Abstract
Post mortem studies suggest protracted myelination of subcortical white matter into the middle age followed by gradual decline in the late adulthood. To date, however, establishing the proposed inverted-U pattern of age-myelin association proved difficult, as the most common method of investigating white matter, diffusion tensor imaging (DTI), usually reveals only linear associations between DTI indices and age among healthy adults. Here we use a novel method of estimating Myelin Water Fraction (MWF) based on modeling the short spin-spin (T2) relaxation component from multi-echo T2 relaxation imaging data and assess subcortical myelin content within six white matter tracts in a sample of healthy adults (N=61, age 18–84 years). Myelin content evidenced a quadratic relationship with age, in accord with the pattern observed postmortem studies. In contrast, DTI-derived indices that are frequently cited as proxies for myelination, fractional anisotropy (FA) and radial diffusivity (RD), exhibited linear or null relationships with age. Furthermore, the magnitude of age differences in MWF varied across the white matter tracts. Myelin content estimated by MWF was unrelated to FA and correlated with RD only in the splenium. These findings are consistent with the notion that myelination continues throughout the young adulthood into the middle age. The results demonstrate that DTI cannot serve as a specific proxy for myelination of white matter tracts.
Keywords: Aging, Brain, White Matter, Multi-exponential T2 relaxation, Fractional Anisotropy
1. Introduction
Postmortem studies in humans and non-human primates have consistently demonstrated life-span differences in white matter structure, including significant regional variations in myelin content (Kaes, 1907; Yakovlev et al., 1966; Peters, 2002), and multiple alterations appearing in older adulthood, including gliosis, loss of myelin, decreased nodes of Ranvier density and deformation of myelin sheaths (Peters, 2002; Tang et al., 1997). These studies suggest progressive myelination continuing into the fourth decade of life, with cortical association regions exhibiting the starkest differences in myelin content between infancy and middle age (Yakovlev et al., 1966, Kaes, 1907). Furthermore, subcortical and intracortical myelination of sensory-motor brain regions appears to progress faster and decline slower than that of the association areas (Flechsig, 1901; Kaes, 1907).
The obvious limitations of post-mortem studies are impossibility of evaluating changes over time and assessing cognitive performance concurrently with the brain measurements. Therefore, there is a need for accurate, valid and safe methods that would allow gauging brain myelin content in vivo. Early studies of age differences in white matter volume suggested non-linear age trends (Bartzokis et al., 2001; Jernigan et al., 2001; Raz et al., 2005; but see Raz et al., 1997; 2004). Gross volume, however, is a very coarse index of white matter properties, as it reflects contributions of multiple components, and proliferation of some types of them, such as astroglia and microglia as well increased number of myelin sheaths and increased axon diameter may offset the loss of myelin.
The advent of diffusion tensor imaging (DTI, Basser et al., 1994) facilitated assessment of white matter macrostructure through examining the anisotropy of water diffusion in the brain tissue. As myelin constitutes a formidable barrier to diffusion of water molecules, it is plausible that degree of myelination and myelin content can be represented by DTI indices such as fractional anisotropy (FA) and radial diffusivity (RD). Indeed, in several studies, these DTI-derived indices have been linked to myelin content (Gulani et al., 2001; Song et al., 2003) and age differences in RD are frequently interpreted as evidence of changes in myelin content (e.g., Lebel et al., 2012). Other DTI-derived indices, such as mean diffusivity (MD, a trace of the diffusion tensor) and axial diffusivity (AD, the principal eigenvalue of the diffusion tensor) are usually not considered proxies for myelin (see Salat, 2014 for a review).
To date, life-span age differences in myelination have been inferred predominantly from DTI-based indices, especially RD, which has been interpreted as a marker of myelin integrity in the context of training-related white matter plasticity (Mackey et al., 2012), schizophrenia (Davis et al., 2003), and age-related cognitive decline (Davis et al., 2009). It is important to note, however, that signal from the very short (10–40 ms) component of the spin-spin (T2) relaxation of water molecules within myelin sheaths is undetected by most DTI studies with TE times that typically exceed 50ms. The reported validation of DTI indices vis a vis myelin is therefore not only limited to regions of uniform fiber directionality but is insensitive to diffusion of water between myelin sheaths. In recent years, there is a growing awareness that although the DTI-derived indices may be sensitive to myelin presence, these measures are unlikely to serve as specific indicators of myelin content or myelin sheath integrity (Jones et al., 2013).
Although multiple cross-sectional studies revealed significant negative associations between age and FA as well as positive associations between age and RD of the subcortical white matter (see Madden et al, 2012 for a review), in the extant literature, association of age with FA and RD has been described by various functional relationships. In adult life span studies FA evidenced linearly declining, flat or accelerating slope with age and RD showed flat or accelerated age differences (Michielse et al., 2010; Westlye et al., 2010; Hasan et al., 2009). The results of cross-sectional investigations of age differences in FA and RD are inconsistent and, when adults are concerned, do not conform to the patterns of protracted life-span myelination and regional differences suggested by the postmortem studies (e.g., Kaes, 1907). DTI-derived indices lack specificity because FA and RD (as well as AD and MD) reflect multiple structural and organizational properties of the white matter, including axon density and caliber, the intra- and extracellular space, and local geometry of crossing and kissing fibers (Beaulieu, 2002; Jeurissen et al., 2010; Jones et al., 2013; Vos et al., 2012). Moreover, whereas recent longitudinal studies showed significant differential changes in local FA and diffusivity components in a wide age range of healthy adults, the lack of neurobiological specificity of DTI-derived indices significantly constrains interpretation of these findings (Barrick et al., 2010; Sexton et al, 2014; Bender and Raz, 2015; Bender, Voelkle and Raz, 2016).
Several alternatives have been proposed to overcome the limitations of DTI-based methods in estimating myelin content. A promising method of myelin assessment is the multi-component driven equilibrium single-component observation of T1 and T2 (mcDESPOT, Deoni et al., 2008). This approach generates whole-brain maps of T1, T2, and myelin fraction by using a combination of spoiled gradient echo recalled (SPGR) and balanced-steady-state free precession (b-SSFP) sequences along with fitting a three-compartment model to the data (Deoni et al., 2013). While whole-brain acquisition with SPGR and b-SSFP sequences is relatively quick, mcDESPOT requires application of multiple flip angles for both sequences (Deoni et al., 2008), which prolongs acquisition times. Moreover, mcDESPOT may be sensitive to magnetization transfer effects (Bieri et al., 2006; Lenz et al., 2010), tends to over-estimate MWF (Deoni et al., 2008; Zhang et al., 2015) and is yet to be validated by quantitative comparison with direct histological measures of myelin (Deoni et al., 2015).
These limitations can be mitigated by a method that has been developed more than two decades ago (Mackay et al., 1994). This approach relies on the multi-exponential T2 decay modeling of multi-echo T2 relaxation imaging data, which directly estimates Myelin Water Fraction (MWF) and thus can provide valid estimates of myelin content. MWF imaging draws on well-known physical properties that determine behavior of water protons within myelin sheaths in magnetic field (Menon & Allen, 1991). Namely, for water trapped between the myelin sheaths the T2 relaxation time is approximately between 10 and 40 ms (short T2 component), whereas the T2 relaxation time of water associated with the intra- and extra-axonal spaces ranges between 60–70 ms (middle T2 component). These T2 relaxation components and their relative contribution to the total water signal can be estimated by modeling the multi-exponential decay as a distribution of T2 components (Mackay et al., 2006; Whittal et al., 1997). Notably, estimates of myelin content derived from MWF have been extensively validated and agree with histological measures of myelin obtained from optical density measurements with luxol fast blue staining (Laule et al., 2006; Laule et al., 2008). In addition, animal models of myelin degeneration (McCreary et al., 2009; Webb et al., 2003) demonstrate sensitivity and utility of MWF in monitoring demyelination and re-myelination. Collectively, these studies provide strong support for the use of MWF imaging for in vivo quantification of myelin content. Moreover, recent MRI sequence development has dramatically reduced the acquisition time (Prasloski et al. 2012), thus improving the feasibility of the histologically validated measures of myelin content in humans. To the best of our knowledge, this approach has been used in only one comprehensive study of white matter diffusion properties in 17–70 year old healthy adults (Billiet et al., 2015). That investigation revealed only a few linear as well as quadratic correlations between MWF and age in some regions, and none of these correlations survived a correction for multiple comparisons. Notably, diffusion-based indices in the same sample evidences curvilinear relationship with age in some regions (Billiet et al., 2015). In two other studies that used multi-echo T2 imaging sequences but were not designed to examine age differences, linear increase in MWF with age was observed (Flynn et al., 2003; Lang et al., 2014). Notably, in these two studies, sample age covered the range from early or late childhood to the middle age: 15–55 years (Flynn et al., 2003) and 5–40 (Lang et al., 2014). These studies suggest an increase in MWF values (and, by implication, myelination) into middle age. Thus, the question of age-related differences in regional myelin content requires further study, with a focus on comparison between putative proxies of brain myelin based on multi-T2 component analysis and DTI.
In this study, we had two main objectives. First, we wanted to characterize the age differences in myelin content within selected regions of subcortical white matter in a life-span sample of healthy adults. In accord with the post-mortem evidence, we hypothesized that across adult life span, age would be quadratically related to MWF and this relationship would vary across white matter tracts. Second, we compared age differences in MWF-based estimates with the most commonly reported DTI indices, FA and RD that are frequently taken as indicators of myelination (e.g., Kumar et al., 2014; Lebel et al., 2012; Madden et al., 2012; Song et al., 2003). To avoid adding multiple statistical tests and inflating Type I error, we did not include other DTI–derived indices (AD and MD) that are usually not viewed as proxies for myelin content or integrity. We hypothesized that DTI-based indices, in accord with the extant literature would exhibit linear associations with age. We expected that because of these differences in their associations with age, DTI indices would be unrelated to MWF estimates of myelin content and thus be deemed unsuitable proxies for myelin.
2. Methods
2.1 Participants
Participants were paid volunteers recruited from the Detroit metropolitan area. They were screened via a telephone interview and a mail-in questionnaire for history of neurological and psychiatric disorders, cardiovascular disease other than medically treated hypertension, metabolic and endocrine disorders, head injury accompanied by loss of consciousness for more than five minutes, use of antiepileptic, anxiolytic, and antidepressant medications. The participants were screened for cognitive impairment with the Mini Mental State Examination (Folstein et al., 1975; cutoff = 26) and for symptoms of depression with the Geriatric Depression Questionnaire (CES-D; Radloff, 1977; cutoff = 15).
The sample consisted of consequently recruited 61 healthy adults (59% women) between the ages of 18 to 84 years (mean age = 52.1 years, SD = 17.9 years) who were enrolled in a longitudinal study currently underway. Men and women did not differ in age, education, MMSE and CES-D scores but higher proportion of men had history of hypertension (see Table 1). Multi-echo T2 relaxation imaging data were collected on all participants; DTI data were missing for one, due to a technical error.
Table 1.
Sample Description
| Total | Women | Men | t or χ2 | p | |
|---|---|---|---|---|---|
| N | 61 | 36 | 25 | ||
| Age (years) | 52.1 ± 17.9 | 52.1 ± 18.6 | 52.0 ± 17.3 | 0.03 | 0.97 |
| Education (years) | 16.0 ± 2.2 | 15.7 ± 2.3 | 16.3 ± 11.9 | −1.07 | 0.30 |
| MMSE | 28.9 ± 0.9 | 29.1 ± 0.8 | 28.7 ± 1.1 | 1.92 | 0.06 |
| Persons with hypertension, Number (%) | 10 (16.4) | 3 (8.3) | 7 (28.0) | 4.16 | 0.04 |
| CES-D | 5.4 ± 5.1 | 6.0 ± 5.2 | 4.6 ± 4.8 | 1.1 | 0.3 |
2.2 MRI acquisition and processing
2.2.1 MRI acquisition protocol
Imaging was performed on a 3T MRI system (Siemens MAGNETOM VerioTM) with a 12-channel RF coil. The acquisition session was part of an ongoing longitudinal study and therefore included multiple MRI sequences, of which the structural T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE), DTI, and multi-echo T2 relaxation imaging were used in this study. Fluid-attenuated inversion recovery (FLAIR) sequence was used to screen for space-occupying lesions and clinically significant white matter abnormalities.
DTI images were acquired in the axial plane with a single-shot echo-planar sequence. The parameters were as follows: repetition time (TR) = 12000 ms, echo time (TE) = 124 ms, Generalized Autocalibrating Partial Parallel Acquisition (GRAPPA) acceleration factor 2, 20 diffusion directions, 2 averages, field of view (FOV) = 256×256 mm2, matrix size = 192×192, slice thickness = 2 mm, number of slices = 50, b = 1000 s/mm2, in-plane resolution = 1.3 × 1.3 mm2. The duration of the DTI sequence was 9 min. Multi-echo T2 relaxation images were acquired in the axial plane with a 3D-gradient and spin-echo (GRASE) sequence generously contributed by Dr. Jongho Lee (Seoul National University), which was based on the sequence developed by Prasloski and colleagues (Prasloski, et al., 2012). Acquisition parameters for the GRASE sequence were as follows: TR = 1100 ms, number of echoes = 32, first echo = 11 ms, inter-echo spacing = 11 ms, FOV = 190×220 mm2, matrix size = 165×192, slice thickness = 5 mm, number of slices = 24, in-plane resolution = 1.15 × 1.15 mm2. The duration of GRASE sequence was 16 min. A T1-weighted MPRAGE sequence was acquired with the following parameters: TE = 4.38 ms, TR =1,600 ms, inversion time (TI) = 800 ms, FOV = 256×256 mm2, voxel size = .67×.67×1.34 mm3, matrix size = 384×384, flip angle = 8°, and GRAPPA factor = 2. The duration of MPRAGE sequence was 5:41 min. To evaluate possible white matter lesions, FLAIR images were acquired with the following parameters: TR = 8,440 ms, TE = 112 ms, TI = 2,200 ms, flip angle = 150°, FOV = 256×256 mm2, voxel size= 1×1×2 mm3, matrix size = 256×256, 50 axial slices. The duration of FLAIR sequence was 3:49 min. All MPRAGE and FLAIR images were inspected for potential pathology and possible incidental findings were reviewed by an experienced radiologist at the scanning facility. No incidental findings were noted in this sample.
2.2.2 Image processing
The principles underlying the post-acquisition processing are illustrated in Figure 1. The 3D GRASE dataset was analyzed using a combination of FMRIB Software Library (FSLM, Jenkinson et al., 2012) and in-house MATLAB scripts to generate MWF images. GRASE images were interpolated to 2.5-mm thickness. The T1-weighted images were co-registered to the first-echo of the GRASE dataset using FSL FLIRT tool (six degrees of freedom) as well as to MNI152 T1 images using FSL FNIRT and the resultant transformation and warp-field maps were saved.
Figure 1.
A summary of the principles of Myelin Water Fraction imaging. The measured MRI signal originates from the myelin, intra-axonal, and extra-axonal (IE) water compartments (A). By decomposing the measured multi-exponential T2 decay signal into the components of T2 relaxation times, the short-T2 water signal from the myelin compartment can be quantified and its fraction in the total signal can be computed (B). Mean values for the short and intermediate components are indicated on the graph. Values on axes Y are in arbitrary units (AU).
Six regions of interest (ROI) were demarcated using the JHU white matter atlas, in template space and included two commissural tracts: genu of the corpus callosum (genu CC), and splenium of the corpus callosum (splenium CC); two association tracts: superior longitudinal fasciculus (SLF) and the inferior fronto-occipital-fasciculus (IFOF); and two projection tracts: anterior (ALIC), and posterior (PLIC) limbs of the internal capsule. These ROIs (illustrated in Figure 2) were selected based on theoretical and practical considerations. First, we strived to sample diverse white matter regions that vary in their onto- and phylogenetic timing properties and functional significance. Second, we took care to select the regions that would contain primarily white matter and would be least prone to partial voluming artifacts and to avoid relatively thin and narrow tracts such as the fornix. The ROIs were mapped back into subject space by inverting and applying the warp-field map generated by FNIRT. The subject-space ROI masks were then applied to the GRASE images using fslmaths .Thus, all analyses were conducted in subject space. All ROIs were examined for errors of misregistration and none were found.
Figure 2.
Regions of interest mapped from standard space onto subject space for myelin quantification. The same regions were used in the DTI analysis. ROIs in subject space for a representative participant are shown below, left to right: (A.) Genu of the corpus callosum (1), Anterior limb of the internal capsule (2), Posterior limb of the internal capsule (3) and Splenium of the corpus callosum (4). The middle axial slice (B.) Superior longitudinal fasciculus (5), and (C.) Inferior fronto-occipital fasciculus (6).
Voxel-wise multi- T2 component analysis was conducted for each ROI using a regularized non-negative least squares (rNNLS) algorithm (Whittal et al., 1997). Generalized cross validation was used to find the optimal regularization parameter (Golub et al., 1979). To account for non-ideal refocusing flip-angles the extended phase graph (EPG) algorithm was implemented as a part of the rNNLS fitting (Hennig, 1988; Prasloski et al., 2012). Distributions of T2 relaxation components were generated using 200 logarithmically spaced T2 values ranging from 10 to 2000 ms. MWF was calculated as the discrete integral for T2 relaxation times from 10 to 40 ms normalized to the discrete integral for T2 times from 10 to 2,000 ms. MWF values were averaged within each ROI. Typical MWF maps are displayed in Figure 3. DTI data were analyzed with FSL tools (Jenkinson et al., 2012). The FSL Eddy Current correction with the first b0 volume as a reference was applied. The b-vectors were rotated after motion correction, followed by application of FSL Brain Extraction Tool to generate a brain mask. The brain mask was used in the FSL DTIFIT tool generate FA images. RD images were generated by averaging the images of two planar components of the diffusion tensor, λ2 and λ3. To enable a comparison between MWF with FA and RD co-registration of the datasets was accomplished in two steps. First, from the GRASE data, the images with an echo-time = 121 ms were co-registered with the DTI b0 image because the latter were collected with a similar echo-time. The b0 image was co-registered to the chosen GRASE volume-using FLIRT (six-degrees of freedom) and the registration matrix was saved for the second step. Next, FA images were co-registered to the first echo from the GRASE dataset using FNIRT. The FLIRT matrix was used as starting point for the FNIRT step. Similar to the MWF analyses, the subject space ROIs were applied to FA and RD images, and mean indices were calculated for each ROI.
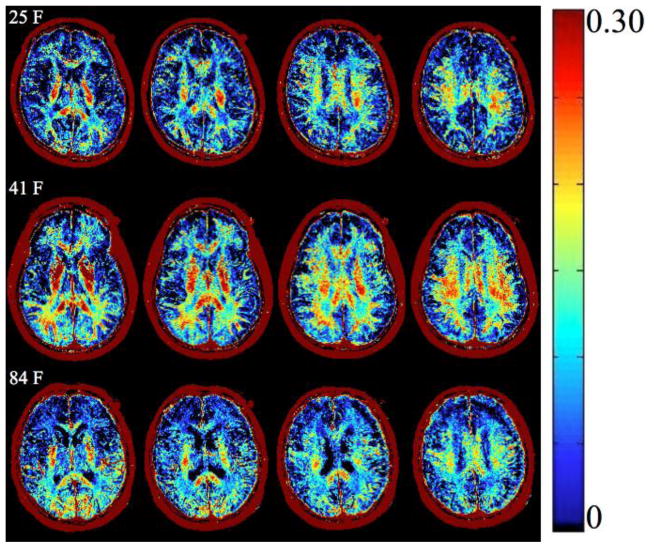
Figure 3.
Representative example of MWF maps of participants in early, mid, and late adulthood. This figure depicts myelin content expressed as MWF values (scale on the right) across the adult life span in three representative individuals. All MWF images, across persons, are from anatomically identical slices. Visual inspection reveals greater myelin content in middle age compared to early and late adulthood. Quantitative analyses are summarized in Table 2.
Lastly, T1-weighted images were segmented voxel-wise into white matter (WM), gray matter (GM) or cerebrospinal fluid (CSF), using FSL FAST module. To minimize partial volume artifacts only those voxels whose WM partial volume fraction was 0.95 or greater were included in the final binarized ROI masks. All ROIs were inspected for gross registration errors for the DTI and GRASE datasets.
2.2.3 Data Conditioning
To minimize rounding errors and avoid scaling artifacts in the analyses, MWF and FA values were multiplied by a factor of 100, and RD was multiplied by a factor of 10,000. To reduce the effect of outliers, all MRI measures were winsorized at the 90th percentile (values above the 95th percentile were set to the 95th percentile and values below the 5th percentile were set to the 5th percentile). Because the extant literature consistently reports no age-related lateral differences (Callaghan et al., 2014; Lebel et al., 2012; Yeatman et al., 2014), we had no reason to hypothesize such effects for any of the variables of interest. Therefore values from each hemispheric ROI (SLF, ILF, ALIC, PLIC) were averaged to yield single measures thus reducing the number of parameters in statistical models, conserving degrees of freedom, and reducing the potential number of post-hoc comparisons.
3. Statistical analysis
To assess age differences in myelin content (operationalized by MWF) and DTI indices across the ROIs we used the repeated measures general linear model (RM-GLM) framework. In each RM-GLM, MWF (or DTI) values were the dependent variable, with ROI being a six-level within-subject factor, sex as a between subject categorical factor and age, centered at the sample mean, and mean-centered aged squared (age2) as continuous independent variables. Within-subject interactions between sex and age and sex with age2 were also included in the model and removed if found nonsignificant (p > 0.05). Reduced models were then re-evaluated without the nonsignificant interaction term. Significant (p < 0.05) interactions were decomposed using post-hoc simple effects analysis, i.e., regressions for each ROI separately. To mitigate violation of sphericity assumption for the RM-GLM, all p-values for within-subject factors were adjusted using the Huynh-Feldt correction. In addition, with six possible regressions in the post-hoc analysis, the nominal α level of 0.05 was adjusted to α’=.008, using Bonferroni correction. For each ROI, in the post-hoc analysis, only those effects, which were significant in the within-subjects analysis, were included (ex: age2 term). For each ROI 95% confidence intervals for the slope of the age2 term were generated using 5000 bootstrapped samples; bias corrected and accelerated confidence intervals reported.
Associations between age and DTI-derived indices were evaluated in the RM-GLM, as used for MWF measures. Bivariate correlations between MWF with FA and RD within each ROI were computed and their significance was adjusted family-wise from nominal α= .05 to α’=. 008, using the Bonferroni correction. All statistical analyses were performed using SPSS Statistics (IBM Corp. IBM Statistics for Mac, Version 21.0).
4. Results
4.1 Age-related regional differences in MWF
After discarding nonsignificant within-subjects interactions ROI × age × sex and ROI × age2 × sex (F<1 for both), and between-subjects interactions age × sex [F(1,55) < 1] and age2 × sex [F(1,55) = 1.233, p = .27], a reduced model was fitted to the data. The results of that analysis revealed significant main effects of sex [F(1,57) = 5.651, p = .021] and age2 [F(1,57) = 16.521, p < 0.001]. Women had higher MWF than the men did, mean ±SE: 14.3 ±.3 % vs 13.3± .3%. The main effect of age was not significant (F<1). The ROI × sex interaction [(F(5,285) = 1.255, p = .29] and ROI × age [F(5,285) = 1.722, p = .42] interaction were not significant. However, the ROI × age2 interaction was significant [F(5,285) = 4.726, p = .0010], indicating that the magnitude of the quadratic effect of age differed across ROIs.
Decomposition of the ROI × age2 interaction revealed a significant effect of age2 for the SLF [F(1,59) = 16.385, p < .001], ALIC [F(1,59) = 22.44, p < .001], PLIC [F(1,59) = 14.83, p < .001], IFOF [F(1,59) = 7.659, p =.0080] and the splenium [F(1,59) = 8.118, p =.0060]. For the genu it was significant only at the unadjusted α [F(1,59) = 5.228, p = .026] (see Table 2 and Figure 4).
Table 2.
A summary of post-hoc analyses of associations between the MWF and age2 for each ROI with bootstrapped confidence intervals.
| ROI | R2 | p | b (MWF/yr2) | 95% CI |
|---|---|---|---|---|
| ALIC | .28 ** | < .001 | −.005 | −.006, −.003 |
| PLIC | .21 ** | < .001 | −.003 | −.004, −.001 |
| Genu CC | .08 * | .026 | −.001 | −.003, .000 |
| Splenium CC | .12 ** | .006 | −.002 | −.004, −.001 |
| SLF | .22 ** | < .001 | −.003 | −.004, −.002 |
| IFOF | .12 ** | .008 | −.002 | −.004, −.001 |
Note: MWF – myelin water fraction , percent. Only age2 was included in the post-hoc models as this was the only term that was significant in the RM-GLM.
significant at the unadjusted α = .05 level;
significant at Bonferroni adjusted α’ = .008 level
Figure 4.
Associations between MWF and age for each ROI. 95% confidence limits (dashed line) and prediction limits (dot-dot-dash line) are drawn around the regression line. Plotted on the same scale, the regional differences in myelin content are apparent.
4.3 Age-related regional differences in DTI-derived indices
4.3.1 Fractional Anisotropy
After discarding nonsignificant within-subjects interactions ROI × age × sex [F(5,270) = 1.083, p = .36] and ROI × age2 × sex [F(5,270) = 1.365, p = .25] and between-subjects interactions age × sex (F<1) and age2 × sex [F(1,54) = 3.603, p = .063], a reduced model was fitted to the FA data. The analysis revealed nonsignificant main effects of sex and age2 (F<1 for both). The ROI × sex [F(5,280) = 1.832, p = .12] and ROI × age2 [F(5,280) = 1.916, p = .11] interactions were not significant, but the ROI × age interaction was: F(5,280) = 6.297, p < .001. After dropping the age2 term the model was re-evaluated. The main effect of sex (F<1) and the ROI × sex interaction [F(5,285) = 1.901, p = .11] were not significant. The main effect of age was significant [F(1,57) = 14.489, p < .0010], but it was qualified by a significant ROI × age interaction [F(5,285) = 5.565, p < .0010]. The interaction indicated that the linear effect of age on FA varied across ROIs. Post-hoc analysis revealed significant linear effects of age for the genu [F(1,58) = 18.091, p <.0010], splenium [F(1,58) = 9.125, p = .0040] and the IFOF [F(1,58) = 35.372, p < .0010]. All other ROIs either did not reach significance at the Bonferroni-adjusted level (see Table 3 and Figure 5).
Table 3.
A summary of post-hoc analyses of associations between the DTI indices and age for each ROI.
| FA | RD | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ROI | R2 | p | b (1/yr) | 95% CI | R2 | p | b(mm2/s* yr) | 95% CI- |
| ALIC | .10* | .022 | −.065 | −.121, −.009 | .05 | .074 | .006 | .001, .013 |
| PLIC | .00 | .668 | −.012 | −.062, .040 | .00 | .780 | .001 | −.004, .006 |
| Genu | .24** | < .001 | −.109 | −.163, −.052 | .23** | < .001 | .016 | .010, .023 |
| Splenium | .14** | .004 | −.073 | −.119, −.026 | .18** | .001 | .008 | .004, .013 |
| SLF | .01 | .420 | −.016 | −.056, −.026 | .02 | .374 | .002 | −.003, .007 |
| IFOF | .40** | < .001 | −.116 | −.149, −.082 | .28** | < .001 | .014 | .010, .018 |
Note: Only age was included in the post-hoc models as this was the only term that was significant in the RM-GLM.
Significant at the unadjusted α = .05 level;
significant at Bonferroni adjusted α’ = .008 level.
Abbreviations: ALIC-anterior limb of the internal capsule; PLIC – posterior limb of the internal capsule; SLF – superior longitudinal fasciculus; IFOF – inferior fronto-occipital fasciculus.
Figure 5.
Association between Fractional Anisotropy (FA) and age for each ROI. 95% confidence limits (dashed line) and prediction limits (dot-dot-dash line) are drawn around the regression line. Note the regional differences in FA.
4.3.3 Radial Diffusivity
After discarding nonsignificant within-subjects interactions ROI × age × sex (F<1) and ROI × age2 × sex [F(5,270) = 1.279, p = .28], respectively, and between-subjects interactions age × sex (F<1) and age2 × sex [F(1,54) = 1.648, p = .21], a reduced model was fitted to the data. The analysis revealed nonsignificant main effects of sex (F<1) and age2 [F(1,56) = 2.508, p = .12]. The ROI × sex [F(5,280) = 2.114, p = .080] and ROI × age2 [F(5,280) = 1.901, p = .11] interactions were not significant. After dropping the age2 term the model was re-evaluated. The main effect of sex (F<1) and the ROI × sex interaction [F(5,285) = 2.3, p = .069] were not significant. The main effect of age was significant [F(1,57) = 14.451, p < 0.0010]. This effect, however, was qualified by the ROI × age interaction: F(5,285) = 14.541, p < 0.001. Post-hoc analysis revealed significant effects of age for the genu [F(1,58) = 23.344, p < .001], splenium [F(1,58) = 12.426, p = .001], and the IFOF [F(1,58) = 32.810, p < .001]. All other ROIs either did not reach significance at the Bonferroni level (see Table 3 and Figure 6).
Figure 6.
Association between Radial Diffusivity (RD) and age for each ROI. 95% confidence limits (dashed line) and prediction limits (dot-dot-dash line) are drawn around the regression line. Plotted on the same scale to illustrate the regional differences in RD values.
4.4 Correlations between MWF and DTI indices across ROIs
Correlations between MWF and FA and between MWF and RD, within each ROI, did not pass the Bonferroni adjusted significance. The correlation between RD and MWF was significant only for the splenium: r = .346, F(1,58) = 8.201, p = .006, whereas for PLIC and the SLF they were significant at the unadjusted significance level of α= .05 (See Table 4 and Figures 7 and 8).
Table 4.
Associations between DTI indices and MWF for each ROI: Summary statistics.
| ROI | FA | RD | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R2 | p | b(MWF) | 95% CI | R2 | p | b(MWF* s/mm2) | 95% CI | |
| ALIC | .03 | .182 | .132 | −.055, .286 | .04 | .119 | −1.185 | −2.52, .345 |
| PLIC | .03 | .178 | .083 | −.043, .193 | .08* | .029 | −1.314 | −2.386, −.014 |
| Genu | .01 | .559 | .031 | −.073, .126 | .01 | .463 | −.282 | −.965, .461 |
| Splenium | .04 | .114 | .122 | −.041, .264 | .12** | .006 | −2.06 | −3.448, −.468 |
| SLF | .02 | .249 | .109 | −.082, .314 | .09* | .018 | −1.9 | −3.407, −.359 |
| IFOF | .00 | .930 | .008 | −.167, .186 | .01 | .392 | −.568 | −1.886, .749 |
ROIs are significant at the unadjusted α = .05 level;
ROIs are significant at the adjusted α’ = .008 level.
Abbreviations: ALIC-anterior limb of the internal capsule; PLIC – posterior limb of the internal capsule; SLF – superior longitudinal fasciculus; IFOF – inferior fronto-occipital fasciculus.
Figure 7.
Association between Myelin Water Fraction (MWF) and Fractional Anisotropy (FA) for each ROI. 95% confidence limits (dashed line) and prediction limits (dot-dot-dash line) are drawn around the regression line.
Figure 8.
Association between Myelin Water Fraction (MWF) and Radial Diffusivity (RD) for each ROI. 95% confidence limits (dashed line) and prediction limits (dot-dot-dash line) are drawn around the regression line.
5. Discussion
5.1 Heterochronic associations between age and myelin content
The main finding in this study is the in vivo demonstration that age differences in myelin content of subcortical white matter tracts conform to the parabolic (inverted U) relationship described in postmortem literature. The results suggest that across examined regions, peak myelin content is found around the fourth-sixth decade of life. The observed positive linear association between age and MWF up to the middle age is in agreement with the reports on samples that included only that part of the adult life span (Flynn et al., 2003; Lang et al., 2014).
We observed no consistent U-shaped age curves for the DTI indices (FA and RD), but even in the studies that report such non-linear relationships, this estimated peak age of myelination derived from DTI indices appears to be driven by inclusion of young children and adolescents (Lebel et al., 2012; Westlye et al., 2010). Peak age of myelination is estimated in these studies at much earlier decades, e.g., 24–33 years of age (Westlye et al., 2010) or 32–39 years (Kochunov et al., 2012), depending on selection of a white matter region. These estimates suggest earlier peak ages than is apparent from Kaes (1907) postmortem data that shows extensive myelin staining in the brains harvested from cadavers of in the 6th decade of age. This discrepancy may reflect differences in development of multiple components and properties of the white matter that are expressed in FA and RD, with myelin contribution being relatively minor. Later ages of peak myelination estimated here may be in a closer correspondence to those estimated from Kaes (1907) myelin staining study, as they reflect virtually exclusive contribution of myelin. Notably, in agreement with a previous report (Billet et al., 2015), we observed only weak if any associations between DTI-derived indices and MWF . Thus, it is unlikely that individual differences in myelin content contributed significantly to variation in FA or RD.
Quadratic effects of age were observed for all ROIs, similar to a recent study, which used R1 (1/T1) as a proxy for myelin content (Yeatman et al., 2014). Furthermore, we found that the magnitude of age differences in myelin content varied across white matter tracts. Projection fibers (ALIC, PLIC) and the SLF (association fiber) evidenced the largest quadratic age effects, with IFOF showing weaker relation to age and the weakest association with age2 was observed in the genu of corpus callosum. This pattern of results is inconsistent with the “first-in-last-out” hypothesis, which posits that white matter tracts connecting the late-to-myelinate association areas would show the greatest effects of age, whereas for the tracts originating or ending in primary sensory-motor cortices, the associations with age would be the weakest.
We also found that women had greater myelin content than men did: Cohen’s d = .62. Although in vivo literature on sex differences in white matter properties is very inconsistent (Salat, 2014), this finding is consistent with the reported higher levels of myelin associated proteins in female rodents (Bayless & Daniel, 2015) and may reflect greater g-ratio in men compared to women (Paus, et al., 2009). The g-ratio, the fraction of the axon radius within its membrane in the its radius including the myelin sheath, is a dimensionless index bracketed between 0 and 1, with values closer to 1 indicating thinner myelin sheathe relative to the axonal radius. Recent study suggested that g-ratio might be sensitive to sex-specific developmental factors such as androgens, which can contribute to expansion of axonal caliber, and ensuing increase of g-ratios (Pesaresi et al., 2015). In our study, direct ascertainment of this hypothesis, however, was not possible due to the absence of relevant data. In addition, higher proportion of men had diagnosis of hypertension and thus it is unclear whether disproportionally higher vascular risk among men has contributed to the observed sex difference. Prior reports on the independent samples drawn from the same population indicated that hypertension was associated with less favorable status on multiple indicators of white matter (Kennedy & Raz, 2009; Burgmans et al, 2010). In this sample, the frequency of hypertension was too low to warrant its inclusion as a covariate.
The biological mechanisms of age-related decline in myelin content remain unclear. Assuming a dynamic equilibrium between myelin production and loss (Peters, 2009), and observing the continuing myelination into the fourth-fifth decade of life, it is plausible that during that period, the myelin generating processes overtake their counterparts that drive myelin attrition. It is plausible to hypothesize that this dynamic equilibrium shifts over time and the decline in myelin content is driven by decreased myelin synthesis, increased myelin degeneration or some combination of the two. Although the mechanisms for such shift in homeostasis are unknown, it is possible that progressive decline in myelin content is just another expression of age-related energy crisis. Synthesis of myelin and maintenance of the oligodendrocyte resting potential is energetically costly (Harris et al., 2012). Aging is associated with decline in mitochondrial respiration (Bratic et al., 2010), decreased glucose and oxygen consumption, and decreased cerebral blood flow (Aanerud et al., 2012; Lin et al., 2014) as well as poor vascular health (Mozaffarian et al., 2015). These age-related factors would be expected to limit myelin synthesis given its energetic costs. Additionally, age related reduction in the number of myelinated axons (Marner et al., 2003; Meier-Ruge et al., 1992; Peters, 2002; Tang et al., 1997) would also contribute to decreased myelin content. Future investigations should assess age differences in both anabolic and catabolic aspects of myelination in conjunction with investigations of age differences in brain energetics, vascular risk and axonal loss.
5.2 Heterochronic age differences in DTI indices: Unrelated to myelin content
In line with previous studies, we found linear associations between age and DTI indices, with the magnitude of association varying across the examined ROIs. We observed a negative linear association between FA and age in the genu and splenium of the corpus callosum, IFOF, and the ALIC. Positive linear associations between RD and age were noted in the genu, splenium and the IFOF. These relationships are in a stark contrast with consistent quadratic effects of age on MWF. Thus, age-related differences in FA or RD that are frequently reported in cross-sectional age-heterogeneous studies should not be interpreted as evidence of age differences in myelin content.
We observed no significant associations between MWF and FA, and MWF was significantly associated with RD only in ROIs containing larger diameter axons such as in the PLIC and Splenium, in accord with previously published work (Mädler et al., 2008). This finding is also consistent with studies demonstrating that the axonal plasma membrane, fiber density and crossing fibers, contribute to diffusion anisotropy and radial diffusivity, thus complicating the biological interpretation of DTI-derived indices (Beaulieu, 2002; Jones et al., 2013; Vos et al., 2012). Therefore, future studies should not interpret changes in diffusion metrics as reflecting myelin changes.
5.3 Limitations
The results of this study should be interpreted within the constraints of several limitations. First and foremost, the cross-sectional design precludes inferring age-related change from observed age differences in myelin content (Baltes and Nesselroade, 1979; Kramer et al., 2000; Lindenberger et al., 2011). Longitudinal and cross-sectional findings in investigation of brain aging are not always in agreement with respect to the mean change (e.g., Raz et al., 2005; Bender and Raz, 2015; Daugherty et al., 2015) and cannot address an important question of individual differences in trajectories of aging. Thus, longitudinal studies are needed to establish whether the nonlinear age-myelin content relationships that we observed indeed hold and whether common age-related risk factors affect their parameters. Moreover, because of its lack of temporal component, cross-sectional design precludes testing hypotheses and reconciling various theoretical accounts of the observed patter of age differences, such as “first-in-last-out” (Raz et al., 1997) and “gain-predicts-loss” (Yeatman et al., 2014). We hope to accomplish this goal in a longitudinal study currently underway.
Second, we used an ROI based analysis, which would not reveal myelin differences within a totality of any white matter tract. The parameters of our DTI images were not optimal for tractographic analyses and we opted for ROI approach in both MWF and DTI. If the resolution of MWF imaging could be further increased, future studies could combine tractography with MWF to investigate differences in myelin content along the tracts. In addition, it should be noted that the differential quadratic effects of age that we observe maybe the result of differential reliability across the ROIs investigated. Assessment of reliability of MWF estimates across white matter regions is currently underway in our laboratory.
Third, although we sampled typical regions of interest that represent three major types of white matter tracts, it is possible that other regions would deviate from the inverted-U pattern of age differences that was consistently observed here. In particular, those may be smaller regions of the myelinated pathways evaluation of which was not feasible with the current 5-mm slice thickness.
Fourth, the shape of age-MWF relationship has been approximated by a quadratic curve. The symmetry of myelination at the younger age and loss of myelin content at the end of adult life span engendered by such regression fit does not necessarily reflect reality. Other, higher order curves could have been fitted to the data if more data points, including observations in young children, were available.
Fifth, it is important to state that the long component is included in the modeling of T2 relaxation. However, according to our analyses, it happens to have negligible contribution, and we can conclude that it contributes no new water signal. Nonetheless, it is indeed the case that MWF is a proportion of the entire T2 spectrum and the concern is that changes in other compartments would change the MWF value. A decrease in MWF is possible if there was an additional water compartment that increased the overall water content. Such a scenario observed in pathological conditions such as multiple sclerosis (Laule et al. 2007a) and phenylketonuria (Laule et al. 2007b) is unlikely in our case as we screened our subjects for white matter abnormalities using FLAIR images.
We have conducted a reliability study for MWF (Arshad, Stanley, Lee & Raz, in preparation), in which we found no differences in reliability among the tracts investigated.
6. Conclusions
Using a novel myelin-specific imaging method, we observed quadratic associations between age and myelin content across all six examined ROIs, in accord with post-mortem studies. Regional differences in myelin content, as expected from postmortem studies, ranged from the largest values in the PLIC, ALIC and splenium to the smallest in the genu. In contrast to MWF, we observed linear associations between DTI indices of white matter macrostructure and age, which also varied in strength across ROIs. We found no correlations between myelin content and FA in any of the examined ROIs, while RD correlated with MWF only in the splenium. Thus, whereas DTI can provide important information about the state of the white matter, commonly used DTI-based descriptors of the white matter diffusion properties do not specifically reflect myelin content and are not suitable for examining age differences therein.
Acknowledgments
Funding
This work was supported by a grant from the National Institute on Aging [R37-AG011230] to Naftali Raz.
The authors thank Dr. Jongho Lee, Seoul National University, for providing the 3D GRASE sequence and Dr. Alex MacKay, University of British Columbia, for providing the MATLAB code for implementing the regularized NNLS and extended phase analysis.
Footnotes
Disclosure statement
The authors of this publication have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jonsdottir KY, et al. Brain energy metabolism and blood flow differences in healthy aging. Journal of Cerebral Blood Flow & Metabolism. 2012;32(7):1177–1187. doi: 10.1038/jcbfm.2012.18. Epub 2012 Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Nesselroade JR. History and rationale of longitudinal research. In: Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. New York: Academic Press; 1979. pp. 1–39. [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51(2):565–577. doi: 10.1016/j.neuroimage.2010.02.033. Epub 2010 Feb 21. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Po HLu, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. JAMA Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Daniel JM. Sex differences in myelin-associated protein levels within and density of projections between the orbital frontal cortex and dorsal striatum of adult rats: implications for inhibitory control. Neuroscience. 2015;300:286–296. doi: 10.1016/j.neuroscience.2015.05.029. Epub 2015 May 19. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basics of anisotropic water diffusion in the nervous system-a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N. Normal-appearing cerebral white matter in healthy adults: mean change over 2 years and individual differences in change. Neurobiology of Aging. 2015;36(5):1834–1848. doi: 10.1016/j.neurobiolaging.2015.02.001. Epub 2015 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Völkle MC, Raz N. Differential aging of cerebral white matter in middle-aged and older adults: A seven-year followup. Neuroimage. 2016;125:74–83. doi: 10.1016/j.neuroimage.2015.10.030. Epub 2015 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri O, Scheffler K. On the origin of apparent low tissue signals in balanced SSFP. Magnetic Resonance in Medicine. 2006;56(5):1067–1074. doi: 10.1002/mrm.21056. [DOI] [PubMed] [Google Scholar]
- Billiet T, Vandenbulcke M, Mädler B, Peeters R, Dhollander T, Zhang H, Deprez S, Van den Bergh BR, Sunaert S, Emsell L. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging. 2015;36(6):2107–21. doi: 10.1016/j.neurobiolaging.2015.02.029. Epub 2015 Mar 7. [DOI] [PubMed] [Google Scholar]
- Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochimica et Biophysica ACTA-Bioenergetics. 2010;1797(6–7):961–967. doi: 10.1016/j.bbabio.2010.01.004. Epub 2010 Jan 11. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MPJ, Gronenschild EHBM, Vuurman EFPM, Hofman P, Uylings HBM, Jolles J, Raz N. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage. 2010;49(3):2083–2093. doi: 10.1016/j.neuroimage.2009.10.035. Epub 2009 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R, et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiology of Aging. 2014;35(8):1862–1872. doi: 10.1016/j.neurobiolaging.2014.02.008. Epub 2014 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Haacke EM, Raz N. Striatal iron content predicts its shrinkage and changes in verbral working memory after two years in healthy adults. Journal of Neuroscience. 2015;35(17):6731–6743. doi: 10.1523/jneurosci.4717-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Archives of General Psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46(2):530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Rutt BK, Arun T, Pierpaoli, Jones DK. Gleaning multicomponent T1and T2 information from steady-state imaging data. Magnetic Resonance in Medicine. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, Matthews L, Kolind SH. One component? Two components? Three? The effect of including a non-exchanging free water component in multicomponent driven equilibrium single pulse observation of T1 & T2 (mcDESPOT) Magnetic Resonance in Medicine. 2013;70(1):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Dean DCD, Remer J, Holly D, O’Muircheartaigh J. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 2015;115:147–161. doi: 10.1016/j.neuroimage.2015.04.058. Epub 2015 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. Epub 2013 Dec 28. [DOI] [PubMed] [Google Scholar]
- Fjaer S, Bo L, Myhr KM, Torkildsen O, Wergeland S. Magnetization transfer ratio does not correlate to myelin content in the brain in the MOG-EAE mouse model. Neurochemistry International. 2015;83–84:28–40. doi: 10.1016/j.neuint.2015.02.006. Epub 2015 Mar 2. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental myelogenetic localization of the cerebral cortex in the human subject. Lancet. 1901:1027–1029. [Google Scholar]
- Flynn SW, Lang DJ, MacKay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Molecular Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golub GH, Heath M, Wahba G. Generalized cross validation as a method for choosing a good ridge parameter. Technometrics. 1979;21(2):215–223. [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magnetic Resonance in Medicine. 2001;45(2):191–195. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The energetics of central nervous system white matter. Journal of Neuroscience. 2012;32(1):356–371. doi: 10.1523/jneurosci.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. Epub 2008 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR in Biomedicine. 2001;14(2):57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- Hennig J. Multiecho imaging sequences with low refocusing flip angles. Journal of Magnetic Resonance. 1988;78(3):397–407. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. Epub 2011 Sep 16. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping. 2013;34(11):2747–2766. doi: 10.1002./hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and dont’s of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. Epub 2012 Jul 23. [DOI] [PubMed] [Google Scholar]
- Kaes T. Ein Gehirnanatomischer Atlas. Jena: Gustav Fischer; 1907. Die Grosshirnrinde des Menschen in ihren Massen und in ihrem Fasergehalt. [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Research. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. Epub 2009 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33(1):9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. Epub 2010 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pham TT, Macey PM, Woo MA, Yan-Go FL, Harper RM. Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep. 2014;37(4):723–732. doi: 10.5665/sleep.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DJM, Yip E, MacKay AL, Thornton AE, Rodriguez FV, MacEwan GW, Kopala LC, Smith GN, Laule C, MacRae CB, Honer WG. (2014) Neuroimage Clinical. 2014;6:408–414. doi: 10.1016/j.nicl.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C, Leung E, Li DKB, Traboulsee AL, Paty DW, MacKay AL, Moore GRW. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Multiple Sclerosis. 2006;12(6):747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Laule C, Kozlowski P, Leung E, Li DKB, MacKay AL, Moore GRW. Myelin water imaging of multiple sclerosis at 7T: Correlations with histopathology. Neuroimage. 2008;40(4):1575–1580. doi: 10.1016/j.neuroimage.2007.12.008. Epub 2008 Mar 5. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicoli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. Epub 2011 Dec 8. [DOI] [PubMed] [Google Scholar]
- Lenz C, Klarhofer M, Scheffler K. Limitations of rapid myelin water quantification using 3D bSSFP. MAGMA. 2010;23(3):139–151. doi: 10.1007/s10334-010-0211-1. Epub 2010 Apr 28. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychological Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lin AL, Rothman DL. Review: What have novel imaging techniques revealed about metabolism in the aging brain? Future Neurology. 2014;9(3):341–354. doi: 10.2217/fnl.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay AL, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine. 1994;31(6):673–677. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- MacKay AL, Laule C, Vavasour I, Bjarnason T, Shannon K, Mädler B. Insights into brain microstructure from the T2 distribution. Magnetic Resonance Imaging. 2006;24(4):515–525. doi: 10.1016/j.mri.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Frontiers in Neuroanatomy. 2012;6:1–9. doi: 10.3389/fnana.2012.00032. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. Epub 2011 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mädler B, Drabycz SA, Kolind SH, Whittal KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magnetic Resonance Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. Epub 2008 Jun 4. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comparitive Neurology. 2003;462(2):144–162. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- McCreary CR, Bjarnason TA, Skihar V, Mitchell JR, Yong VW, Dunn JF. Multiexponential T2 and magnetization transfer MRI of demyelination and remyelination in murine spinal cord. 2009;45(4):1173–1182. doi: 10.1016/j.neuroimage.2008.12.071. Epub 2009 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ruge A, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of NY Academy Science. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Menon RS, Allen PS. Application of continuous relaxation time distributions to the fitting of data from model systems and excised tissue. Magnetic Resonance in Medicine. 1991;20(2):214–227. doi: 10.1002/mrm.1910200205. [DOI] [PubMed] [Google Scholar]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52(4):1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. Epub 2010 May 17. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. Epub 2014 Dec 17. [DOI] [PubMed] [Google Scholar]
- Paus T, Toro R. Could sex differences in white matter be explained by g ratio? Frontiers in Neuroanatomy. 2009;3:1–7. doi: 10.3389/neuro.05.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Axon diameter and axonal transport: In vivo and in vitro effects of androgen. Neuroimage. 2015;115:191–201. doi: 10.1016/j.neuroimage.2015.04.048. Epub 2015 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytology. 2002;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Frontiers in Neuroanatomy. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasloski T, Rauscher A, MacKay AL, Hodgson M, Vavasour IM, Laule C, Mädler B. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage. 2012;63(1):533–539. doi: 10.1016/j.neuroimage.2012.06.064. Epub 2012 Jul 6. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain JM, Briggs SD, Thornton AE, Loken WJ, Acker JD. Selective aging of human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Rodrigue K, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging. 2004;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Salat DH. Diffusion Tensor Imaging in the study of aging and age-associated neural disease. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: From Quantitative Measurement to in-vivo Neuroanatomy. 2014. pp. 257–281. [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. Accelerated changes in white matter microstructure during aging: A longitudinal diffusion tensor imaging study. Journal of Neuroscience. 2014;34(46):15425–15436. doi: 10.1523/jneurosci.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJG. Age-induced white matter changes in the human brain: A stereological investigation. Neurobiology of Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Vavasour IM, Laule C, Li DKB, Traboulsee AL, MacKay AL. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis. Journal Magnetic Resonance Imaging. 2011;33(3):713–718. doi: 10.1002/jmri.22441. Epub 2011 Feb 1. [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage. 2012;59(3):2208–2216. doi: 10.1016/j.neuroimage.2011.09.086. Epub 2011 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Munro CA, Midha R, Stanisz GJ. Is multicomponent T2 a good measure of myelin content in periphernal nerve? Magnetic Resonance in Medicine. 2003;49(4):638–645. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DKB, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine. 1997;37(1):34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain Early in Life. Blackwell Scientific Publications Inc; Oxford, UK: 1966. pp. 3–70. [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nature Communications. 2014;5:4932. doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kolind SH, Laule C, MacKay AL. Comparison of myelin water fraction from multiecho T2 decay curve and steady-state methods. Magnetic Resonance in Medicine. 2015;73(1):223–232. doi: 10.1002/mrm.25125. Epub 2014 Feb 11. [DOI] [PubMed] [Google Scholar]