Abstract
Nicotine can be metabolized by the enzyme CYP2B; brain CYP2B is higher in rats and monkeys treated with nicotine, and in human smokers. A 7-day nicotine treatment increased CYP2B expression in rat brain but not liver, and decreased the behavioral response and brain levels (ex vivo) to the CYP2B substrate propofol. However, the effect of CYP2B induction on the time course and levels of circulating brain nicotine in vivo has not been demonstrated. Using brain microdialysis, nicotine levels following a subcutaneous nicotine injection were measured on day one and after a 7-day nicotine treatment. There was a significant time x treatment interaction (p = 0.01); peak nicotine levels (15–45 minutes post-injection) were lower after treatment (p = 0.04) consistent with CYP2B induction. Following a two-week washout period, brain nicotine levels increased to day one levels (p = 0.02), consistent with brain CYP2B levels returning to baseline. Brain pretreatment of the CYP2B inhibitor, C8-xanthate, increased brain nicotine levels acutely and after 7-day nicotine treatment, indicating the alterations in brain nicotine levels were due to changes in brain CYP2B activity. Plasma nicotine levels were not altered for any time or treatment sampled, confirming no effect on peripheral nicotine metabolism. These results demonstrate that chronic nicotine, by increasing brain CYP2B activity, reduces brain nicotine levels, which could alter nicotine’s reinforcing effects. Higher brain CYP2B levels in smokers could lower brain nicotine levels; as this induction would occur following continued nicotine exposure it could increase withdrawal symptoms and contribute to sustaining smoking behavior.
Keywords: brain, CYP2B, metabolism, microdialysis, nicotine, rat
Introduction
Nicotine is the main component in cigarettes that leads to the development of smoking dependence (Stolerman and Jarvis, 1995). Humans (Henningfield et al., 1983) and animals, including rats (Corrigall and Coen, 1989), will dose-dependently self-administer nicotine, indicating that nicotine reinforces addictive behavior. Addictive behavior is thought to arise from many neurobiological changes in the brain after nicotine exposure (Markou, 2008). In rodents, acute nicotine administration elicits dopamine release from the ventral tegmental area (VTA) to the nucleus accumbens in the mesolimbic pathway (Di Chiara and Imperato, 1988). Persistent activation of this system from continued nicotine administration leads to adaptive changes in the brain, such as enhanced dopamine signalling (Marshall et al., 1997) and upregulation of nicotinic acetylcholine receptors (nAchR) (Lapchak et al., 1989). These changes have also been observed in humans, where smokers display greater dopamine release following nicotine exposure using positron emission tomography (PET) (Takahashi et al., 2008) and post-mortem brains of smokers show greater nAchR binding (Benwell et al., 1988).
Another change in the brain resulting from chronic nicotine exposure might be induction of the drug metabolizing enzyme CYP2B6, as higher CYP2B6 protein levels were found in the post-mortem brains of smokers (Miksys et al., 2003). CYP2B6 can convert nicotine to its major metabolite cotinine (Yamazaki et al., 1999), as well as another metabolite, nornicotine (Yamanaka et al., 2005); therefore it is possible that nicotine from cigarettes may lead to induction of CYP2B6 in the brain. Higher CYP2B6 levels could increase nicotine metabolism and decrease local brain nicotine levels. Thus, lower nicotine levels in the brains of smokers following continued nicotine exposure may alter smoking behaviors.
Animals have higher brain CYP2B expression after repeated nicotine treatment (Lee et al., 2006; Miksys et al., 2000), suggesting that nicotine can induce this enzyme in the brain. CYP2B protein levels in monkey brain increased following three weeks of subcutaneous (s.c.) nicotine injections (0.6 mg/kg/s.c. bid) (Lee et al., 2006), while protein levels of the rat homolog CYP2B1 increased in rat brain following seven daily injections of nicotine in doses ranging from 0.1 to 1.0 mg/kg/s.c. (Miksys et al., 2000). This increase in CYP2B expression was found in the brain but not in the liver of both rats and monkeys, consistent with that seen in the post-mortem tissue of smokers (Hesse et al., 2004; Miksys, 2003). Given that these nicotine doses are similar to the range of daily nicotine intake in smokers [0.2 to 1.1 mg/kg] (Benowitz and Jacob, 1984), the elevated brain CYP2B6 protein levels in smokers may be due to chronic nicotine exposure.
We have recently shown that selective inhibition of CYP2B in the brain by intracerebral injection of a CYP2B inhibitor can increase nicotine levels in the brain, but not in the plasma, following a single intravenous nicotine injection (Garcia et al., 2015). Thus, reducing brain CYP2B activity can influence nicotine levels within the brain without influencing systemic levels or hepatic metabolism. Induction of brain CYP2B may also influence local brain nicotine levels by increasing CYP2B activity and localized nicotine metabolism. This change in brain nicotine levels could alter nicotine’s rewarding effects.
The highest dose of nicotine (1.0 mg/kg/s.c.) tested in the 7-day nicotine treatment paradigm in rats resulted in the greatest increase in brain CYP2B mRNA and protein levels (Miksys et al., 2000). This nicotine treatment can decrease the anaesthestic effect and brain levels (measured upon sacrifice), of propofol, another CYP2B substrate (Khokhar and Tyndale, 2011), providing indirect evidence to suggest that this degree of induction is sufficient to alter drug response.
While effects of CYP2B induction have been shown ex vivo and behaviorally, the impact of induction on in vivo brain levels of CYP2B substrates in a living animal has not yet been characterized. If CYP2B induction specifically in the brain can alter brain drug levels in vivo, it would be the first evidence that enzyme induction had a functional impact on the amount of available drug within this tissue. Furthermore, this induction occurs in the brain but not in the liver (Miksys et al., 2000), suggesting that brain-specific induction may alter the ability of peripheral drug levels to predict brain drug levels. Contrasting peripheral and central drug levels could subsequently influence the efficacy of drugs that act within the brain.
CYP2B induction with 1.0 mg/kg/s.c. 7-day nicotine treatment was observed for at least 24 hours after the last nicotine injection with brain CYP2B protein levels returning to baseline within seven days (Khokhar et al., 2010), indicating that higher brain CYP2B levels are prolonged after nicotine administration had ceased. Therefore, it is possible that increases in brain CYP2B levels could decrease peak brain levels and/or alter the time course of brain levels of its substrates. The effect of CYP2B induction on both of these potential changes in brain drug levels can be investigated using brain microdialysis, where brain drug levels can be repeatedly measured. We have previously shown that acutely inhibiting brain CYP2B activity can increase local brain nicotine levels (Garcia et al., 2015), thus this study aimed to investigate the effect of altering CYP2B activity on brain nicotine levels through enzyme induction using microdialysis to measure brain nicotine levels over time (Garcia et al., 2015). We expect that CYP2B induction in the brain, by increasing CYP2B activity, would decrease brain nicotine levels.
Materials and Methods
Animals and housing conditions
Adult male Wistar rats (250–300 g, Charles River Laboratories, Quebec, Canada) were individually housed after surgery on a 12 hr light/dark cycle. Water and food was provided ad libitum.
ICV cannulation surgery
Animals were anaesthetized with Isofluorane and EMLA cream (Lidocaine[2.5%]/Prilocaine[2.5%]) was applied as a local anaesthetic at the incision site. Anafen (Merial Canada, Baie D’Urfé, QC, Canada; 5 mg/kg, s.c.) was given as an analgesic after animals were anaesthetized. Animals were placed in a Stereotaxic apparatus where guide cannulas (MD-2250; Bioanalytical Systems, Inc.,West Lafayette, IN) were implanted into the right lateral ventricle [anterior-posterior 0.9 mm, lateral 1.4 mm, and dorsoventral 2.6 mm from Bregma (Paxinos and Watson, 1986)]. Implantation involved drilling a small hole into the skull, fixing the cannula in place with dental cement and securing the cement cap to the skull with jeweler’s screws. Stylets were inserted into cannulas after surgery. Animals were given seven days to recover from surgery before microdialysis experiments were initiated.
Drug preparation
Sterile nicotine solution (Sigma-Aldrich, Oakville, ON, Canada) was prepared on each experiment day as a concentration of nicotine base in saline, pH 6.8–7.2. The nicotine dose was 1 mg/kg/s.c. (expressed as base) given in a 1 ml/kg volume. This dose was based on two studies from our group, where rat brain CYP2B mRNA and protein increased after 7-day treatment (Khokhar et al., 2010; Miksys et al., 2000) and the time course of protein induction was determined (Khokhar et al., 2010). C8-xanthate (C8X, Toronto Research Chemicals, Toronto, ON, Canada), the CYP2B-specific inhibitor (Yanev, Kent et al. 2000), was dissolved in sterile artificial cerebrospinal fluid (ACSF) to create a 5 μg/μl solution. Rats were injected ICV with 4 μl of C8X (20 μg) over 1 minute and the injector was removed after 1 minute. Injections of sterile ACSF (4 μl) or C8X were made with a 30-gauge injector connected to a 10-μL Hamilton syringe. This ICV C8X dose was previously optimized to inhibit CYP2B in the brain but not the liver (Garcia et al., 2015; Khokhar and Tyndale, 2011, 2012).
In vivo nicotine brain microdialysis procedures
Nicotine microdialysis
Each microdialysis session was performed as outlined in Figure 1a. At the start of each session, concentric silica coated microdialysis probes with 2 mm membranes (MD-2200; Bioanalytical Systems, Inc., West Lafayette, IN) were placed in the right lateral ventricle. Perfusion medium (Ringer’s solution: 147 mM Na+, 2 mM Ca2+, 4mM K+, 155 mM Cl−, pH 6.0) was run through fluorinated ethylene propylene (FEP) Teflon tubing attached to a syringe infusion pump at a flow rate of 2 μl/minute. Dialysate was collected every 15 minutes into microcentrifuge tubes kept on ice. Dialysate was collected for 15 minutes intervals starting before nicotine (1 mg/kg s.c. injection) was administered (baseline) and then every 15 minutes for a total of 2 hours and 15 minutes (135 minutes). Blood (200–300 μl) was collected from the saphenous vein at 22.5 and 112.5 minutes post-nicotine injection to measure plasma nicotine levels at the midpoint of the 15–30 minute and 105–120 minute dialysate collections, respectively. The first time point for assessment of plasma nicotine was chosen based on pilot data where 15–30 minutes post-injection corresponded to peak brain nicotine levels after a single s.c. nicotine injection (data not shown) and the second time point was chosen to measure plasma nicotine at the end of the microdialysis session.
Figure 1.
Effect of ICV C8X pre-treatment on brain nicotine levels
To determine whether brain CYP2B activity influences brain nicotine levels following a nicotine s.c. injection, C8X was administered centrally prior to microdialysis (Figure 1b). Animals were first given an ICV injection of ACSF (vehicle for the C8X) and 22 hours later underwent one nicotine microdialysis session following one s.c injection of nicotine. On the following day they were given an ICV injection of C8X and 22 hours later a second nicotine microdialysis session was performed.
Effect of 7-day nicotine treatment on brain nicotine levels
To determine if increasing brain CYP2B activity would decrease brain nicotine levels, another cohort of animals underwent 7-day nicotine treatment (1 mg/kg sc/day), which is known to induce brain CYP2B expression (Khokhar et al., 2010; Miksys et al., 2000) (Figure 1c). Animals underwent nicotine microdialysis on day 1 and day 7 of nicotine treatment, which were labeled “acute” condition and “chronic” conditions respectively.
Effect of a washout period on brain nicotine levels after 7-day nicotine treatment
To determine if brain nicotine levels returned to baseline after terminating nicotine treatment, the same animals were left in their home cages for a two-week period following their chronic (day 7) microdialysis session (Figure 1d). This period was labeled “washout” because animals were not given nicotine. After this washout period, animals underwent a third microdialysis session.
Nicotine quantification
For brain nicotine quantification, each dialysate sample (30 μl) was diluted to 100 μl with deuterium-labeled internal nicotine and cotinine standards (20 ng/ml in 0.01M HCl). For plasma quantification, 100 μl plasma samples were prepared as previously described (Craig et al., 2014; Vieira-Brock et al., 2013). The prepared dialysate and plasma samples were then analyzed by LC-MS as previously described (Craig et al., 2014). Cotinine dialysate levels were near and at the limit of detection; thus, cotinine levels were not reliably quantifiable.
Statistical Analysis
Mean brain nicotine levels from baseline to 135 minutes post-injection were compared within and between treatments using repeated measures ANOVA. Simple effects of treatment within each time point were analyzed using multivariate ANOVA. Brain nicotine levels collected within 15–45 minutes post-injection, the sum of nicotine levels collected from 15–30 and 30–45 minutes post-injection, were compared between nicotine treatments using paired t tests. Brain nicotine levels collected within 15–135 minutes post-injection were compared between ACSF and C8X treatments using a paired t test. Mean plasma nicotine levels between treatments were compared using t tests. Significance level for all comparisons was p = 0.05.
Results
Altering CYP2B activity in the brain by inhibition can increase brain nicotine levels
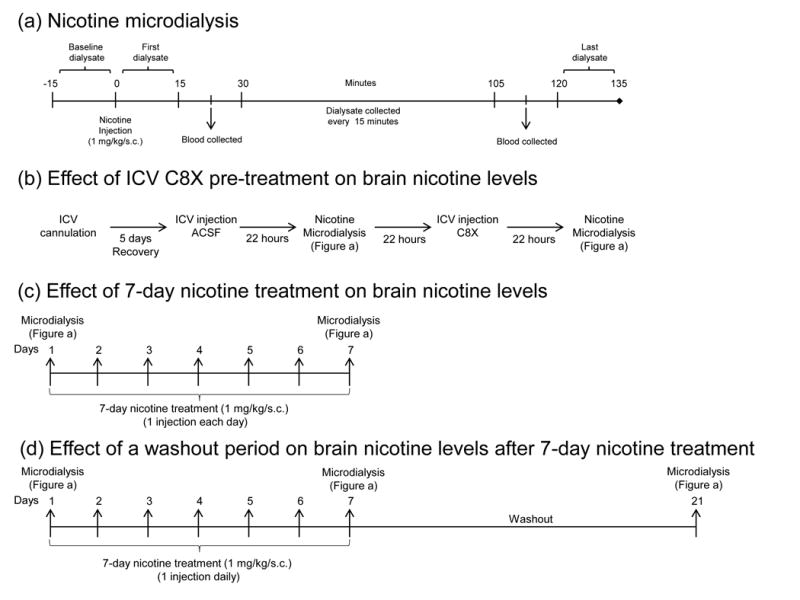
Brain nicotine levels were collected at baseline and over 135 minutes following a nicotine injection (1 mg/kg/s.c.) and were compared between ICV C8X and ACSF treatments (Figure 2a). Baseline dialysate (−15 to 0 minutes post-injection) did not contain nicotine, indicating that nicotine was not present in brain CSF prior to the nicotine injection. Mean nicotine levels were significantly higher after C8X treatment compared to ACSF (F[1, 15] = 7.28, p = 0.02). There was also a significant interaction between ICV treatment and time (F[2.8, 42.15] = 5.34, p < 0.01), indicating that brain nicotine levels over time were different with C8X treatment compared to ACSF. Multivariate analysis examining ACSF and C8X treatments at each time point revealed that the difference in brain nicotine levels between ACSF and C8X groups trended towards significance at 30–45 minutes post injection (p = 0.05) and was significantly different at all time points following 45 minutes post-injection (p < 0.05).
Figure 2.
Total brain nicotine levels in dialysates collected from 15 to 45 minutes post-injection, during the peak of nicotine levels, were compared between ICV treatments within-animal (Figure 2b). C8X treatment increased brain nicotine levels within this time period; however this difference only trended towards significance (t[15] = −1.95, p = 0.07). Nicotine levels in plasma from blood samples collected at 22.5 and 112.5 minutes post-injection were not different between C8X and ACSF ICV treatments (Figure 2c), indicating that systemic levels of nicotine were the same in both conditions.
Using 7-day nicotine treatment to induce brain CYP2B decreased brain nicotine levels
Brain nicotine levels, collected over the 135 minute period post-injection, on the first (acute) and the 7th day (chronic) of nicotine treatment are shown in Figure 3a. There was no detectable nicotine in baseline dialysate from either microdialysis day, indicating that nicotine was not present in brain CSF before the nicotine injection for each session. A repeated measures ANOVA did not reveal differences in brain nicotine levels between acute and chronic treatments (F[1,14] = 0.13, p = 0.72). However, there was a significant interaction between treatment condition (acute vs. chronic) and time (F[2.8, 39.1] = 4.1, p = 0.01), indicating that nicotine levels collected between the two conditions were different over time. Multivariate analysis examining acute and chronic treatments at each time point revealed a significant difference in brain nicotine levels between treatments at 120–135 minutes post-injection (p = 0.03). Separate analysis of brain nicotine levels within each treatment, using repeated measures ANOVA, showed that nicotine levels from 30–75 minutes post-injection were not significantly different from each other in the acute condition (p > 0.05), while nicotine levels at all time points were significantly different from each other in the chronic condition (p < 0.05).
Figure 3.
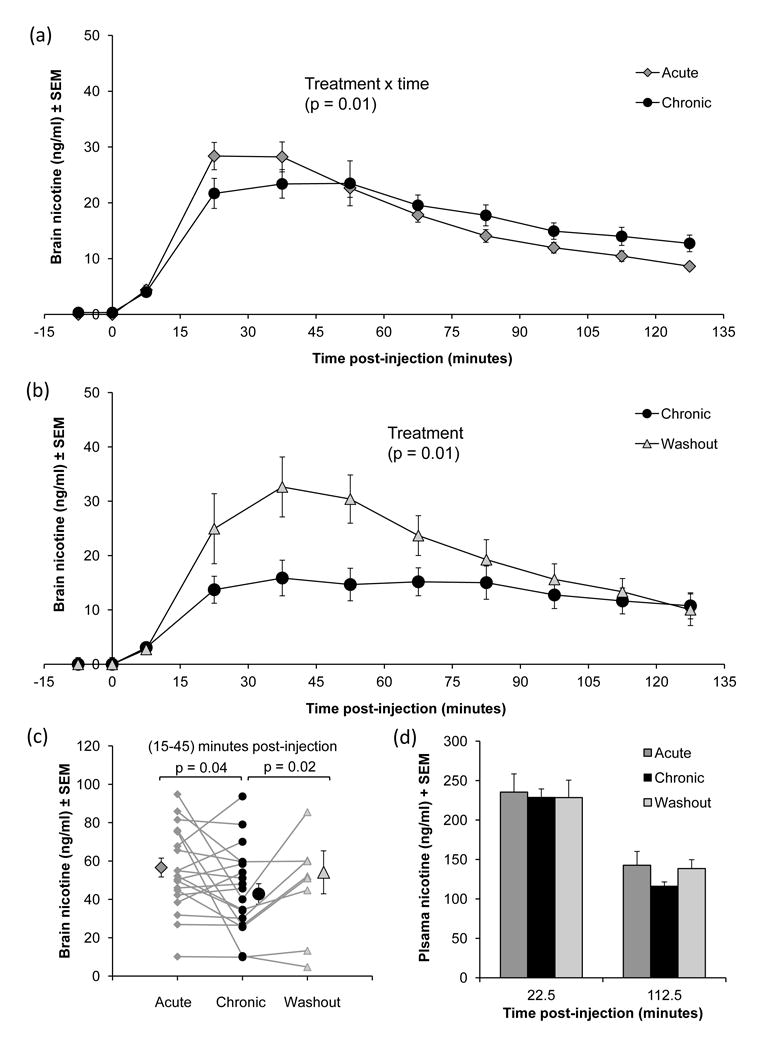
Brain nicotine levels were highest in the acute condition between 15–45 minutes post-injection, after which they decreased over subsequent time points (Figure 3a). During this peak time frame (15–45 minutes post-injection), nicotine levels were lower in the chronic condition. Mean brain nicotine levels accumulated over this time frame were compared within-animal (Figure 3b), where paired t-tests revealed that brain nicotine levels in the chronic condition were lower (t[19]= 2.1, p = 0.04).
Plasma nicotine levels from blood collected at 22.5 and 112.5 minutes post-injection were also compared between conditions (Figure 3d). There was no difference in plasma nicotine levels between acute and chronic conditions at either time point, one of which was during this 15–45 minute time frame, indicating that systemic levels of nicotine were the same in both conditions.
Brain nicotine levels increased as brain CYP2B returns to baseline following a washout period
Figure 3 also shows brain nicotine levels over time following an injection of nicotine in the subset of animals that had undergone nicotine microdialysis after the washout period. Within this subset of animals, the effect of 7-day nicotine treatment (acute vs. chronic) on brain nicotine levels was consistent with that seen in the whole group of animals (acute data not shown), although brain nicotine levels collected between 15–45 minutes post-injection only trended towards significance (t[7] = 2.29, p = 0.06) in this subset (n = 8). Following the washout period, brain nicotine levels were higher than that seen in the chronic condition (Figure 3b). A repeated measures ANOVA comparing brain nicotine levels between chronic and washout conditions revealed a significant effect of treatment (F[1,7] = 11.44, p = 0.01), demonstrating that brain nicotine levels were higher after the washout period. There was also a significant interaction between condition (chronic vs. washout) and time (F[8,56]=6.15, p < 0.01), demonstrating that the time course of brain nicotine levels was different between condition. Multivariate analysis comparing both conditions during each time point post-injection revealed a significant difference in brain nicotine levels at 45–60 minutes post-injection (p = 0.04) and a trend towards significance in at 30–45 minutes post-injection (p = 0.05). Separate repeated measures ANOVA for brain nicotine levels over time within each condition revealed a significant effect of time in both conditions (chronic: F[8, 56] = 9.29, p < 0.01; washout: F[8, 56] = 3.76, p < 0.01). Differences in the time course for each condition were found, where brain nicotine levels collected 45–60 minutes post-injection were significantly different from dialysate collected at 120–135 minutes post-injection (p = 0.04) within the chronic condition, while there were no significant differences in brain nicotine levels between time points within the washout condition, although brain nicotine levels collected 0–15 minutes post-injection compared to 90–120 and 120–135 minutes post-injection trended towards significance (p = 0.06).
In the same time frame (15–45 minutes post-injection) where brain nicotine levels within-animal were lower in the chronic compared to acute conditions, nicotine levels in the washout condition were significantly higher than the chronic (t[7] = 2.71, p = 0.02) (Figure 3c). Brain nicotine levels in the washout condition were similar to the acute (Figure 3c), indicating that nicotine levels returned to pre-treatment levels. Plasma nicotine levels in acute, chronic and washout conditions did not differ between conditions, indicating that neither chronic nicotine nor washout had any effect on plasma nicotine levels, and presumably hepatic CYP2B activity (Figure 3d).
Reduction in brain nicotine levels following 7-day nicotine treatment was reversed with C8X pretreatment
C8X was given after 7-day nicotine treatment in a small group of animals (n=3) to test whether induced CYP2B activity was responsible for the reduction in brain nicotine levels (Figure S1). Animals were given ACSF and then C8X injections after 7-day nicotine treatment (Figure S1a). The day after chronic nicotine microdialysis (day 8), animals were given an additional (8th) injection of nicotine followed 2 hours later by an ICV injection of C8X. Another microdialysis session was performed 22 hours after the ICV injection (day 9). Mean brain nicotine levels within 15–45 minutes post-injection were lower after 7-day nicotine treatment (acute vs. chronic + ACSF) (Figure S1b), consistent with the previous animal cohort (Figure 3b). After receiving C8X, brain nicotine levels within 15–45 minutes post-injection were higher compared to chronic + ACSF condition (Figure S1b). Plasma nicotine levels in both groups were not different between ACSF- and C8X-treatments (data not shown), indicating that C8X ICV injections did not inhibit peripheral nicotine metabolism.
Discussion
This is the first study demonstrating that CYP enzyme induction specifically in the brain, by 7-day nicotine treatment, is sufficient to change brain drug levels in vivo. Previous studies by our group have shown that this 7-day nicotine treatment regimen can increase CYP2B mRNA and protein levels in the brain (Khokhar et al., 2010; Miksys et al., 2000) and increase the drug response to propofol, another CYP2B substrate (Khokhar and Tyndale, 2011); however, the effect of enzyme induction on substrate levels in the brain had not been directly tested in a live animal, nor was the time course understood. Using microdialysis, we were able to show that brain nicotine levels were lower following brain CYP2B induction. Furthermore, this reduction in brain nicotine was significant within-animal as well as between-animal, demonstrating the impact of induction on brain CYP2B substrate levels in the same animal, which had not been measured in our earlier studies.
First, we showed that inhibiting brain CYP2B activity could increase brain nicotine levels after a single s.c. injection of nicotine, which demonstrated that altering CYP2B activity can influence nicotine levels measured by the microdialysis technique. Then, using the established 7-day nicotine treatment paradigm, we demonstrated that CYP2B induction was able to reduce nicotine levels locally in the brain without altering systemic nicotine levels. This decrease in brain nicotine levels was reversed following (1) a washout period without nicotine and (2) using a CYP2B inhibitor. These data suggest that a 7-day nicotine treatment induced CYP2B levels sufficiently to result in reduced nicotine levels within the brain.
Inhibiting brain CYP2B activity using C8X was able to increase brain nicotine levels following one s.c. injection of nicotine. These results are in line with the previous finding that inhibiting CYP2B activity can increase brain nicotine levels after an i.v. injection of nicotine (Garcia et al., 2015), and confirm that inhibiting CYP2B activity consistently increases brain nicotine levels at different nicotine test doses and routes of administration. The few studies which have examined nicotine levels in the brain by microdialysis (Chang et al., 2005; Katner et al., 2014; Woods et al., 2006) have focused on the time course of drug levels (Chang et al., 2005; Woods et al., 2006) or dose-response analyses (Katner et al., 2014). The current study, and previous report (Garcia et al., 2015), are the first to demonstrate that pharmacological manipulation, by inhibiting enzyme activity in the brain, can alter drug levels using this technique. Together these findings directly show that altering brain CYP2B activity can impact circulating levels of nicotine within the brain, even after peripheral administration of nicotine.
Brain nicotine levels were lower after 7-day nicotine treatment, consistent with higher brain CYP2B expression (Khokhar et al., 2010; Miksys et al., 2000). The changes in nicotine levels were found in the brain and not in the plasma, which is also consistent with increased CYP2B expression observed in rat brain but not in the liver (Miksys et al., 2000), and with similar plasma nicotine and cotinine levels reported in rats after one vs. seven nicotine injections at the same nicotine dose (1 mg/kg/s.c.) (Micu et al., 2003). Our results with microdialysis demonstrate that 7-day nicotine treatment, previously shown to induce brain-specific CYP2B protein, can alter brain levels of nicotine without altering peripheral levels.
Reducing brain, but not plasma, drug levels by CYP2B induction could reduce the efficacy of the centrally-acting drugs given at therapeutic doses. This was demonstrated by Khokhar et al. (2011), where the same 7-day nicotine treatment in rats reduced propofol-mediated anesthesia; anesthesia measurements correlated with brain but not plasma propofol levels. Thus, while plasma drug levels may be within the therapeutic range, brain drug levels may not be enough to elicit its effect. The effect of increasing brain CYP2B activity by induction on nicotine-mediated behavior has not been tested, but we have shown that inhibiting brain CYP2B activity can increase acquisition and motivation in intravenous nicotine self-administration (Garcia et al., 2015), suggesting that increasing brain CYP2B activity could have the opposite effect of inhibition in this behavior. As many CYP2B substrates are drugs that act in the brain, such as bupropion (Hesse, 2000), ketamine (Yanagihara et al., 2001), and methadone (Totah et al., 2008), or drugs that could enter the brain such as efavirenz (Ward et al., 2003), a functional increase in expression and resulting activity of CYP2B selectively in the brain could have the potential to alter the efficacy of these substrates.
Brain nicotine levels were consistent with changes in CYP2B activity. The reduction in brain nicotine levels following 7-day nicotine treatment did not persist after a two-week washout period with no nicotine, as brain nicotine levels from a single nicotine injection were higher in this washout compared to the chronic condition. Although brain nicotine levels in the washout condition appear higher than that seen in the acute condition, this was not significantly different. This is in agreement with CYP2B protein levels returning to baseline by seven days after the last (7th) nicotine treatment (Khokhar et al., 2010), suggesting that CYP2B expression was similar to baseline levels during microdialysis in the washout condition. Also, brain nicotine levels were higher when C8X was given after 7-day nicotine treatment. Together, these data provide strong support that increased or decreased CYP2B activity centrally alters nicotine levels in the brain.
If brain CYP2B induction by chronic nicotine exposure occurs in humans such as smokers, it could lead to a reduction in brain nicotine levels. Higher CYP2B6 protein levels in the brains of smokers have been previously reported (Miksys et al., 2003), suggesting the possibility of human brain CYP2B induction by nicotine. In contrast to nicotine’s ability to induce brain CYP2B levels, human hepatic CYP2B activity is not different in people with a smoking history (Hesse et al., 2004), which suggests that CYP2B activity in the liver is not influenced by chronic nicotine exposure from cigarettes. These two findings are consistent with CYP2B induction by nicotine in the brain but not the liver in rats (Miksys et al., 2000) and monkeys (Lee et al., 2006).
Altered brain nicotine levels after CYP2B induction, from chronic nicotine exposure during smoking, could alter smoking behaviours including nicotine withdrawal. Chronic nicotine exposure in rats and in smoking leads to adaptive changes, such as nAchR upregulation (Benwell et al., 1988; Lapchak et al., 1989), which is thought to be compensatory mechanisms in response to the continued presence of nicotine in the brain (De Biasi and Dani, 2011). In nicotine withdrawal, these receptors are unoccupied when nicotine is no longer present in the brain (De Biasi and Salas, 2008), possibly disrupting the activity of the neurotransmitter systems that express these receptors (Koob et al., 2004). Altered neurotransmitter activity in the absence of nicotine may be involved in the cognitive disturbances seen in withdrawal (McClernon et al., 2015) and subsequent nicotine intake may be a response to alleviate these symptoms (Koob et al., 2004). If brain CYP2B induction occurs along with these adaptive receptor and neurotransmitter changes, lower brain nicotine levels may lead to insufficient activation of the nAchRs, which could reduce nicotine’s attenuation of withdrawal symptoms. The diminished effect of nicotine could give rise to continued smoking in order to increase brain nicotine levels sufficiently to reduce negative withdrawal symptoms. Thus, smoking over time could increase brain nicotine metabolism and reduce brain nicotine levels, which may then contribute to persistent smoking and withdrawal behavior.
This is the first study to directly measure brain drug levels following induction of a drug metabolizing enzyme, such as CYP2B, in order to demonstrate that local enzyme induction could alter drug levels in the brain in vivo. One limitation of the study is that protein levels were not assessed to confirm that CYP2B was induced in the brains of our animals, however our microdialysis results are consistent with protein induction of brain CYP2B previously characterized (Khokhar et al., 2010; Miksys et al., 2000) and the in vivo effects on other CYP2B substrates such as propofol (Khokhar and Tyndale, 2011). Also, Khokhar et al. (2010) reported a time course of CYP2B protein levels up to seven days from the last (7th) injection. Our study complements these findings on CYP2B protein levels, demonstrating an effect on brain drug levels due to the increase or inhibition of the enzyme within the brain.
Another limitation was the use of only two time points for plasma nicotine assessment. It is difficult to obtain multiple samples using the procedure used for blood sampling (saphenous vein puncture), therefore we were unable to conduct a full time course of plasma nicotine levels in conjunction with microdialysis. However, plasma nicotine and cotinine levels were also measured after 4 hours in rats treated with 1 vs. 7 nicotine injections (Micu et al., 2003), where no difference for either the parent or metabolite was found, yielding similar findings to these current results at earlier time points. Thus, the plasma data from this work and Micu et al. (2003) indicate that 7-day nicotine treatment did not alter peripheral nicotine metabolism.
Free circulating nicotine levels were measured in the right lateral ventricle using microdialysis. This method allowed for the repeated sampling of brain nicotine concentrations over time in the same animal. As nicotine is known to accumulate in the brain following chronic nicotine treatment (Ghosheh et al., 2001), it was particularly important to assess the microdialysate immediately before the nicotine injection. In all testing conditions, there was no detectable nicotine in baseline dialysate samples collected within 15 minutes before the nicotine injection, indicating that nicotine was not in the brain CSF at detectable levels at the start of microdialysis collection. Thus, the changes in brain nicotine levels observed in our experiments were not likely affected by accumulated nicotine in brain tissue.
These experiments demonstrate that an established 7-day nicotine CYP2B induction paradigm could reduce nicotine levels in the brain without influencing peripheral nicotine levels. Additionally, this reduction in nicotine levels was reversed two weeks after the termination of the induction treatment and by pretreatment with a CYP2B inhibitor. Changes in brain levels of free nicotine may lead to altered behavioral response to nicotine, and this change over time with repeated nicotine exposure from smoking could be a contributing factor to increased consumption of cigarettes and the precipitation of withdrawal. Thus, drug metabolism in the brain, by influencing local drug levels, can play a role in subsequent drug response. In the case of nicotine, which has reinforcing properties thought to mediate tobacco dependence, altered drug metabolism in the brain could influence nicotine’s impact in these processes.
Supplementary Material
Acknowledgments
We would like to thank Fariba Baghai Wadji, Maryam Ghiasvand, and Sharon Miksys for their assistance with surgery and microdialysis collection and Maria Novalen and Bin Zhao for their technical assistance. This work was supported by CIHR (MOP97751) and NIDA R21 (DA029160–01) grants, the Centre for Addiction and Mental Health (CAMH), the Campbell Family Mental Health Research Institute of CAMH, the CAMH foundation, an Endowed Chair in Psychiatry (RFT), the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation.
Footnotes
Disclosure/Conflicts of Interest
RFT has consulted for Apotex. KLPG and ADL have no conflict of interest to declare.
Authors Contributions
KLPG performed all experiments; KLPG and RFT designed experiments and prepared the manuscript. ADL provided comments and edited the manuscript.
References
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clinical Pharmacology & Therapeutics. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. Journal of neurochemistry. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Chang Y-L, Tsai P-L, Chou Y-C, Tien J-H, Tsai T-H. Simultaneous determination of nicotine and its metabolite, cotinine, in rat blood and brain tissue using microdialysis coupled with liquid chromatography: Pharmacokinetic application. Journal of Chromatography A. 2005;1088:152–157. doi: 10.1016/j.chroma.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, Novalen M, Miksys S, Tyndale RF. Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug Metabolism and Disposition. 2014;42:1447–1455. doi: 10.1124/dmd.114.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, Addiction, Withdrawal to Nicotine. Annual review of neuroscience. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Experimental Biology and Medicine (Maywood) 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KL, Coen K, Miksys S, Le AD, Tyndale RF. Effect of Brain CYP2B Inhibition on Brain Nicotine Levels and Nicotine Self-Administration. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2’-(14)C]nicotine. Drug Metabolism and Disposition. 2001;29:645–651. [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR. Cigarette smokers self-administer intravenous nicotine. Pharmacology Biochemistry and Behavior. 1983;19:887–890. doi: 10.1016/0091-3057(83)90099-0. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, Moltke LL, Greenblatt DJ. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics and genomics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metabolism and Disposition. 2000;28:1176–1183. [PubMed] [Google Scholar]
- Katner SN, Toalston JE, Smoker MP, Rodd ZA, McBride WJ, Engleman EA. Time-course of extracellular nicotine and cotinine levels in rat brain following administration of nicotine: effects of route and ethanol coadministration. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar JY, Miksys SL, Tyndale RF. Rat brain CYP2B induction by nicotine is persistent and does not involve nicotinic acetylcholine receptors. Brain Research. 2010;1348:1–9. doi: 10.1016/j.brainres.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Tyndale RF. Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects. Neuropsychopharmacology. 2011;36:692–700. doi: 10.1038/npp.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar JY, Tyndale RF. Rat brain CYP2B-enzymatic activation of chlorpyrifos to the oxon mediates cholinergic neurotoxicity. Toxicological Sciences. 2012;126:325–335. doi: 10.1093/toxsci/kfs029. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Quirion R, Collier B. Effect of chronic nicotine treatment on nicotinic autoreceptor function and N-[3H]methylcarbamylcholine binding sites in the rat brain. Journal of neurochemistry. 1989;52:483–491. doi: 10.1111/j.1471-4159.1989.tb09146.x. [DOI] [PubMed] [Google Scholar]
- Lee AM, Miksys S, Palmour R, Tyndale RF. CYP2B6 is expressed in African Green monkey brain and is induced by chronic nicotine treatment. Neuropharmacology. 2006;50:441–450. doi: 10.1016/j.neuropharm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. Journal of neurochemistry. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Addicott MA, Sweitzer MM. Smoking abstinence and neurocognition: implications for cessation and relapse. Current topics in behavioral neurosciences. 2015;23:193–227. doi: 10.1007/978-3-319-13665-3_8. [DOI] [PubMed] [Google Scholar]
- Micu AL, Miksys S, Sellers EM, Koop DR, Tyndale RF. Rat hepatic CYP2E1 is induced by very low nicotine doses: an investigation of induction, time course, dose response, and mechanism. J Pharmacol Exp Ther. 2003;306:941–947. doi: 10.1124/jpet.103.052183. [DOI] [PubMed] [Google Scholar]
- Miksys S. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Miksys S, Hoffmann E, Tyndale RF. Regional and cellular induction of nicotine-metabolizing CYP2B1 in rat brain by chronic nicotine treatment. Biochemical pharmacology. 2000;59:1501–1511. doi: 10.1016/s0006-2952(00)00281-1. [DOI] [PubMed] [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45 doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego: 1986. [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y, Kanno I, Ito H, Suhara T. Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. International Journal of Neuropsychopharmacology. 2008;11:413–417. doi: 10.1017/S1461145707008103. [DOI] [PubMed] [Google Scholar]
- Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- Vieira-Brock PL, Andrenyak DM, Nielsen SM, Fleckenstein AE, Wilkins DG. Age-related differences in the disposition of nicotine and metabolites in rat brain and plasma. Nicotine & Tobacco Research. 2013;15:1839–1848. doi: 10.1093/ntr/ntt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The Cytochrome P450 2B6 (CYP2B6) Is the Main Catalyst of Efavirenz Primary and Secondary Metabolism: Implication for HIV/AIDS Therapy and Utility of Efavirenz as a Substrate Marker of CYP2B6 Catalytic Activity. Journal of Pharmacology and Experimental Therapeutics. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- Woods J, II, C K, Solomon B, Gunaratna C, Duda C, Kissinger C. Simultaneous determination of nicotine and cotinine pharmacokinetics in the blood and brain using a combination of automated blood sampling and in vivo microdialysis in Sprague Dawley rats. Current Separations and Drug Development. 2006;21:85–90. [Google Scholar]
- Yamanaka H, Nakajima M, Fukami T, Sakai H, Nakamura A, Katoh M, Takamiya M, Aoki Y, Yokoi T. CYP2A6 and CYP2B6 are involved in nornicotine formation from nicotine in humans: Interindividual differences in these contributions. Drug Metab Dispos. 2005;33:1811–1818. doi: 10.1124/dmd.105.006254. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Inoue K, Hashimoto M, Shimada T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Archives in Toxicology. 1999;73:65–70. doi: 10.1007/s002040050588. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, Iga T. Involvement of CYP2B6 in N-Demethylation of Ketamine in Human Liver Microsomes. Drug Metabolism and Disposition. 2001;29:887–890. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


