Abstract
In eukaryotic cells, cellular homeostasis requires that different organelles respond to intracellular as well as environmental signals and modulate their behavior as conditions demand. Understanding the molecular mechanisms required for these changes remains an outstanding goal. On such organelle is the lysosome/vacuole, which undergoes alterations in size and number in response to environmental and physiological stimuli. Changes in the morphology of this organelle are mediated in part by the equilibrium between fusion and fission processes. While the fusion of the yeast vacuole has been studied intensively, the regulation of vacuolar fission remains poorly characterized by comparison. In recent years, a number of studies have incorporated genome-wide visual screens and high-throughput microscopy to identify factors required for vacuolar fission in response to diverse cellular insults, including hyperosmotic and endoplasmic reticulum (ER) stress. Available evidence now demonstrates that the rapamycin-sensitive TOR network, a master regulator of cell growth, is required for vacuolar fragmentation in response to stress. Importantly, many of the genes identified in these studies provide new insights into potential links between the vacuolar fission machinery and TOR signaling. Together these advances both extend our understanding of the regulation of vacuolar fragmentation in yeast as well as underscore the role of analogous events in mammalian cells.
Keywords: TORC1, ER Stress, Vacuolar Fission
Introduction
Eukaryotic cells are defined by the presence of distinct membrane-bound organelles that carry out specialized cellular functions. Proper homeostasis requires that these organelles respond to diverse metabolic and stress-related signals to modulate their behavior and maintain proper cellular function. Understanding these responses and the mechanisms involved is thus important for a full description of the behavior of normal cells as well as for an understanding of the basis of different human diseases. In this regard, studies in model organisms continue to play an indispensible role in the advance of our understanding of these important events within cells.
In budding yeast, Saccharomyces cerevisiae, the vacuolar compartment is analogous to the mammalian lysosome and is the degradative compartment of the cell, specifically as the site of proteolysis as well as micro- and macroautophagy. As such, the vacuole/lysosome functions in processes critical to maintenance of cellular homeostasis, including detoxification of the cytoplasm, maintenance of cellular pH, response to osmotic shock and nutrient depravation, and storage of ions and metabolites (Li and Kane 2009). Vacuolar morphology is intimately linked to its function as the size and volume of this dynamic organelle change in response to environmental and stress conditions (Bonangelino, et al. 2002; Kim, et al. 2012; Stauffer and Powers 2015) (Figure 1). Similarly, loss of proper lysosomal function has been implicated in various human disorders including neurodegenerative diseases and cancer (Kirkegaard and Jaattela 2009; Zhang, et al. 2009).
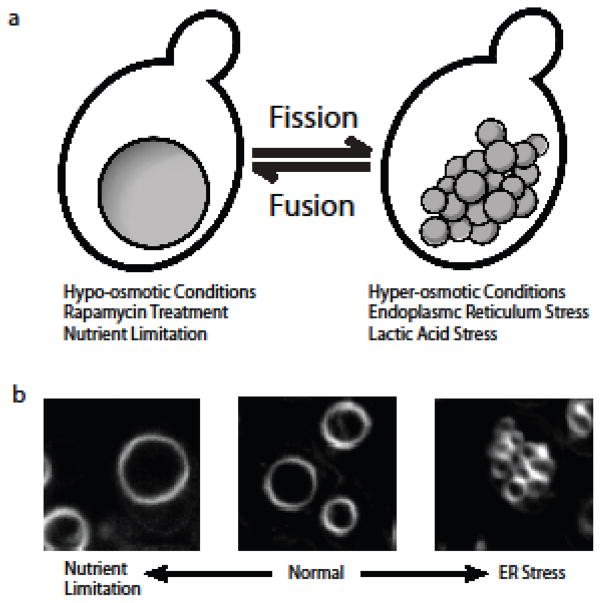
Fig. 1.
Vacuolar morphology is responsive to cellular stress. (A) Model of changes in vacuolar morphology mediated by an equilibrium between fission and fusion processes. Under hypo-osmotic conditions, rapamycin treatment, or nutrient limitation, vacuoles are one large organelle. In response to Hyper-osmotic, ER, and Lactic acid stress, vacuoles fragment into multiple, smaller organelles. (B) Visualization of vacuolar morphology using FM4-64. WT (W303 ) cells were treated overnight with FM4-64, then live cells were imaged following treatment with DMSO (Normal), 1μg/mL Tm (ER Stress), and 200nM Rapamycin (Nutrient Limitation)
The vacuole/lysosome are multi-copy organelles, where wild type yeast contains between one and four vacuoles, depending upon strain background, while mammalian cells contain several hundred lysosomes under normal growth conditions (Banta, et al. 1988; Luzio, et al. 2007). Upon nutrient limitation or hypotonic conditions, both vacuoles and lysosomes become enlarged and their copy number reduced, with yeast containing essentially one large vacuole (Baba, et al. 1994) (Figure 1). Conversely, under hyperosmotic or acid stress, vacuoles undergo asymmetric fragmentation resulting in smaller and more numerous organelles (Bonangelino, et al. 2002; Suzuki, et al. 2012; Zieger and Mayer 2012) (Figure 1). In yeast, alterations in vacuolar size and number are suggested to be regulated by an equilibrium between vacuolar fusion and fission processes (Baars, et al. 2007). While the steps and molecular players involved in vacuolar fusion have been well characterized, the vacuolar fission process remains poorly defined and the molecular machinery responsible for fission remains largely uncharacterized. In addition, for the few components that have been identified, their requirement for vacuolar fragmentation appears to vary depending on the precise stimuli, further complicating our understanding of this process (Bonangelino, et al. 2002; Zieger and Mayer 2012). Recent studies are now addressing this important area of research. For example, novel stimuli that induce vacuolar fission have been uncovered as we recently demonstrated that vacuolar fission occurs in response to stress within the endoplasmic reticulum (ER). Furthermore, a role for TORC1 in the vacuolar fission process has been solidified by recent findings that ER stress-induced as well as hyperosmotic stress- induced vacuolar fragmentation require components of the rapamycin-sensitive TOR signaling network (Michaillat, et al. 2012; Ho and Gasch 2015; Stauffer and Powers 2015). Additionally, by employing in vivo genome-wide visual screens, recent studies have identified several new molecular players involved in vacuolar fission (Michaillat and Mayer 2013; Stauffer and Powers 2015). Importantly, these studies provide evidence that ER-stress stimulates fission in a manner that is overlapping yet distinct in comparison to other forms of cellular stress (Stauffer and Powers 2015).
Inducers of vacuolar fragmentation
Vacuolar fission is known to occur in response to hyperosmotic stress, acid stress, and at distinct stages of the cell cycle (Bonangelino, et al. 2002; Weisman 2003; Suzuki, et al. 2012). In response to hyperosmotic shock, water is release from the vacuolar compartment to maintain osmotic homeostasis following the increased solute concentration in the cytoplasm (Bonangelino, et al. 2002; Weisman 2003). Fission of the vacuolar membrane under these conditions maintains the proper surface area to volume ratio of the vacuolar compartment and occurs rapidly, within ten minutes of hyperosmotic shock. Addition of lactic and hydrochloric acid to yeast growth medium also results in vacuolar fragmentation, although the significance of fragmentation in response to these conditions remains unknown (Suzuki, et al. 2012). Proper vacuolar inheritance is dependent upon correct partitioning of the vacuolar compartment into the daughter cell. Many early insights into vacuolar fission were gained following screens that identified mutants unable to undergo vacuolar fission, and subsequently, vacuolar inheritance (Weisman, et al. 1990; Shaw and Wickner 1991; Gomes de Mesquita, et al. 1996; Wang, et al. 1996).
Recent studies have demonstrated that vacuolar fragmentation also occurs in response to ER stress, as cells treated with tunicamycin (Tm), a drug that inhibits N-linked glycosylation within the ER, also produced fragmented vacuoles (Kim, et al. 2012; Stauffer and Powers 2015). Although the specificity and molecular basis for this observation was not determined in the first report, our subsequent study utilized both pharmacological and genetic approaches to determine if vacuolar fragmentation was indeed a general result of ER stress or, rather, whether it was a specific response to treatment with tunicamycin. ER stress was induced using a different chemical agent, dithiothreitol (DTT), as well as a temperature sensitive allele of ER oxidoreductin I, ERO1, and vacuolar morphology was examined. Disruption of Ero1 activity, as well as treatment with either Tm or DTT results in vacuolar fragmentation, signifying that fragmentation is indeed a general response to ER stress (Stauffer and Powers 2015). Consistent with this conclusion, the kinetics of fragmentation was observed to be very similar to the kinetics of activation of the ER stress signaling pathway, the so-called unfolded protein response (UPR). Although we don’t yet understand the physiological significance of fragmentation in response to ER stress, an inability to undergo fragmentation in response to Tm treatment was observed to enhance the ability of yeast cells to undergo cell death (Kim, et al. 2012).
These findings raise the question of how ER stress signals to the vacuole to induce fragmentation. Initially, it was reasoned that one of the previously identified ER stress response pathways would likely be involved, including the UPR. However, we determined that mutants that are unable to induce the UPR, specifically a deletion of the ER transmembrane sensor kinase/nuclease IRE1 (Sidrauski and Walter 1997; Okamura, et al. 2000), still undergo vacuolar fragmentation in response to ER stress (Stauffer and Powers 2015). Similarly, mutants in several additional ER stress-specific pathways, including the ER Associated Degradation (ERAD) and ER Surveillance (ERSU) pathways, are also able to respond to inducers and carryout fragmentation. Ultimately, components of the TOR signaling network were identified that provide the link between ER stress and vacuolar dynamics (Stauffer and Powers 2015).
TORC1 mediates vacuolar fragmentation
TOR is a Ser/Thr kinase that regulates cellular growth and is conserved in all eukaryotes examined to date. Budding yeast possess two TOR proteins, Tor1 and Tor2, that provide the mechanistic center of two distinct protein complexes, termed TOR complexes 1 and 2 (TORC1 and TORC2, respectively) (Loewith and Hall 2011). TORC1 is composed of either Tor1 or Tor2, Tco89, Kog1, and Lst8, and is enriched at the vacuolar membrane, where it regulates a diverse range of cellular processes in response to nutritional cues as well as stress conditions (Barbet, et al. 1996; Powers and Walter 1999; Urban, et al. 2007; Binda, et al. 2009; Michaillat, et al. 2012; Betz and Hall 2013; Jin, et al. 2014; Jiang 2016). TORC1 signaling is mediated through at least two major effector branches that include (i) the AGC kinase Sch9 and (ii) a protein phosphatase complex that contains an essential regulatory protein Tap42 (Urban, et al. 2007; Huber, et al. 2009).
TORC1 has been implicated in vacuolar fragmentation, as multiple studies have found that treatment with rapamycin, a specific inhibitor of TORC1, blocks both hyperosmotic, and ER stress-induced vacuolar fragmentation (Michaillat, et al. 2012) (Stauffer and Powers 2015). In response to ER stress, loss of function mutations within both downstream effector branches, including deletion of SCH9 or temperature sensitive alleles of the essential TAP42 gene, also block vacuolar fragmentation in the presence of ER stress, suggesting that both of these TORC1 branches are required for fragmentation. Conversely, Sch9 and Tap42 are not necessary for hyperosmotic stress-induced vacuolar fragmentation, although the phosphatase downstream of Tap42, Sit4 is required for fragmentation under both conditions (Michaillat, et al. 2012; Stauffer and Powers 2015). Based on these findings, it is likely that TORC1 is involved in a distinct ER stress-induced signaling pathway that is required for vacuolar fragmentation.
An important question is whether TORC1 signaling is activated under ER stress conditions, as has been proposed for salt-induced vacuolar fragmentation (Michaillat, et al. 2012). Results obtained thus far suggest that ER stress does not in fact affect TORC1 activity, indicating that ER stress and TORC1 may function in parallel to influence vacuolar morphology (Stauffer and Powers 2015) (Figure 2). Similar findings have also been reported recently by the Cunningham group (Kim and Cunningham 2015).
Fig. 2.
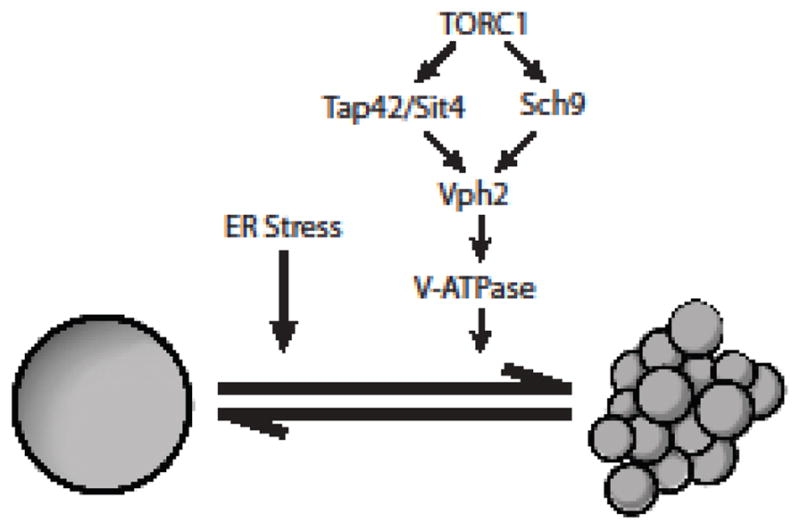
Model for ER stress-induced vacuolar fragmentation. ER stress and TORC1 are likely to act in parallel to influence vacuolar morphology. While components downstream of ER stress remain to be identified, both TORC1 effector branches mediated by Tap42/Sit4 and Sch9 are known to be required for vacuolar fragmentation incited by ER stress. Additionally, Vph2, an assembly factor for the V-ATPase, as well as the V-ATPase itself are also required for ER stress-induced vacuolar fragmentation. Interestingly, the V-ATPase is required for vacuolar fission in response to ER stress; however, this role appears to be distinct from its role in maintaining acidification of the vacuolar compartment. At present, the potential role of Sch9 and/or Tap42/Sit4 in Vph2 regulation remains speculative, and is based upon TORC1-dependent changes in Vph2 localization in response to ER stress in a TORC1 dependent manner
Vacuolar membrane localization of TORC1
Cellular fractionation and localization studies of TORC1 components have shown that TORC1 is enriched at the vacuolar membrane (Reinke, et al. 2004; Aronova, et al. 2007; Urban, et al. 2007; Sturgill, et al. 2008; Binda, et al. 2009). Because the vacuole is known to be a major compartment for nutrient storage and TORC1 responds to nutritional cues, it has become appreciated that localization of TORC1 to this compartment likely represents an important aspect of nutrient regulation within this pathway. Likewise, activation of mammalian TORC1 (mTORC1) by amino acids has been shown to require the regulated movement of mTORC1 from an endosome-like compartment to the lysosomal membrane, a process that requires the activity of the Rag GTPases (Sancak, et al. 2010).
In yeast, association of TORC1 with the vacuolar membrane does not appear to change in response to nutrient status (Binda, et al. 2009). Therefore, the possible regulation of TORC1 activity at the vacuole in yeast remains somewhat of a puzzle. It has been observed that TORC1 dissociates from the vacuolar membrane during heat stress, which is proposed to negatively regulate its function (Takahara and Maeda 2012). However, we observed that TORC1 remains associated with fragmented vacuoles under ER stress conditions (Stauffer and Powers 2015). This observation leads us to speculate that TORC1 functions at the vacuolar membrane to facilitate fission in response to ER stress.
A recent study has revealed that components of TORC1 are localized to so-called ER-vacuole membrane contact sites (MCS) (Murley, et al. 2015), suggesting the possibility that direct communication between these organelles may convey information regarding ER stress to TORC1 and/or the vacuolar membrane directly. A comprehensive understanding of TORC1 localization at the vacuole and its possible connection to ER-vacuole MCS is an area of ongoing study.
Identification of factors involved in vacuolar fission
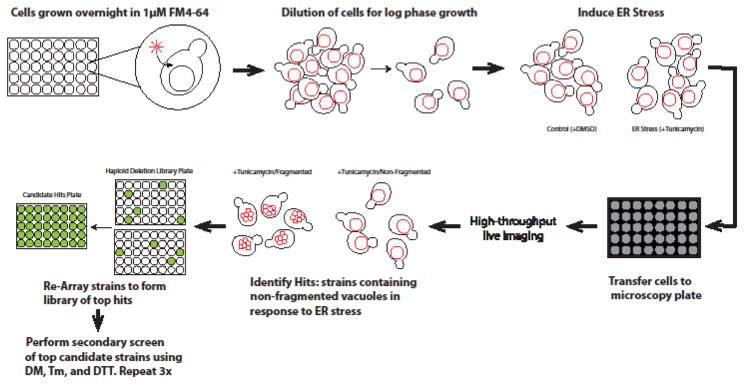
To gain insight into the molecular machinery required for vacuolar fragmentation, two groups have performed forward in vivo genome-wide visual screens to identify factors that are involved in fragmentation following ER and hyperosmotic stress (Michaillat and Mayer 2013; Stauffer and Powers 2015). Protocols were used that allowed sampling of vacuolar morphology using a high-throughput fluorescence microscope in a time resolved manner following the addition of ER stress- and hyperosmotic stress- inducing agents (Figure 3). Using this technique, the yeast haploid deletion collection (Giaever, et al. 2002) was analyzed and mutants deficient in fragmentation following ER stress and hyperosmotic stress were identified. A comparison of the results of these screens by meta-analysis yielded an overlapping yet non-identical set of proteins required for both ER stress- and hyperosmotic stress-induced vacuolar fragmentation.
Fig. 3.
Genome-wide screen for genes involved in ER stress-induced vacuolar fragmentation. Approximately 5000 strains contained in the yeast haploid deletion collection were grown overnight (16h) in YPD medium containing 1μM FM4-64. Cells were diluted 1:25 in fresh medium for 3h to allow for logarithmic phase growth, after which ER stress was induced by adding YPD medium containing 1μg/mL Tm. Treated cells were transferred to glass bottomed microscopy plates treated with concanavalin A and imaged using the CellVoyager CV1000 confocal system, using a 60Å~ water immersion objective. Deletion strains with 50% or more of cells within the population containing a defect in vacuolar fragmentation (non-fragmented) were considered to be candidate hits. Potential hits from the initial pass were re-arrayed using a RoToR robot to form a new library of candidate hits that was assayed twice more as described above and, in addition, following treatment with 25μM DTT. 315 strains that contained cells with >50% non-fragmented vacuoles were considered hits and manually grouped according to their cellular function.
Our screen identified 315 genes required for vacuolar fragmentation following ER stress (Stauffer and Powers 2015). These candidate genes were ranked according to the severity of their fragmentation defects, and the top “hits”, along with a number of other candidate genes known to be involved in cellular signaling, were subjected to a more rigorous individual inspection of vacuolar morphology. We identified genes implicated in vacuolar fission in response to hyperosmotic stress, including the vacuolar ATPase (V-ATPase), TORC1 signaling components, members of the AP-3 adaptor complex, as well as genes involved in phosphatidylinositol 3,5 bisphosphate (PI(3,5)P2 biosynthesis (Michaillat and Mayer 2013; Stauffer and Powers 2015). Additionally, we identified genes that appear to be unique to ER stress, including genes involved in assembly of the V-ATPase as well as novel proteins localized to the ER and Golgi.
Interplay between TORC1 and the vacuole
Many of the genes required for vacuolar fragmentation identified in these visual screens have been implicated independently in the regulation of TORC1, suggesting they may form part of the pathway that linkes ER stress to TORC1 activity. Conversely, these screens also identified factors that TORC1 has been proposed to regulate. These findings underscore the extent to which TORC1 function and vacuolar dynamics are likely to be interconnected.
Regulation of TORC1 activity is remarkably complex and influenced by many factors, including nutrient availability, temperature, osmotic conditions, redox stress, and caffeine (Kuranda, et al. 2006; Reinke, et al. 2006; Urban, et al. 2007; Binda, et al. 2009). The vacuolar GTPase EGO complex, Ego3, was identified in our screen as important for vacuolar fragmentation in response to ER stress (Stauffer and Powers 2015). The EGO complex consists of members of the Ras-like family of small GTPases Gtr1 and Gtr2, as well as Ego1, Ego2, and Ego3, which have unknown molecular functions but are probably orthologous to mammalian Ragulator components (see below) (Gao and Kaiser 2006; Binda, et al. 2009; Powis, et al. 2015). Discovery of this complex has provided insight into the regulation of TORC1 activity and has been proposed to couple amino acid signals to TORC1 (Gao and Kaiser 2006; Binda, et al. 2009; Powis, et al. 2015). Similarly, the mammalian Ragulator complex consists of components that recruit mTORC1 in mammalian cells to the lysosomal membrane for activation. TORC1 activity is sensitive to the nucleotide binding state of Gtr1, where Gtr1GTP/Gtr2GDP activates TORC1, while the opposite GTP/GDP-bound configuration is proposed to inactivate TORC1 (Binda, et al. 2009). The EGO complex is anchored to the vacuolar membrane by both palmitoylation and myristolation of Ego1, as well as by the fact that Ego3 is a transmembrane protein. Interactions between EGO and TORC1 have been identified as Gtr1GTP associates with both Tco89 and Kog1 in a nutrient-sensitive manner (Binda, et al. 2009). How the EGO complex, specifically Ego3, influences TORC1 to influence vacuolar morphology remains to be determined.
Interestingly, Vam6, the guanine exchange factor (GEF) for Gtr1, is also a component of the homotypic vacuolar fusion and protein sorting (HOPS) tethering complex utilized during homotypic vacuolar fusion (Binda, et al. 2009) and, moreover, is suggested to function upstream of TORC1 (Takahara and Maeda 2012; Kingsbury, et al. 2014). Similarly, components of the class C core vacuole-endosome transport (CORVET) endolysosomal membrane trafficking complex, which has been implicated in vacuolar fission, is also thought to regulate TORC1 activity (Kingsbury, et al. 2014). The link between the vacuole, TORC1, and the HOPS and CORVET complexes is extended by evidence that deletion of these complexes leads to vacuolar acidification defects, as well as the inability of the cell to resume growth after rapamycin treatment (Kingsbury, et al. 2014). Interestingly, both of these phenotypes are rescued by overexpression of Vph2, a V-ATPase assembly factor that is also implicated in vacuolar fission (Bachhawat, et al. 1993; Kingsbury, et al. 2014). We demonstrated that Vph2 is required for ER stress-induced vacuolar fragmentation, and that this protein undergoes changes in localization in a TORC1-dependent manner (Stauffer and Powers 2015). The molecular mechanism of TORC1 regulation by these complexes, as well as the relationship of Vph2 to TORC1, remain to be characterized.
Members of the vacuolar ATPase (V-ATPase), which acidifies the vacuole, were also identified as required for ER stress-induced vacuolar fragmentation. The V-ATPase has been implicated in both vacuolar fission and fusion processes as deletion of specific components results in either fusion or fission defects (Baars, et al. 2007). However, whether the role of the V-ATPase is simply to provide acidification of the organelle during fission and fusion or an alternative process that requires its presence at the vacuolar membrane remains somewhat controversial (Coonrod, et al. 2013). Additionally, recent studies in mammalian cells and yeast have shown that the V-ATPase influences TORC1 activity. In mammalian cells, the V-ATPase is necessary for mTORC1 activation in response to amino acids (Zoncu, et al. 2011). Interestingly, in yeast, the V-ATPase is thought to be stabilized by phosphatidylinositol 3,5 bisphosphate PI(3,5)P2 (Li, et al. 2014), which also influences TORC1 activity.
PI(3,5)P2 is a low abundant phospholipid that increases in response to hyperosmotic shock (Bonangelino, et al. 2002) and, moreover, is required for vacuolar fragmentation in response to hyperosmotic and ER stress as well as in response to changes in the cell cycle. Recently, PI(3,5)P2 has also been shown to influence TORC1 activity in both yeast and mammalian cells. Deletion of genes that mediate PI(3,5)P2 synthesis in yeast (FAB1, VAC7, VAC14) or their counterparts in mammalian cells (PIKFYVE, VAC14 or FIG4) have decreased TORC1 activity as assessed by hypersensitivity to rapamycin, and decreased phosphorylation of the TORC1 substrates Sch9, Npr1, Atg13, and mammalian S6K (Bridges, Ma, et al. 2012; Jin, et al. 2014). Increased PI(3,5)P2 levels restores TORC1 activity of these mutants in yeasts, clarifying the role of PI(3,5)P2 in regulation of TORC1 activity (Jin, et al. 2014). Additionally, insulin and amino acid stimulation increase PI(3,5)P2 levels in mammalian cells (Bridges, Ma, et al. 2012). Although phosphorylated phosphoinositides often recruit proteins to specific membranes to modify their function, yeast TORC1 components remain localized to the vacuolar membrane regardless of PI(3,5)P2 levels (Jin, et al. 2014). Interestingly, while PI(3,5)P2 –dependent changes in localization of the Kog1 were not evident, artificial tethering of this TORC1 subunit to the vacuolar membrane partially suppressed rapamycin hypersensitivity of PI(3,5)P2- deficient strains (Jin, et al. 2014). Similary, interactions between Raptor, the mammalian Kog1 homolog, and PI(3,5)P2 may partially mediate its interaction of with the lysosomal membrane as well as play a role in activation of mTORC1 (Bridges, Ma, et al. 2012). Additionally, in certain cell lines, mTORC1changes localization in response to altered PI(3,5)P2 levels, suggesting differences between yeast and mammalian cells (Bridges, Ma, et al. 2012). Moreover, PI(3,5)P2 levels modulate recruitment of TORC1 effectors to the vacuolar membrane as depletion of PI(3,5)P2 compromises Sch9 vacuolar localization, while elevated levels of PI(3,5)P2 restores localization (Urban, et al. 2007; Jin, et al. 2014).
Vps9 is the sole guanine nucleotide exchange factor in yeast that mediates GDP exchange for the Rab5 GTPases (Vps21, Ypt52, Ypt53), and is required for correct localization of the CORVET complex to endosomes (Balderhaar, et al. 2013). We found that Vps9 is also required for vacuolar fission in response to ER stress (Stauffer and Powers 2015). Interestingly, deletion of Vps9 results in rapamycin hypersensitivity, which is rescued by overexpression of a Rab5 GTPase, Vps21 (Bridges, Fisher, et al. 2012). Vps9 overexpression also results in rapamycin resistance, a phenotype characteristic of many upstream TORC1 regulators (Bridges, Fisher, et al. 2012). A role for Rab5 family members and their GEF’s in TORC1 regulation is conserved as Rab5 activity changes mTORC1 activity in response to insulin and amino acid signaling (Bridges, Fisher, et al. 2012). Additionally, Rab5 expression influences mTORC1 localization (Bridges, Fisher, et al. 2012). Although proteins found to regulate TORC1 activity have been identified, we are still lacking a clear understanding of how TORC1 activity is controlled, particularly in the context of association with membranes.
As described above, not only do vacuolar proteins regulate TORC1, but TORC1 also regulates many vacuolar processes. TORC1 negatively regulate autophagy, the catabolic process where portions of the cytoplasm are carried to the vacuole for turnover. Similarly, piecemeal microautophagy of the nucleus – a process that degrades portions of the nuclear membrane at nuclear-vacuole junctions, is also proposed to be regulated by TORC1 (Roberts, et al. 2003). A recent study found that TORC1 promotes vacuolar membrane permeabilization and cellular death in response to simultaneous ER stress initiation and calcineurin inhibition (Kim and Cunningham 2015). However, the precise relationship between TORC1 and vacuolar function with respect to ER stress remains to be defined.
Conclusions and perspective
The physiological consequence of the inability of cells to undergo fragmentation in response to environmental conditions remains to be addressed comprehensively. In response to increased solute concentration in the cytoplasm, the vacuole releases water to balance osmotic pressure that results in a shriveling of the vacuolar membrane (Bonangelino, et al. 2002). Fragmentation of the vacuolar is thought to occur in order to maintain the correct surface area to volume ratio of the vacuolar membrane. However, fragmentation during the cell cycle occurs to allow proper vacuolar inheritance into the daughter cell (Weisman 2003). While we don’t fully understand why vacuoles undergo fragmentation in response to ER stress, preliminary evidence suggests that cells unable to undergo division under these conditions are more susceptible to cell death (Kim, et al. 2012). More insight into the process of vacuolar fission, including the signaling networks that regulate the fusion and fission equilibrium, the timing and location of division events, and molecular machinery involved is needed. Remarkably, our examination of ER stress signaling pathways and vacuolar morphology excludes involvement of known ER stress pathways and instead identifies a signaling pathway that includes TORC1. Ongoing studies are thus aimed at further characterization of this pathway in yeast, as well as exploring the relationship between cellular stress, TOR signaling, and lysosomal dynamics in mammalian cells.
Acknowledgments
This work was supported by National Institutes of Health Grant GM086387 to Ted Powers.
References
- Aronova S, Wedaman K, Anderson S, Yates J, 3rd, Powers T. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Molecular biology of the cell. 2007;18:2779–2794. doi: 10.1091/mbc.E07-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars TL, Petri S, Peters C, Mayer A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Molecular biology of the cell. 2007;18:3873–3882. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. The Journal of cell biology. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhawat AK, Manolson MF, Murdock DG, Garman JD, Jones EW. The VPH2 gene encodes a 25 kDa protein required for activity of the yeast vacuolar H(+)-ATPase. Yeast. 1993;9:175–184. doi: 10.1002/yea.320090208. [DOI] [PubMed] [Google Scholar]
- Balderhaar HJ, Lachmann J, Yavavli E, Brocker C, Lurick A, Ungermann C. The CORVET complex promotes tethering and fusion of Rab5/Vps21-positive membranes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3823–3828. doi: 10.1073/pnas.1221785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta LM, Robinson JS, Klionsky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. The Journal of cell biology. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Molecular biology of the cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C, Hall MN. Where is mTOR and what is it doing there? The Journal of cell biology. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Molecular cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. The Journal of cell biology. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Fisher K, Zolov SN, Xiong T, Inoki K, Weisman LS, Saltiel AR. Rab5 proteins regulate activation and localization of target of rapamycin complex 1. The Journal of biological chemistry. 2012;287:20913–20921. doi: 10.1074/jbc.M111.334060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Ma JT, Park S, Inoki K, Weisman LS, Saltiel AR. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Molecular biology of the cell. 2012;23:2955–2962. doi: 10.1091/mbc.E11-12-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod EM, Graham LA, Carpp LN, Carr TM, Stirrat L, Bowers K, Bryant NJ, Stevens TH. Homotypic vacuole fusion in yeast requires organelle acidification and not the V-ATPase membrane domain. Developmental cell. 2013;27:462–468. doi: 10.1016/j.devcel.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nature cell biology. 2006;8:657–667. doi: 10.1038/ncb1419. [DOI] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, van den Hazel HB, Bouwman J, Woldringh CL. Characterization of new vacuolar segregation mutants, isolated by screening for loss of proteinase B self-activation. Eur J Cell Biol. 1996;71:237–247. [PubMed] [Google Scholar]
- Ho YH, Gasch AP. Exploiting the yeast stress-activated signaling network to inform on stress biology and disease signaling. Curr Genet. 2015;61:503–511. doi: 10.1007/s00294-015-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes & development. 2009;23:1929–1943. doi: 10.1101/gad.532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. Regulation of TORC1 by ubiquitin through non-covalent binding. Curr Genet. 2016 doi: 10.1007/s00294-016-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Mao K, Jin Y, Tevzadze G, Kauffman EJ, Park S, Bridges D, Loewith R, Saltiel AR, Klionsky DJ, et al. Roles for PI(3,5)P2 in nutrient sensing through TORC1. Molecular biology of the cell. 2014;25:1171–1185. doi: 10.1091/mbc.E14-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Cunningham KW. A LAPF/phafin1-like protein regulates TORC1 and lysosomal membrane permeabilization in response to endoplasmic reticulum membrane stress. Molecular biology of the cell. 2015;26:4631–4645. doi: 10.1091/mbc.E15-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim A, Cunningham KW. Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast. The Journal of biological chemistry. 2012;287:19029–19039. doi: 10.1074/jbc.M112.363390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury JM, Sen ND, Maeda T, Heitman J, Cardenas ME. Endolysosomal membrane trafficking complexes drive nutrient-dependent TORC1 signaling to control cell growth in Saccharomyces cerevisiae. Genetics. 2014;196:1077–1089. doi: 10.1534/genetics.114.161646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard T, Jaattela M. Lysosomal involvement in cell death and cancer. Biochimica et biophysica acta. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kuranda K, Leberre V, Sokol S, Palamarczyk G, Francois J. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol Microbiol. 2006;61:1147–1166. doi: 10.1111/j.1365-2958.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- Li SC, Diakov TT, Xu T, Tarsio M, Zhu W, Couoh-Cardel S, Weisman LS, Kane PM. The signaling lipid PI(3,5)P(2) stabilizes V(1)-V(o) sector interactions and activates the V-ATPase. Molecular biology of the cell. 2014;25:1251–1262. doi: 10.1091/mbc.E13-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochimica et biophysica acta. 2009;1793:650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature reviews Molecular cell biology. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Michaillat L, Baars TL, Mayer A. Cell-free reconstitution of vacuole membrane fragmentation reveals regulation of vacuole size and number by TORC1. Molecular biology of the cell. 2012;23:881–895. doi: 10.1091/mbc.E11-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaillat L, Mayer A. Identification of genes affecting vacuole membrane fragmentation in Saccharomyces cerevisiae. PloS one. 2013;8:e54160. doi: 10.1371/journal.pone.0054160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley A, Sarsam RD, Toulmay A, Yamada J, Prinz WA, Nunnari J. Ltc1 is an ER-localized sterol transporter and a component of ER-mitochondria and ER-vacuole contacts. The Journal of cell biology. 2015 doi: 10.1083/jcb.201502033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochemical and biophysical research communications. 2000;279:445–450. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Molecular biology of the cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis K, Zhang T, Panchaud N, Wang R, Virgilio CD, Ding J. Crystal structure of the Ego1-Ego2-Ego3 complex and its role in promoting Rag GTPase-dependent TORC1 signaling. Cell Res. 2015;25:1043–1059. doi: 10.1038/cr.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A, Anderson S, McCaffery JM, Yates J, 3rd, Aronova S, Chu S, Fairclough S, Iverson C, Wedaman KP, Powers T. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. The Journal of biological chemistry. 2004;279:14752–14762. doi: 10.1074/jbc.M313062200. [DOI] [PubMed] [Google Scholar]
- Reinke A, Chen JC, Aronova S, Powers T. Caffeine targets TOR complex I and provides evidence for a regulatory link between the FRB and kinase domains of Tor1p. The Journal of biological chemistry. 2006;281:31616–31626. doi: 10.1074/jbc.M603107200. [DOI] [PubMed] [Google Scholar]
- Roberts P, Moshitch-Moshkovitz S, Kvam E, O’Toole E, Winey M, Goldfarb DS. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Molecular biology of the cell. 2003;14:129–141. doi: 10.1091/mbc.E02-08-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Wickner WT. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. The EMBO journal. 1991;10:1741–1748. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Stauffer B, Powers T. Target of rapamycin signaling mediates vacuolar fission caused by endoplasmic reticulum stress in Saccharomyces cerevisiae. Molecular biology of the cell. 2015;26:4618–4630. doi: 10.1091/mbc.E15-06-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin DE, Hall MN. TOR1 and TOR2 have distinct locations in live cells. Eukaryotic cell. 2008;7:1819–1830. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sugiyama M, Wakazono K, Kaneko Y, Harashima S. Lactic-acid stress causes vacuolar fragmentation and impairs intracellular amino-acid homeostasis in Saccharomyces cerevisiae. Journal of bioscience and bioengineering. 2012;113:421–430. doi: 10.1016/j.jbiosc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Takahara T, Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Molecular cell. 2012;47:242–252. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Molecular cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhao H, Harding TM, Gomes de Mesquita DS, Woldringh CL, Klionsky DJ, Munn AL, Weisman LS. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Molecular biology of the cell. 1996;7:1375–1389. doi: 10.1091/mbc.7.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS. Yeast vacuole inheritance and dynamics. Annual review of genetics. 2003;37:435–460. doi: 10.1146/annurev.genet.37.050203.103207. [DOI] [PubMed] [Google Scholar]
- Weisman LS, Emr SD, Wickner WT. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sheng R, Qin Z. The lysosome and neurodegenerative diseases. Acta Biochim Biophys Sin (Shanghai) 2009;41:437–445. doi: 10.1093/abbs/gmp031. [DOI] [PubMed] [Google Scholar]
- Zieger M, Mayer A. Yeast vacuoles fragment in an asymmetrical two-phase process with distinct protein requirements. Molecular biology of the cell. 2012;23:3438–3449. doi: 10.1091/mbc.E12-05-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]