Chung, 2007).
Suppression of excitability of dMHb neurons after an aversive experience
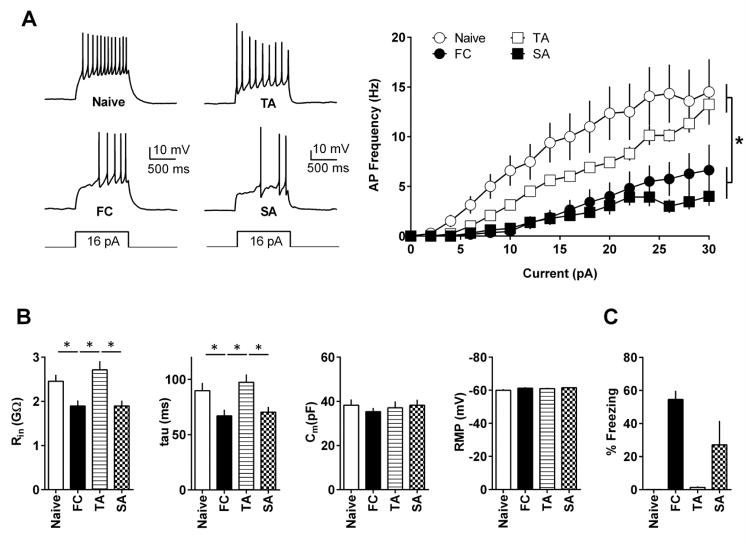
Earlier studies have suggested the role of the MHb in aversive memory (Agetsuma et al., 2010; Soria-Gomez et al., 2015; Yamaguchi et al., 2013). However, it is unknown whether aversive experiences affect MHb neurons. Therefore, we first studied whether fear conditioning (FC) or simple foot shock (SA) exposure affect the excitability of dMHb neurons. Mice received fear conditioning or foot shock alone on day 1 and we measured the excitability of dMHb neurons 1–2 hours after the memory test on day 2. As expected, the FC group showed significantly higher freezing on day 2 (Figure 4C). dMHb neurons from the FC and SA groups fired significantly fewer APs in response to current injections compared with naïve and TA animals (main effect of treatment: F(3,812) = 79.86, p < 0.0001; main effect of current: F(15,812) = 22.49, p < 0.0001; interaction F(45,812) = 1.65, p = 0.005, two-way ANOVA with Tukey’s post hoc test) (Figure 4A). TheFC group presented significantly reduced Rin [p < 0.05, one-way ANOVA (F(3,55) = 7.57, p = 0.0003) with Tukey’s post hoc test]) and tau values [p < 0.05, one-way ANOVA (F-(3,55) = 5.73, p = 0.002) with Tukey’s post hoc test] compared with the naive and TA groups (Figure 4B). In the SA group, reductions in Rin and tau values were significant compared with the TA group (p < 0.05) but did not reach significance compared with the naïve group (p > 0.05). Membrane capacitance and RMP values remained unaffected by the treatment (p > 0.05). These results suggest that an aversive experience results in the suppression of dMHb neuron excitability.
Figure 4.
Aversive experience suppresses excitability of dMHb neurons. A) dMHb neurons of the FC (17 cells/7 mice) and SA (13 cells/4 mice) groups fired fewer APs in response to the same current intensities compared with neurons of naïve (15 cells/5 mice) and TA (14 cells/4 mice) animals. Left panel shows example traces. B) The FC group showed reduced Rin and tau values compared to the naïve and TA group. The SA group showed reduced Rin and tau values compared with the TA group. C) Average freezing responses on day 2. Naïve: 10 mice, FC: 10 mice, TA: 4 mice, SA: 4 mice. Asterisk denotes statistical significance.
Suppression of GABAergic but not glutamatergic transmission in dMHb neurons after an aversive experience
Given the suppression of excitability of dMHb neurons in both the SA and FC groups, we tested whether fear conditioning affects GABAergic transmission in dMHb neurons. We compared the frequency and amplitude of spontaneous GABAergic currents in FC and naïve groups. Brain slices were prepared 2 and 24 hrs after fear conditioning. We observed a reduction in the amplitude of spontaneous GABAergic currents in the FC group compared to the naïve group (Figure 5A,B; t(20) = 2.17, p = 0.04 and t(22) = 2.24, p = 0.03, respectively, for 2 and 24hrs experiments). The effect of fear conditioning on the frequency of spontaneous GABAergic currents was not statistically significant (t(20) = 1.39, p = 0.18 and t(22) = 1.28, p = 0.21, respectively, for 2 and 24hrs experiments). The suppression of GABAergic transmission after fear conditioning was confirmed using an optogenetic stimulation of GABAergic terminals in the dMHb of VGAT-ChR2-EYFP mice 24 hrs after fear conditioning. There was a significant reduction in the amplitude of evoked GABAergic currents in dMHb neurons of FC mice compared with the naïve group (main effect of treatment: F(1,104) = 9.8, p = 0.0005; main effect of light duration: F(3,104) = 11.0, p = 0.003; interaction F(3,104) = 0.5, p = 0.7) (Figure 5C). Paired-pulse ratio (PPR) remained unaffected by fear conditioning (Figure 5C, 50 ms: t(12) = 0.12, p = 0.91; 100 ms: t(12) = 0.11, p = 0.91). These results confirm a reduction in GABAergic transmission in dMHb neurons following fear conditioning.
To test whether dMHb glutamatergic plasticity is involved in fear conditioning, we compared the frequency and amplitude of spontaneous glutamatergic currents 24 hrs after fear conditioning. Neither frequency (0.2±0.03Hz and 0.19±0.05Hz for naïve and FC groups, respectively, n=11 cells/4 mice for both groups) nor amplitude (10.25±0.93pA and 9.16±0.8pA for naïve and FC groups, respectively) was affected in the FC group, suggesting a selective effect of aversive experience on the GABAergic synapses in the dMHb.
DISCUSSION
In the current study, we describe the development of the electrophysiological properties of dMHb neurons and the modulation of these properties by aversive experience. While the elevated glutamatergic transmission in the pre-juvenile stage undergoes a downregulation during the maturation to adulthood, we observed a simultaneous increase in GABAergic transmission during this developmental period. The elevated glutamatergic transmission during the pre-juvenile stage is primarily mediated by CPARs. Earlier studies have shown a dominant CPAR-mediated glutamatergic transmission in other brain areas during the early postnatal stages (Kumar et al., 2002). Strikingly, the developmental shift from glutamatergic to GABAergic-predominant transmission is unprecedented in the CNS. Measurements of both spontaneous and evoked GABAergic currents strongly suggest fewer GABAergic inputs in the dMHb of pre-juvenile mice. The concurrent increase in GABAergic transmission in adults indicates its dominant role in the adult dMHb. The MHb is one of the brain areas that express the highest levels of GABAB receptors (Jones et al., 1998). Congruent with the excitatory role of GABA in the MHb, the MHb does not express any detectable levels of KCC2, the co-transporter responsible for chloride efflux (Kim and Chung, 2007). Consistently, blocking GABAA receptors reduced spontaneous spiking in adult dMHb neurons suggesting that the GABAA receptor-mediated transmission positively regulates the excitability of adult dMHb neurons. Given the diminished glutamatergic transmission in the adult dMHb, the ability of GABA to exert both excitatory and inhibitory action might play a key role in dMHb function (Kim and Chung, 2007).
Our current studies revealed that aversive experience results in an attenuation of both the excitability and GABAergic transmission in dMHb neurons. This suppression of neuronal excitability might involve calcium-activated potassium channels or voltage-gated potassium channels (Dong et al., 2005; Rosenkranz et al., 2010). It is unclear whether the high expression of GABAB receptors in the MHb plays a role in suppression of excitability and the reduction of GABAA receptor mediated currents (Jones et al., 1998; Lüscher et al., 1997; Luscher and Slesinger, 2010; Takeda et al., 2004). Interestingly, a recent study suggested that the GABAB receptors on MHb terminals play a unique excitatory role to enhance neurotransmitter release in the IPN and its activation is critical for fear extinction (Zhang et al., 2016). Therefore, it is plausible that the suppression of the MHb activity by aversive experiences might result in reduced MHb-IPN transmission and hence impair extinction of aversive memories.
The MHb acts as an interface between the limbic system and the midbrain (Viswanath et al., 2013). The MHb is connected to the MRN directly and indirectly via the IPN (Contestabile et al., 1987; Groenewegen et al., 1986; Herkenham and Nauta, 1979; Ogawa et al., 2014). The downstream connectivity of the MHb leading to a potential modulation of the MRN strongly suggests its role in regulating cognitive and emotional behaviors (Deakin, 2013; Herkenham and Nauta, 1979; Hsu et al., 2014; Ogawa et al., 2014; Szonyi et al., 2014; Varga et al., 2009). A recent study implicated the dMHb in the regulation of fear behavior (Yamaguchi et al., 2013). Consistently, a genetic disruption of a brain region analogous to the dMHb in zebrafish increased fear behavior (Agetsuma et al., 2010; Lee et al., 2010). Our findings that fear conditioning results in a reduction of dMHb activity suggest the possibility that a diminished dMHb output might interfere with MRN function. Activation of the MRN reduces both hippocampal ripple activity and the consolidation of fear memory (Wang et al., 2015). Therefore, future studies will be necessary to understand whether the MHb-MRN pathway is involved in the consolidation of aversive memories.
In conclusion, our current study describes the developmental regulation of membrane and synaptic properties of dMHb neurons. Furthermore, we demonstrate that aversive experience results in an attenuation of both excitability and GABAergic transmission in the dMHb neurons. These cellular and synaptic adaptations could alter downstream MRN function and facilitate consolidation of aversive memories which could play a role in an impaired inhibition of conditioned fear as observed in post-traumatic stress disorder (Desmedt et al., 2015; Liberman et al., 2006).
Highlights.
dMHb neurons show stable membrane properties throughout development.
dMHb neurons exhibit a predominant glutamatergic transmission during pre-juvenile period which is downregulated during the maturation to adulthood.
Excitatory GABAergic transmission is more pronounced in adult dMHb neurons.
Aversive experience suppresses dMHb neuronal activity.
Acknowledgments
FUNDING AND DISCLOSURE
This work was supported by NIH (HD076914 to I.N.). Authors declare no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, Sassa T, Amo R, Shiraki T, Kawakami K, Hosoya T, Higashijima S, Okamoto H. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nature neuroscience. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- Aizawa H. Habenula and the asymmetric development of the vertebrate brain. Anat Sci Int. 2013;88:1–9. doi: 10.1007/s12565-012-0158-6. [DOI] [PubMed] [Google Scholar]
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. The Journal of comparative neurology. 1999;407:130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fonnum F. Cholinergic and GABAergic forebrain projections to the habenula and nucleus interpeduncularis: surgical and kainic acid lesions. Brain Res. 1983;275:287–297. doi: 10.1016/0006-8993(83)90989-7. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Deakin J. The origins of '5-HT and mechanisms of defence' by Deakin and Graeff: a personal perspective. J Psychopharmacol. 2013;27:1084–1089. doi: 10.1177/0269881113503508. [DOI] [PubMed] [Google Scholar]
- Desmedt A, Marighetto A, Piazza PV. Abnormal Fear Memory as a Model for Posttraumatic Stress Disorder. Biological psychiatry. 2015;78:290–297. doi: 10.1016/j.biopsych.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. The Journal of comparative neurology. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. The Journal of comparative neurology. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Hsu YW, Wang SD, Wang S, Morton G, Zariwala HA, de la Iglesia HO, Turner EE. Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. J Neurosci. 2014;34:11366–11384. doi: 10.1523/JNEUROSCI.1861-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature reviews. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Chung LY. Dual GABAergic synaptic response of fast excitation and slow inhibition in the medial habenula of rat epithalamus. Journal of neurophysiology. 2007;98:1323–1332. doi: 10.1152/jn.00575.2007. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, Kawasaki H, Kanba S, Lipp HP, Murphy NP, Wolfer DP, Itohara S. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppensteiner P, Aizawa S, Yamada D, Kabuta T, Boehm S, Wada K, Sekiguchi M. Age-dependent sensitivity to glucocorticoids in the developing mouse basolateral nucleus of the amygdala. Psychoneuroendocrinology. 2014;46:64–77. doi: 10.1016/j.psyneuen.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lee A, Mathuru AS, Teh C, Kibat C, Korzh V, Penney TB, Jesuthasan S. The habenula prevents helpless behavior in larval zebrafish. Current biology : CB. 2010;20:2211–2216. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, Changeux JP. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, Uchida N, Watabe-Uchida M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 2014;8:1105–1118. doi: 10.1016/j.celrep.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Quina LA, Wang S, Ng L, Turner EE. Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development. J Neurosci. 2009;29:14309–14322. doi: 10.1523/JNEUROSCI.2430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biological psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gomez E, Busquets-Garcia A, Hu F, Mehidi A, Cannich A, Roux L, Louit I, Alonso L, Wiesner T, Georges F, Verrier D, Vincent P, Ferreira G, Luo M, Marsicano G. Habenular CB1 Receptors Control the Expression of Aversive Memories. Neuron. 2015;88:306–313. doi: 10.1016/j.neuron.2015.08.035. [DOI] [PubMed] [Google Scholar]
- Szonyi A, Mayer MI, Cserep C, Takacs VT, Watanabe M, Freund TF, Nyiri G. The ascending median raphe projections are mainly glutamatergic in the mouse forebrain. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Kadoi J, Matsumoto S. Activaton of GABA(B) receptor inhibits the excitability of rat small diameter trigeminal root ganglion neurons. Neuroscience. 2004;123:491–505. doi: 10.1016/j.neuroscience.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science (New York, NY. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Frontiers in human neuroscience. 2013;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Yau HJ, Broker CJ, Tsou JH, Bonci A, Ikemoto S. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nature neuroscience. 2015;18:728–735. doi: 10.1038/nn.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, Zhou J, Hu F, Ren J, Wei C, Yu T, Zhuang Y, Bettler B, Wang F, Luo M. Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell. 2016;166:716–728. doi: 10.1016/j.cell.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]