Abstract
A little studied lesion, the resolving lesion, is described in multiple sclerosis (MS). Unusual features of early resolving lesions comprised a fibrous astrogliotic parenchyma replete with lipid-laden (foamy) microglia/macrophages widely scattered throughout and lined up at the edge, separating demyelinated plaque from myelinated white matter. Ongoing myelin breakdown was absent, as was remyelination. Later resolving lesions displayed the unusual coexistence of macrophages and remyelination within the gliotic parenchyma. Collectively, these observations may provide for the first time evidence for a role in MS for mitigating factors like alternatively-activated (M2) microglia/macrophages, known to have an anti-inflammatory phenotype and to be associated with wound-healing and repair.
Keywords: Multiple sclerosis, microglia, foamy macrophages, remyelination, resolving plaque, alternative activation, regulatory mechanisms
Introduction
This overview will highlight structural events, some of them novel, occurring at the margins of the resolving lesion in multiple sclerosis (MS). From a neuropathologic standpoint, two long-pondered questions are posed: first, why does the typical MS plaque have a sharp edge?; and second, why does the plaque not progress relentlessly throughout the central nervous system (CNS)? Through the eyes of the resolving lesion, this overview will engage these questions, notwithstanding that they have already been addressed to some extent in the literature in studies which concluded that events at the lesion edge were related to astroglial scarring and/or oligodendroglial responses. However, when framed against current opinions on lesion pathogenesis and macrophage dynamics, such explanations appear inadequate. In this regard, this account will break the mold and propose that in addition to glial cells, activated microglia/macrophages (currently a hot-button topic in neuroimmunology), a prominent feature in resolving lesions, play seminal roles and may orchestrate reparatory and regulatory functions.
Results
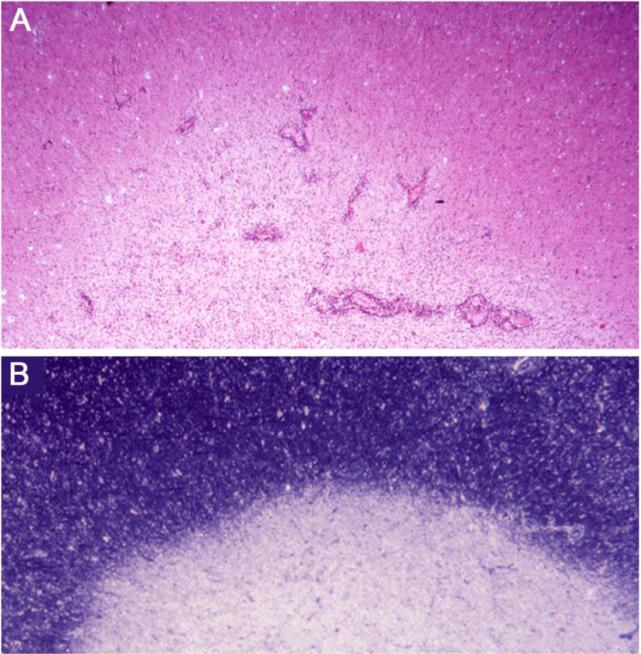
For fuller coverage of the pathology associated with the spectrum of lesion types and stages in MS, a major chronic debilitating neurologic condition of young adults, the reader is referred to the classic accounts of Adams and Sidman (1968), Lumsden (1970), and Greenfield and Norman (1971), in addition to the more recent, detailed analyses of Prineas et al. (2002); Lassmann and Wekerle (2005); Frohman et al. (2006); Ludwin and Raine (2008); and Moore and Stadelmann-Nessler (2015). Although resolving lesions per se in MS have been largely overlooked, one study by Prineas et al. (1993a) on CNS remyelination in nascent MS lesions did mention them briefly. For the purposes of the present discourse, drawn from early autopsy and biopsy CNS tissue from cases of acute and primary progressive MS, it might be helpful to the reader to reiterate the salient features of acute and chronic MS lesions for comparison with the more transient resolving lesion. In the acute MS lesion, the edge is broad and indistinct as demyelinated CNS parenchyma transitions gradually into normal-appearing white matter (NAWM) – Figure 1A. This zone, as well as most of the body of the lesion, is heavily infiltrated perivascularly and parenchymally by hematogenous cells (small lymphocytes, plasma cells and monocytes), the phenotypic analysis of which has been extensively investigated – see Ludwin and Raine (2008), inter alia. At the other end of the spectrum, the demyelinated, chronic (silent) MS lesion has a sharp edge, is strikingly less cellular, is composed mainly of fibrous astrocytes and microglia, is essentially free of cellular infiltration, and stands out prominently from NAWM (Figure 1B).
Figure 1.
A. Low magnification of the margin of an acute MS plaque. Note the broad, indistinct edge where ongoing inflammatory activity is seen around blood vessels and within the hypercellular parenchyma. Normal appearing white matter (NAWM) lies above, demyelinated plaque, below. From a case of acute MS with a 2 week clinical history; paraffin section, H&E stain.
B. The margin of a chronic (silent) MS lesion displays a characteristic sharp edge between NAWM above and demyelinated plaque below. Secondary progressive MS, 11 year clinical history. Epoxy section, toluidine blue stain.
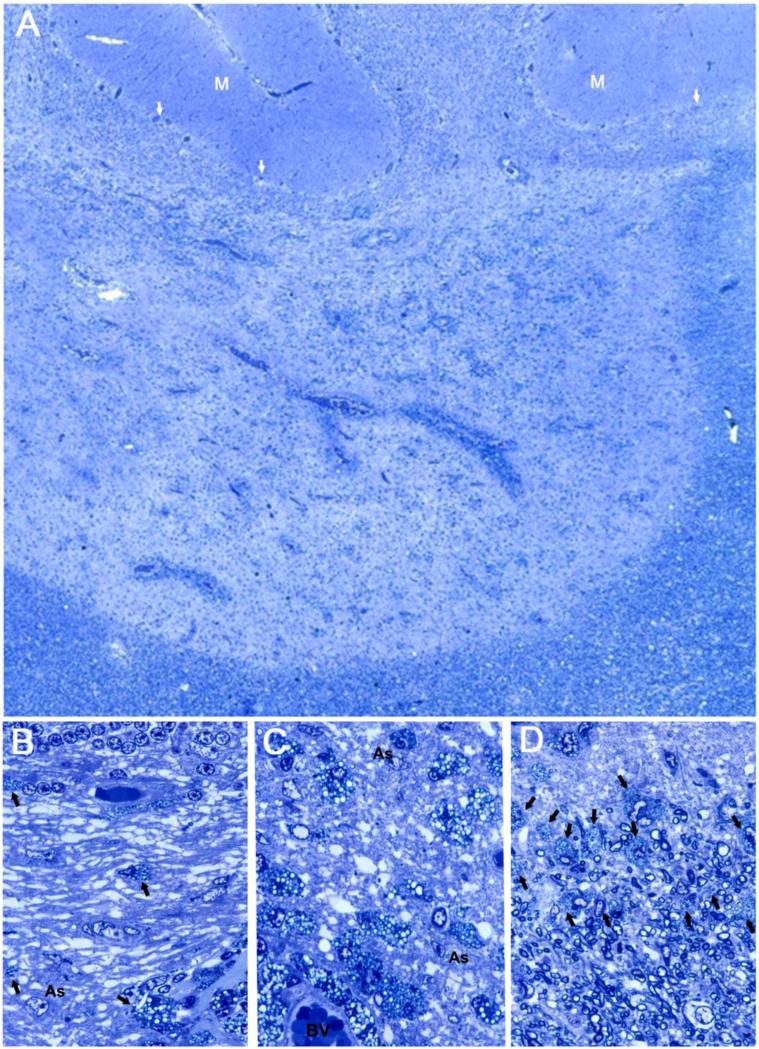
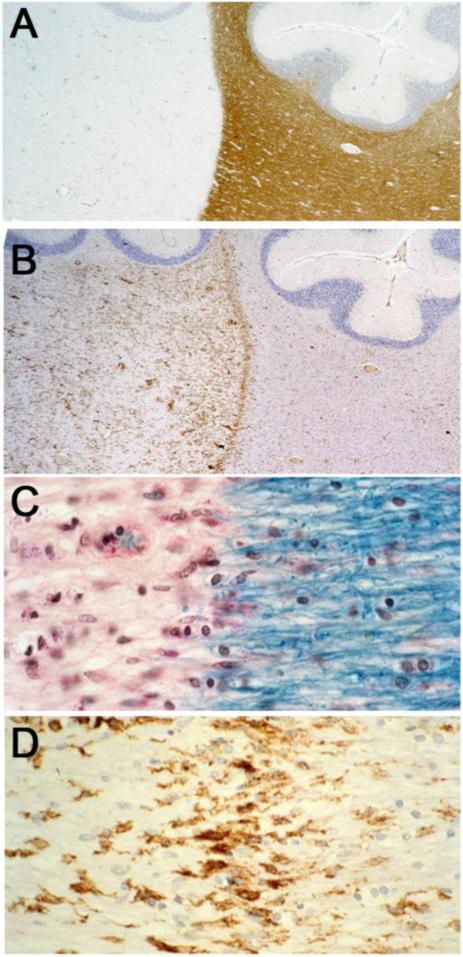
Resolving lesions arise within or adjacent to late-stage acute lesions and in areas of recent activity related to chronic lesions (usually in cases of primary progressive MS), by which time, active demyelination has subsided and infiltrating hematogenous cells have departed, leaving in their place an homogeneous population of foamy microglia/macrophages. In comparison to acute and chronic lesions (vide supra), early resolving lesions examined in one micron thick epoxy sections displayed a mixed phenotype ostensibly possessing the hypercellularity and perivascular infiltrates of an acute lesion and the sharp edge and fibrous astrogliosis of a chronic MS lesion (Figure 2A). Closer examination, however, revealed that the hypercellularity was due not to active inflammation (indeed, evidence of lymphocytic infiltration could not be found), but to diffusely scattered, foamy microglia/macrophages throughout a fibrous astrogliotic parenchyma (Figure 2B). Surprisingly, the perivascular infiltrates were due not to hematogenous elements but to aggregates of lipid-laden macrophages (Figure 2C), perhaps in the process of exiting the tissue. The microglia/macrophages lacked evidence of myelin phagocytosis and were filled with neutral lipid droplets. In the study by Prineas et al. (1993a), on nascent lesions in MS, similar macrophages stained positively by Sudan Black and Oil-red-O and like the present study, were not associated with myelin breakdown products. Along the margin of the resolving lesion where demyelinated astrogliotic parenchyma and myelinated white matter met abruptly, a layer of foamy microglia/macrophages could be discerned (Figure 2D). This alignment of cells was highly reminiscent of images from another case of acute MS in which staining of whole brain paraffin sections for myelin revealed sharp-edged demyelinated plaques (Figure 3A), the edges of which in matching sections showed, a prominent zone of class II MHC-positivity (Figure 3B). Detail of the same lesion edge in Luxol-fast-blue/periodic-acid-Schiff stained preparations revealed intact myelin internodes extending from the NAWM to terminate abruptly at the lesion edge and microglia/macrophages with rod-shaped nuclei to be aligned along the interphase (Figure 3C). Thus, totally demyelinated lesion and myelinated white matter were separated by little more than the width of a node of Ranvier. Myelin debris was notably absent in these early resolving lesions, as was remyelination. Detail from matching preparations revealed the class II MHC-positive cells to exhibit a mixed macrophage/microglial phenotype (Figure 3D), and to be arranged in a linear fashion, tapering off diffusely towards the lesion proper. The presence of such a zone of cells with an activated phenotype may betray the presence of local regulatory/protective mechanisms, perhaps to restrict lesion expansion and/or to set the stage for repair (discussed below).
Figure 2.
A. Low magnification of an early resolving lesion from the cerebellum of a case of primary progressive MS, 3 year clinical history. Note cerebellar folia above, with molecular layer at (M) and Purkinje cells at arrows, overlying the granule cell layer. The lesion edge along the NAWM (bottom and right), is well demarcated. Perivascular infiltrates surround blood vessels within the demyelinated lesion. The background parenchyma is replete with cells identified at higher magnification as foamy microglia/macrophages.
B. Detail of (A) to show the demyelinated white matter lying beneath the granule cell layer at top, with a demyelinated astrogliotic matrix in which lie scattered foamy macrophage/microglia (arrows), the occasional astrocyte cell body (As), and blood vessels.
C. Detail of an area deeper in the lesion to show the abundance of rounded foamy microglia/macrophages in the demyelinated, gliotic background, some of which surround a blood vessel (BV), below.
D. Detail of the edge of the same early resolving lesion with lesion above and NAWM containing myelinated nerve fibers, below. A zone of foamy microglia/macrophages (arrows) lies along the interphase. A-D are from the same one micron epoxy section, toluidine blue stain.
Figure 3.
A. Low magnification of a cerebellar lesion similar to Figure 2 displaying myelin-stained NAWM (lower right) extending upwards between two folia and a demyelinated plaque to the left. Cerebellar folia lie above. Note the sharp margin to the lesion. Acute MS, 10 month clinical history. Paraffin section, immunostained with anti-MBP, hematoxylin counterstain. This image and Figures 3B, C and D are from paraffin sections from the same case and are reproduced with the kind permission of Dr. John Prineas, Sydney, Australia.
B. Same lesion, stained for class II MHC. Note the abundant immunoreactivity within the lesion (left) and the intense line of MHC II staining along the edge. Low level reactivity also exists in the NAWM (right). Cerebellar folia counterstained with hematoxylin lie above.
C. Detail of the edge of the above lesion, stained for myelin with Luxol-fast-blue/periodic-acid-Schiff. Note how the myelin sheaths of fibres entering from the right terminate abruptly as they meet lesion area. Note also the lining-up of microglia/macrophages with rod-shaped nuclei at the interphase, the lack of myelin debris, and the decrease in oligodendroglial cell nuclei within the demyelinated area.
D. Detail of a matching field to (C), immunoreacted for class II MHC. Note the prominent class II MHC positivity on microglia/macrophages along the edge.
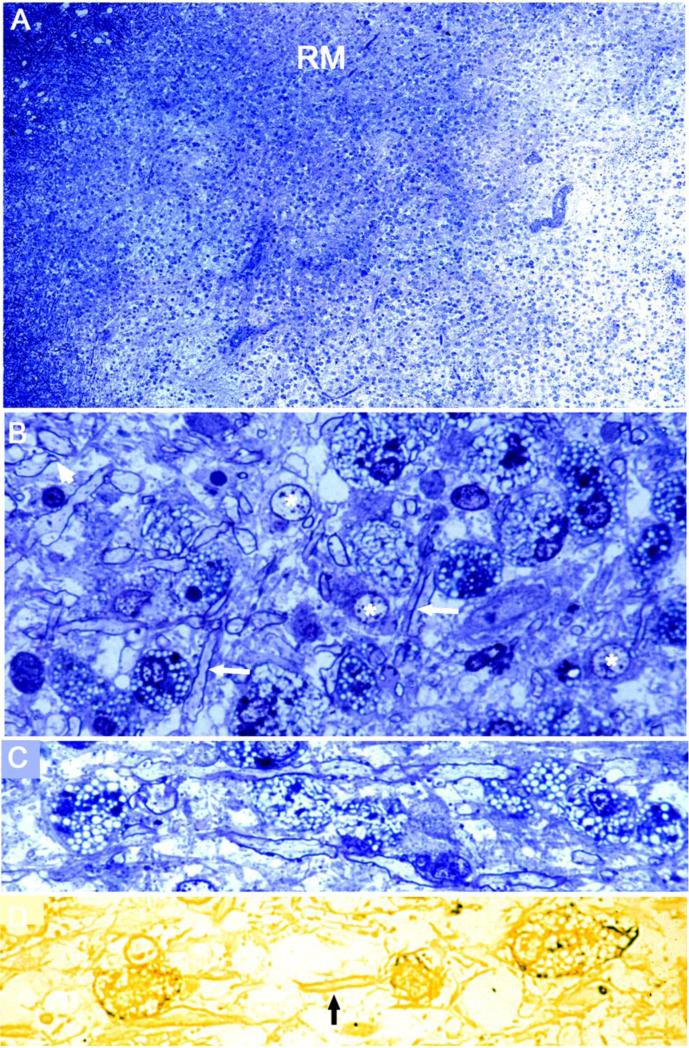
What happens next in the resolving lesion remains somewhat speculative since intermediate images bridging early demyelinated to later remyelinated resolving stages have been difficult to document. However, examples of resolving lesions displaying remyelination were quite common (Figure 4A), and have been mentioned previously Prineas et al. (1993a). At the margin, abutting myelinated white matter and extending into the lesion area, a broad zone of thinly myelinated (remyelinated) nerve fibers occurred, the density of which decreased towards deeper regions. The lesion center was rich in foamy macrophages, some of which formed perivascular cuffs (Figure 4A). The edge of the lesion was indistinct and lining-up of macrophages could not be documented. The remyelinated area underlaid NAWM like a broad shadow and at higher magnification was seen to be densely populated by rounded foamy macrophages, as well as scattered oligodendrocytes, axonal spheroids and fibrous astrocytes amongst thinly myelinated (remyelinated) axons (Figures 4B and C). The lipid-filled, foamy macrophages lacked myelin debris, a feature also mentioned previously e.g. Prineas et al. (1993a); Boven et al. (2006); Mehta et al. (2013). Although presence of macrophages commonly presages tissue damage, in the context of a gliotic CNS undergoing repair, this was apparently not the case. Moreover, there was no evidence of recent inflammation but microglia/macrophages did express class II MHC, indicative of a readiness to interact with immune cells (Figure 4D). Recurrent episodes of inflammation and demyelination in remyelinated lesions were not encountered.
Figure 4.
A. The margin of a late resolving lesion in the cerebral white matter (NAWM upper left, demyelinated plaque lower right) is broad, shadow-like and contains an abundance of remyelination (RM), see below. The demyelinated area is studded with macrophages, some of which are in perivascular cuffs. Primary progressive MS, 5 years. One micron epoxy section, toluidine blue stain.
B. Detail of the remyelinated border in (A), showing the multitude of thinly myelinated nerve fibers (arrows), rounded lipid-laden, foamy microglia/macrophages, a few densely staining axonal spheroids, oligodendrocytes (*), and an absence of ongoing demyelination.
C. In another case of primary progressive MS, clinical history 11 years, an area of resolving lesion in the brainstem displays rounded foamy macrophages which flank longitudinally-sectioned remyelinated fibers.
D. An adjacent epoxy section to C is reacted for class II MHC. Note the class II MHC positive foamy macrophages (left and right), and the pale background-staining of remyelinated fibers (arrow).
Discussion
The burgeoning literature on the microglial cell in recent years has elevated its status from that of merely the resident macrophage of the CNS to that of a multifaceted citizen with a wide range of duties, detrimental and beneficial. Of some significance is its unique signature surface expression of CX3CR1, the receptor for the chemokine, fractalkine (CX3CL1), a chemokine produced in the CNS by nerve cells, thus implicating neuronal control of microglial function and molecular cross-talk (Ransohoff and El Khoury, 2015). Among its repertoire of characteristics, along with its variation in activation state (see below), is that microglial cells are a self-renewing cell population with no contribution from blood monocytes, are long-lived with a highly plastic phenotype, and have major house-keeping functions, viz. synaptic editing (or pruning) and clearance of apoptotic cells during development (Davis and Carson, 2013). Also important is the microglial cell's role in surveillance within the CNS, elegantly demonstrated in situ by Nimmerjahn et al. (2005), whereby normal ramified “resting” microglia were seen constantly to extend highly motile cell processes and protrusions into the surrounding environment, surveying the neighborhood, a phenomenon greatly accentuated after injury. Related to this function was the possibility in the present study that the accumulations of microglia/macrophages in perivascular spaces may have also been related to surveillance, in this case, the screening of elements entering from the bloodstream, rather than simply representing cells leaving the tissue. Together with its role in innate immunity within the CNS and its ability to interact with immune cells, in addition to the rest of its repertoire, microglia cell is certainly well-qualified as a major player in homeostatic mechanisms in the CNS.
From the observations on later resolving lesions, one is immediately struck by the incongruity of the coexistence of potentially damaging elements (microglia/macrophages) and a highly vulnerable, notoriously fragile repair process (remyelination). This raises questions whether microglia/macrophages might sustain a beneficial/neuroprotective environment for oligodendrocytes and myelin and whether they represent so-called “alternatively activated” microglia/macrophages expressing an anti-inflammatory (M2) phenotype (see below), and capable of producing a number of growth factors, important in oligodendrocyte development (Rawji and Wee Yong (2013). The release of such growth factors, perhaps resulting from neuronal signaling via CX3CL1 and CX3CR1 on microglia might be mechanistically related to the CNS remyelination. Of considerable significance to the present discourse is that while microglial activation has traditionally been a term synonymous with cytotoxicity and damage, microglia/macrophages are today defined according to a spectrum of activation states covered by two nomenclatures – classical activation spectrum (or MI, with a pro-inflammatory, Th1-type phenotype), and alternative activation spectrum (or M2, with an anti-inflammatory, Th2-type phenotype) - Colton et al., (2006); Rawji and Wee Yong (2013). A hybrid population of microglia/macrophages Type II has also been described with a mixture of M1 and M2 characteristics. That the microglia/macrophage phenomena described in the resolving lesion might well bespeak a regulatory role for these cells is possible, especially in light of the existence of M1 and M2 spectrums and other reports implicating similar foamy macrophages in MS with anti-inflammatory profiles. For example, one account shows these cells to express IL-6 and IL-1ra, among other molecules - Boven et al. (2006); and another mannose receptor, known for its ability to initiate an anti-inflammatory response - Mehta et al., (2013).
The macrophages described herein were unusual, differed in many regards from acute, inflammatory monocytic macrophages and have been previously identified in MS by the present author as “activated microglia” (Raine, 1997), based, among other criteria, on their nuclear morphology, staining patterns and ability to persist within lesions for indefinite periods. Microglia/macrophages display wide phenotypic variation based on activation state (Davis and Carson, 2013) and are capable of cycling between activation states (Colton et al., 2006), although according to Ransohoff and El Khoury (2015), a “crucial” need still exists for further definition of these cells. Conventional macrophages are commonly of monocytic origin, have a distinctive reniform nucleus, enter the tissue from the blood and are acutely infiltrating after injury, engage in phagocytosis and depart within days to weeks. The foamy microglia/macrophages featured here resolving MS lesions most probably correspond to the “fat granule cells” of Dawson (1916) in “early cerebral areas” in MS. Dawson attributed to them the function of “clearing”, and was the first to comment on their becoming confined to the peripheral zone of the “clearing” lesion. Similar cells have been reported by other authors to persist in MS lesions for many months (e.g. Seitelberger, 1973; Allen, 1984.) However, precisely why the present homogeneous population of microglia/macrophages cells predominated in the resolving lesion, and apparently demarcated demyelinated lesion from myelinated white matter, remain intriguing questions with important implications yet to be investigated.
The propensity for later resolving lesions to display extensive myelin repair in the face of an abundance of microglial/macrophages, is remarkable. Remyelination in the CNS, usually identified in MS on the basis of thinly myelinated nerve fibers at the margins of chronic lesions in the absence of inflammation and macrophages, has been known from the classic German literature in the early nineteenth century (see Dawson, 1916), although definitive proof had to await more refined structural technologies – reviewed in Ludwin and Raine (2008). It is now widely held that the best outcome for myelin repair in MS, the so-called shadow plaque, is the result of a single episode of acute demyelination and that such repair is long-lasting - see Prineas et al., (1993b). On the other hand, it is also held that repeated episodes of acute demyelination and inflammation do not bode well for myelin repair and can lead to failed remyelination. The lesions described herein existed within a spectrum of ongoing CNS lesion activity from cases of late acute and primary progressive MS in which the risk of recurrent activity was probably quite high, particularly in a condition in which the blood-brain barrier is known to be compromised. Therefore, the likelihood of the myelin repair being permanent was probably small. Even though the microglia/macrophages among remyelinated fibers appeared non-aggressive, it is worrisome that they are known to persist for long intervals, possess an innate ability to vary and cycle phenotype (M1 to M2), and express molecules like class II MHC. Cumulatively, these propensities denote an immunologic readiness and strong potential for future interactions with the immune system which might have negative consequences for new myelin and oligodendrocytes. Nevertheless, the coexistence of such cells with myelin repair and their unusual alignment along the lesion edge, even if a transient, short-term phenomenon, might bespeak expression of regulatory pathways. Taken in concert, while not exactly answering the questions posed in the Introduction, the present descriptive account does touch on a number of promising avenues of therapeutic relevance that may supplement the list of beneficial drugs for MS, many of which incidentally, possess proclivities to bolster anti-inflammatory responses - recently reviewed by Rawji and Wee Yong (2013). Specifically, Copaxone (glatiramer acetate), increases levels of a number of anti-inflammatory cytokines and promotes an M2 macrophage/microglial phenotype; Fingolimod (Gilenya), induces a Th-2 type response in activated macrophages; and interferon-β (Avonex), reduces proliferation of macrophages and expression of MHC II. Thus, the resolving lesion in MS may provide an interesting novel paradigm for the examination of anti-inflammatory pathways within microglia/macrophages that may be applicable to current protocols or yet-to-be-developed treatments.
Acknowledgements
Supported in part by a research grant from the National Multiple Sclerosis Society – NMSS RG 1001-K-11, and the Cumming Foundation. CSR was the Wollowick Family Professor of Multiple Sclerosis Research at this institution. The scientific advice and assistance of Dr. Celia Brosnan, the expert technical assistance of Miriam Pakingan, and superb administrative assistance of Ms. Patricia Cobban-Bond in the preparation of the manuscript, are gratefully acknowledged. The author also thanks Dr. Ross Gruber for Photoshop assistance and is particularly grateful to his colleague, Dr. John Prineas, University of Sydney, Australia, for long-term collaboration and permission to use the images in Figure 3.
The material covered was the subject of a presentation delivered to attendees of a meeting entitled “Forty Years of Neuroimmunology”, held in Warrenton, VA in April, 2015. The event marked the reunion of clinical and scientific staff, fellows and colleagues, former and present, who had passed through the Neuroimmunology Branch (NIB) of the National Institutes of Health (NIH), in Bethesda, MD from its inception in 1975 by its Founding Chief and my close colleague, the late Dr. Dale E. McFarlin, to the present day. It was during Dale's watch at NIH (he passed away in 1992), that “neuroimmunology” became an accepted term and largely due to the NIB's influence and the establishment of the Journal of Neuroimmunology in 1981, the field acquired an identity.
This report is dedicated to the memory of Dr. Dale E. McFarlin from whom the author learned so much and through whom he was able to collaborate with many outstanding NIB investigators, namely Drs. Lesley Barnett, Avery Brown, Richard McCarron, Anne Cross, Farooz Mokhtarian, Robert Fallis, Suhayl Dhib-Jalbut, Henry McFarland, Steven Jacobson, Tanya Lehky, David Katz, Norbert Sommer, Roland Martin, Michael Racke, Betsy Smith and Rhonda Voskuhl.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RD, Sidman RL. In: Introduction to Neuropathology. Adams RD, Sidman RL, editors. McGraw-Hill; New York: 1968. pp. 149–170. [Google Scholar]
- Allen IV. Demyelinating Diseases. In: Adams JH, Corsellis JAN, Duchen LW, editors. Greenfield's Neuropathology. IVth Edition Wiley; New York: 1984. pp. 338–384. [Google Scholar]
- Boven LA, Van Meurs M, Van Zwan M, Wierengar-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflamm. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DS, Carson MJ. An introduction to CNS-resident microglia. Definitions, assays, and functional roles in health and disease. In: Cur C, Grandison L, Noroha A, editors. Neural immune interactions in brain function and alcohol-related disorders. Springer (Publisher); New York: 2013. pp. 3–29. [Google Scholar]
- Dawson JW. The histology of disseminated sclerosis. Trans. Roy. Soc. Edinburgh. 1916;50:517–740. [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple Sclerosis: The plaque and its pathogenesis. N. Engl. J. Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Greenfield JG, Norman RM. Demyelinating diseases. In: Blackwood W, McMenemey WH, Meyer M, Norman RM, Russell DS, editors. Greenfield's Neuropathology. 2nd Edition Arnold; London: 1971. pp. 474–519. [Google Scholar]
- Lassmann H, Wekerle H. The pathology of multiple sclerosis. In: Compston A, Confavreux C, Lassmann H, McDonald I, Miller D, Noseworthy J, Smith K, Wekerle H, editors. McAlpine's Multiple Sclerosis. Fourth Edition Churchill/Livingston/Elsevier (Publishers); London: 2005. pp. 557–599. [Google Scholar]
- Ludwin SK, Raine CS. The neuropathology of multiple sclerosis. In: Raine CS, McFarland HF, Hohlfeld R, editors. Multiple Sclerosis: A comprehensive text. Elsevier/Saunders; London: 2008. pp. 151–177. [Google Scholar]
- Lumsden CE. The neuropathology of multiple sclerosis. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 9. North Holland Publishing Company; Amsterdam: 1970. pp. 217–309. [Google Scholar]
- Mehta V, Pei W, Yang G, Li S, Swamy E, Boster A, Schmelbrock P, Pitt D. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. Plos ONE. 2013;8:e57573. doi: 10.1371/journal.pone.0057573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GRW, Stadelmann-Nessler C. Demyelinating diseases. In: Love S, Perry A, Ironside J, Budka H, editors. Greenfield's Neuropathology. 9th Edition CRC Press; Boca Raton, FL: 2015. pp. 1297–1412. [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Remyelination of nascent lesions. Ann. Neurol. 1993(a);33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Bernard RO, Ervesz T, Kwon EE, Sharer L, Cho ES. Multiple Sclerosis: pathology of recurrent lesions. Brain. 1993(b);116:681–693. doi: 10.1093/brain/116.3.681. [DOI] [PubMed] [Google Scholar]
- Prineas JW, McDonald WI, Franklin RJM. Demyelinating Diseases. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathology. 7th Edition Vol. 2. Arnold; London: 2002. pp. 471–535. [Google Scholar]
- Raine CS. The neuropathology of multiple sclerosis. In: Raine CS, McFarland HF, Tourtellotte WW, editors. Multiple Sclerosis: Clinical and pathogenetic basis. Chapman and Hale; London: 1997. pp. 151–171. [Google Scholar]
- Ransohoff RM, El Khoury J. Microglia in health and disease. Cold Spring Harb. Perspect. Biol. 2015 doi: 10.1101/cshperspect.a020560. Doi: 10.1101/cshperspect.a020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji K, Wee Yong V. The benefits and detriments of microglia/macrophages in models of multiple sclerosis. Clin. Devp. Immunol. 2013 doi: 10.1155/2013/948976. http://dx.doi.org/10.1155/2013/948976. [DOI] [PMC free article] [PubMed]
- Seitelberger F. Pathology of multiple sclerosis. Ann. Clin. Res. 1973;5:337–344. [PubMed] [Google Scholar]