Abstract
Cell damage and death releases “alarmins”, or self-derived immunomodulatory molecules, that recruit and activate the immune system. Unfortunately, numerous processes critical to the transplantation of allogeneic materials result in the destruction of donor and recipient cells and may trigger alarmin release. Alarmins, often described as damage-associated molecular patterns (DAMPs), together with the exogenous pathogen-associated molecular patterns (PAMPs), are potent orchestrators of immune responses. Yet the precise role that alarmins play in the alloimmune responses remains relatively undefined. Here, we examine evolving concepts regarding how alarmins impact solid organ and allogeneic hematopoietic cell transplantation outcomes and the mechanisms by which self-molecules are released. We describe how once released, alarmins may act alone, or in conjunction with non-self materials, to contribute to cytokine networks controlling alloimmune responses and their intensity. It is becoming appreciated that this class of molecules has pleotropic functions and certain alarmins can promote both inflammatory and regulatory responses in transplant models. Emerging evidence indicates that alarmins and their receptors may be promising transplantation biomarkers. Developing the therapeutic ability to support alarmin regulatory mechanisms and use the predictive value of alarmin pathway biomarkers for early intervention may provide opportunities to benefit graft recipients.
Introduction
Transplant-associated procedures and alloimmune responses cause tissue and cell damage that can free endogenous (self-derived) molecules and exogenous (non-self) materials, which are perceived by the immune system. Pathogen-associated molecular patterns (PAMPs) found in non-self materials are recognized by pattern-recognition receptors (PRRs), including Toll-like receptors (TLRs), lectin receptors, Nod-like receptors (NLRs), and RIG-I-like receptors (1). Cells of the body sense and respond to invading pathogens when PRRs signaling pathways are activated (1). While innate immune cells are viewed as the most influential PRR expressers, adaptive immune cells and non-leukocytes also express PRRs and respond to PAMPs (2, 3). Self-derived materials, such as extracellular matrix proteins, heat-shock proteins, and high-mobility group box 1 (HMGB1), are often classified as damage-associated molecular patterns (DAMPs), which can also activate the immune system (1, 4, 5) (Table 1). However, most DAMPs engage the same set of receptors and/or activate the same signaling pathways as PAMPs. Thus, DAMPs and PAMPs are further grouped as a broader class of “danger-associated molecules patterns” (4, 5). Yet, the molecular and functional overlaps between DAMPs (danger- or damage-associated) and PAMPs have complicated establishing the importance of self- vs. non-self derived material in initiating the alloimmune response. Likewise, many self-molecules, such as cytokines and chemokines, can carry out DAMP-like functions when they alert and activate the immune system to tissue damage. Thus, to avoid confusion when describing self- vs. non-self molecule functions in this review, we will define immune modifying self-molecules using the more general term, “alarmins”. This term was coined by Dr. Joost Oppenheim to define endogenous molecules that signal tissue and cell damage (4). While the impact of non-self (viral, bacterial, or alloantigen) on solid organ transplantation (SOTx) and transplant (Tx) tolerance has been documented (6–8), how alloresponses are influenced by alarmins following transplantation has only recently begun to be considered. Likewise, how PAMPS and alarmins interface to direct transplant outcomes is poorly appreciated.
Table 1.
Proposed Alarmins and Their Impact on Alloimmunity
| Alarmin | Receptor | Impact | References |
|---|---|---|---|
| Nuclear Proteins | |||
|
High-mobility group box 1 (HMGB1) |
TLR2, TLR4, RAGE |
Promotes Alloreactive T cell responses, GHVD, and Tx rejection Pre-conditioning protects against I/R injury |
(34) (40) (39) (35) (36) (37) (38) (46) |
| IL-1α | IL-1R | (9) | |
| IL-33 | ST2 | Increases GVHD via augmentation of Type 1 Immunity Supports protection of HTx via Treg expansion |
(25) (26, 29) |
| Cytosolic Materials | |||
| Heme | TLR4 | Implicated in innate immune cell activation leading to increased alloimmune responses |
(12) (19) |
| Haptoglobin | MyD88-dependent Receptor |
Donor Haptoglobin Activates DC to stimulate allograft rejection |
(13) |
| Mitochondria | Formyl Peptide Receptor-1; TLR9 |
Proposed to activate innate responses after Tx |
(11) |
| ATP | P2X7 | Promotes GVHD | (15) |
| Heat Shock Proteins (HSP) | Promotes alloreactive T cell proliferation and GVHD Modulation of Treg may be protective against I/R |
(15) (48) |
|
| Extracellular Matrix Materials | |||
| Heparan Sulfate | TLR4-My88 | Mediates activation of DC to promote alloimmune responses |
(15) |
| Hyaluronan | TLR2/4-My88 | Low molecular weight hyaluronan degradation products activates APC to stimulate chemokine and cytokine production, which drives alloimmunity High molecular weight hyaluronan protects against epithelial cell apoptosis and attenuates allograft inflammation. |
(51) (52) (53) (50) (51) (53) |
Alarmin sources during transplantation
Multiple processes involved in SOTx, such as surgical procedures and their associated ischemia/reperfusion (I/R) injury, as well as recipient alloimmune responses, initiate a sterile inflammatory process that would not be associated with release of PAMPs (9). Likewise, SOTx and allogeneic hematopoietic cell transplantation (AlloHCT) may utilize induction protocols containing irradiation or therapeutic agents that cause dramatic cell death in recipient tissues, particularly the epithelium (10). Immunosuppressants, as well as leukocyte targeting antibodies, mediate the destruction of recipient leukocytes, as well as other recipient cells. As such, numerous proposed alarmins would be expected to arise from damaged or dying host cells during transplantation. While significant attention has been placed on how PAMPs initiate the immune response, how self-molecules shape immune responses is only beginning to be uncovered. Suggested alarmins include several nuclear proteins, such as high-mobility group box 1 (HMGB1), IL-1α, IL-33, and histones, as well other cellular components such as mitochondria and mitochondria DNA (11), ATP, Uric Acid, Heat shock proteins (HSP), heme (12) and haptoglobin (13) (Table 1). Multiple extracellular cellular matrix (ECM) fragments also have been suggested to act as alarmins, particularly heparan sulfate proteoglycan, fibronectin, fibrinogen hyaluronate, biglycan, tenascin, and hyaluronan. Details of many proposed alarmins (Table 1) have been provided in recent reviews, with several discussing their impact on the alloimmune responses leading to graft-versus-host disease (GVHD) (10, 14, 15). Rather than focusing on the general characterization of individual alarmins, we instead discuss how an evolving understanding of alarmin Immunobiology is being revealed in recent rodent studies, -primarily examining the impact of HMGB1 or IL-33 on AlloHCT and SOTx.
Although AlloHCT to generate mixed chimerism for the purpose of tolerance induction in SOTx is not a new concept, the procedure has recently gained significant momentum. Yet this procedure is still associated with greater incidence of early complications compared to traditional immunosuppression (16) and GVHD remains a major potential complication following AlloHCT (8, 17). We take this opportunity to summarize new findings and historically important publications characterizing how alarmins modulate alloimmune responses after both AlloHCT and SOTx.
Alarmins as pro-inflammatory mediators after transplantation
Alarmins were originally defined as proinflammatory endogenous molecules that mobilize and activate immune cells after cellular damage (4, 18). A significant component of the proinflammatory capacity of alarmins has been attributed to the ability of many alarmins, including HMGB1, ATP and heme, to drive the stimulatory capacity of macrophages and dendritic cells (DC) (19, 20). Like PAMPs, alarmins can promote the production of pro-inflammatory cytokines, particularly the IL-12 family cytokines IL-12p70 and IL-23. IL-12p70 signaling via IL-12Rβ1 and -β2 is key for CD4+Th1 cell development and directly promotes CD8+ T-cell IFNγ production (21, 22). In allotransplantation, IL-12 drives detrimental Type 1 alloimmune responses by IFNγ+ CD8+ T-cells, which are critical for GVHD and implicated in acute and chronic heart Tx (HTx) rejection, as well as lost Tx tolerance. Likewise, IL-23 stabilizes Th17 cells, also implicated in GVHD and inferior SOTx outcomes (21, 22).
An underappreciated capacity of IL-12p70 is its profound ability to synergize with IL-1 family members, including IL-1α, -β, -18, and -33, for the induction of IFNγ production by Type 1 effector cells. IL-18 was initially identified and described as Interferon-gamma inducing factor for its ability to enhance IFNγ production by T cells and NK cells, particularly in conjunction with IL-12 (23, 24). The prototypical IL-1 family member, IL-1, and the nuclear protein and alarmin, IL-1α, both promote IFNγ production by NK cells, though to a lesser degree than IL-18. Likewise, the IL-1 family member and classified alarmin, IL-33, has emerged as a critical factor controlling Type 1 cytokine production post-transplantation. IL-33 is a nuclear protein upregulated under recipient inflammatory conditions and once released promotes detrimental Type 1 donor alloimmunity and acute GVHD lethality (25). Initially, this was surprising given contrary evidence in other models that IL-33 is a potent mediator of Type 2 and regulatory immune responses (26, 27). However, utilizing IL-33R (a.k.a. ST2; IL1RL1) deficient donors and IL-33 deficient mice, as well as IL-33 antagonists, we demonstrated that IL-33, released early post-transplant, drives Type 1 immunity and lethal acute GVHD (25). Interestingly, ST2 expression was necessary for upregulation of T-cell IL-18R expression and IL-12 is appreciated as a potent inducer of ST2 on CD8+ T-cells (25, 28, 29). Likewise, IL-1β can increase ST2+ on leukocytes, including myeloid antigen presenting cells (APC) (30). Thus, there is an amplification network involving alarmins, including the IL-1 alarmins, IL-1α and IL-33, other IL-1 cytokines, and IL-12 family members, which shape the pro-inflammatory environment post-transplantation (Fig. 1). Further work in transplant models is necessary to establish the precise relationships in this network and the ideal targets to limit alloimmunity. For instance, we have demonstrated that the IL-33 antagonist, sST2-Fc, given on days 0, 3, 6, and 9 after allo-HCT was highly effective in preventing GVHD (25).
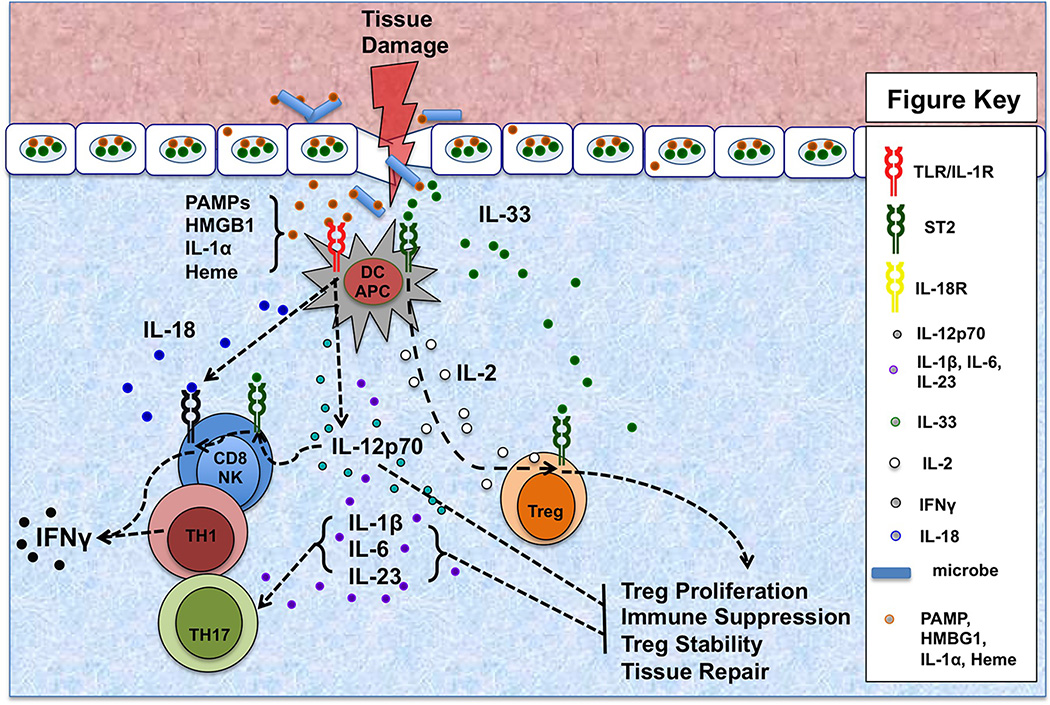
Figure 1. Cytokine Networks Shaping Alarmin Functions in Allotransplantation.
Tissue damage and cell death, particularly of the barrier tissues, results in the release of alarmins, including HMGB1, IL-1α, IL-33, and free heme. In the presence of PAMPs arising from infiltrating pathogens or commensal microbes, alarmins augment IL-12p70, IL-1β, IL-6, IL-18, and IL-23 production by TLR/IL-1R stimulated antigen-presenting cells (APC), including dendritic cells (DC). IL12p70 stimulates Th1 polarization and immune cell IFNγ production. In addition, IL-12p70 induces ST2 on NK and CD8+ T-cells, thus enabling them to respond to IL-33 with augmented IFNγ. IL-33 signaling may also facilitate CD8+ T-cells IFNγ secretion by supporting their responses to IL-18 by upregulating the IL-18 receptor. In addition to stabilizing the Th17 polarization of CD4+ T-cells, IL-23 also renders ST2+ Treg less responsive to IL-33. IL-12p70-stimulated T cells are resistant to regulation. However, in the absence of PAMPs, IL-33 does not cause DC pro-inflammatory cytokine production, but instead stimulates them to secrete IL-2 which works with IL-33 to expand Treg populations, particularly the subset expressing ST2, which is both suppressive and implicated in tissue repair.
Like IL-1α or IL-33, HMGB1 is a nuclear protein that acts as an alarmin when released from damaged or dying cells contributing to the pathogenesis of various inflammatory diseases (31). Similar to IL-1α, HMGB1 also turns myeloid APC into potent T-cell stimulators by upregulating APC co-stimulatory molecules and inducing pro-inflammatory cytokine secretion by APC, particularly IL-12p70 (20). As expected from these properties, released HMGB1 supports I/R injury (32, 33) and the alloimmune response leading to GVHD (34) and transplant rejection (35–38). Zou et al. has delivered anti-HMGB1 antibody to C57BL/6 recipients for 4 weeks post heterotopic transplantation of MHC class II disparate bm12 hearts and demonstrated that HMGB1 release supports chronic cardiac rejection (38). Further assessments of heart allografts and sera both revealed increased HMGB1 levels post-transplantation and decreased sera levels after anti-HMGB1 antibody delivery (38). Mechanistic studies indicate that decreases in systemic HMGB1 results in less activated DC and hence reduced Th1 and Th17 responses (38). Li et al. established that anti-HMGB1 antibodies suppressed B-cell activation and reduced IFNγ and IL-17A production after xenotransplantation of rat hearts into BALB/c mice (36). Anti-HMGB1 antibody delivery promoted allogeneic islet survival (39, 40). Matsuoka et al. found that HMGB1 triggered TLR2 and RAGE on monocytes to induce DC CD40 and IL-12 production, -leading to islet rejection due to NKT cell support of Gr-1+ CD11b+ cell IFNγ production (40). Treatment with IL-12– or CD40L-specific antibody could prevent early islet graft loss (40). Similarly, anti-IL-6 antibody prevented HMGB1-induced NKT cell IFNγ production and ameliorated islet rejection (39).
Experiments by Im et al. revealed a profound increase in circulating HMGB1, as well as increased HMGB1 protein in the spleen of AlloHCT mice during GVHD (34). In addition, HMGB1 promoted alloreactive T-cell proliferation ex vivo (34). Interestingly, the indole-derived antioxidant cyclopentyla-mino-carboxymethylthiazolylindole-7(NecroX-7) reduced target-tissue HMGB1 levels, stifled HMGB1-driven alloreactive T-cell response ex vivo, and decreased Th1 and Th17 generation in vivo after AlloHCT (34). NecroX-7 improved GVHD outcomes, and this was ascribed to NecroX-7 capacity to reduce reactive oxygen species (ROS). The authors suggest that ROS support HMGB1 translocation from the nucleus and subsequent release. It will be important, however, to further separate the impact of ROS reduction on HMGB1 directly as HMGB1 is inactivated in vivo by ROS-induced oxidation (41).
In total, there is significant evidence using anti-HMGB1 agents to support the conclusion that extracellular HMGB1 promotes heart and islet rejection via augmentation IFNγ and IL-17 dominated immune responses. Moving forward, it will be important to verify a direct detrimental role for HMGB1 in SOTx and AlloHCT using recently generated conditional knock-out mice (42). The use of the Cre-Lox system to target HMGB1 in GVHD-target tissues or the allograft should be especially informative. The positive outcomes through the use of anti-HMGB1, or anti-IL-6, or anti-IL-12 antibodies suggest that blocking HMGB1 or disruption of the cytokine networks initiated by HMGB1 may be warranted in allotransplantation.
Alarmins are alarming but not necessarily inflammatory
Notwithstanding the accumulating evidence that alarmins promote alloimmunity as described above, there is a building consensus that some alarmins may be instrumental in stimulating tissue repair and immune regulation. Indeed some alarmins are appear poorly pro-inflammatory, especially in the absence of pathogens. Instead, they may primarily stimulate low-level local inflammation, while orchestrating efforts to regulate local inflammatory responses and return the organism to homeostasis by mediating damaged tissue repair (27, 29, 43–45). In support of this view, there are multiple reports demonstrating that preconditioning rodent recipients by systemic administration of alarmins, such as HMGB1 or IL-33, has a protective effect against I/R injury, can prolong survival in experimental transplant models, and expands multiple regulatory cell populations including T regulatory cells (Treg) and regulatory myeloid cells (RMC) (26, 29, 46, 47). Heat shock protein HSP70 can also mediate protective functions against ischemic kidney injury through modulation of Tregs (48). Hyaluronan (HA), a major ECM component, is a nonsulfated glycosaminoglycan that exists as an extremely high-molecular-weight polymer (>4,000 KDa) under normal physiological conditions (49, 50). During tissue injury and local inflammation, including lung transplant rejection, hyaluronan is degraded to lower molecular weight HA species (≈100–400 KDa) that activate DC and macrophages via TLR2 and TLR4 (50–52). Accumulated lower-molecular-weight HA species are potent stimulators of inflammation after lung injury and drive lung transplant rejection, especially if they can not be removed via the lymphatic vessels (50, 52). Yet, consistent with the dual roles displayed by other alarmins in inflammation and its resolution, the presence of high-molecular-weight HA supports lung epithelial cell integrity (51) and attenuates allograft inflammation (53).
High doses of many alarmins do stimulate immune cell production of pro-inflammatory cytokines, including IL-1β, IL-6, IL-12p70, IL-23, as well as facilitate Type 1 immunity (12, 13, 15, 20). Yet, alarmin release that is part of normal cell turnover must not reach a level required to sustain a pro-inflammatory environment. We have found that IL-33 alone does not induce significant DC secretion of IL-1β, IL-6, IL-12p70, or IL-23, nor does it support DC generation of allogeneic Th1 or Th17 responses (29). Instead, IL-33 endows DC with the capacity for Th2 polarization and Treg expansion (29). The capacity of IL-33 to expand Treg is due, at least in part, to its capacity to stimulate DC and mast cell secretion of IL-2 (29, 54). Expansion of Treg in vivo by IL-33 requires CD11c+ DC and Treg expanded through IL-33 delivery predominantly are an ST2-expressing population (29). IL-33 stimulation of ST2+ Treg in the lung has recently been demonstrated to support tissue repair after viral infection (44). Thus, alarmins such as IL-33 and HSP70 have the capacity to support Treg expansion and functions.
Interestingly, while IL-33 does not induce significant macrophage pro-inflammatory cytokine production, it does not impede, and actually augments IL-6 secretion upon subsequent exposure to LPS (55). Thus, during tissue damage, while released IL-33 would not stimulate, it also would not be expected to limit DC activation by PAMPs derived from infiltrating pathogens. DC response to PAMPs is typified by IL-12p70 secretion leading to ST2 upregulation on CD8+ T-cells (25, 28). ST2 expression allows CD8+ T-cells to respond to IL-33 and TCR stimulation with profoundly augmented IFNγ production (25, 28, 29). In addition to IL-12p70, IL-1β, IL-6, and IL-23 are also commonly produced by PAMP-stimulated myeloid cells. In addition to promoting Type 1 and Th17 immune responses, these cytokines each have been demonstrated to limit Treg functions, (56). Thus, PAMP-activated DC may propagate pro-inflammatory states by secreting cytokines that differentiate T effectors, but also counter the function of Treg expanding in response to alarmins.
We suggest that the local presence of other pro-inflammatory cytokines is a critical switch controlling alarmin regulatory versus pro-inflammatory functions. For example, IL-12 has been shown to support effector T-cell proliferation and cytokine production even in the presence of Treg (22). Similarly, IL-23 negatively regulates the differentiation of CD4+ T-cells into Treg (22) and blocks IL-33 stimulation of ST2+ Treg (43). IL-12/23 domination of IL-33 and other alarmins would make sense for a process of pathogen removal and subsequent tissue repair. The presence of alarmins in the absence of IL-12/23 would signify the lack of pathogens and free ST2+ Treg and other ST2+ reparative cells to initiate repair of the damaged tissue, thereby releasing IL-33 (27). However, any PAMP-activated myeloid cells producing IL-23 would suppress ST2+ Treg responses to IL-33 and drive Th17 immunity (Fig. 1). Likewise, IL-12p70-induced expression of ST2 on CD8+-T-cells would switch IL-33 functions towards those supporting pathogen removal via CD8 and CD4 production of IFNγ (Fig. 1). Such mechanisms, while logical, need to be tested directly. Initial studies in mice using an anti-IL-12p40 antibody that targets the shared subunit of both IL-12 and IL-23 have been promising. These studies found anti-IL-12p40 antibodies reduced Th1 and Th17 T-cell polarization in vivo during GVHD (57, 58) and following heart allograft transplantation in mice (59, 60). There was little or no impact on Th2 or Treg populations. This suggests that countering IL-12 and IL-23 in vivo will limit the pro-inflammatory functions of certain alarmins thereby permitting the regulatory and/or tissue reparative pathways to prevail. While most of these studies have focused on the outcome of T-cell polarization, further experiments that identify mechanisms underlying these effects are needed to fully understand how to successfully manipulate these cytokine pathways for the benefit of transplantation outcome.
Mechanisms of alarmin release and their relationship to pro-inflammation
Recipient conditioning, as well as donor organ I/R injury and post-transplant anti-alloimmune responses results in cell death and tissue damage that will provide a source of released alarmins and potentially accompanying alloantigens. While much still needs understood regarding how the simultaneous release of intracellular alarmins and alloantigen from dying cell impacts on alloimmune responses and transplant outcomes, recent publications suggest the importance of this knowledge. In the case of AlloHCT, the rapidly dividing cells of mucosal barrier tissues are particularly susceptible to death during conditioning regimens. Barrier tissue breaches result in the release of commensal bacteria-associated PAMPs that instigate proinflammatory cytokine generation supporting GVHD development. A growing body of evidence also has implicated PAMP signaling in several regulated cell death (RCD) pathways (61). Apoptosis is a common RCD pathway that involves the apoptotic caspases -3, -7, and -8 and is viewed as non-inflammatory cell death (61). Necroptosis, however, is caspase- independent and requires receptor-interacting protein kinase (RIP3K) activity (61). Unlike apoptosis, which is regarded as a non-inflammatory mechanism of cell death, necroptosis, as well as other non-apoptotic RCD pathways like ferroptosis or pyroptosis, result in membrane rupture and release of cell contents such that they trigger an inflammatory response (61). A detrimental impact of alarmins released by necroptosis after HTx has been suggested in rodents (62). Interestingly, PAMP signaling also increases cellular alarmin levels (63, 64). Thus, PAMP-driven signaling could both increase expression of these potential pro-inflammatory mediators, while facilitating their release into the extracellular space by triggering RCD. This may be especially important pathway for nuclear alarmins, including IL-33 and HMGB1, to exit the cell in an inflammatory context and contribute to systemic immune responses. Once released IL-33 can be subjected to proteolytic cleavage by neutrophil elastases that enhance its bioactivity (65). The fact that neutrophils are typically the first responders following gut tissue injury suggests that neutrophil elastase cleavage of IL-33 may be a significant mechanism supporting inflammation (65).
Interesting work assessing how dying cells initiate adaptive immunity indicates that alarmins are not inherently able to promote adaptive immunity (66). In fact, the release of HMGB1 and ATP from a dying cell alone could not promote robust cross-priming and CD8 responses to released antigens unless NF-κB and RIP1K were also activated in dying cells. Moving forward, it can be expected that the study of how RCD, especially of alloantigen expressing graft cells, shapes alarmin function and alloimmune responses will be a critical area of research in transplantation.
Alarmins and their receptors as diagnostic and prognostic biomarkers
Biomarkers that can readily identify pathogenic alloimmune response are needed to improve outcomes after both SOTx and AlloHCT. As alarmin expression is often driven by local inflammation or pro-inflammatory cytokines, alarmins may have the potential to act as sensitive transplant biomarkers. Serum levels of HMGB1 and the heme binding protein, haptoglobin, are indicative of solid organ damage (13, 67) and GVHD (68, 69). While IL-33 serum levels increase during numerous inflammatory diseases (70), there are no reports that serum IL-33 is meaningfully increased during alloimmune responses. Conversely, there are now several examples suggesting that measuring serum levels of the IL-33 antagonist, soluble ST2 (sST2) may have value in transplantation. sST2 is an IL-33 decoy receptor generated through alternative RNA splicing and processing of the same RNA that also yields ST2, the membrane-bound, signaling form (71, 72). ST2 and sST2 are induced or augmented in leukocytes and stromal cells by pro-inflammatory cytokines, TLR ligands, and mechanical stress. Building upon our early rodent studies that established that ST2 is increased locally, but not systemically during rejection of heterotopic heart transplants (26), we showed that the alloimmune-associated pro-inflammatory cytokines increase graft ST2 during rejection episodes in pediatric SOTx recipients (73). Graft ST2 increases were reflected in increased serum sST2 levels that signified rejection in cohorts of small bowel or heart transplant recipients (73). Similarly, high serum sST2 acts as a reliable biomarker predicting resistance in GVHD patients to corticosteroid therapy and non-relapse mortality (74). Additional studies found high sST2 levels at day 28 post-Tx with increased transplant-related mortality and acute GVHD (75). Further supporting the value of ST2 monitoring after AlloHCT was the report that ST2, with TNFR1 and Reg3a could classify patients at GVHD onset into those at risk of treatment failure and non-relapse mortality (76). In total, these studies suggest that routine serum sST2 quantification, especially combined with other biomarkers, will have value in supporting biopsy-free detection of early allograft rejection in SOTx, as well as aiding effective therapeutic resolution of alloimmune responses causing graft rejection or GVHD.
Conclusions
The study of alarmins and their influence on alloimmune responses suggests that how cells die and the way they release both these potentially immunostimulatory contents and alloantigens is of critical importance to AlloHCT and SOTx outcomes. There is mounting evidence from transplant, as well as other disease and infection models, that alarmins can be both inflammatory and regulatory. Such observations suggest that developing an understanding of the processes that control alarmin functions may allow us to harness alarmins by countering their pro-inflammatory triggers and optimizing their regulatory properties.
Acknowledgments
We thank Carla Forsythe for excellent administrative support in the generation of this manuscript. The authors are supported by multiple grants from the National Institutes of Health (R01HL122489 and R21AI121981: HRT); R01AI34495 and R01HL56067: BRB); T32HL007062 (DKR) and T32AI074490 (BMM). Further funding was derived from an American Society of Transplantation/Pfizer Basic Science Faculty Development Grant (HRT) and American Heart Association Grant (#14GRNT20400004; HRT).
Abbreviations
- AlloHCT
allogeneic hematopoietic cell transplant
- APC
antigen presenting cells
- DAMPS
damage-associated molecular patterns
- DC
dendritic cells
- GVHD
graft-versus-host disease
- HMGB1
high-mobility group box 1
- HSP
heat shock protein
- I/R
ischemia/reperfusion
- NLR
nod-like receptor
- PAMPs
pathogen-associated molecular patterns
- PRR
pattern recognition receptor
- RCD
regulated cell death
- RMC
regulatory myeloid cells
- ROS
reactive oxygen species
- SOTx
solid organ transplantation
- sST2
soluble ST2
- TLR
toll-like receptor
- Treg
T regulatory cells
- Tx
transplantation
Footnotes
Supplemental Material: None
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod H, Wetzler LM. T cell activation by TLRs: a role for TLRs in the adaptive immune response. Science's STKE : signal transduction knowledge environment. 2007;2007(402):e48. doi: 10.1126/stke.4022007pe48. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(32):13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 5.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological reviews. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 6.Alegre ML, Mannon RB, Mannon PJ. The microbiota, the immune system and the allograft. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(6):1236–1248. doi: 10.1111/ajt.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberbarnscheidt MH, Lakkis FG. Innate allorecognition. Immunological reviews. 2014;258(1):145–149. doi: 10.1111/imr.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono H, Onda A, Yanagida T. Molecular determinants of sterile inflammation. Current opinion in immunology. 2014;26:147–156. doi: 10.1016/j.coi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Brennan TV, Rendell VR, Yang Y. Innate immune activation by tissue injury and cell death in the setting of hematopoietic stem cell transplantation. Frontiers in immunology. 2015;6:101. doi: 10.3389/fimmu.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Current opinion in immunology. 2016;38:94–100. doi: 10.1016/j.coi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Song Y, Colangelo CM, Wu T, Bruce C, Scabia G, et al. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. The Journal of clinical investigation. 2012;122(1):383–387. doi: 10.1172/JCI58344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramadan A, Paczesny S. Various forms of tissue damage and danger signals following hematopoietic stem-cell transplantation. Frontiers in immunology. 2015;6:14. doi: 10.3389/fimmu.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova P, Zeiser R. The role of danger signals and ectonucleotidases in acute graft-versus-host disease. Human immunology. 2016 doi: 10.1016/j.humimm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Current opinion in organ transplantation. 2013;18(4):402–407. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]
- 17.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Science translational medicine. 2015;7(280):280rv282. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Wei F, Tewary P, Howard OM, Oppenheim JJ. Alarmin-induced cell migration. European journal of immunology. 2013;43(6):1412–1418. doi: 10.1002/eji.201243138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(39):E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. The Journal of experimental medicine. 2012;209(1):157–171. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32(12):603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nature medicine. 2015;21(7):719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 23.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerdiles Y, Ugolini S, Vivier E. T cell regulation of natural killer cells. The Journal of experimental medicine. 2013;210(6):1065–1068. doi: 10.1084/jem.20130960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125(20):3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187(9):4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42(6):1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8(+) T cells. European journal of immunology. 2011;41(11):3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, et al. IL-33 Is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol. 2014;193(8):4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J Immunol. 2008;181(1):62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nature reviews Rheumatology. 2012;8(4):195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 32.Kaczorowski DJ, Nakao A, Vallabhaneni R, Mollen KP, Sugimoto R, Kohmoto J, et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;87(10):1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H, Li J, Wang S, Liu K, Wang L, Huang L. Hmgb1-TLR4-IL-23-IL-17A axis promote ischemia-reperfusion injury in a cardiac transplantation model. Transplantation. 2013;95(12):1448–1454. doi: 10.1097/TP.0b013e318293b7e1. [DOI] [PubMed] [Google Scholar]
- 34.Im KI, Kim N, Lim JY, Nam YS, Lee ES, Kim EJ, et al. The Free Radical Scavenger NecroX-7 Attenuates Acute Graft-versus-Host Disease via Reciprocal Regulation of Th1/Regulatory T Cells and Inhibition of HMGB1 Release. J Immunol. 2015;194(11):5223–5232. doi: 10.4049/jimmunol.1402609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan L, Wang CY, Chen J, Gong Q, Zhu P, Zheng F, et al. High-mobility group box 1 promotes early acute allograft rejection by enhancing IL-6-dependent Th17 alloreactive response. Laboratory investigation; a journal of technical methods and pathology. 2011;91(1):43–53. doi: 10.1038/labinvest.2010.141. [DOI] [PubMed] [Google Scholar]
- 36.Li JH, Zhao B, Zhu XH, Wang L, Zou HJ, Chen S, et al. Blockade of Extracellular HMGB1 Suppresses Xenoreactive B Cell Responses and Delays Acute Vascular Xenogeneic Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(8):2062–2074. doi: 10.1111/ajt.13275. [DOI] [PubMed] [Google Scholar]
- 37.Moser B, Szabolcs MJ, Ankersmit HJ, Lu Y, Qu W, Weinberg A, et al. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(2):293–302. doi: 10.1111/j.1600-6143.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 38.Zou H, Yang Y, Gao M, Zhang B, Ming B, Sun Y, et al. HMGB1 is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(8):1765–1777. doi: 10.1111/ajt.12781. [DOI] [PubMed] [Google Scholar]
- 39.Itoh T, Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, et al. HMGB1-Mediated Early Loss of Transplanted Islets Is Prevented by Anti-IL-6R Antibody in Mice. Pancreas. 2015;44(1):166–171. doi: 10.1097/MPA.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 40.Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. The Journal of clinical investigation. 2010;120(3):735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. The Journal of experimental medicine. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanai H, Matsuda A, An J, Koshiba R, Nishio J, Negishi H, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513(7519):564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162(5):1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nature immunology. 2015;16(3):276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Steenstra R, de Boer EC, Zhao CY, Ma J, van der Stelt JM, et al. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney international. 2014;85(4):824–832. doi: 10.1038/ki.2013.475. [DOI] [PubMed] [Google Scholar]
- 47.Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer research. 2014;74(20):5723–5733. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, et al. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney international. 2014;85(1):62–71. doi: 10.1038/ki.2013.277. [DOI] [PubMed] [Google Scholar]
- 49.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 50.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. The Journal of clinical investigation. 2015;125(11):4255–4268. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature medicine. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 52.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(11):2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 53.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43(1):175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno T, Oboki K, Morita H, Kajiwara N, Arae K, Tanaka S, et al. Paracrine IL-33 stimulation enhances lipopolysaccharide-mediated macrophage activation. PLoS One. 2011;6(4):e18404. doi: 10.1371/journal.pone.0018404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell DJ. MyD88 and IL-1: loosening T(reg) cells' firm grip. Trends Immunol. 2014;35(3):95–96. doi: 10.1016/j.it.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto S, Fujiwara H, Nishimori H, Matsuoka K, Fujii N, Kondo E, et al. Anti-IL-12/23 p40 antibody attenuates experimental chronic graft-versus-host disease via suppression of IFN-gamma/IL-17-producing cells. J Immunol. 2015;194(3):1357–1363. doi: 10.4049/jimmunol.1400973. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Bastian D, Schutt S, Nguyen H, Fu J, Heinrichs J, et al. Essential Role of Interleukin-12/23p40 in the Development of Graft-versus-Host Disease in Mice. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Xu X, Xie A, Li J, Ye P, Liu Z, et al. Anti-interleukin-12/23p40 antibody attenuates chronic rejection of cardiac allografts partly via inhibition gammadeltaT cells. Clinical and experimental immunology. 2012;169(3):320–329. doi: 10.1111/j.1365-2249.2012.04612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie A, Wang S, Zhang K, Wang G, Ye P, Li J, et al. Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation. 2011;91(1):27–34. doi: 10.1097/tp.0b013e3181fdd948. [DOI] [PubMed] [Google Scholar]
- 61.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nature reviews Immunology. 2014;14(11):759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 62.Pavlosky A, Lau A, Su Y, Lian D, Huang X, Yin Z, et al. RIPK3-mediated necroptosis regulates cardiac allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(8):1778–1790. doi: 10.1111/ajt.12779. [DOI] [PubMed] [Google Scholar]
- 63.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, et al. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189(1):50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350(6258):328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14(10):1517–1525. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- 68.McGuirk J, Hao G, Hou W, Abhyankar S, Williams C, Yan W, et al. Serum proteomic profiling and haptoglobin polymorphisms in patients with GVHD after allogeneic hematopoietic cell transplantation. Journal of hematology & oncology. 2009;2:17. doi: 10.1186/1756-8722-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kornblit B, Masmas T, Petersen SL, Madsen HO, Heilmann C, Schejbel L, et al. Association of HMGB1 polymorphisms with outcome after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(2):239–252. doi: 10.1016/j.bbmt.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nature reviews Immunology. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 71.Lipsky BP, Toy DY, Swart DA, Smithgall MD, Smith D. Deletion of the ST2 proximal promoter disrupts fibroblast-specific expression but does not reduce the amount of soluble ST2 in circulation. European journal of immunology. 2012;42(7):1863–1869. doi: 10.1002/eji.201142274. [DOI] [PubMed] [Google Scholar]
- 72.Mueller T, Jaffe AS. Soluble ST2--analytical considerations. Am J Cardiol. 2015;115(7 Suppl):8B–21B. doi: 10.1016/j.amjcard.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Mathews LR, Lott JM, Isse K, Lesniak A, Landsittel D, Demetris AJ, et al. Elevated ST2 Distinguishes Incidences of Pediatric Heart and Small Bowel Transplant Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(3):938–950. doi: 10.1111/ajt.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. The New England journal of medicine. 2013;369(6):529–539. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ponce DM, Hilden P, Mumaw C, Devlin SM, Lubin M, Giralt S, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199–205. doi: 10.1182/blood-2014-06-584789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A Prognostic Score for Acute Graft-Versus-Host Disease Based on Biomarkers: A Multicenter Study. The Lancet Haematology. 2015;2(1):e21–e29. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]