Abstract
AIM
To analyses the current literature regarding the urogenital functional outcomes of patients receiving robotic rectal cancer surgery.
METHODS
A comprehensive literature search of electronic databases was performed in October 2015. The following search terms were applied: “rectal cancer” or “colorectal cancer” and robot* or “da Vinci” and sexual or urolog* or urinary or erect* or ejaculat* or impot* or incontinence. All original studies examining the urological and/or sexual outcomes of male and/or female patients receiving robotic rectal cancer surgery were included. Reference lists of all retrieved articles were manually searched for further relevant articles. Abstracts were independently searched by two authors.
RESULTS
Fifteen original studies fulfilled the inclusion criteria. A total of 1338 patients were included; 818 received robotic, 498 laparoscopic and 22 open rectal cancer surgery. Only 726 (54%) patients had their urogenital function assessed via means of validated functional questionnaires. From the included studies, three found that robotic rectal cancer surgery leads to quicker recovery of male urological function and five of male sexual function as compared to laparoscopic surgery. It is unclear whether robotic surgery offers favourable urogenital outcomes in the long run for males. In female patients only two studies assessed urological and three sexual function independently to that of males. In these studies there was no difference identified between patients receiving robotic and laparoscopic rectal cancer surgery. However, in females the presented evidence was very limited making it impossible to draw any substantial conclusions.
CONCLUSION
There seems to be a trend towards earlier recovery of male urogenital function following robotic surgery. To evaluate this further, larger well designed studies are required.
Keywords: Rectal neoplasms, Robotic surgical procedures, Colorectal surgery, Sexual dysfunction, Physiological, Urinary bladder, Neurogenic, Humans
Core tip: Urogenital dysfunction is a significant problem following rectal cancer surgery that significantly affects quality of life. Despite laparoscopic total mesorectal excision becoming the standard approach in much of the developed world, the incidence of post-operative urogenital dysfunction remains high. Robotic surgery allows for precision surgery in the pelvis, therefore enabling better preservation of the pelvic autonomic nerves. Current studies examining the urogenital outcomes following robotic rectal cancer surgery have several limitations, but suggest that robotic surgery may offer favourable outcomes when compared to laparoscopic and open surgery. Larger scale prospective studies are required to validate these results.
INTRODUCTION
Colorectal cancer is one of the most common cancers in the developed world[1-3] with rectal cancers making up a third of those cancers[2-4]. The aim of rectal cancer surgery is to radically resect the cancer in order to achieve oncological cure and avoid local recurrence. During the past three decades significant improvements have been made to combat this predicament. These advances include earlier diagnosis, advanced surgical techniques and the improvement of adjuvant and neoadjuvant treatment[4-8]. These developments were not only aimed to improve the patients’ survival but also directed to improve the quality of life after cancer rectal surgery.
Urogenital function is one of the most important aspects of quality of life and rectal cancer may have adverse effects on it[5,9-13]. Although urogenital dysfunction is considered to be multifactorial, intra-operative damage to the pelvic autonomic nerves is the primary cause[14-16]. This is mainly due to the close proximity of the mesorectum to the autonomic nerves, and the difficulty in identifying such small structures such as the autonomic nerves in a narrow operative space such as the pelvis[13,17]. Damage to the sympathetic nerves results in urinary incontinence, ejaculation disorders in men and decreased orgasmic intensity in women[13,18]. Damage to the parasympathetic nerves leads to a lack of detrusor muscle function and subsequent voiding disorder, as well as erectile problems and lubrication dysfunction in men and women respectively[13,18]. These are significant post-operative and life changing events that jeopardise patients quality of life[9].
It is logical to assume that better visualisation of the structures of the pelvis, such as offered from laparoscopic or robotic surgery, can aid preservation of the autonomic nerves. Nevertheless, there is a debate as to whether laparoscopic surgery offers improved urogenital functional outcomes when compared to open surgery[19], as some studies have shown improved outcomes[20] while other advocate the contrary[21]. A probable reason for the disparate results is due to laparoscopic rectal surgery being technically difficult[22], as evident from its long learning curve[23] and the high conversion rate demonstrated in the CLASSICC and COLOR II trials[24,25]. Existing laparoscopic instruments have a restricted range of movement compared with that of the surgeons hand and are difficult to use in confined spaces such as the pelvis[26,27].
Robotic surgical systems were introduced to overcome the technical limitations of laparoscopic surgery[28]. They provide a superior three dimensional view, tremor filtering and superior ergonomic instrumentation[26,29]. These chattels enable precise dissection in narrow surgical fields such as the pelvis and help preserve the autonomic nerves. Even though multiple studies have examined the pathological, oncological and postoperative outcomes of robotic rectal surgery, there are only a few studies that have investigated the urological and sexual outcomes of robotic rectal cancer surgery and these tend to be predominantly about male patients.
Therefore the aim of this systematic review is to examine the available literature on the postoperative urogenital outcomes of robotic rectal cancer surgery on both male and female patients.
MATERIALS AND METHODS
A comprehensive literature search of electronic databases was performed in October 2015 by using the Discovery search engine tool (for more info refer to: http://www.port.ac.uk/library/infores/discovery/). Discovery is Portsmouth University’s search engine tool and it simultaneously searches over 200 scientific electronic databases including MEDLINE (PubMed), Google Scholar and Science Direct. The following search terms were applied: “rectal cancer” or “colorectal cancer” and robot* or “da Vinci” and sexual or urolog* or urinary or erect* or ejaculat* or impot* or incontinence. All original studies that reported the urological and/or sexual outcomes of patients having robotic rectal cancer surgery were included. Reference lists of all retrieved articles were manually searched for further relevant articles. A flow diagram of the selection process is given in Figure 1. Abstracts were independently searched by two authors. Fifteen full text articles fulfilled the inclusion criteria.
Figure 1.
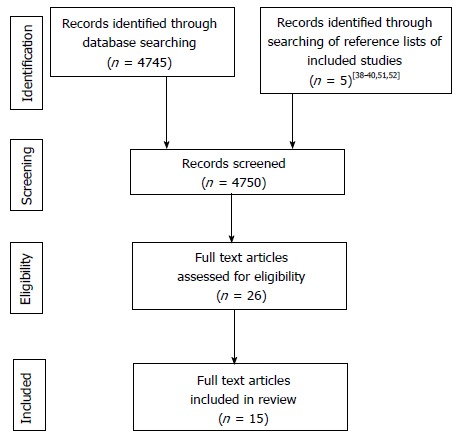
Selection process flow diagram.
RESULTS
Original studies
A total of 1338 patients were included in the reviewed studies (818 received robotic, 498 laparoscopic and 22 open rectal cancer surgery). The characteristics of all the original studies reporting either urinary or sexual outcomes are outlined in Tables 1 and 2. Of the 15 studies that met the inclusion criteria, 14 were cohort studies[5,6,9,18,30-39] and one a randomised control trial[40]. Nine of the cohort studies were comparing robotic rectal cancer surgery to either laparoscopic[9,30-33,35,38,40] or open[18] rectal cancer surgery.
Table 1.
Characteristics of original studies
| Ref. | Country | Study design | Control group | No. of cases for urogenital outcomes | Study specifically examines urogenital outcomes |
| Hellan et al[34] | United States | Retrospective | No control group | 39 | No |
| Patriti et al[40] | Italy | RCT | Robot vs lap | 29 rob vs 37 lap | No |
| Luca et al[6] | Italy | Prospective | No control group | 74 | Yes |
| Kim et al[31] | South Korea | Prospective | Robot vs lap | 30 rob vs 39 lap | Yes |
| Park et al[39] | United States | Prospective | No control group | 30 | No |
| Leung et al[5] | Hong Kong | Prospective | No control group | 33 | Yes |
| Park et al[32] | South Korea | Retrospective | Robot vs lap | 14 rob vs 15 lap | No |
| D'Annibale et al[33] | Italy | Retrospective | Robot vs lap | 30 vs 30 | No |
| Stănciulea et al[37] | Romania | Retrospective | No control group | 78 | No |
| Erguner et al[38] | Turkey | Prospective | Robot vs lap | 27 rob vs 37 lap | No |
| Park et al[9] | South Korea | Retrospective | Robot vs lap | 32 vs 32 | Yes |
| Ozeki et al[18] | Japan | Prospective | Robot vs open | 15 rob vs 22 open | Yes |
| Cho et al[35] | South Korea | Retrospective | Robot vs lap | 278 vs 278 | No |
| Alecu et al[36] | Romania | Retrospective | No control group | 79 | No |
| Morelli et al[30] | Italy | Retrospective | Robot vs lap | 30 vs 30 | Yes |
These include: (1) the studies country of origin; (2) the study design (prospective, retrospective or randomised control trial); (3) the control group (if present) used to compare with the robotic rectal surgery, this was either laparoscopic or open rectal surgery cases; (4) the number of cases included in each study whose urogenital outcomes were evaluated; and (5) whether the study was specifically designed to investigate the urogenital outcomes of robotic surgery or not. RCT: Randomised control trial; Robot: Robotic; lap: Laparoscopic.
Table 2.
Further characteristics of original studies
| Ref. | Fully or hybrid robotic procedure | Functional scores applied | Follow up in months | No. of surgeons performing cases | SIGN score |
| Hellan et al[34] | Hybrid | No | Median f/u 13 mo | Not stated | + |
| Patriti et al[40] | Hybrid | No | Mean f/u 12 mo | Not stated | + |
| Luca et al[6] | Fully | Yes | 1, 6, 12 | 2 surgeons | ++ |
| Kim et al[31] | Hybrid | Yes | 1, 3, 6, 12 | 1 surgeon | ++ |
| Park et al[39] | Reverse hybrid | No | Not stated | Not stated | + |
| Leung et al[5] | Mixture | Yes | 3 | Not stated | ++ |
| Park et al[32] | Hybrid | Yes | 3, 6, 12 | 1 surgeon | ++ |
| D'Annibale et al[33] | Fully | Yes | 1, 12 | 1 surgeon | ++ |
| Stănciulea et al[37] | 93% fully | Yes | Once b/n 6 and 12 mo | 3 surgeons | + |
| Erguner et al[38] | Mixture | No | Not stated | Not stated | + |
| Park et al[9] | Hybrid | Yes | 3, 6, 12 | 1 surgeon | ++ |
| Ozeki et al[18] | Fully | Yes | 3, 6, 12 | 2 for robot cases | ++ |
| Cho et al[35] | Fully | No | 1 | 3 surgeons did 97.1% cases | ++ |
| Alecu et al[36] | Hybrid | Yes | Not stated | Not stated | + |
| Morelli et al[30] | Not stated | Yes | 1, 6, 12 | 1 surgeon | ++ |
These include: (1) whether the surgeons used the hybrid or robotic approach for their study; (2) whether urogenital function was assessed by means of functional scores or not; (3) the follow up period during which data for urogenital outcomes was collected; (4) the number of surgeons performing the cases in each study; and (5) the studies SIGN score. f/u: Follow up; SIGN: Scottish Intercollegiate Guidelines Network.
Out of the 15 studies only six[5,6,9,18,30,31] were specific to urogenital outcomes; the rest reported urogenital outcomes amongst a multitude of outcomes examined in those studies.
Outcome assessment
Functional questionnaire scores were used in ten[5,6,9,18,30-33,36,37] of these studies to access the urological and sexual function of patients. These questionnaires are validated tools that have been used in a multitude of previous studies to access urinary and sexual function in males and females[41-45]. Out of the 1338 patients included in this review, only 726 (54%; 442 robotic, 262 laparoscopic, 22 open) had their urogenital function assessed via functional questionnaires.
To assess male urological function the majority of studies used the International Prostatic Symptoms Score (IPSS) or a slight modification of it. This is a subjective scoring system examining seven categories[41]. These include incomplete bladder emptying, frequency, intermittency, urgency, weak stream, straining and nocturia. Patients score each category and assign a higher score for increasing severity of symptoms. Alternative questionnaires used to assess urological function were the the International Consultation on Incontinence Questionnaire - Male Lower Urinary Tract Symptoms[44], and the International Consultation on Incontinence Questionnaire - Female Lower Urinary Tract Symptoms[45] questionnaire.
Male sexual function was assessed in ten studies by the international index of erectile function (IIEF)[42] score. The IIEF is a 15-item score that analyses five factors: Erectile function, orgasmic function, libido, intercourse satisfaction and overall satisfaction. Unlike the IPSS score for urinary function, a high IIEF score is associated with good sexual function and the lower the IIEF score the greater the degree of sexual dysfunction.
Female sexual function was assessed in three studies[6,30,37] via the Female Sexual Function Index (FSFI)[43]. This is a validated questionnaire that is in many ways the female version of the IIEF questionnaire.
The studies that did not use validated scoring tools to assess functional outcomes simply reported the incidence of dysfunction. The limitations present in this method of reporting are the inability to quantify dysfunction and the difficulty in defining what makes a case.
Finally, one study[31] assessed urological function by performing urodynamic studies as well as using a validated functional questionnaire, making it the only study to report urinary outcomes with both subjective and objective measurement tools.
Pre-operative assessment and follow up
The studies assessing functional outcomes via validated questionnaires asked their participants to fill the questionnaires pre-operatively in order to establish their baseline urogenital function. In this way post-operative scores were assessed against the pre-operative scores for each patient, allowing the change of function from baseline to be assessed. Reporting the change of function from baseline is a more accurate way of assessing the impact of the intervention, rather than reporting the postoperative functional scores alone.
It was unclear across several of the studies[6,18,30,32] how many patients were sexually inactive pre-operatively and whether they were included in the analysis. Adding sexually inactive patients in the analysis will result in skewing of the data and it is therefore important to report how many patients were sexually inactive and whether they were included in the analysis or not.
In contrast to the studies applying validated functional scores, most of the studies that simply reported the incidence of urogenital dysfunction did not mention the pre-operative state of their participants. This makes it difficult to assess whether any cases of dysfunction became cases because of the intervention or not.
Follow up was fairly variable between the different studies and the follow up intervals for each study are summarised in Table 2. The majority of the studies followed up their patients in more than one occasion following surgery. The commonest follow up intervals were 3, 6 and 12 mo post-operatively.
Quality of included original studies
The Scottish Intercollegiate Guidelines Network critical appraisal tool for cohort studies was used to evaluate the original studies included in this review. However, none of the studies met the majority of the criteria for a high quality study. Most of the studies fell between the acceptable and low quality bracket (Table 2). The majority of studies were retrospective in nature, included a small number of patients, were subject to selection bias in terms of patient selection and made no adjustments for confounding factors.
The studies included in this review have significant differences in terms of outcome reporting and methodology. In addition, almost all of them are non-randomised in nature. Considering this and because of the heterogeneity of the data in these studies it was not appropriate to perform a meta-analysis. There are only a few studies whose data were homogeneous enough to permit a meta-analysis. However, this has already been performed by two previous systematic reviews[46,47] which combined the data of three studies. We discuss these systematic reviews in our discussion.
Male urological function
Out of the 15 original studies included, 12 studies reported male urological functional outcomes. The characteristics of these studies plus a summary of their results are present in Table 3.
Table 3.
Original studies reporting male urological function
| Ref. | Males assessed independently of females | Functional scores applied | Control group | No. of cases examining male urological function | Follow up in months | Outcome summary |
| Kim et al[31] | No | Yes | Robot vs lap | 30 rob vs 39 lap | 1, 3, 6, 12 | Urological function recovered faster in robotic group (3 mo vs 6 mo) IPSS change from baseline lower in robotic group at 3 mo (P = 0.036) Mean voiding volume deterioration lower in 3 and 6 mo in robotic group (P = 0.007, P = 0.049) Similar outcomes at 12 mo in both groups |
| Park et al[9] | Yes | Yes | Robot vs lap | 32 vs 32 | 3, 6, 12 | IPSS scores elevated post-operatively in both groups At 12 mo IPSS change from baseline lower in robotic group but non-significant (P = 0.051) |
| Park et al[32] | Yes | Yes | Robot vs lap | 14 rob vs 15 lap | 3, 6, 12 | Deterioration of IPSS scores in 3 mo which recovered by 6 mo in both groups |
| D'Annibale et al[33] | Yes | Yes | Robot vs lap | 30 vs 30 | 1, 12 | Deterioration of IPSS scores in 3 mo which recovered by 12 mo in both groups |
| Ozeki et al[18] | Yes | Yes | Robot vs open | 15 rob vs 22 open | 3, 6, 12 | No statistical deterioration of IPSS scores in either group |
| Morelli et al[30] | Yes | Yes | Robot vs lap | Not available | 1, 6, 12 | Voiding and incontinence worse 1 mo in both groups, incontinence recovered by 6-12 mo in both groups |
| Leung et al[5] | Yes | Yes | No control group | 33 | 3 | No significant male urological function deterioration |
| Luca et al[6] | Yes | Yes | No control group | 38 | 1, 6, 12 | No significant male urological function deterioration |
| Stănciulea et al[37] | No | Yes | No control group | 78 | Once b/n 6 and 12 | No deterioration in IPSS scores but no data presentation in results |
| Hellan et al[34] | No | No | No control group | 39 | median F/U 13 mo | One patient (2.56%) developed bladder dysfunction post operatively |
| Park et al[39] | No | No | No control group | 30 | Not stated | No patients developed bladder dysfunction post operatively |
| Cho et al[35] | No | No | Robot vs lap | 278 vs 278 | 1 | Voiding dysfunction rate higher in the laparoscopic group (4.3% lap vs 0.7% rob; P = 0.012) |
The following study characteristics are described: (1) whether male patients were assessed independently of female patients or not, in studies that this was not the case data from male and female patients was combined; (2) whether functional scores were used to assess urogenital outcomes or not; (3) the control group used in the study if applicable; (4) the number of cases examining male urological function; (4) the follow up periods in months; and (5) a brief summary of the study’s findings regarding male urological function. Robot: Robotic; lap: Laparoscopic; f/u: Follow up; IPSS: International Prostatic Symptoms Score.
Validated functional scores were used in nine of the above studies. Six of those compared the scores of patients undergoing robotic surgery with those undergoing laparoscopic or open surgery. Most studies[18,30,32,33] showed that urological function tended to deteriorate in the early postoperative phase (1-3 mo) but later recovered with time (6-12 mo) irrespective of surgical modality. One study[9] found that IPSS score change from baseline was less in the robotic group at 12 mo after surgery, but failed to reach statistical significance (P = 0.051).
Kim et al[31] reported IPSS scores in favour of the robotic group. They found that IPSS scores significantly increased 1 mo after surgery; but then recovered in 3 mo in the robotic group and 6 mo in the laparoscopic group with a statistically significant lesser deterioration of scores from baseline in the 3 mo follow up period in the robotic group (P = 0.036). It is worth noting that Kim et al[31]’s study was the only one to assess urinary function by means of urodynamic studies in conjunction with a functional score. He reported that the deterioration in mean voiding volume from baseline was statistically less in 3 and 6 mo post-op in favour of the robotic group (P = 0.007, P = 0.049). The only other study to report urological outcomes in favour of the robotic group was Cho et al[35]’s study; reporting a higher voiding dysfunction rate in the laparoscopic group (4.3% vs 0.7%; P = 0.012). However, this study did not use any functional scores to assess urological function.
Female urological function
Seven studies reported female urological functional outcomes (Table 4). However, there are only two studies that report female urological dysfunction independently to that of males.
Table 4.
Original studies reporting female urological function
| Ref. | Females assessed independently of males | Functional scores applied | Control group | No. of cases examining female urological function | Follow up in months | Outcome summary |
| Morelli et al[30] | Yes | Yes | Robot vs lap | Not available | 1, 6, 12 | No difference between the pre- and post-operative scores in both groups |
| Luca et al[6] | Yes | Yes | No control group | 36 | 1, 6, 12 | Worse female urological function at 1 mo with full recovery by 12 mo in both groups |
| Kim et al[31] | No | Yes | Robot vs lap | 30 rob vs 39 lap | 1, 3, 6, 12 | As in Table 3 |
| Stănciulea et al[37] | No | Yes | No control group | 78 | Once b/n 6 and 12 | As in Table 3 |
| Hellan et al[34] | No | No | No control group | 39 | Median f/u 13 mo | As in Table 3 |
| Park et al[39] | No | No | No control group | 30 | Not stated | As in Table 3 |
| Cho et al[35] | No | No | Robot vs lap | 278 vs 278 | 1 | As in Table 3 |
This table describes the same study characteristics included in Table 3 but for female instead of male patients. Robot: Robotic; lap: Laparoscopic; f/u: Follow up.
Both studies used approved functional scores to assess urinary function and both studies compared robotic surgery patients with laparoscopic surgery patients. Morelli et al[30] found no difference between the pre-operative and post-operative scores concerning voiding and filling symptoms in both groups. Conversely, Luca et al[6] reported worsening of symptoms one month post operatively with full recovery by 12 mo in both robotic and laparoscopic groups.
Male sexual function
Fourteen original studies reported male sexual functional outcomes (Table 5). Ten of those assessed male sexual function via the IIEF[42] questionnaire.
Table 5.
Original studies reporting male sexual function
| Ref. | Males assessed independently of females | Functional scores applied | Control group | No. of cases examining male sexual function | Follow up in months | Outcome summary |
| Kim et al[31] | Yes | Yes | Robot vs lap | 18 rob vs 20 lap | 1, 3, 6, 12 | Quicker recovery of male sexual function in robotic group (6 mo vs 12 mo) No difference in IIEF change from baseline between two groups at any stage Erectile function and libido deteriorated significantly more in lap group at 3 mo |
| Park et al[9] | Yes | Yes | Robot vs lap | 20 vs 20 | 3, 6, 12 | Quicker recovery of male sexual function in robotic group (6 mo vs 12 mo) IIEF deterioration significantly higher in lap group at 6 mo (P = 0.03) |
| Park et al[32] | Yes | Yes | Robot vs lap | 14 rob vs 15 lap | 3, 6, 12 | Better male sexual function scores at 3 and 6 mo in robotic group No difference in IIEF change from baseline between two groups at any stage |
| D'Annibale et al[33] | Yes | Yes | Robot vs lap | 18 rob vs 23 lap | 1, 12 | Erectile function restored 1 yr post-operatively in robotic group (P = 0.066) and partially in lap group (P = 0.048) No statistical comparison of IIEF change from baseline b/n 2 groups at any stage |
| Ozeki et al[18] | Yes | Yes | Robot vs open | 15 rob vs 22 open | 3, 6, 12 | IIEF scores unchanged at 3, 6 and 12 mo in both groups |
| Morelli et al[30] | Yes | Yes | Robot vs lap | Not available | 1, 6, 12 | Quicker recovery of erectile and orgasmic function in robotic group (6 mo vs 12 mo) No difference in IIEF change from baseline between two groups at any stage |
| Leung et al[5] | Yes | Yes | No control group | 15 | 3 | No significant difference between post- and pre-operative IIEF scores |
| Luca et al[6] | Yes | Yes | No control group | 38 | 1, 6, 12 | Male sexual function scores decreased at 1 and 6 mo, recovered at 12 mo |
| Stănciulea et al[37] | Yes | Yes | No control group | 31 | Once b/n 6 and 12 | No difference of pre- and post-op IIEF scores with exception of 3 patients (9.68%) with severe erectile dysfunction |
| Alecu et al[36] | No | Yes | No control group | 79 | Not stated | 3 patients (3.79%) developed important sexual dysfunction. No mention of IIEF scores in results |
| Patriti et al[40] | Yes | No | Robot vs lap | 11 rob vs 12 lap | Mean f/u 12 mo | No difference in the incidence of sexual dysfunction between the 2 groups |
| Erguner et al[38] | No | No | Robot vs lap | 27 rob vs 37 lap | Not stated | No difference in the incidence of sexual dysfunction between the 2 groups |
| Cho et al[35] | No | No | Robot vs lap | 278 vs 278 | 1 | No difference in the incidence of sexual dysfunction between the 2 groups |
| Park et al[39] | Yes | No | No control group | 16 | Not stated | 1 patient (6.25%) developed ejaculatory dysfunction, no patients developed erectile dysfunction |
Six of the ten studies using the IIEF scores compared the scores of patients receiving robotic rectal cancer surgery with that of a control. Park et al[9]’s study showed that sexual function recovers faster in the robotic group. At 6 mo the IIEF scores in the robotic group were higher than in the laparoscopic group and showed a significantly smaller decrease from baseline (P = 0.03). Kim et al[31] also found that sexual function recovered quicker in the robotic group (6 mo vs 12 mo), but unlike Park et al[9]’s study, when comparing the change of total IIEF scores from baseline no significant difference was detected. However, erectile function and libido had deteriorated significantly more in the laparoscopic group 3 mo post op. Park et al[32] showed similar results, with significantly higher mean IIEF scores at 3 and 6 mo post op in favour of the robotic group. Like Kim et al[31]’s study, the change of scores from baseline did not statistically favour either intervention. In Morelli et al[30]’s study erectile and orgasmic function was significantly worse 1 mo after RobTME while it was significantly worse after 1 and 6 mo after LapTME, with erectile and orgasmic function normal at 12 mo in both groups. The other components of the IIEF score deteriorated 1 and 6 mo following surgery in both groups, with normalisation of the scores at 12 mo. D’Annibale et al[33] reported better restoration of erectile function 1 year after surgery in the robotic group; however, there is no mention of the actual IIEF scores or their change from baseline in the study so any results need to be interpreted with caution. Overall, the above comparative studies seem to report a trend towards quicker recovery of sexual function in the robotic group. However, Park et al[9]’s study was the only one to reveal an interval change in IIEF scores in favour of the robotic group that was statistically significant.
Female sexual function
In contrast to male sexual function, only a few studies have investigated sexual function in females (Table 6). Only three studies have examined female sexual dysfunction independently with that of males[6,30,37] and only one of those compared robotic outcomes to those of a control group[30]. All three studies assessed female sexual function via the FSFI.
Table 6.
Original studies reporting female sexual function
| Ref. | Females assessed independently of males | Functional scores applied | Control group | No. of cases examining female sexual function | Follow up in months | Outcome summary |
| Morelli et al[30] | Yes | Yes | Robot vs lap | not available | 1, 6, 12 | Female sexual function worse at 1 and 6 mo and restored by 12 mo, in both groups |
| Luca et al[6] | Yes | Yes | No control group | 36 | 1, 6, 12 | Female sexual function worse at 1 and 6 mo and restored by 12 mo |
| Stănciulea et al[37] | Yes | Yes | No control group | 13 | Once b/n 6 and 12 | No difference between pre- and post-operative FSFI scores (but data not provided in results section) |
| Alecu et al[36] | No | Yes | No control group | 79 pts | Not stated | As in Table 5 |
| Erguner et al[38] | No | No | Robot vs lap | 27 rob vs 37 lap | Not stated | As in Table 5 |
| Cho et al[35] | No | No | Robot vs lap | 278 vs 278 | 1 | As in Table 5 |
Morelli et al[30] reported worsening of sexual outcomes in both groups 1 and 6 mo following surgery, but sexual outcomes were restored by 12 mo. There were no differences between the robotic and laparoscopic groups. Luca et al[6] demonstrated similar results in their robotic group as in Morelli et al[30]’s study, whereas Stănciulea et al[37] reported no difference between pre- and post-operative FSFI scores.
DISCUSSION
This literature review highlights the fact that the impact of robotic rectal surgery on urogenital functional outcomes is yet to be established. There are number of limitations in the current studies. These include poor study design, small number of participants, lack of stringent follow up and limitations to the methods and types of data collected.
The main limitations of the primary studies were the lack of randomisation, retrospective design and small number of cases in the majority of studies (Tables 1 and 2). As for the prospective studies, most of them failed to mention the number of patients excluded during recruitment, the number of patients refusing to participate and the number of drop outs. There was one RCT but randomisation was abandoned early on as the operating surgeon quickly favoured the robotic approach for low rectal tumours. In terms of participant selection only nine studies reported their outcomes against those of a control, with the other studies essentially only describing their case series rather than comparing them to alternative treatment methods.
Case matching was performed in 2 of the comparative studies[9,35], but in the remaining studies patient selection was susceptible to selection bias due to the method of patient selection and allocation. In a number of studies patients were only able to receive robotic surgery if they covered the extra costs themselves, leaving the patients that couldn’t afford it opting for laparoscopic or open surgery instead. Therefore the validity of the data may be skewed since patients that opted for robotic surgery were more likely to be from a higher socio-economic background, which is a potential confounding factor. Moreover, two studies compared their robotic cases with an equivalent number of their first laparoscopic cases[30,33]. This selection method was done to eliminate the confounding factor of a learning curve from either method. However, the learning curve for each method is not equal[48] and since in both studies all cases were performed by one surgeon only, it is possible that many of the skills acquired from the laparoscopic method were transferable to the robotic one. This way, results in favour of the robotic group could simply represent advancement in the surgeon’s operative technique rather than superiority for the robot.
Patients in the robotic cohort either had a fully robotic procedure or a hybrid procedure (Table 2). The main difference between the two approaches is that in the hybrid approach robotic rectal dissection is preceded by laparoscopic mobilisation of the left colon and ligation of the inferior mesenteric vessels. It is possible that the difference in approach could influence urogenital outcomes. Supporters of the fully robotic approach would advocate that robotic dissection around the inferior mesenteric artery pedicle is an essential step of the procedure for identification and preservation of the periaortic nerves[49], which is where the superior hypogastric plexus lies. Moreover, the paired hypogastric nerves are susceptible to injury during mobilisation of the rectosigmoid colon from the gonadals and the ureter[13]; a step performed laparoscopically during the hybrid approach. Since injury to those nerves can lead to urogenital dysfunction, the hybrid approach might not exploit the full potential of the robotic system.
Five studies did not use functional scores to assess urogenital outcomes. The challenge with only reporting the incidence of urological or sexual dysfunction is not only the inability to quantify the level of dysfunction but also to define what makes a case. Furthermore, where studies fail to report how many of the patients were sexually active pre-operatively, observational bias may be present.
It is important to mention that even though iatrogenic nerve injury is the primary cause of urogenital dysfunction[14-16], this group of symptoms is probably multifactorial in origin. Ozeki et al[18] utilised univariate analysis and found that age and post-operative complications significantly affected urinary function and sexual function respectively at 12 mo follow up. Sexual function in comparison to urological function is reported as being influenced by psychological factors and this is the case more so in women[4,6]. Luca et al[6] showed that whereas the presence of an ileostomy in men did not influence sexual function, it deeply affected it in women. Furthermore, poor body image, fatigue, depression, loss of independence and changes in relationships have all been identified as important factors in women’s sexual dysfunction[4]. In addition, radiation induced ovarian failure in premenopausal women can further worsen sexual symptoms[4]. Since the above are potentially important confounding factors, it is important for the control group to be as similar to the experimental group as possible or control for these confounders in the analysis, something absent in the studies examined in this review.
In this review we did not perform a meta-analysis due to the heterogeneity of the included studies. Nevertheless, it should be mentioned that two review articles have performed meta-analyses on male urological and sexual function scores of patients receiving robotic vs laparoscopic rectal surgery[46,47]. For male urological function, the reviews pooled the data from three studies and found that at 3 mo there was a significant difference of IPSS scores in favour of the robotic group. However, this was not the case at 6 mo following surgery and at 12 mo the two meta-analyses reported contradictory results, one showing favourable IPSS scores for the robotic group[46] whilst the other demonstrated no difference between the two groups[47]. Regarding male sexual function, the meta-analyses pooled the data for erectile function only. By including three and two studies respectively[46,47], both reviews demonstrated favourable erectile function scores for the robotic group at 3 and 6 mo following surgery. Weighing these results one should note that as a rule, the overall quality of a meta-analysis is limited to the quality of its primary studies, and since the quality of the evidence available is low, the results of the available meta-analysis are of equally low quality.
There is a degree of inconsistency of results across the research examined in this review and the potential for bias amongst the various studies on the subject. There is a lack of high level evidence supporting any particular approach for preservation of urogenital function following rectal surgery. Nevertheless, the current evidence suggests that robotic surgery might lead to a quicker recovery of male urological and sexual function when compared to alternative methods. It is less clear whether robotic surgery makes any difference in male urogenital outcomes 1 year following surgery. In females the evidence on urogenital function following robotic rectal surgery is further limited. Again functional outcomes seem to improve with time but this is regardless of operative approach.
Larger randomised controlled trials such as the ROLARR trial[50] might provide more insight into this matter. However, even though the ROLARR trial is underway, urogenital outcomes are not one of its primary ends points and urogenital outcomes are only assessed once following surgery, at six months. Therefore, to answer whether robotic rectal cancer surgery truly offers superior urogenital outcomes further randomised control trials specifically designed to evaluate urogenital function with appropriate short and long term follow up are recommended. In addition, urogenital dysfunction should be rigorously assessed through appropriate validated functional scores and males should be analysed separately to females.
COMMENTS
Background
Urological and sexual dysfunctions are unfortunate sequela of rectal cancer surgery. They occur due to iatrogenic injury to the pelvic autonomic nerves during the surgical process and cause significant quality of life limitations for patients. Better visualisation of the pelvis such as during laparoscopy has failed to address this issue due to the stiff, fixed tip instruments used for laparoscopy being hard to use in narrow spaces such as the pelvis. Robotic surgical systems overcome many of the limitations of laparoscopic surgery but whether robotic rectal surgery can lead to superior urological and sexual functional outcomes remains to be determined.
Research frontiers
Robotic surgical systems possess several advantages over conventional laparoscopy such as flexible wristed instruments that mimic the surgeon’s hands. They eliminate the surgeon’s tremor and offer far superior ergonomics and dexterity. In addition, the surgeon, rather than the assistant, controls a 3-D, high definition stable camera, an important aspect for co-ordinated surgery. These advantages allow for precision surgery in narrow spaces such as the pelvis, where other methods have failed and in rectal surgery could enable preservation of the pelvic autonomic nerves and therefore increase the quality of life for these patients.
Innovations and breakthroughs
There are only a few studies that have investigated the urological and sexual outcomes of robotic rectal surgery and these tend to be predominantly about male patients. This study differs by critically reviewing the available literature on the postoperative urological and sexual outcomes of robotic rectal surgery on both male and female patients. As such, this review is unique in that it examines the largest number or relevant studies to date; it focuses solely on the urogenital outcomes of robotic rectal surgery and examines the evidence on both males and females.
Applications
This review critically analyses the literature examining the urogenital outcomes of robotic rectal cancer surgery. Readers will be able to have a concise understanding of the available literature on this subject. Furthermore, this review leads to clear conclusions indicating a paucity of evidence of whether robotic rectal surgery offers favourable urogenital functional outcomes and establishes quality of life differences. Nevertheless, the authors identify that robotic surgery might lead to a quicker recovery of male urological and sexual function when compared to alternative methods of surgery and recommend the direction of further research.
Terminology
Urogenital function is a term referring to the combination of urological and sexual function. Laparoscopic and robotic surgeries are forms of minimally invasive surgery which offer several advantages over open surgery, such as smaller wounds and quicker postoperative recovery.
Peer-review
The manuscript is a comprehensive review addressing pelvic functions (rectal and sexual) after robotic surgery. Content coverage is adequate and focus. Language quality and flow of idea are excellent.
Footnotes
Conflict-of-interest statement: The authors of this manuscript have no conflicts of interest to declare.
Data sharing statement: The technical appendix, statistical code and dataset are available from the corresponding author at sofoklis_p@hotmail.com.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: May 25, 2016
First decision: July 6, 2016
Article in press: September 8, 2016
P- Reviewer: Barreto S, Kayaalp C, Sangkhathat S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Panjari M, Bell RJ, Burney S, Bell S, McMurrick PJ, Davis SR. Sexual function, incontinence, and wellbeing in women after rectal cancer--a review of the evidence. J Sex Med. 2012;9:2749–2758. doi: 10.1111/j.1743-6109.2012.02894.x. [DOI] [PubMed] [Google Scholar]
- 5.Leung ALH, Chan W-H, Cheung HYS, Lui GKL, Fung JTK, Li MKW. Initial experience on the urogenital outcomes after robotic rectal cancer surgery. Surg Pract. 2013;17:13–17. [Google Scholar]
- 6.Luca F, Valvo M, Ghezzi TL, Zuccaro M, Cenciarelli S, Trovato C, Sonzogni A, Biffi R. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg. 2013;257:672–678. doi: 10.1097/SLA.0b013e318269d03b. [DOI] [PubMed] [Google Scholar]
- 7.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 8.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Yun SH. Urinary and erectile function in men after total mesorectal excision by laparoscopic or robot-assisted methods for the treatment of rectal cancer: a case-matched comparison. World J Surg. 2014;38:1834–1842. doi: 10.1007/s00268-013-2419-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim NK, Aahn TW, Park JK, Lee KY, Lee WH, Sohn SK, Min JS. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum. 2002;45:1178–1185. doi: 10.1007/s10350-004-6388-5. [DOI] [PubMed] [Google Scholar]
- 11.Chang PL, Fan HA. Urodynamic studies before and/or after abdominoperineal resection of the rectum for carcinoma. J Urol. 1983;130:948–951. doi: 10.1016/s0022-5347(17)51589-x. [DOI] [PubMed] [Google Scholar]
- 12.Havenga K, DeRuiter MC, Enker WE, Welvaart K. Anatomical basis of autonomic nerve-preserving total mesorectal excision for rectal cancer. Br J Surg. 1996;83:384–388. doi: 10.1002/bjs.1800830329. [DOI] [PubMed] [Google Scholar]
- 13.Kim NK, Kim YW, Cho MS. Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: Expert technical tips for robotic surgery. Surg Oncol. 2015;24:172–180. doi: 10.1016/j.suronc.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol. 2011;8:51–57. doi: 10.1038/nrurol.2010.206. [DOI] [PubMed] [Google Scholar]
- 15.Havenga K, Enker WE. Autonomic nerve preserving total mesorectal excision. Surg Clin North Am. 2002;82:1009–1018. doi: 10.1016/s0039-6109(02)00044-0. [DOI] [PubMed] [Google Scholar]
- 16.Masui H, Ike H, Yamaguchi S, Oki S, Shimada H. Male sexual function after autonomic nerve-preserving operation for rectal cancer. Dis Colon Rectum. 1996;39:1140–1145. doi: 10.1007/BF02081416. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey I, Guy RJ, Warren BF, Mortensen NJ. Anatomy of Denonvilliers’ fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000;87:1288–1299. doi: 10.1046/j.1365-2168.2000.01542.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozeki S, Maeda K, Hanai T, Masumori K, Katsuno H, Takahashi H. Effects of robotic rectal surgery on sexual and urinary functions in male patients. Surg Today. 2016;46:491–500. doi: 10.1007/s00595-015-1217-0. [DOI] [PubMed] [Google Scholar]
- 19.Lim RS, Yang TX, Chua TC. Postoperative bladder and sexual function in patients undergoing surgery for rectal cancer: a systematic review and meta-analysis of laparoscopic versus open resection of rectal cancer. Tech Coloproctol. 2014;18:993–1002. doi: 10.1007/s10151-014-1189-x. [DOI] [PubMed] [Google Scholar]
- 20.McGlone ER, Khan O, Flashman K, Khan J, Parvaiz A. Urogenital function following laparoscopic and open rectal cancer resection: a comparative study. Surg Endosc. 2012;26:2559–2565. doi: 10.1007/s00464-012-2232-5. [DOI] [PubMed] [Google Scholar]
- 21.Jones OM, Stevenson AR, Stitz RW, Lumley JW. Preservation of sexual and bladder function after laparoscopic rectal surgery. Colorectal Dis. 2009;11:489–495. doi: 10.1111/j.1463-1318.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 22.Katsios CG, Baltogiannis G. Laparoscopic sphincter-preserving rectal cancer surgery: a highly demanding procedure. Surg Endosc. 2010;24:3241–3243. doi: 10.1007/s00464-010-1025-y. [DOI] [PubMed] [Google Scholar]
- 23.Schwab KE, Dowson HM, Van Dellen J, Marks CG, Rockall TA. The uptake of laparoscopic colorectal surgery in Great Britain and Ireland: a questionnaire survey of consultant members of the ACPGBI. Colorectal Dis. 2009;11:318–322. doi: 10.1111/j.1463-1318.2008.01601.x. [DOI] [PubMed] [Google Scholar]
- 24.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 25.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 26.deSouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, Abcarian H. Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum. 2010;53:1611–1617. doi: 10.1007/DCR.0b013e3181f22f1f. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–248. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, Berger DH, Albo DA, Artinyan A. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg. 2013;206:509–517. doi: 10.1016/j.amjsurg.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Xiong B, Ma L, Zhang C, Cheng Y. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res. 2014;188:404–414. doi: 10.1016/j.jss.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Morelli L, Ceccarelli C, Di Franco G, Guadagni S, Palmeri M, Caprili G, D’Isidoro C, Marciano E, Pollina L, Campani D, et al. Sexual and urinary functions after robot-assisted versus pure laparoscopic total mesorectal excision for rectal cancer. Int J Colorectal Dis. 2016;31:913–915. doi: 10.1007/s00384-015-2301-z. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485–2493. doi: 10.1245/s10434-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48–55. doi: 10.1007/s00464-012-2405-2. [DOI] [PubMed] [Google Scholar]
- 33.D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–1895. doi: 10.1007/s00464-012-2731-4. [DOI] [PubMed] [Google Scholar]
- 34.Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168–3173. doi: 10.1245/s10434-007-9544-z. [DOI] [PubMed] [Google Scholar]
- 35.Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 2015;94:e522. doi: 10.1097/MD.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alecu L, Stănciulea O, Poesina D, Tomulescu V, Vasilescu C, Popescu I. Robotically performed total mesorectal excision for rectal cancer. Chirurgia (Bucur) 2015;110:137–143. [PubMed] [Google Scholar]
- 37.Stănciulea O, Eftimie M, David L, Tomulescu V, Vasilescu C, Popescu I. Robotic surgery for rectal cancer: a single center experience of 100 consecutive cases. Chirurgia (Bucur) 2013;108:143–151. [PubMed] [Google Scholar]
- 38.Erguner I, Aytac E, Boler DE, Atalar B, Baca B, Karahasanoglu T, Hamzaoglu I, Uras C. What have we gained by performing robotic rectal resection? Evaluation of 64 consecutive patients who underwent laparoscopic or robotic low anterior resection for rectal adenocarcinoma. Surg Laparosc Endosc Percutan Tech. 2013;23:316–319. doi: 10.1097/SLE.0b013e31828e3697. [DOI] [PubMed] [Google Scholar]
- 39.Park IJ, You YN, Schlette E, Nguyen S, Skibber JM, Rodriguez-Bigas MA, Chang GJ. Reverse-hybrid robotic mesorectal excision for rectal cancer. Dis Colon Rectum. 2012;55:228–233. doi: 10.1097/DCR.0b013e31823c0bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13:176–183. [PMC free article] [PubMed] [Google Scholar]
- 41.Park YW, Lee JH. Correlation between the visual prostate symptom score and international prostate symptom score in patients with lower urinary tract symptoms. Int Neurourol J. 2014;18:37–41. doi: 10.5213/inj.2014.18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 43.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 44.Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schäfer W. Scoring the short form ICSmaleSF questionnaire. International Continence Society. J Urol. 2000;164:1948–1955. [PubMed] [Google Scholar]
- 45.Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol Female Lower Urinary Tract Symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol. 2004;191:73–82. doi: 10.1016/j.ajog.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Broholm M, Pommergaard HC, Gögenür I. Possible benefits of robot-assisted rectal cancer surgery regarding urological and sexual dysfunction: a systematic review and meta-analysis. Colorectal Dis. 2015;17:375–381. doi: 10.1111/codi.12872. [DOI] [PubMed] [Google Scholar]
- 47.Lee SH, Lim S, Kim JH, Lee KY. Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res. 2015;89:190–201. doi: 10.4174/astr.2015.89.4.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pai A, Melich G, Marecik SJ, Park JJ, Prasad LM. Current status of robotic surgery for rectal cancer: A bird’s eye view. J Minim Access Surg. 2015;11:29–34. doi: 10.4103/0972-9941.147682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aly EH. Robotic colorectal surgery: summary of the current evidence. Int J Colorectal Dis. 2014;29:1–8. doi: 10.1007/s00384-013-1764-z. [DOI] [PubMed] [Google Scholar]
- 50.Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27:233–241. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]
- 51.Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56:253–262. doi: 10.1097/DCR.0b013e3182694595. [DOI] [PubMed] [Google Scholar]
- 52.Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc. 2006;20:1521–1525. doi: 10.1007/s00464-005-0855-5. [DOI] [PubMed] [Google Scholar]


