Abstract
Interleukin (IL)-18 is an IL-1 family cytokine expressed by macrophages, dendritic cells, epithelial cells, and keratinocytes and is implicated in various aspects of both the innate and adaptive immune systems. IL-18 signals similar to IL-1β intracellularly to activate gene transcription. Since its discovery, IL-18 has been demonstrated to play a key role in pathogen defense from helminths and some bacteria. Recently however, evidence has accumulated that IL-18 expression is increased in many presentations of allergic disease. A pathologic role for IL-18 includes stimulating mast cell and basophil degranulation, recruiting granulocytes to sites of inflammation, increasing cytotoxic activity of natural killer (NK) and NK-T cells, inducing Immunoglobulin (Ig)E production and isotype switching, and affecting a broad range of T cells to promote a type II helper T cell (Th2) response. Evidence and importance of these effects are presented, including novel results from our lab implicating IL-18 in the direct expansion of mast cells, basophils, and other myeloid-lineage cells from bone-marrow precursors. The development of urticaria, asthma, dermatitis, rhinitis, and eosinophilic disorders all have demonstrated correlations to increased IL-18 levels either in the tissue or systemically. IL-18 represents a novel site of immune regulation in not only allergic conditions, but also autoimmune diseases and other instances of aberrant immune functioning. Diagrammatic summarized abstract for readers convinanceis presented in figure 1.
Keywords: Allergy, Eosinophils, Interleukin-18, iNKT cells, Mast cells
1. Introduction
Interleukin (IL)-18, discovered only 20 years ago, was first known as interferon (IFN)-γ inducing factor due to its ability to induce Type 1 helper T (Th1) cells to release IFN-γ [1]. Since then, this novel cytokine has been found to be expressed by macrophages, dendritic cells (DCs), epithelial cells, and keratinocytes and to activate a wide variety of lymphoid- and myeloid-derived cell types [2–4]. Induced IL-18 is now identified in a number of disorders, like autoimmunity, atopic and allergen-induced allergic responses, and defense against pathogens, most notably helminths. IL-18 is also known to be an IL-1 family cytokine implicated in various aspects of the innate and adaptive immune system, with some analogy to IL-1β. Like IL-1β, IL-18 is produced in an inactive precursor form and requires processing from IL-1β converting enzyme (ICE), or caspase-1, before activation and secretion [5]. Caspase-1 is induced by inflammasomes, multimeric complexes in the cytosol that are in turn activated by mediators such as the nucleotide binding and oligomerization domain-like receptor (NLR) sensor molecules [6,7]. The inflammasomes generally consist of a pattern recognition receptor (PRR) and an apoptosis-associated speck-like protein (ASC) [8]. Characterization of inflammasomes has demonstrated similarities to the apoptosome and has shown the ability to induce pyroptosis, a novel type of cell death distinct from apoptosis [9]. Additional pathways mediated by inflammatory cells lead to the production of active IL-18. Chymase, a secreted enzymatic product of mast cells, can process pro-IL-18 into an active IL-18 fragment extracellularly that is unique from caspase-1-derived IL-18 [10]. Additionally, IL-18 is also reported to be secreted by epithelial cells in vitro in response to neutrophil proteinase-3 (PR3) independent of caspase-1 [11]. Similar to the receptor for IL-1 molecules, the IL-18 receptor is a complex of a primary IL-18Rα protein that binds IL-18 with low affinity, and an accessory protein IL-18Rβ that imparts high-affinity binding [12,13]. The downstream signaling process is analogous to IL-1β signaling, including recruitment of myeloid differentiation 88 (MyD88) and IL-1R-associated kinase (IRAK) and eventual translocation of nuclear factor-kappa B (NF-κB) [14–16
2. Role of IL-18 in Allergic Disease
An allergic disease is a hypersensitive state mediated by specific immunologic mechanisms. Classical allergic diseases are mediated by immunoglobulin (Ig)E, although other cell-mediated processes can occur as well [17]. Although a classic allergic response is mediated by IgE, a vast array of leukocyte types are involved in both the sensitization and later response to allergens.
2.1 IL-18 Stimulates the Differentiation and Activation of Mast Cells and Basophils
The classical definition of allergy describes diseases driven primarily by Immunoglobulin E (IgE)–dependent mechanisms [18]. Basophils and mast cells, myeloid-derived leukocytes that contain granules of vasoactive and pro-inflammatory molecules, degranulate in response to Immunoglobulin E cross-linking with the high affinity IgE receptor (FcεRI) and are well-established as the primary mediators of allergic responses [19,20]. In addition, evidence shows that basophils and mast cells can be stimulated and degranulate in response to different immunoglobulins such as IgG and IgM as well as non-immunoglobulin signals [21,22]. Basophils and mast cells, while serving similar roles, are different in more than just primary location (mature basophils in the blood and mature mast cells resident in tissue). Traditional IgE-mediated anaphylaxis, the most severe presentation of allergy, appears to be primarily dependent on mast cells, with little contribution from basophils [23]. However, IgG-mediated anaphylaxis-like reactions appear to be primarily basophil-driven [23,24]. Mast cells and basophils, as the primary effectors of allergic processes, release a wide variety of vasoactive and inflammatory molecules. Studies support the ability of both mast cells and basophils to selectively release some of these molecules in a process of “activation” without full degranulation [25–28]. Histamine and leukotrienes, two main targets of most commercial anti-allergy treatments, are some of the many molecules released by both mast cells and basophils. Histamine and leukotrienes have immediate effects like increasing vascular permeability and bronchoconstriction [29,30] and long-term effects mediated by regulation of other inflammatory cells, including eosinophil recruitment and adhesion and Th2 promoting DCs [31,32]. The ability of basophils to independently recruit eosinophils and neutrophils in chronic allergic inflammation was demonstrated in a mouse model, and the low abundance (<2%) of basophils in the infiltrates suggested that basophils initiate rather than effect chronic inflammation [33]. In addition, mast cells have the ability to secrete serotonin, enzymes like chymase and tryptase, peptides, growth factors, cytokines, and chemokines, which act as powerful chemoattractants, regulate migration of mast cells, and activate platelets, among many other effects [34–37]. Important cytokines produced by both mast cells and basophils include thymic stromal lymphopoetin (TSLP), IL-4, IL-5, IL-13, IL-25, and granulocyte/macrophage colony stimulating factor (GM-CSF) [38–42]. Additionally, mast cells produce IL-3 and IL-31 [43,44].
Recent research now supports the direct effects of IL-18 on mast cells and basophils. Mature basophils and to a lesser extent mast cells have been shown to express the IL-18Rα chain, but primary bone marrow cells were previously demonstrated to require incubation in IL-3, a known differentiation factor for basophils and mast cells, in order to be responsive to IL-18 [45]. After an incubation period of at minimum a week, differentiated basophils were demonstrated to secrete the Th2 cytokines IL4 and IL13 in the absence of FcεRI stimulation, and mast cells produced IL13 as well in response to IL3 or IL-18 alone. The combination of IL3 and IL-18 greatly increased the production of Th2 cytokines [45]. Later investigation showed the IL-18-induced release of cytokines from basophils was MyD88 and p38α dependent and that IL-18 increased basophil survival over IL3 alone. The same study demonstrated expression of IL1β and IL-18 transcripts in mast cells, but only IL1β in basophils [46].
In addition to stimulating the release of Th2 cytokines, IL-18 induces changes in gene expression and affects the release of other mediators of allergic symptoms. In mucosal, but not connective-tissue, mast cells (MMC and CTMC respectively), stimulation by IL-18 greatly increases chemokine expression. The chemokine Ccl1 was greatly increased by IL-18 alone, in contrast to the co-requirement of IL-3 for high levels of IL-13 release, and increases were shown in IgE-activated MMCs as well [47]. This chemokine is important for the recruitment of T cells and DCs in atopy [48]. In contrast, however, cord-derived mast cells did not increase release of prostaglandin D2 or tryptase in response to treatment with IL-18 [49]. Basophils increase IgE- and A23187-induced release of leukotrienes B4 and C4 after culture in IL-3 or IL-18 (but not IL-4, IL-6, or IL-13) in a dose dependent manner, with the effects of IL-3 exceeding the effects of IL-18. These increases were not accompanied by increases in mRNA transcripts for leukotriene synthases, however mRNA and protein levels of 5-lipooxygenase (5-LO), a critical enzyme in leukotriene synthesis, were significantly increased [50]. Taken together, IL-18 upregulates machinery for leukotriene synthesis in basophils transcriptionally and potentially post-transcriptionally, and this most likely accounts for the increase in leukotriene release.
We recently found an important role for IL-18 in the generation and maturation of mast cells and basophils from bone marrow progenitors. Bone marrow from Balb/c mice was isolated and cultured in stem cell factor (SCF) with IL-3, IL-18, or both IL-3 and IL-18 (all at 20ng/mL concentration). At time points between one and four weeks, cultures were analyzed by flow cytometry for CD34, FceRI, CD49b, and c-kit. The total FceRI+ population in the isolated bone marrow was less than 1%, of which most cells were c-kit negative. In all three conditions, basophil lineage (FceRI+c-kit−) and mast cell lineage (FceRI+c-kit+) cells developed by the end of one week. The fraction of basophils began to drop after week 2 while the fraction of mast cells continued until the majority of cells were mast cells by the end of week 4. While containing a much lower percentage of basophils overall, the proportion of basophils expressing CD49b, a marker of matured basophils, was highest in the IL-18 only culture. This is the first study to demonstrate mast cell and basophil development by IL-18 without added IL-3, and we duplicated these results in subsequent experiments. In the combined IL-3 and IL-18 cultures, the fraction of cells that were CD34+ as well as the fraction of mature basophil and mast cell lineage populations were higher than the sums of those seen in either just IL-3 or IL-18, suggesting synergistic effects on generation. In the IL-18 cultures, the total cell concentration fell steadily after week 1 until less than 10% of the starting cell concentration was left at week 3, although the addition of IL-3 from week 3 onward produced slight increases in cell counts. Additionally, to test the maturity of the generated cells, semi-quantitative PCR was run for mouse mast cell proteases (mMCP)-1, -4, and -6 using previously described primers [51,52]. mMCP-1 and -6 are proteases expressed only in mucosal and connective-tissue mast cells, respectively, and MCP-4 is one of the first chymases to be expressed in mast cells. Mast cells from IL-3 culture express mMCP-4 but no mMCP-1 or -6, in line with many studies showing IL-3 generates immature mast cells. In contrast, mast cells from the IL-18 cultures expressed detectable levels of all proteases. Our experiments support a hypothesis that IL-18 is able to induce generation and maturation of both basophils and mast cells, but does not have the pro-survival effects that IL-3 has. This is supported by the fact that when we switched the IL-3 or IL-3 and IL-18 cultures to only IL-18 at week 2 the cell counts dropped by about 50% in the next week and by almost 90% after two weeks.
2.2 Eosinophilia, Neutrophilia, and IL-18
IL-5 is a lineage-specific cytokine that induces rapid proliferation of eosinophils from their progenitors. A wide variety of cytokines and chemokines induce the recruitment of eosinophils into the tissue such as eotaxin. Th2 cytokines such as IL-4 and IL-13 as well as mast cell-derived histamine and prostaglandin D2 promote tissue eosinophilia. Eosinophils can activate other granulocytes, participate in humoral immunity and host defenses, act in antigen presentation, and promote tissue remodeling. Aside from eosinophil disorders, the role of eosinophils and neutrophils in allergic disease are best described for allergic airway diseases like asthma. Eosinophil counts correlate to clinical indicators of asthma, and the release of inflammatory granules of basic proteins, lipid mediators, and various cytokines. Neutrophils are the first infiltrating cells after airway allergen challenge and are established as mediators of non-allergic asthma. Nocturnal and fatal asthma have been correlated to neutrophil counts in the airway, and neutrophils are observed in the sputum and bronchoalveolar lavage of acute and chronic persistent asthma.
The eosinophil marker eosinophilic cationic protein (ECP) increases in humans parallel to pollen exposure and the increases are associated with increased IL-18 levels [53]. IL-18 administered during allergic sensitization increased IL-5 levels and produced airway eosinophilia in response to antigen challenge in mice. However, IL-18 administered only during challenge in the sensitized mouse did not affect IL-5 levels and decreased eosinophilia [54]. A possible mechanism for the eosinophilia induced by IL-18 is eotaxin-dependent, as IL-18 administration induced peribronchial eosinophilia that was diminished in eotaxin knock-out mice [55]. In addition to effects on eosinophil recruitment, IL-18 dose-dependently increases IL-8 expression by eosinophils [56]. Along with promoting eosinophilia, IL-18 administration can promote in vivo neutrophil accumulation, and anti-IL-18 treatment reduces neutrophil accumulation [57,58]. Both studies demonstrated reduced TNF-α production with neutralizing IL-18. IL-18 also has the capacity to induce cytokine and chemokine release, upregulate CD11b, and induce granule release from neutrophils in vitro, and this effect was magnified when the neutrophils had previously been activated in vivo [57]. While a previous study indicated no effect of IL-18 on neutrophil survival [57], a later study demonstrated dose-dependent decreases in neutrophil apoptosis due to IL-18 [59]. Given the very short half-life of neutrophils in vivo, pro-survival effects of IL-18 have the potential to greatly enhance neutrophil-mediated allergic inflammation. In the bone marrow cultures described previously, we also ran flow cytometry at week 2 for Gr-1 and CD11c and found almost 60% of cells grown in IL-18 were Gr-1+, of which about one-fifth were CD11c+, and 10% were CD11c+GR-1−. In contrast, less than 5% of cells grown in IL-3 were either Gr-1 or CD11c positive. The combined IL-3 plus IL-18 treatment had almost no Gr-1+ cells but the same CD11c+Gr-1− population as the IL-18 group. These preliminary results suggest IL-18 induces the differentiation of granulocytes other than just basophils and mast cells and deserves further investigation.
2.3 IL-18 and T helper cell activation
The “Th2 hypothesis of allergy” has implicated T helper 2 (Th2) cells in the pathogenesis of allergic diseases for many years. Th1 cells, which produce IL-2, IL-12, TNF-β, and IFN-γ, induce phagocytic mechanisms and thus are active in cell-mediated immunity [60,61]. Th1 responses mediate autoimmune disorders in addition to responses driven by Th17 cells, T helper cells that produce IL-17 [62]. The IL-12 and IFN-γ produced by Th1 cells drive autoimmunity from arthritis and encephalitis to the non-obese diabetic mouse model [63]. In contrast, Th2 cytokines like IL-4, IL-5, IL-9, IL-10, and IL-13 mediate both allergen-induced and atopic allergic responses, and produce a wide range of effects on inflammatory cells as is discussed in other sections of this review [60,61]. Th1 and Th2 responses are traditionally thought of as mutually antagonistic, as it is generally accepted that Th1 and Th2 cells reciprocally regulate the development of the other. Th1-derived IFN-γ drives the further expansion of Th1 cells and suppresses the development of the Th2 lineage, and the reverse is true for Th-2 derived IL-4 [64]. This has been coined the “Th1/Th2 paradigm” of both autoimmune and allergic diseases.
IL-18 was first described for its ability to induce a Th1 response, resulting in the production of IFN-γ and subsequently inhibiting a Th2 response [65,66]. It is now appreciated that IL-18 can have opposing effects on the balance of Th1 and Th2 activation and cytokine production [67]. Th1 cells abundantly express IL-18Rα while there is little expression on Th2 cells, and IL-18 can also accelerate but not induce differentiation of Th1 cells [68,69]. When administered with IL-12, IL-18 stimulates Th1 activity and inhibits IgE production in an IFN-γ dependent manner [66]. When administered alone, IL-18 increases IgE levels independent of IFN-γ [45]. A later study determined this induction by IL-18 to be dependent on IL-4, and STAT-6 [70]. IL-18 in combination with IL-2 activates naïve T helper cells to produce large amounts of IL-13, and these cells are able to differentiate into Th2 cells [70]. IL-18 alone also has the ability to induce Th1 cells to begin producing the Th2 cytokines IL-3, IL-9, and IL-13 [71], and the authors later coined the term super Th1 cell to describe cells with this Th2 cytokine capability [72]. IL-18 activated these super Th1 cells in passive Th1 mice, resulting in allergen-induced asthma mediated in part by IL-13 and IFN-γ [71].
2.4 IL-18 activates Cytotoxic, Regulatory, and Natural-Killer T Cells
Cytotoxic (CD8+), regulatory, and natural-killer T cells all play a role in allergic responses. CD8+ T cells have paradoxical effects, as they have been reported to be the mediators of palladium and amoxicillin allergies, while also able to non-specifically inhibit allergic sensitization and promote a Th1 response during a Th2 allergic response. In both examples of drug allergy, CD8+ cells were enriched upon sensitization and were still able to effect an allergic response after adoptive transfer to naïve mice, and CD8+-derived IFN-γ was implicated as a key mediator [73,74]. In the studies demonstrating an inhibition of allergic sensitization, memory CD8+ cells stimulated DCs to promote Th1 cells, but in this response the IFN-γ derived from Th1 cells [75,76]. Regulatory T cells attenuate allergic responses in a broad variety of ways, ranging from suppressing Th2 proliferation, to inhibiting allergy-specific IgE production by B cells, to directly suppressing mast cell, basophil, and eosinophil activation [77]. Invariant natural killer T cells (iNKT) amplify eosinophil recruitment as well as Th2 cytokine and IgE production following allergic challenge [78]. Additionally, mice deficient in NKT cells were unable to produce airway hyperresponsiveness despite normal Th2 cell function [79].
A study of fatal asthma indicated that both CD8+ T cells and IL-18 were strongly upregulated in lung samples versus control, and that the CD8+ count greatly exceeded the CD4+ counts in the asthma lungs [80]. Other studies have directly examined the effect of IL-18 on both effector and memory CD8+ cells. IL-18 was shown to greatly enhance effector CD8+ T cell expansion, contributing to development of graft versus host disease [81]. IL-18 was able to stimulate in vivo memory CD8+ T cell proliferation in an IFN-γ dependent manner, which was hypothesized to be IL15-dependent due to a lack of stimulation in vitro [82]. DC-derived IL-18 also was demonstrated at the DC-T cell interface in antigen-stimulated memory CD8+ expansion in immunized mice [83]. Similar to the ability of IL-18 to have opposing effects on T helper cells, IL-18 is able to stimulate or retard T regulatory conversion under differing conditions. In the engraftment study on memory CD8+ mentioned previously, IL-18 decreased Treg cells in peripheral blood and prevented the suppressive effect of T regulatory cells on GVHD [81]. However, the bacterium Helicobacter pylori is known to protect against allergic and autoimmune disorders, and this effect was shown to be IL-18-dependent via Treg expansion [84]. This is consistent with the “hygiene hypothesis”, the idea that early exposure to microbes decreases later aberrant immune responses via Treg induction, possibly through lasting effects on IL-18 [85]. iNKT cells have the capacity to release Th1 and Th2 cytokines. IL-12 is able to increase IFN-γ (Th1) production and IL-7 is able to increase IL-4 production (Th2) dependent on stimulation of the T cell receptor (TCR) [86,87]. IL-18, in combination with IL-12, activates NKT cells to release IFN-γ independent of TCR stimulation [88]. IL-18 with the NKT ligand a-galactosylceramide increases IL-4 production regardless of IL-12 administration but did not affect NKT cell proliferation [89]. The data from our group indicates that direct exposure of IL-18 to invariant NKT (iNKT) cells in vitro releases eosinophilia-promoting cytokines like IL-4, Il-5 and IL-13, and promotes eosinophilic inflammation in tissue [90]. Additionally, we showed that transgenic mice overexpressing IL-18 are capable of promoting pulmonary and gastrointestinal eosinophilic disorders presumably via this iNKT activation [91].
2.5 IL-18 and NK Cells
Natural killer (NK) cells are a diverse repertoire of cytotoxic cells and play crucial roles in viral defense and control of cancerous cells among other functions [92]. NK cells serve important regulatory roles as they function both in innate immunity as well as both humoral and cell-mediated adaptive responses, and important sources of a number of cytokine and chemokines [93]. While it was known that NK cells function in the development of eosinophilic airway inflammation [94], the role of NK cells in allergic diseases was not heavily investigated until very recently. NK cells can be stimulated to produce Th1 or Th2 and Th17 cytokines as well as induce isotype switching in B cells [95,96]. It has also been proposed that the regulatory role of an NK-subset that maintains Th1 responses is impaired in allergic diseases [97].
When IL-18 was first isolated as IFN-γ-inducing factor, it was observed to increase NK cell activity [98]. It was later observed to upregulate Fas-ligand-mediated cytotoxic activity [99], and IL-18-deficient mice had a three-fold reduction in killing activity that both further reduced by IL-12 deficiency and restored via IL-18 administration [100]. IL-18 stimulation of NK cells results in greatly increased IFN-γ and GM-CSF release when combined with IL-12 or IL-15, and it was shown that IL-12 and IL-15 greatly upregulated IL-18 receptor expression, suggesting the synergism is in part is due to increased sensitivity to IL-18 [101].
2.6 IL-18 and IgE
B cells can initiate an allergic response in multiple ways. B cells can act as antigen presenting cells (APCs) and induce Th2 expansion and this has been implicated in chronic lung inflammation [102–104]. Most importantly, B cells produce the antibodies necessary for immunoglobulin-mediated allergic responses. The cytokines IL-4 and IL-13 are the primary stimulus for class switching to activate IgE-secreting plasma cells [105]. Increased IgE levels in turn upregulate the expression of FcεRI, making mast cells more responsive to IgE [106]. B cells are also able to differentiate into cytokine producing cells, whereby Th1-stimulated cells release Th1 cytokines and Th2-stimulated cells produce Th2 cytokines [107]. Multiple studies have demonstrated correlations between serum IL-18 and IgE levels [108–110]. In vivo mice studies support this correlation, showing increased serum IgE, IgG1/2a, and IgM after IL-18 administration [111]. The mature B2 subset marginal-zone B cell (MZB), innate resident B cells in the spleen [112], expanded in vivo after IL-18 administration independent of CD4+ T cells, as did regulatory B cells in a CD4+ dependent manner. In addition, MZB-like cells developed in MZB-deficient cells after IL-18 administration [111]. The same study found accelerated mature B cell development and greatly increased antibody production from IL-18 in NKT-deficient mice. The combination of IL-18 and IL-12 induces B cells to produce IFN-γ in vitro and in vivo, inhibiting IgE and IgG1 and enhancing IgG2a production [113]. This further supports the previously presented data supporting IL-12 plus IL-18 induces an anti-allergic Th1-type response.
3. IL-18-induced Allergic Diseases
Hypersensitivity allergic reactions are often atopic, or in response to routine allergens and dependent on IgE sensitization, and a few disease states are classically associated with atopic allergy: urticaria, asthma, rhinitis, and dermatitis, as well as the life-threatening acute reaction anaphylaxis. Food and drug hypersensitivities are also common classes of allergy. [17]
3.1 Urticaria: a Classic Allergic Response
Urticaria, or hives, is a symptom often seen in anaphylaxis and is an example of a classic response to histamine released by basophils and mast cells [114]. Patients with both acute urticarial episodes as well as those with chronic spontaneous urticaria, a form of recurrent hives, have significantly higher serum IL-18 levels than controls [115,116]. Correlations between IL-18 levels and disease severity in chronic idiopathic urticaria have also been observed [117].
3.2 Anaphylaxis
Anaphylaxis is a life-threatening allergic reaction mediated by mast cells and basophils. Many reserve the term to only describe IgE-dependent mechanisms, but other “anaphylactoid” reactions can be IgG and IgM mediated as well [118]. As presented above, IL-18 has the ability to increase levels of IgE, IgG, and IgM, and as such IL-18 could play a role in these life-threatening reactions. In line with the antiallergenic effect from the combination of IL-18 and IL-12, co-administration of these cytokines inhibits the IL-4-induced enhancement of anaphylaxis [119]. There is not substantial evidence covering the role of IL-18 in anaphylactic and anaphylactoid reactions. However, IL-18 polymorphisms have been associated with latex and penicillin allergies [120,121]. Additionally, activation of the NLRP3 inflammasome is known to increase IL-18 and IL-1β processing [122], and functional NLRP3 polymorphisms are linked to food-induced anaphylaxis [123].
3.3 Allergic Rhinitis
Both seasonal allergic rhinitis (SAR) and perennial allergic rhinitis (PAR) are associated with increased IL-18 levels. Multiple studies have associated different single nucleotide polymorphisms (SNPs) in the IL-18 promoter with increased sensitization and risk of allergic rhinitis [124,125], with an additional study showing significantly increased risk in patients with multiple SNPs [126]. Increases in serum IL-18 and IL-1β followed the time course of pollen exposure with a delay in SAR patients and remained elevated weeks after the end of the season. The same study found significantly higher IL-18 in PAR patients compared to both SAR and controls [53]. This suggests IL-18 in the chronic inflammatory response compared to the acute phase after exposure to an allergen. In allergic rhinitis patients, the presence of additional symptoms was associated with higher levels of IL-18 as well, with examples of bronchial hyperresponsiveness in SAR and asthma in PAR [127,128]. Elevations of IL-18 levels were observed in lung but not nasal lavage from an animal model allergic rhinitis, and systemic corticosteroid administration returned IL-18 levels to baseline [129]. More recently, the NLRP3 inflammasome, a caspase-1 activator, showed increased expression alongside elevations in IL-18 and IL-1β in a model of allergic rhinitis [130].
3.4 Allergic Dermatitis (Eczema)
The IL-18 promoter polymorphisms 137G/C and 607C/A that decrease IL-18 transcription have been linked to a decreased risk of allergic dermatitis in a meta-analysis [131]. IL-18 was first implicated in allergic dermatitis when transgenic mice for caspase-1 developed dermatitis with spontaneous apoptosis, and high levels of IL-18 were found in the skin [132]. A later study demonstrated IL-18 transgenic mice exhibited mastocytosis and dermatitis with elevations in serum IgE. Interestingly, the same study examined caspase-1 transgenic mice showed that a STAT-6 deficiency inhibited IgE increases but did not prevent dermatitis, while IL-18 deficient transgenic mice developed neither [133]. Further studies of an atopic dermatitis model showed severe dermatitis with elevations in serum IL-18 but not IgE, and IL-18 blockade prevented dermatitis development [134]. IL-3 blockade decreased the severity of dermatitis developed. Taken together these studies indicate an IgE-independent, IL-18-mediated allergic mechanism that in part involves IL-18 induced IL-3 release that accelerates mastocytosis. Human studies supported a correlation between IL-18 and disease severity and serum IgE [135].
3.5 Asthma
The 137G allele in the IL-18 gene was identified as a risk factor for atopic asthma [136], and the previously mentioned Cheng et al meta-analysis found a significantly decreased risk of allergic asthma in certain polymorphisms [131]. In one study, the combination of IL-18 an IL-12 decreased airway hyper-responsiveness, airway eosinophilia, and serum IgE [66]. In contrast, IL-18 administered with antigenic sensitization increases serum IgE, Th2 cytokines, and airway eosinophilia in murine allergic asthma [54]. Interestingly, IL-18 administered during antigen challenge inhibited all of these responses. In acute asthma exacerbations, IL-18 is significantly elevated and inversely correlated to clinical measures, but without IFN-γ elevation [137]. In cases of fatal asthma, post-mortem biopsies have shown very high levels of IL-18 and IL-18Rα expression in the epithelium and smooth muscle, compared to very little in controls [80]. In a study of IL-18 transgenic mice, airway hyper-responsiveness and infiltration of inflammatory cells was greatly increased over wild-type mice in induced asthma. This was shown to be in a CD4+ T cell and IL-13 dependent manner [138].
3.6 Eosinophilic esophagitis, gastroenteritis and colitis
As mentioned previously, IL-18 has been linked to increased ECP levels, increases the eosinophil-inducing cytokine IL-5, can induce eotaxin-dependent eosinophilia, and increases IL-8 expression by eosinophils [53–56]. It follows that IL-18 may play a role in eosinophilic gastrointestinal disorders. Our early results showed increased levels of the IL-18 receptor transcript in humans [139]. We have recently demonstrated induction of IL-18 and its receptor in a mouse model of eosinophilic esophagitis (EoE) [91]. We have also demonstrated correlations between IL-18 and eosinophilia in human EoE patients in addition to the previously mentioned results of iNKT cells [90].
4. Conclusion
IL-18 participates in both humoral and cell-mediated immune responses. IL-18 works in synergy with various other cytokines, and the response induced often depends on the identity of these cytokines. With IL-12, IL-18 induces a Th1 response, while with IL-2 a Th2 response is initiated. IL-18 is able to recruit granulocytes including mast cells, eosinophils, and neutrophils into target tissues in order to initiate an inflammatory response. We have also demonstrated the ability of IL-18 alone to induce mast cells, basophils, and other granulocytes from bone marrow precursors. IL-18 alone stimulates B cell expansion and isotype switching, while this is inhibited with added IL-12. IL-18 induces opposite effects on T regulatory and cytotoxic T cells depending on the stimulus, being able to exacerbate or inhibit allergic inflammation. Cytotoxic activity of and cytokine release from NK and NKT cells is enhanced in the presence of IL-18. The ability to induce a wide range of leukocytes allows IL-18 to participate in allergic responses from rhinitis and dermatitis to asthma and eosinophilic disorders.
All of the physiologic effects of IL-18 on various leukocytes presented give the impression that IL-18 is essential to both normal immune functioning and the abnormal functioning seen in allergic and autoimmune diseases. This is supported by the observations that IL-18 is protective against tuberculosis and Legionnaire’s disease, that lower baseline IL-18 levels were associated with more severe episodes of the common cold, and the plethora of evidence supporting the crucial role of IL-18 in helminth expulsion [140–143]. Even in allergic disease, IL-18 has differential effects during sensitization and challenge, increasing inflammation and eosinophilia when administered during sensitization and decreasing immunoglobulin production and eosinophilia when administered during challenge [54]. Many authors have speculated on the possible therapeutic applications of monoclonal IL-18 antibodies or IL-18 binding protein, the endogenous antagonist of IL-18, to treat autoimmune conditions, boost immune function for chemotherapy, and various others [144,145]. However, given the many dichotomous effects of IL-18, the number of unintended consequences from IL-18 blockade should be studied. Additionally, we provide prospective summarized pathway involved in IL-18 associated inflammatory cytokines and cells that are influenced by the induction of IL-18 in innate and adaptive immunity is presented in figure 2.
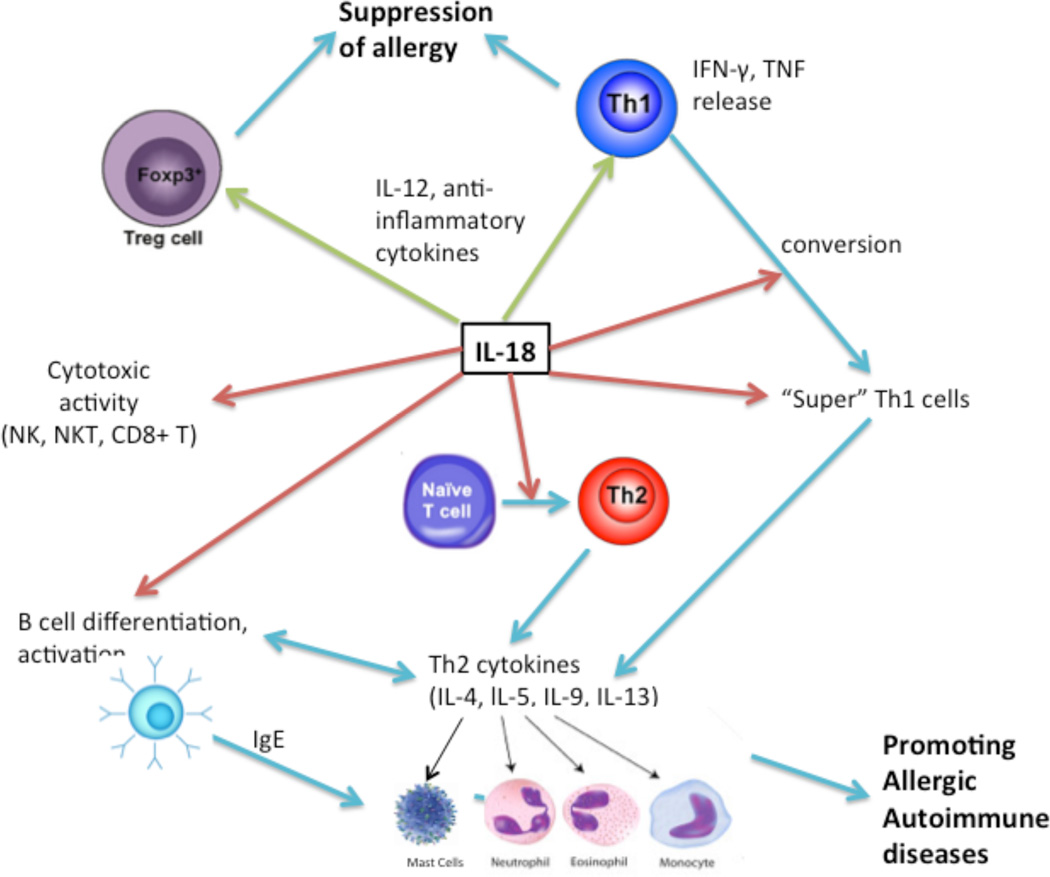
Figure 2. Diagrammatic representation of IL-18 involvement in innate and adaptive immunity.
Pro-inflammatory pathway of IL-18 in the maturation and activation of T cell subsets are demonstrated by red arrows and anti-inflammatory pathway by green arrows, and blue arrows indicates that IL-18 has a role in a number of inflammatory cells activation to promote autoimmune and allergic diseases. The presented summary indicates that IL-18 is involved in natural killer T cells activation, induces B cell differentiation, and conversion of naïve T cells to Th2 cells. IL-18 can stimulate the conversion in vitro of Th1 cells to “super Th1 cells” that produce Th2 cytokines that promotes autoimmune and allergic diseases and in synergy with IL-12 activates T regulatory cell activity and Th1 cytokine to release anti-inflammatory cytokines.
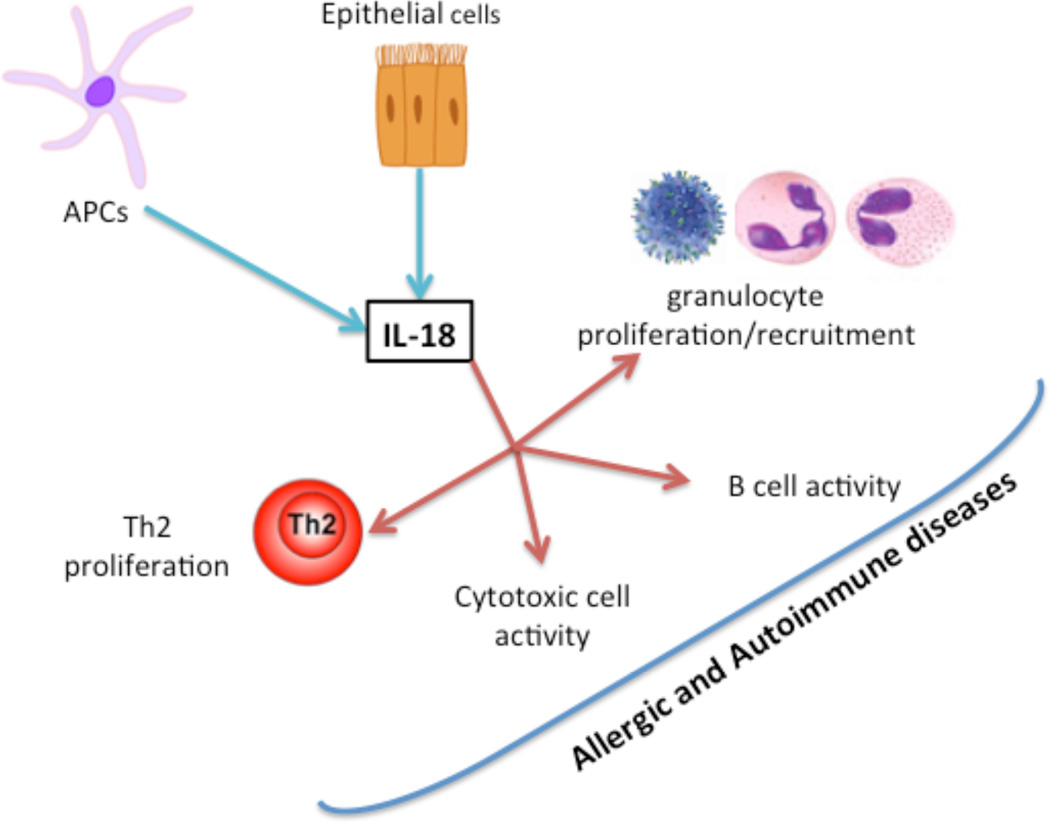
Figure 1. Abstract Summary.
Diagrammatic presentation of review summary on IL-18 secretion by the epithelial and antigen presenting cells and its role in promoting allergic and autoimmune diseases by activating inflammatory cells.
Highlights.
IL-18 is an IL-1 family cytokine that has recently been demonstrated to be pro-inflammatory and contribute to the production of allergic conditions.
IL-18 induces a Th2-type response by directly stimulating both T and B lymphocytes as well as by stimulating granulocyte expansion and recruitment and natural killer activity.
IL-18 have been implicated in classic atopic conditions such as urticarial, dermatitis, rhinitis, and asthma, may play a role in life-threatening anaphylaxis, and has been demonstrated in eosinophilic gastrointestinal disorders
Acknowledgments
This work was supported by grants from….
Abbreviations
- 5-LO
5-lipooxygenase
- APC
antigen-presenting cell
- ASC
apoptosis-associated speck-like protein
- CTMC
connective-tissue mast cell
- DC
dendritic cell
- ECP
eosinophil cationic protein
- EoE
eosinophilic esophagitis
- FcεRI
high affinity Immunoglobulin E receptor
- GM-CSF
granulocyte/macrophage colony stimulating factor
- ICE
IL-1β converting enzyme
- IFN-γ
interferon-γ
- Ig
immunoglobulin
- IL
interleukin
- IL-18Ra
interleukin-18 receptor-α
- iNKT
invariant natural killer T cell
- IRAK
interleukin-1 receptor-associated kinase
- MMC
mucosal mast cell
- mMCP
mouse mast cell protease
- MyD88
myeloid differentiation 88
- MZB
marginal zone B cell
- NF-κB
nuclear factor-kappa B
- NK
natural killer cell
- NKT
natural killer T cell
- PAR
perennial allergic rhinitis
- PRR
pattern recognition receptor
- PR3
neutrophil proteinase-3
- SAR
seasonal allergic rhinitis
- SCF
stem cell factor
- SNP
single nucleotide polymorphism
- STAT6
signal transducer and activator of transcription 6
- TCR
T cell receptor
- Th1
type I helper T cell
- Th2
type II helper T cell
- Th17
T helper 17
- Treg
regulatory T cell
- TSLP
thymic stromal lymphopoetin
Biographies

Nathan L. Sanders is a Presidential Scholar at Tulane University, graduating in 2017 with a B.S. degree in Neuroscience and Biological Chemistry. He previously conducted research and continues consulting work at Duke University under Drs. William Parker and Shu Lin studying acute rejection in lung transplantation and intestinal immunity. He has co-authored a business model for sustainable development of treatments for rare diseases recognized by GlaxoSmithKline. His focus at Tulane is understanding the pathways involved in inflammatory cells development and maturation. His current research focuses on the differentiation and maturation of murine mast cells, specifically examining the role of IL-18 on mast cell development, maturation and protease gene transcription. His long term goal is to become a physician scientist.

Dr. Anil Mishra, PhD is Professor of Medicine and Endowed Chair, Edward G. Schlieder Educational Foundation in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. He is also the Director of Eosinophilic Disorder Center at Tulane School of Medicine, New Orleans, LA. Dr. Mishra had an outstanding background, beginning with his distinguished education at Rajasthan University, Jaipur, India where he graduated with Bachelor of Science. Having excelled in science education, he then undertook PhD training at Kanpur University, Kanpur, India. Dr. Mishra gained inhalation toxicology training from a prestigious Institute of Toxicology, Lucknow, India and further at GSF Institute of Inhalation at Munchen, Germany. Dr. Mishra completed his postdoctoral training at Northestern Universities college of Medicine, Rootstown, OH, Tunale Universtity School of Medicien, New Orleans, LA, and at Indian University Purdu Institute of Medical College, Indianapolis, IN. He served as a faculty member at Allergy and Immunology Division, Cincinnati Childrens Hospital Medical Center, Cincinnati, OH and Case Western Reserve University Medical College, OH. Currently Dr. Mishra is Endowed Chair and Professor of Medicine at Tulane University School of Medicine, New Orleans, LA. Dr. Mishra’s most important contribution was to establish that eosinophils are the resident cell that home prenatally in the gastrointestinal tract (Mishra et al. J. Clin. Invest. 1999, 103, 1719–27) developed a first murine model of asthma associated eosinophilic esophagitis (Mishra et al. J. Clin. Invest. 2001, 107, 83–90). His basic and translational research is focused on newly emerging esophageal disease termed as “Eosinophilic Esophagitis (EoE).” Dr. Mishra’s is an elected fellow of the American Academy of Allergy Asthma Immunology (FAAAAI), and American Gastrointestinal Association (FAGA). Dr. Mishra was the active member of Cincinnati Center for Eosinophilic Disorder (CCED) from the start of the center. He has over a 70 articles on molecular mechanisms of the pulmonary gastrintestinal toxicity and allergic responses. Some of his publications are cited more than 300 to 400 till 2015. Dr. Mishra’s research has been supported by the 2-different intitutes of National Institutes of Health (NIDDK and NIAID). He is serving in a number of study sections as member in NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
We declare that we have no professional or personal affiliations with any organization or entity with a financial or non-financial interest related to the topics presented in this manuscript that could influence the positions stated.
References
- 1.Okamura H, Nagata K, Komatsu T, et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63(10):3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron LA, Taha RA, Tsicopoulos A, et al. Airway epithelium expresses interleukin-18. Eur Respir J. 1999;14(3):553–559. doi: 10.1034/j.1399-3003.1999.14c12.x. [DOI] [PubMed] [Google Scholar]
- 3.Stoll S, Muller G, Kurimoto M, et al. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159(1):298–302. [PubMed] [Google Scholar]
- 4.Stoll S, Jonuleit H, Schmitt E, et al. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28(10):3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386(6625):619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 7.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi S, Sagara J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Semin Immunopathol. 2007;29(3):231–238. doi: 10.1007/s00281-007-0082-3. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky IE, Medzhitov R. Pyroptosis: macrophage suicide exposes hidden invaders. Curr Biol. 2011;21(2):R72–R75. doi: 10.1016/j.cub.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Omoto Y, Tokime K, Yamanaka K, et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177(12):8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara S, Uehara A, Nochi T, et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167(11):6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 12.Torigoe K, Ushio S, Okura T, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272(41):25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 13.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273(45):29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 14.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 15.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7(6):837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Tsuji-Takayama K, Aizawa Y, et al. Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochem Biophys Res Commun. 1997;234(2):454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 17.Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242(1):31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 19.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25(5):266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcepsilonRI beta-chain in allergic diseases. Int Arch Allergy Immunol. 2004;135(1):62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 21.Bernardini R, Novembre E, Lombardi E, et al. Pseudoallergies. Pediatr Med Chir. 2001;23(1):9–16. [PubMed] [Google Scholar]
- 22.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146(1–2):1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115(3):449–457. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 25.Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol. 1995;153(3 Pt 1):629–636. doi: 10.1097/00005392-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Letourneau R, Pang X, Sant GR, Theoharides TC. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Br J Urol. 1996;77(1):41–54. doi: 10.1046/j.1464-410x.1996.08178.x. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak AM, McLeod RS, Onderdonk A, et al. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int Arch Allergy Immunol. 1992;99(1):74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- 28.Mustafa FB, Ng FS, Nguyen TH, Lim LH. Honeybee venom secretory phospholipase A2 induces leukotriene production but not histamine release from human basophils. Clin Exp Immunol. 2008;151(1):94–100. doi: 10.1111/j.1365-2249.2007.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328(1):89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 30.Devi NS, Doble M. Leukotriene c4 synthase: upcoming drug target for inflammation. Curr Drug Targets. 2012;13(8):1107–1118. doi: 10.2174/138945012802009053. [DOI] [PubMed] [Google Scholar]
- 31.Caron G, Delneste Y, Roelandts E, et al. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167(7):3682–3686. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- 32.Foster AP, Cunningham FM. Histamine-induced adherence and migration of equine eosinophils. Am J Vet Res. 1998;59(9):1153–1159. [PubMed] [Google Scholar]
- 33.Mukai K, Matsuoka K, Taya C, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23(2):191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 34.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159(12):6216–6225. [PubMed] [Google Scholar]
- 35.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125(7):1491–1500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation. 2008;31(2):112–120. doi: 10.1007/s10753-007-9056-9. [DOI] [PubMed] [Google Scholar]
- 37.Misiak-Tloczek A, Brzezinska-Blaszczyk E. IL-6, but not IL-4, stimulates chemokinesis and TNF stimulates chemotaxis of tissue mast cells: involvement of both mitogen-activated protein kinases and phosphatidylinositol 3-kinase signalling pathways. Apmis. 2009;117(8):558–567. doi: 10.1111/j.1600-0463.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 38.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 39.Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42(5):712–737. doi: 10.1111/j.1365-2222.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider E, Thieblemont N, De Moraes ML, Dy M. Basophils: new players in the cytokine network. Eur Cytokine Netw. 2010;21(3):142–153. doi: 10.1684/ecn.2010.0197. [DOI] [PubMed] [Google Scholar]
- 42.Okayama Y, Okumura S, Sagara H, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34(2):425–435. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 43.Law M, Morales JL, Mottram LF, Iyer A, Peterson BR, August A. Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int J Biochem Cell Biol. 2011;43(8):1228–1239. doi: 10.1016/j.biocel.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niyonsaba F, Ushio H, Hara M, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184(7):3526–3534. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimoto T, Tsutsui H, Tominaga K, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96(24):13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86(4):769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiener Z, Pocza P, Racz M, et al. IL-18 induces a marked gene expression profile change and increased Ccl1 (I-309) production in mouse mucosal mast cell homologs. Int Immunol. 2008;20(12):1565–1573. doi: 10.1093/intimm/dxn115. [DOI] [PubMed] [Google Scholar]
- 48.Gombert M, Dieu-Nosjean MC, Winterberg F, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174(8):5082–5091. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- 49.Maccauro G, Tripodi D, Saggini A, Conti F, Cianchetti E, Angelucci D, Rosati M, Toniato E, Fulcheri M, Tete S, et al. Calcium ionophore A23187 and compound 48/80 induce PGD2 and tryptase in human cord blood-derived mast cells: lack of effect of IL-18. Eur J Inflamm. 2012;10(1):33–43. [Google Scholar]
- 50.Zaitsu M, Yamasaki F, Ishii E, Midoro-Horiuti T, Goldblum RM, Hamasaki Y. Interleukin-18 primes human basophilic KU812 cells for higher leukotriene synthesis. Prostaglandins Leukot Essent Fatty Acids. 2006;74(1):61–66. doi: 10.1016/j.plefa.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Wastling JM, Knight P, Ure J, et al. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153(2):491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J Biol Chem. 1996;271(5):2851–2855. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]
- 53.Verhaeghe B, Gevaert P, Holtappels G, et al. Up-regulation of IL-18 in allergic rhinitis. Allergy. 2002;57(9):825–830. doi: 10.1034/j.1398-9995.2002.23539.x. [DOI] [PubMed] [Google Scholar]
- 54.Wild JS, Sigounas A, Sur N, et al. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164(5):2701–2710. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 55.Campbell E, Kunkel SL, Strieter RM, Lukacs NW. Differential roles of IL-18 in allergic airway disease: induction of eotaxin by resident cell populations exacerbates eosinophil accumulation. J Immunol. 2000;164(2):1096–1102. doi: 10.4049/jimmunol.164.2.1096. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Tanaka T, Okamura H, et al. Interleukin-18 enhances the production of interleukin-8 by eosinophils. Eur J Immunol. 2001;31(4):1010–1016. doi: 10.1002/1521-4141(200104)31:4<1010::aid-immu1010>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167(5):2879–2886. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 58.Netea MG, Fantuzzi G, Kullberg BJ, et al. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164(5):2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 59.Hirata J, Kotani J, Aoyama M, et al. A role for IL-18 in human neutrophil apoptosis. Shock. 2008;30(6):628–633. doi: 10.1097/SHK.0b013e31817c0c69. [DOI] [PubMed] [Google Scholar]
- 60.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 61.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15(3):121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 62.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995;7(6):793–798. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 64.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148(7):2142–2147. [PubMed] [Google Scholar]
- 65.Kohno K, Kataoka J, Ohtsuki T, et al. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158(4):1541–1550. [PubMed] [Google Scholar]
- 66.Hofstra CL, Van Ark I, Hofman G, Kool M, Nijkamp FP, Van Oosterhout AJ. Prevention of Th2-like cell responses by coadministration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J Immunol. 1998;161(9):5054–5060. [PubMed] [Google Scholar]
- 67.Xu D, Trajkovic V, Hunter D, et al. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30(11):3147–3156. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 68.Xu D, Chan WL, Leung BP, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188(8):1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson D, Shibuya K, Mui A, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7(4):571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1(2):132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 71.Sugimoto T, Ishikawa Y, Yoshimoto T, Hayashi N, Fujimoto J, Nakanishi K. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J Exp Med. 2004;199(4):535–545. doi: 10.1084/jem.20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakanishi K, Tsutsui H, Yoshimoto T. Importance of IL-18-induced super Th1 cells for the development of allergic inflammation. Allergol Int. 2010;59(2):137–141. doi: 10.2332/allergolint.10-RAI-0208. [DOI] [PubMed] [Google Scholar]
- 73.Rozieres A, Vocanson M, Rodet K, et al. CD8+ T cells mediate skin allergy to amoxicillin in a mouse model. Allergy. 2010;65(8):996–1003. doi: 10.1111/j.1398-9995.2009.02307.x. [DOI] [PubMed] [Google Scholar]
- 74.Kawano M, Nakayama M, Aoshima Y, et al. NKG2D(+) IFN-gamma(+) CD8(+) T cells are responsible for palladium allergy. PLoS One. 2014;9(2):e86810. doi: 10.1371/journal.pone.0086810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leggat JA, Gibbons DL, Haque SF, et al. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J Allergy Clin Immunol. 2008;122(5):1014–1021. e1014. doi: 10.1016/j.jaci.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang Y, Guan SP, Chua BY, et al. Antigen-specific effector CD8 T cells regulate allergic responses via IFN-gamma and dendritic cell function. J Allergy Clin Immunol. 2012;129(6):1611–1620. e1614. doi: 10.1016/j.jaci.2011.12.976. [DOI] [PubMed] [Google Scholar]
- 77.Braga M, Schiavone C, Di Gioacchino G, et al. Environment and T regulatory cells in allergy. Sci Total Environ. 2012;423:193–201. doi: 10.1016/j.scitotenv.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171(4):1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 79.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 80.Oda H, Kawayama T, Imaoka H, et al. Interleukin-18 expression, CD8(+) T cells, and eosinophils in lungs of nonsmokers with fatal asthma. Ann Allergy Asthma Immunol. 2014;112(1):23–28. e21. doi: 10.1016/j.anai.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Carroll RG, Carpenito C, Shan X, et al. Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells. PLoS One. 2008;3(9):e3289. doi: 10.1371/journal.pone.0003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tough DF, Zhang X, Sprent J. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J Immunol. 2001;166(10):6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 83.Iwai Y, Hemmi H, Mizenina O, Kuroda S, Suda K, Steinman RM. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS One. 2008;3(6):e2404. doi: 10.1371/journal.pone.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oertli M, Sundquist M, Hitzler I, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mojtahedi Z, Ghaderi A. Role of interleukin-18 in allergy and autoimmunity: an explanation for the hygiene hypothesis. Med Hypotheses. 2005;65(2):305–307. doi: 10.1016/j.mehy.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 86.Hameg A, Gouarin C, Gombert JM, et al. IL-7 up-regulates IL-4 production by splenic NK1.1+ and NK1.1- MHC class I-like/CD1-dependent CD4+ T cells. J Immunol. 1999;162(12):7067–7074. [PubMed] [Google Scholar]
- 87.Leite-De-Moraes MC, Moreau G, Arnould A, et al. IL-4-producing NK T cells are biased towards IFN-gamma production by IL-12. Influence of the microenvironment on the functional capacities of NK T cells. Eur J Immunol. 1998;28(5):1507–1515. doi: 10.1002/(SICI)1521-4141(199805)28:05<1507::AID-IMMU1507>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 88.Leite-De-Moraes MC, Hameg A, Arnould A, et al. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol. 1999;163(11):5871–5876. [PubMed] [Google Scholar]
- 89.Leite-De-Moraes MC, Hameg A, Pacilio M, et al. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J Immunol. 2001;166(2):945–951. doi: 10.4049/jimmunol.166.2.945. [DOI] [PubMed] [Google Scholar]
- 90.Niranjan R, Rajavelu P, Ventateshaiah SU, et al. Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis. Clin Immunol. 2015;157(2):103–113. doi: 10.1016/j.clim.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutt P, Shukla JS, Ventateshaiah SU, et al. Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice. Immunol Cell Biol. 2015;93(10):849–857. doi: 10.1038/icb.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whiteside TL, Herberman RB. Role of human natural killer cells in health and disease. Clin Diagn Lab Immunol. 1994;1(2):125–133. doi: 10.1128/cdli.1.2.125-133.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korsgren M, Persson CG, Sundler F, et al. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;189(3):553–562. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satoskar AR, Stamm LM, Zhang X, et al. NK cell-deficient mice develop a Th1-like response but fail to mount an efficient antigen-specific IgG2a antibody response. J Immunol. 1999;163(10):5298–5302. [PubMed] [Google Scholar]
- 96.Deniz G, Akdis M, Aktas E, Blaser K, Akdis CA. Human NK1 and NK2 subsets determined by purification of IFN-gamma-secreting and IFN-gamma-nonsecreting NK cells. Eur J Immunol. 2002;32(3):879–884. doi: 10.1002/1521-4141(200203)32:3<879::AID-IMMU879>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 97.Scordamaglia F, Balsamo M, Scordamaglia A, et al. Perturbations of natural killer cell regulatory functions in respiratory allergic diseases. J Allergy Clin Immunol. 2008;121(2):479–485. doi: 10.1016/j.jaci.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 98.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 99.Tsutsui H, Nakanishi K, Matsui K, et al. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157(9):3967–3973. [PubMed] [Google Scholar]
- 100.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8(3):383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 101.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162(8):4511–4520. [PubMed] [Google Scholar]
- 102.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183(3):891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Macaulay AE, DeKruyff RH, Goodnow CC, Umetsu DT. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158(9):4171–4179. [PubMed] [Google Scholar]
- 104.Lindell DM, Berlin AA, Schaller MA, Lukacs NW. B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. PLoS One. 2008;3(9):e3129. doi: 10.1371/journal.pone.0003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39(6):440–456. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]
- 106.Gould HJ, Sutton BJ, Beavil AJ, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 107.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 108.Ando M, Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol. 2007;17(1):14–19. [PubMed] [Google Scholar]
- 109.Aral M. The Relationship Between Serum Levels of Total IgE, IL-18, IL-12. 2006;2006(4) doi: 10.1155/MI/2006/73098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trzeciak M, Glen J, Bandurski T, Sokolowska-Wojdylo M, Wilkowska A, Roszkiewicz J. Relationship between serum levels of interleukin-18, IgE and disease severity in patients with atopic dermatitis. Clin Exp Dermatol. 2011;36(7):728–732. doi: 10.1111/j.1365-2230.2011.04113.x. [DOI] [PubMed] [Google Scholar]
- 111.Enoksson SL, Grasset EK, Hagglof T, et al. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc Natl Acad Sci U S A. 2011;108(51):E1399–E1407. doi: 10.1073/pnas.1107830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin Immunol. 2005;114(1):17–26. doi: 10.1016/j.clim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 113.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc Natl Acad Sci U S A. 1997;94(8):3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ring J, Brockow K, Ollert M, Engst R. Antihistamines in urticaria. Clin Exp Allergy. 1999;29(Suppl 1):31–37. doi: 10.1046/j.1365-2222.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 115.Machura E, Szczepanska M, Mazur B, Barc-Czarnecka M, Kasperska-Zajac A. Interleukin 1-beta, interleukin-1 receptor antagonist, and interleukin 18 in children with acute spontaneous urticaria. Biomed Res Int. 2013;2013:605262. doi: 10.1155/2013/605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurt E, Aktas A, Aksu K, et al. Autologous serum skin test response in chronic spontaneous urticaria and respiratory diseases and its relationship with serum interleukin-18 level. Arch Dermatol Res. 2011;303(9):643–649. doi: 10.1007/s00403-011-1144-x. [DOI] [PubMed] [Google Scholar]
- 117.Rasool R, Ashiq I, Shera IA, Yousuf Q, Shah ZA. Study of serum interleukin (IL) 18 and IL-6 levels in relation with the clinical disease severity in chronic idiopathic urticaria patients of Kashmir (North India) Asia Pac Allergy. 2014;4(4):206–211. doi: 10.5415/apallergy.2014.4.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002;110(3):341–348. doi: 10.1067/mai.2002.126811. [DOI] [PubMed] [Google Scholar]
- 119.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170(7):3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 120.Brown RH, Hamilton RG, Mintz M, Jedlicka AE, Scott AL, Kleeberger SR. Genetic predisposition to latex allergy: role of interleukin 13 and interleukin 18. Anesthesiology. 2005;102(3):496–502. doi: 10.1097/00000542-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 121.Ming L, Wen Q, Qiao HL, Dong ZM. Interleukin-18 and IL18 and −607A/C and −137G/C gene polymorphisms in patients with penicillin allergy. J Int Med Res. 2011;39(2):388–398. doi: 10.1177/147323001103900206. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt RL, Lenz LL. Distinct licensing of IL-18 and IL-1beta secretion in response to NLRP3 inflammasome activation. PLoS One. 2012;7(9):e45186. doi: 10.1371/journal.pone.0045186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hitomi Y, Ebisawa M, Tomikawa M, et al. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124(4):779–785. e776. doi: 10.1016/j.jaci.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 124.Kruse S, Kuehr J, Moseler M, et al. Polymorphisms in the IL 18 gene are associated with specific sensitization to common allergens and allergic rhinitis. J Allergy Clin Immunol. 2003;111(1):117–122. doi: 10.1067/mai.2003.43. [DOI] [PubMed] [Google Scholar]
- 125.Lee HM, Park SA, Chung SW, et al. Interleukin-18/-607 gene polymorphism in allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2006;70(6):1085–1088. doi: 10.1016/j.ijporl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 126.Sebelova S, Izakovicova-Holla L, Stejskalova A, Schuller M, Znojil V, Vasku A. Interleukin-18 and its three gene polymorphisms relating to allergic rhinitis. J Hum Genet. 2007;52(2):152–158. doi: 10.1007/s10038-006-0093-2. [DOI] [PubMed] [Google Scholar]
- 127.Kurt E, Aksu K, Dokumacioglu A, Alatas O. Bronchial hyperresponsiveness in seasonal allergic rhinitis patients is associated with increased IL-18 during natural pollen exposure. Cytokine. 2012;60(1):100–103. doi: 10.1016/j.cyto.2012.06.240. [DOI] [PubMed] [Google Scholar]
- 128.Zhang M, Liu L. The role of IL-18 in allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2004;18(5):257–258. [PubMed] [Google Scholar]
- 129.Kim SW, Jeon YK, Won TB, et al. Effects of corticosteroids on expression of interleukin-18 in the airway mucosa of a mouse model of allergic rhinitis. Ann Otol Rhinol Laryngol. 2007;116(1):76–80. doi: 10.1177/000348940711600113. [DOI] [PubMed] [Google Scholar]
- 130.Wan H, Su H, Wu Y, Zhao Y, Zhou M. Expression and significance of NLRP3 inflammasome and its downstream factors IL-1beta/IL-18 in rat model of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;50(2):145–150. [PubMed] [Google Scholar]
- 131.Cheng D, Hao Y, Zhou W, Ma Y. The relationship between interleukin-18 polymorphisms and allergic disease: a meta-analysis. Biomed Res Int. 2014;2014:290687. doi: 10.1155/2014/290687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamanaka K, Tanaka M, Tsutsui H, et al. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J Immunol. 2000;165(2):997–1003. doi: 10.4049/jimmunol.165.2.997. [DOI] [PubMed] [Google Scholar]
- 133.Konishi H, Tsutsui H, Murakami T, et al. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proc Natl Acad Sci U S A. 992002:11340–11345. doi: 10.1073/pnas.152337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Terada M, Tsutsui H, Imai Y, et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci U S A. 2006;103(23):8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kou K, Aihara M, Matsunaga T, et al. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch Dermatol Res. 2012;304(4):305–312. doi: 10.1007/s00403-011-1198-9. [DOI] [PubMed] [Google Scholar]
- 136.Imboden M, Nicod L, Nieters A, et al. The common G-allele of interleukin-18 single-nucleotide polymorphism is a genetic risk factor for atopic asthma. The SAPALDIA Cohort Study. Clin Exp Allergy. 2006;36(2):211–218. doi: 10.1111/j.1365-2222.2006.02424.x. [DOI] [PubMed] [Google Scholar]
- 137.Tanaka H, Miyazaki N, Oashi K, et al. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol. 2001;107(2):331–336. doi: 10.1067/mai.2001.112275. [DOI] [PubMed] [Google Scholar]
- 138.Sawada M, Kawayama T, Imaoka H, et al. IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13. PLoS One. 2013;8(1):e54623. doi: 10.1371/journal.pone.0054623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sugawara I, Yamada H, Mizuno S, Takeda K, Akira S. Mycobacterial infection in MyD88-deficient mice. Microbiol Immunol. 2003;47(11):841–847. doi: 10.1111/j.1348-0421.2003.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 141.Brieland JK, Jackson C, Hurst S, et al. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect Immun. 2000;68(12):6567–6573. doi: 10.1128/iai.68.12.6567-6573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jackson DJ, Glanville N, Trujillo-Torralbo MB, et al. Interleukin-18 is associated with protection against rhinovirus-induced colds and asthma exacerbations. Clin Infect Dis. 2015;60(10):1528–1531. doi: 10.1093/cid/civ062. [DOI] [PubMed] [Google Scholar]
- 143.Yoshimoto T, Nakanishi K. Roles of IL-18 in basophils and mast cells. Allergol Int. 2006;55(2):105–113. doi: 10.2332/allergolint.55.105. [DOI] [PubMed] [Google Scholar]
- 144.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alagkiozidis I, Facciabene A, Tsiatas M, et al. Time-dependent cytotoxic drugs selectively cooperate with IL-18 for cancer chemo-immunotherapy. J Transl Med. 2011;9:77. doi: 10.1186/1479-5876-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]