Abstract
Heart disease remains one of the leading causes of death in industrialized nations with myocardial infarction (MI) contributing to at least one fifth of the reported deaths. The hypoxic environment eventually leads to cellular death and scar tissue formation. The scar tissue that forms is not mechanically functional and often leads to myocardial remodeling and eventual heart failure. Tissue engineering and regenerative medicine principles provide an alternative approach to restoring myocardial function by designing constructs that will restore the mechanical function of the heart. In this review, we will describe the cellular events that take place after an MI and describe current treatments. We will also describe how biomaterials, alone or in combination with a cellular component, have been used to engineer suitable myocardium replacement constructs and how new advanced culture systems will be required to achieve clinical success.
Keywords: : cardiac tissue engineering, cardiac patch, acellular scaffolds, extracellular matrix scaffolds, myocardial infarction, heart repair
Introduction
Heart disease remains one of the leading causes of death in industrialized nations with myocardial infarction (MI) contributing to at least ∼20% of the reported deaths.1 An acute MI occurs when a coronary artery that feeds oxygenated blood to the right and left ventricles gets occluded, thus resulting in areas of hypoxia. The hypoxic environment, if maintained for sufficient amount of time, eventually leads to cellular death due to lack of oxygen and nutrients triggering an inflammatory response. The inflammatory environment is responsible for the clearing of dead cells and stimulating neighboring cells to increase matrix production ultimately leading to scar tissue formation.
The scar tissue that forms after an MI is unable to contract and, due to the high stresses present during the normal pumping action of the heart, the infarct area deforms over time leading to myocardial remodeling and reduced cardiac output. Although reperfusion and pharmacological treatments have shown some improvements in patients after an MI, the scar tissue is not completely removed and cardiac output is not restored to pre-MI levels.2 If significant damage is sustained, a heart transplant is a potential treatment option. However, heart transplantation remains limited by low availability and the need for life immunosuppression of the transplant recipient.
Tissue engineering and regenerative medicine principles provide an alternative approach to restoring myocardial function after an MI. By combining the expertise of multiple fields, such as engineering, biology, medicine, biochemistry, and pharmacology, tissue engineers try to create suitable tissue replacements capable of restoring function and improving quality of life. This review will first describe the cellular events that take place after an MI with emphasis on the host tissue response dominated by inflammatory cells such as macrophages. The review then will describe how biomaterials, alone or in combination with a cellular component, have been used to engineer suitable myocardium replacement constructs. Given the complexity of the myocardial tissue, we will also discuss ideas for new advanced culture systems that can help assemble and test the new generation of engineered cardiac devices.
Myocardial Infarction
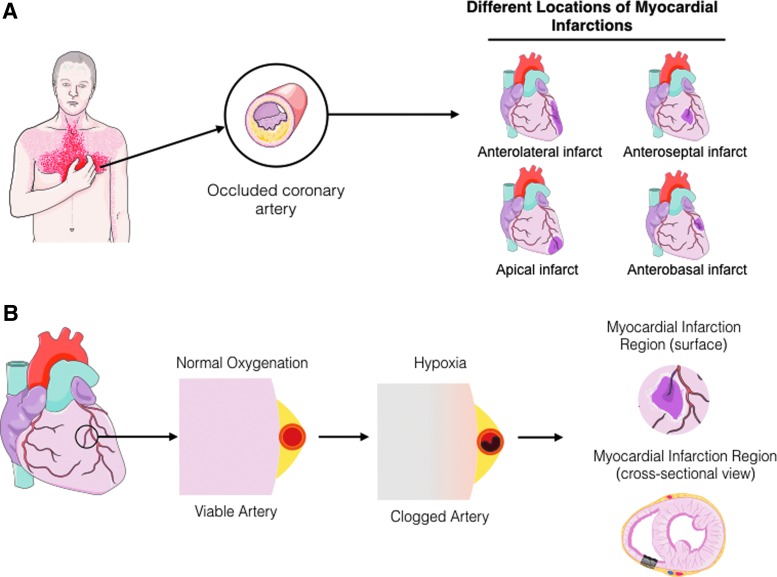
A heart attack or MI is caused by the stenosis and/or occlusion of a coronary artery leading to improper delivery of oxygenated blood to regions of the heart. This condition is classified based on the extent of occlusion into ST-Elevation myocardial infarction (STEMI) when occlusion is completely blocked, or non-ST-Elevation myocardial infarction (NSTEMI) when occlusion is not complete. The physiological and clinical description of MI is described in details elsewhere2; in brief, the occlusion can affect the major coronary arteries such as the left anterior descending, left circumflex, and the right coronary artery. Once the artery is sufficiently occluded, oxygenation and nutrient deficit downstream of the occlusion result in the gradual death of the myocardial tissue (Fig. 1). The infarct site refers to the portion of the necrotic myocardium that is damaged or in the process of being damaged by the hypoxic conditions. Most infarcts involve the death of the full thickness of the ventricular wall (transmural infarction), although in some cases perfusion from neighboring vessels can help delay and/or minimize injury. At the early stages of the infarct there is a decrease in aerobic glycolysis, an increase in anaerobic glycolysis (accumulation of lactic acid), production of high-energy phosphates, and reduced contractility.2
FIG. 1.
Diagrammatic representation of an MI. (A) When symptoms of a heart attack are felt, it represents the occlusion of one or multiple coronary arteries supplying oxygen and nutrients to the heart. (B) Once the artery is occluded, there is hypoxia due to the limited diffusion of oxygen, resulting in the death of myocardial tissue. Artwork provided by Servier Medical Arts. MI, myocardial infarction. Color images available online at www.liebertpub.com/teb
Necrosis and apoptosis begin to occur, leading to the activation of the inflammatory response through the recruitment of neutrophils and subsequently monocytes from peripheral blood. Recent studies have shown the active recruitment of circulating peripheral blood monocytes and a monocyte population resident in the spleen following an MI.3 In addition, studies have shown waves of pro- and anti-inflammatory monocytes circulating at different times after the infarct with classically activated monocytes found soon after the ischemic injury and then followed by alternatively activated monocytes.4 Once recruited to the infarct, monocytes differentiate toward macrophages and begin the labor of removing cellular debris.5,6
During the initial phase of the inflammatory response after the recruitment of the monocytes, there is a strong presence of classically activated macrophages or M1 macrophages within the myocardial tissue. These are macrophages associated with the removal of pathogens and cellular debris and express proinflammatory cytokines such as IL-1β, TNF-α, and IL-6. These macrophages are present at high numbers during the first week after an infarct in mice with a gradual decrease over time.4,5,7,8 Following this initial proinflammatory phase, there is a gradual shift in the type of macrophage toward more alternatively activated macrophages (M2), which are characterized by the secretion of CCL-17, TIMP-1, and IL-10. These macrophages are typically associated with wound healing responses and are thought to activate fibroblasts, smooth muscle, and endothelial cells.5,6,9–11
Myocardial regeneration is the process by which the injured myocardium is restored to its original structure and function. As described above, the normal healing process for postinfarction cardiac tissue involves the generation of a fibrous scar, which provides mechanical support but is devoid of functional cardiomyocytes. Most treatment strategies focus on improving the performance of an already damaged tissue and not in regenerating the myocardium to restore or establish normal function. More recently, new therapeutic approaches based on progenitor cells have been developed with the goal of regenerating tissue to its normal structure and function.
Current Cardiac Treatments
Advancements in medical interventions have improved the prognosis post-MI considerably, but the incidence of heart failure is still increasing, likely as a result of the increasing number of patients who now survive the initial attack. Currently, the only approved treatments for end-stage heart failure post-MI are left ventricular assist devices and heart transplantation. The first is plagued by the complications of a chronic external assist device, which include bleeding (30%), right ventricular failure (20–30%), thromboembolism (3–35%), primary device failure (6% −6 months, 64% −2 years), and infection (18–59%).12 The second is a limited resource in which proper matching of the donor organ to the patient is a great challenge, limiting even further its use. A number of surgical approaches have been developed as preventative measures to improve patient survival and their quality of life, and which can be an option for patients excluded from cardiac transplantation lists. The most common include angioplasty, left ventricular reconstruction, and cellular cardiomyoplasty.
Ischemic tissue revascularization
There is agreement that initial treatment for STEMI is restoring blood flow to the ischemic tissue via tissue reperfusion. The main alternatives for reperfusion can be classified into pharmacologic, surgical, or mechanical. The pharmacological breakdown of blood clots (thrombus) in stenotic coronary arteries is known as thrombolysis. The mechanical alternative to reperfusion is known as primary percutaneous surgical alternative coronary intervention or primary coronary angioplasty, where the occlusion is mechanically expanded to allow blood flow to resume. The surgical alternative is known as coronary artery bypass graft (CABG) surgery, which when compared with angioplasty is highly invasive (requiring open heart surgery) and requires extra surgery to obtain the vein graft.
The use of primary angioplasty for the treatment of STEMI was first described as a rescue treatment in the case of failed intracoronary thrombolysis and was studied extensively as an adjunctive therapy. In general terms, the procedure consists of feeding a deflated balloon or other device (e.g., stent) on a catheter from the inguinal femoral artery or radial artery up through blood vessels until they reach the site of blockage in the heart. At the blockage, the balloon is inflated to open the artery, allowing blood to flow. Primary angioplasty has been shown to be more effective to thrombolysis for treatment of patients with acute STEMI in randomized trials.13–16 The use of angioplasty requires the procedure to be performed preferably within 90 min of the patient presenting to the emergency room, which most hospitals cannot provide.
There is strong evidence that with increasing duration and severity of ischemia, more cardiac tissue damage can develop, allowing a variety of reperfusion-associated pathologies, known as reperfusion injury. This condition results in cardiac tissue damage through myocardial stunning, microvascular and endothelial injury, and irreversible cell damage, necrosis, apoptosis, autophagy, or necroptosis.17,18 Reperfusion injury has been observed in each of the cardiac tissue revascularization strategies mentioned above and under certain conditions can be lethal. There are various pharmacological and nonpharmacological interventions used to reduce reperfusion injury. In the case of pharmacological interventions, the use of drugs such as cyclosporine-A, metoprolol, and glucose modulators has shown some promising results, but a long list of failed examples makes them a weak alternative. In contrast, nonpharmacological interventions have focused on limiting the infarct size as means to reduce reperfusion injury.
Left ventricular reconstruction
After MI, the formation of scar tissue leads to changes in left ventricular (LV) size, shape, structure, and physiology through a process known as myocardial remodeling.19 During this process, there is thinning of the LV walls, with the elliptical LV becoming more spherical and dilated.20 A number of different surgical techniques and modifications have been developed to restore LV shape and reduce its volume to improve LV function and are collectively known as LV reconstruction.21–24 This is a specific surgical procedure developed for the management of heart failure with LV remodeling caused by coronary artery disease.25 Despite its success, these procedures have not found general acceptance in the medical community. Possible reasons include a lack of robust prospective randomized data showing the mortality benefit of this technique in patients with ischemic cardiomyopathy and dilated ventricles that were referred for CABG. To address these concerns, the Surgical Treatment for Ischemic Heart Failure (STICH) trial was developed to evaluate the role of cardiac surgery in the treatment of patients with coronary artery disease and LV systolic dysfunction.26 A major question addressed by this study was if left ventricular reconstruction improved patient outcome when combined with CABG. The results of this clinical trial showed no significant difference between performing CABG alone or when combined with LV reconstruction.26 These surgical techniques, and the use of nonbioactive materials as tissue replacements, helped spark the interest in exploring innovative use of biomaterials and tissue engineering constructs.
Cellular cardiomyoplasty
Cell transplantation is an area of growing interest in clinical cardiology, as a potential means of treating patients after acute MI. Cellular cardiomyoplasty is a therapeutic strategy in which progenitor cells are used to repair regions of damaged or necrotic myocardium. The ability of transplanted progenitor cells to improve function within the failing heart has been shown in experimental animal models and in some human clinical trials.27 The progenitor cells involved in these new therapeutic approaches include bone marrow or adipose tissue-derived mesenchymal stem cells (MSCs), hematopoietic precursor cells, endothelial progenitor cells, endogenous cardiac stem cells, and skeletal muscle-derived cells.28,29
Three mechanisms have been proposed to describe how cardiomyoplasty improves myocardial function: (1) transdifferentiation of the administered stem cells into cardiomyocytes, endothelial cells, and smooth muscle cells,30,31 (2) fusion between the stem cell and endogenous cardiac myocytes,32 and (3) release of paracrine factors that stimulate endogenous cardiac repair mechanisms.33,34 Conflicting results showing a lack of transdifferentiation have put into question its role in cardiomyoplasty and motivated the search of alternative hypotheses like fusion and paracrine signaling.30,35 Further studies suggested that the lack of transdifferentiation shown was related to differences in experimental procedures, but the exact mechanism remains unknown.36 In addition, it has been consistently reported that the level of fusion between stem cells and cardiomyocytes remains low,32,37 suggesting that additional mechanisms may be involved. Current results support paracrine signaling as the principal mechanism for the improvement of myocardial function. In it, stem cells release cytokines and chemokines to stimulate other cells into the regenerative process. In fact, MSCs have been shown to stimulate host myocardial precursor cells to amplify and differentiate into cardiomyocytes in vivo.38
The clinical application of cellular cardiomyoplasty for the treatment of the ischemic tissues after acute MI involves tissue revascularization, isolation of autologous stem cells from the patient, and implementation through repeat cardiac catheterization or intramyocardial injection.39 A major limitation for the application of cellular cardiomyoplasty as a treatment option is stem cell retention and engraftment after intramyocardial implantation.40 A significant proportion of the transplanted cells leak out through the needle track that is made by the puncturing needle or enter systemic circulation.41,42 Cell retention is normally less than 10%, regardless of the delivery route within the first 24 h.43 Although it was initially thought that cell death by apoptosis was the reason for low engraftment,44,45 it has been demonstrated that venous drainage and the contraction of the beating heart are the main reasons for cell loss.40,46 Short-term cell retention is necessary for subsequent long-term engraftment and cardiac tissue functional improvement after acute MI. Other unresolved issues include cell delivery method and route, cell distribution, time transplantation, cell type, cell number, and viability. There are new therapeutic approaches involving engineering culture systems, the use of novel biomaterials for mechanical support of the cells and for controlled release of therapeutics, and tissue engineering (Fig. 2).
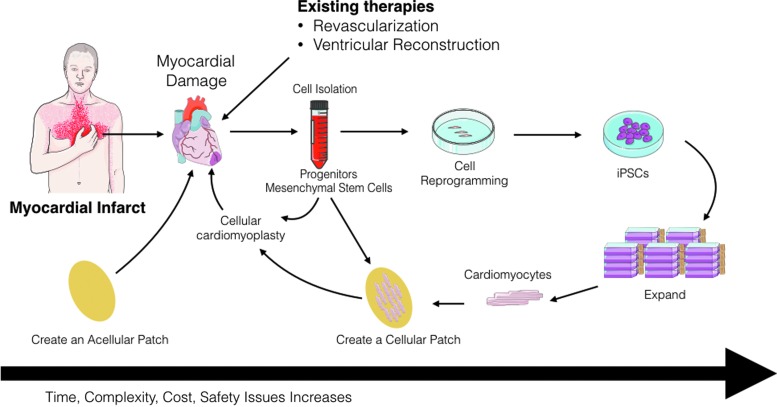
FIG. 2.
Diagrammatic representation of the different approaches that can be used to repair infarcted myocardial tissue. An acellular patch can be used as an off-the-shelf product that can be implanted soon after myocardial infarction. Alternatively, cells can be harvested (i.e., progenitor cells) and injected back into the patient. Another approach can be the isolation of somatic cells (i.e., blood cells), reprogrammed, expanded, differentiated, and assembled into a bioengineered cardiac tissue that can then be implanted back into the patient as an autologous patch. These approaches have different timing and expenses associated with them that can have potential impact on their clinical use. Artwork provided by Servier Medical Arts. Color images available online at www.liebertpub.com/teb
Engineered Cardiac Patch
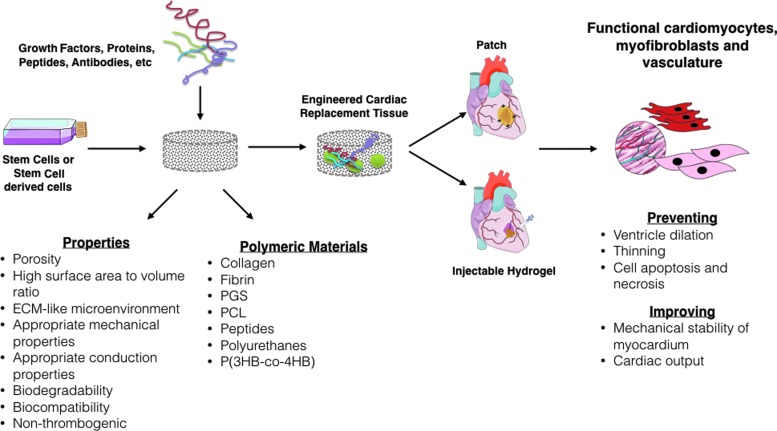
Engineered cardiac patch is fabricated to mimic the native extracellular matrix (ECM) and offer mechanical support and cell delivery into the region of infarction. Its application helps to limit LV remodeling, prevent dilatation and thinning of the infarct zone, enhance mechanical properties of ventricle, and reduce cardiomyocyte apoptosis. In addition, it aids to retain viable transplanted stem cells, which stimulate the formation of vasculature, myofibroblasts, and cardiomyocytes. Hence, the optimal properties of a scaffold involve high porosity, microenvironment similar to ECM, good mechanical properties, biodegradability, and biocompatibility. Natural polymers (i.e., collagen, fibrin, chitosan, alginate, natural ECM, peptides) and synthetic polymers (i.e., polycaprolactone [PCL], polyglycerol sebacate [PGS], and polyurethanes) are a choice of materials to fabricate scaffolds47,48 (Fig. 3). The Tables 1 and 2 summarize some characteristics of natural and synthetic polymers used for cardiac patch, respectively.
FIG. 3.
Diagrammatic representation of tissue engineering strategies using cellular and acellular scaffolds. The goal is to find the optimal configuration that restores myocardial function to preinfarct levels. Color images available online at www.liebertpub.com/teb
Table 1.
Some Natural Polymers for Cardiac Patch
| Material | Stem cells | Porosity and/or pore size | In vivo model | Signaling | Ref. |
|---|---|---|---|---|---|
| Collagen | hMSCs | — | Male CDF rats | α-SMA | 118 |
| Autologous stem cells | 400–600 μm | Male C57/BL6 mouse | Anti-sarcomeric actin antibody and Anti-vWF | 121 | |
| hMSCs hESC-MC |
— | Male athymic RNU nude rats | CD105, CD73, Angiogenin, PDGF-B, VEGF, and CXCL1 | 105 | |
| Autologous mesenchymal stem cells | — | Wistar rats | CD44+, CD90+, CD45−, CD34−, Desmine, and α-smooth muscle actinin | 119 | |
| hESC-derived cardiovascular progenitors Autologous r-ADSCs |
— | Female Wistar rats Rowett nude female rats | Tbx5 and Nkx 2.5 | 116 | |
| Natural ECM | BM-MSCs | 91.2% ± 1.3%; 130.5 ± 25.3 mm | Male syngeneic Lewis rats | α-SMA, bFGF, vWF, PDGF-B, IGF-1, HGF, MEF2D, MYH6, Type I collagen, SMMHC, CD68, and IL-6 | 139 |
| hMSCs | — | Adult mongrel dogs | Sarcomeric α-actinin, Atrial natriuretic peptide Cardiotin, Subunit of the Cav 1.2, and cardiac troponin-T |
137 | |
| Cardiac progenitor cells | — | — | α-MHC, Troponin T, Troponin C, GATA-4, nkx 2.5, α-SMA, Smooth muscle 22α, Fibroblast-specific protein 1, vWF, tie2 | 135 | |
| BMMCs | 19.5 ± 17.9 μm | — | Sarcomeric α-actinin, Myosin heavy chain Cardiac troponin T, and vWF |
134 |
ADSC, adipose tissue-derived stem cell; ECM, extracellular matrix; hMSC, human mesenchymal stem cell; hESC-MC, human embryonic stem cell-derived mesenchymal cells; α-SMA, α-smooth muscle actin.
Table 2.
Other Polymeric Material for Cardiac Patch
| Stem cell | Polymer | Porosity/pore size | Elastic modulus (MPa) Tensile stress Tensile strain (MPa) Tensile strain at break | In vivo model | Signaling | Ref. |
|---|---|---|---|---|---|---|
| Cardiomyocytes differentiated from hESC | Poly(glycerol sebacate) | — | — | Adult male Sprague Dawley rats | — | 144 |
| cSca-1, BMMCs, and ADSCs | Nanopeptides (cell–PuraMatrix™) | — | — | Wild mice (C57Bl/6J) | Anti-vWF), anti-SMA, anti-α-sarcomeric actinin anti-CD31,VEGF, bFGF, and PDGF-bb | 152 |
| BM-MSCs | PPC, PU, and [P(3HB-co-4HB)] | — | — | — | CD34, CD45, CD90, CD73, CX43, and cTn T | 153 |
| Neonatal cardiomyocytes | PGS/collagen | — | 2.06 MPa (TS) 57.87% (TSA) 83.65% (TSB) 4.24 (EM). |
— | Cardiac-specific marker proteins α-actinin, Troponin, β-MHC, and cx43 | 146 |
| BM-MSC | PCL/Gelatin | 83.6% ± 0.8%/0.83 ± 0.15 μm | — | Female Sprague Dawley rats | CD31, cTnT, and Cx43 | 47 |
| Cardiac progenitor cells | PCL/CNT | — | 11 MPa (EM) 1.3 MPa (TS), 131% (TSB) |
— | sca-1, CD34, GATA-4, CD44, CD29, and CD31 | 150 |
| BMMSCs | PGS/Fibrinogen | — | — | Farm pigs (Sus scrofa) Yorkshire Swine | α-sarcomeric actinin, troponin T, CD68, and CD31 | 117 |
| hMSCs | PEG/Alginate | — | — | Male nude rats (Crl:NIH-Foxn1rnu) | anti-vWF, anti-BRDU | 154 |
| Neonatal rat cardiac cell | Fibrin | — | 86.0 ± 3.8 (EM) 75.7 ± 11.5 (UTS) |
Female Fisher F344 syngeneic immune-competent rats | SMA +, CD31, Type I collagen and Type IV collagen | 48 |
PCL, poly(caprolactone); PEG, poly(ethylene glycol); PGS, polyglycerol sebacate; TS, tensile stress; TSA, tensile strain; TSB, tensile strain at break; EM, elastic modulus.
Acellular biomaterial approach to cardiac repair
Cardiac tissue has limited self-renewal capacity, which limits its ability to regenerate and repair itself after injury. Due to the challenges and limitations on the use of biomaterials with exogenous cells, acellular injectable biomaterials and patches have been evaluated as mechanical supports for MI. Acellular scaffolds have several advantages compared to cellular scaffolds such as (1) their off-the-shelf availability for immediate implantation (e.g., SynerGraft®, AlloDerm®, DermaMatrix®), (2) their limited immune reaction,49 and (3) low cost and extended shelf life.50 Cardiac tissue scaffolds should exhibit elasticity matching the myocardium, host cell integration and vascularization, mechanical stability, and nonimmunogenicity to support tissue function and regeneration. The following sections briefly discuss the advantages and limitations of major biomaterials used as acellular constructs for myocardial repair and regeneration with emphasis given to naturally occurring biomaterials.
Collagen
Due to its abundance in connective tissue, collagen type I scaffolds are increasingly being used in tissue repair applications. Collagen type I provides a tissue-like environment for cell attachment and growth, which facilitates host cell integration. Its main attributes include biocompatibility, biodegradability, and fibrous contractile structure.51,52 Collagen type I comprises about 80% of the collagen matrix in cardiac tissue,53 making it the choice of preference for cardiac tissue scaffolds. Gaballa et al.54 grafted a three-dimensional (3D) collagen type I scaffold onto infarcted myocardium in rats and found that the scaffold induced neovessel formation and reduced LV remodeling 3 weeks after implantation. However, solid porous collagen has a lower elastic modulus,55 which can limit its mechanical integration to the cardiac tissue. Serpooshan et al. optimized the elastic modulus of collagen type I gel to improve myocardial contractility in the injured heart.56 Collagen type I was molded using a plastic compression technique to generate dense tissue scaffolds with a high elastic modulus. Four weeks post-MI in mice, collagen type I patches showed host cell infiltration and new blood vessel formation. Echocardiography showed significant improvement in cardiac function, diminished fibrosis, and inhibition of LV dilation and wall thinning.
Collagen in combination with other biomaterials such as chitosan has shown an increase in compression modulus, which makes it more suitable for the stabilization of the ventricular wall.57 Incorporation of angiogenic factors, such as thymosin β4, in composite collagen–chitosan hydrogels has been shown to induce cell migration and improve angiogenesis in vivo.58 Other forms of collagen such as gelatin have shown good cardiac cell attachment and viability, but the tensile strength and degradation rates are inferior to collagen, making it a less attractive option for cardiac tissue implants. These results indicate that collagen scaffolds can exert beneficial effects on cardiac remodeling and function after injury. Cardiac cell integration and function should be further evaluated to determine its long-term clinical potential.
Hyaluronic acid
Hyaluronic acid (HA), also known as hyaluronan, is a natural linear polysaccharide abundantly found in the ECM of several tissues. Its structure and ligand binding properties have been linked to angiogenesis59 and tissue repair.60 Thus, HA has become an important component in scaffolds used for tissue repair and regeneration. In cardiac tissue, HA has shown modest results for cardiac function recovery postinfarction. Yoon et al. were one of the first groups that demonstrated the regenerative potential of HA for heart tissue. A significant decrease in both infarcted area and apoptotic index as well as an increase in local vasculature were observed in rats injected with an acrylated HA hydrogel into the epicardium of the infarcted region.61 Similar results have been observed by Abdalla et al. by evaluating the recovery of cardiac function in the infarcted heart of rats postinjection of HA gel into the peri-infarct region.62 Transthoracic echocardiography revealed a significant increase of about 18% in ejection fraction in HA-injected groups compared to the control group. Decreased collagen deposition and increased levels of VEGF were also observed supporting reduced scarring and new vasculature formation in response to HA.
The molecular weight of HA has been shown to affect its regenerative potential in the myocardium. Evaluation of different molecular weights of HA-based hydrogels (50, 130, and 170 kDa) showed that the lowest molecular weight had the most significant regeneration and function recovery of the infarcted myocardium.63 The regenerative potential of HA-based hydrogel was markedly reduced in chronic models of MI, indicating that the injection time is a major determinant for cardiac repair. The compressive modulus of HA gels is also an important variable for stabilization of the infarcted myocardium. Use of hydrogels with high compression modulus (43 kPa) significantly reduced LV remodeling and improved function when compared with lower modulus hydrogel and control groups in an ovine model.64 Due to the increase in wall stress during systole, a high compression modulus may be more suitable to reduce myocardial stress distribution. Thus, two major factors, the molecular weight of HA and injection time, are main determinants for HA-mediated repair in the infarcted myocardium. Overall, several studies support the use of HA-based hydrogels as a promising novel therapy to reduce scarring and promote vasculature formation in the infarcted myocardium.
Alginate
Alginate is an anionic polysaccharide present in brown seaweed. It is biocompatible and has been used for food and pharmaceutical applications.65 Its capacity for in situ gelation and nonthrombogenic properties makes it attractive for cardiac tissue applications. In heart tissues, intramyocardial injections of alginate hydrogels have shown significant clinical potential for the improvement of LV function and reduced remodeling potentially by providing mechanical support to the damaged ECM. Yu et al. showed that acellular alginate hydrogels could improve cardiac function, reduce remodeling, and increase neovascularization in a chronic rodent model of ischemic cardiomyopathy.66 The angiogenic effect of alginate can be further enhanced by incorporating RGD peptides into the biopolymer, but the structural changes reduce the therapeutic effects of the hydrogel.67 Landa et al. evaluated the effect of alginate hydrogel in the recovery of cardiac function of rats post-MI.68 There was an increase in scar thickness, and reduced LV systolic and diastolic dilatation was typically observed even after injection 60 days postinfarction. These results were comparable to those achieved by neonatal cardiomyocyte transplantation.
The use of alginate hydrogels has also been shown to improve cardiac function in large animal models. Intracoronary injection of alginate hydrogel prevented LV remodeling and increased scar thickness in a swine model of MI.69 The alginate hydrogel was replaced by myofibroblasts, which support local tissue restoration while limiting general myocardial remodeling. Implantation of alginate hydrogel in dogs with heart failure produced by intracoronary microembolizations (LV ejection fraction <30%) significantly improved ventricular wall stability and function.70 Injection of alginate hydrogel expanded the LV wall and improved the LV systolic and diastolic functions at levels comparable to those observed in dogs in long-term therapy with beta-blockers.71 These observations motivated evaluation in patients with ischemic (n = 4) and nonischemic (n = 2) dilated cardiomyopathy. Patients that received alginate implants showed improvements in LV size and function as early as 3 days. Reductions in LV volumes and an increase in ejection fractions were sustained for over 3 months. Due to their promising results, alginate hydrogels are to date the only and first injectable biomaterial in clinical trials for treating MI. However, lack of integration between alginate and cardiac cells might be the major limitation for tissue regenerative applications.
Chitosan
Chitosan is a cationic hydrophilic polysaccharide derivate from chitin commonly found in crustacean shells. It has been extensively used in biomedical applications, including wound healing,72,73 drug delivery systems,74 and surgical adhesives.75 The porosity of chitosan can be controlled as a function of freeze-drying,76 which is important for host cell migration and tissue integration. However, chitosan alone is noncell adhesive and has a high compressive modulus, which requires its chemical modification and/or mixing with other biomaterials to obtain optimal mechanical and physiological properties for cardiac tissue. Pok et al. evaluated a multilayered scaffold formed by a gelatin–chitosan hydrogel around a self-assembled PCL core for use as a cardiac patch.77 Gelatin and chitosan ratios of 50:50 and 25:75 significantly improved cell adhesion while retaining the mechanical strength of PCL. Mixtures of chitosan and collagen have also shown potential to improve cardiac function. Deng et al. combined chitosan with collagen to increase the compressive modulus of collagen as a potential implant for stabilization of the ventricular wall.57 Ahmadi et al. investigated the effects of a collagen chitosan matrix on cardiac remodeling.78 Mice received local injection of collagen–chitosan matrix 2 weeks post-MI. LV ejection fraction was improved only in collagen–chitosan-treated mice over a 3-week follow-up period. Thus, combination of porous chitosan with lower compressive moduli and cell-adherent biomaterials may have the potential to increase tissue integration and therefore increase the mechanical stability of the ventricle postinfarction.
Fibrin
Fibrin-based scaffolds are biopolymer gels formed from fibrinogen, a glycoprotein that contains two Arginine–Glycine–Aspartic acid (RGD) sequences in each amino acid chain and is converted by thrombin into fibrin during blood clot formation simulating the last step of the blood coagulation cascade.79,80 Fibrin polymerizes in situ upon the combination of fibrinogen and thrombin. Fibrin glue has been tested as an injectable scaffold for cardiac tissue repair. Christman et al. examined the effects of injectable fibrin glue as a scaffold and wall support in the ischemic myocardium in rats. Fibrin glue alone or with skeletal myoblasts was injected into the LV 1–2 days after left coronary artery occlusion. Five weeks after injection, fibrin glue alone or with cells preserved infarct wall thickness, reduced infarct size, increased blood flow, and improved cardiac ejection function.81,82 Huang et al. performed a comparative study to determine the therapeutic potential of fibrin, collagen type I, and Matrigel as injectable biomaterials for MI repair. Injection of each individual biomaterial into the infarct zone significantly increased vascularization compared to the saline solution control group at 5 weeks post-treatment in rats. The angiogenic potential was similar for all three polymers probably due the shared common binding sites for avb3 integrin, which is associated to angiogenesis. The major disadvantage of fibrin gels is poor mechanical properties, not able to support the stresses generated during myocardial contraction.83 Therefore, fibrin should be combined with other high-compression moduli components such as chitosan or collagen to increase mechanical strength and reduce degradation rates.
Decellularized extracellular matrices
Decellularized matrices are derived from biological tissues in which cells have been removed, but the architecture and components of the ECM are preserved. The main advantages of decellularized matrices are preserved structure, size, and components of native ECM without the presence of cellular antigens that could induce an immune reaction.84 In cardiac tissue studies, decellularized matrices have shown to promote endothelial cell and cardiac cell infiltration. Decellularized urinary bladder ECM (UB-ECM) has been evaluated as an epicardial patch for repairing the infarcted LV.85 At 6–8 weeks postinfarction, pigs received either a UB-ECM or expanded polytetrafluoroethylene (ePTFE) patch in the LV. At 3 months, the decellularized matrix was resorbed showing a highly vascularized tissue enriched in collagen and myofibroblasts. At the same time point, ePTFE had a foreign body response and calcification. Similarly, Robinson et al.85 used a urinary bladder matrix (UBM) scaffold for repairing the infarcted LV in pigs. Results showed that after 3 months the constructs had a significant increase in cardiac marker expression (i.e., α-smooth muscle actin (SMA)+ myofibroblasts, α-sarcomeric actin, myosin-HC, tropomyosin, and connexin 43) and the number of contractile cells (i.e., expressing α-SMA). It has been well established that UBM scaffolds are superior to synthetic Dacron in regenerating myocardial tissue. This fact is due to their potential capacity to promote cardiomyocyte differentiation and/or migration, allowing the ventricular wall to approach its normal thickness, and finally resulting in improved regional mechanical function.86,87 Moreover, natural matrices from small intestine submucosa (SIS)88 and porcine sternum89 used in rats models have showed to be a good alternative to promote angiogenesis, enhance cardiac function, and decrease apoptosis, through the recruitment of c-kit+ cells, myofibroblasts, and macrophages after MI. Tan et al. used a decellularized SIS patch with MSCs on a MI rabbit model90 and showed significant improvements in LV function, wall thickness, and vasculature. Decellularized ventricular and pericardial matrices are two additional options for MI repair.91–93 Singelyn et al. have shown that decellularized porcine ventricular tissue can be solubilized and self-assembled in situ upon injection into myocardial tissue. Smooth muscle cells and endothelial cells were able to infiltrate the decellularized matrix both in vitro and in vivo with a significant increase in blood vessel density. These studies were performed in the healthy rat myocardium, which have better recovery rates than in MI.
Synthetic materials
Synthetic materials have been widely used in tissue engineering applications due to improved mechanical properties, material uniformity, and low risk of infection compared to natural biomaterials. Synthetic polymers can be modified with high precision to meet tissue-specific properties such as appropriate degradation rates, porosity, and mechanical strength. Several synthetic polymers have been evaluated for cardiac tissue implants, including poly(ethylene glycol)(PEG), polyvinyl alcohol, poly(caprolactone)(PCL), polypropylene, polyester, and poly(N-isopropylacrylamide) (PNIPAM).94,95 Meshes made of polyester and poly(propylene) have been successfully used as LV restraints to prevent LV remodeling and dilation in animal models and human patients.96–100 Injection of a thermosensitive hydrogel containing PCL and PNIPAM into the myocardium 4 days postinfarction in rabbits was found effective to prevent ventricular wall thinning and reduce systolic and diastolic dilatation after 30 days of treatment.95 Similarly, PEG hydrogels have prevented LV remodeling and dilation, but lack vascularization unless codelivered with cells.101 While the mechanical properties and stability of synthetic polymers are superior to natural polymers, cell integration is a major limitation. Blends of synthetic and natural polymers with or without growth factors are often preferred to support cell migration and tissue replacement of the implant.102,103
Engineered cellular constructs for cardiac repair
Although acellular scaffolds have many advantages over cellular scaffolds, the use of cells has also been shown to improve healing and tissue regeneration. In many cases, the addition of a cellular component showed improvement over the material alone.104 The use of an engineered cellular construct is a therapeutic strategy to regenerate the myocardium lost after an MI by supporting the function of the myocardium via a mechanically suitable material and the surviving cells using an appropriate exogenous repair cell. The cardiac patch is a 3D carrier for cell delivery fabricated in vitro and implanted over the infarcted tissue47 with the goals of improving repair cell retention and engraftment, limiting LV remodeling, preventing LV dilatation and thinning, enhancing the mechanical properties of ventricle, and reducing cardiomyocyte apoptosis.48 In addition, it can also provide the means to stimulate angiogenesis, release of cytokines, and myocardial perfusion.48,105 All these properties depend on the choice of scaffold material and repair cells that will ultimately couple with the native cells.
One of the first patches developed for delivering cells to an MI site consisted of a cardiomyocyte-enriched extract from fetal rat ventricular muscle dispersed in a commercially available gelatin scaffold (i.e., Gelfoam, Merck, Co.).106 Although this cardiac patch showed improved cell survival and retention, it was not able to demonstrate any improvement in cardiac function. In contrast, a similar type of patch based on alginate and fetal rat cardiomyocytes showed improvement on both cell survival and cardiac function in a rat MI model.107 This variability in results is common and needs to be considered. These are mainly due to differences in degree of injury to the heart due to the infarct procedure used.108 Additional studies in the development of cardiac patches have shown a limited use of cardiomyocytes. This is because these cell types are terminally differentiated, thus limiting their proliferative capacity.109
As an alternative, many researchers are studying a recently detected small population cardiac progenitor cells (CPCs) negative for blood lineage markers (Lin−) and positive for stem cell surface markers (i.e., c-kit, Sca-1, and MDR-1), which have the potential for myocardial regeneration.110 A potential treatment option could be to attract this cell population to the infarct site and provide them with the appropriate environment to stimulate myocardial regeneration. This environment should promote not only the migration of CPCs but also of any other cell types that might promote myocardial regeneration (e.g., macrophages, MSCs).
It is important to first differentiate between the term repair, which defines the natural healing process that replaces the damaged tissue with a scar, and regeneration (our therapeutic objective), in which the damaged tissue rebuilds itself to its normal structure and function.111 The cardiac repair process involves an inflammatory response, where macrophages remove dead cardiomyocytes and secrete angiogenic and profibrotic cytokines, chemokines, and proteases.112 This is followed by fibrosis, in which macrophages and myofibroblasts work together in reinforcing the ventricular wall through the secretion of connective tissue, eventually forming the scar tissue. This structure is so dense that it prevents the migration of CPCs, essential for cardiac regeneration, making fibrosis an important hurdle in mammals’ capacity to regenerate myocardium after MI.113 In addition, it has been found that acute inflammation is necessary to activate the regenerative response in the neonatal mouse heart.114
Another type of progenitor cells that have been tested for myocardial regeneration are MSCs loaded into scaffolds made of natural and synthetic polymers.115 Collagen, fibrin, PGS, PCL, nanopeptides, polyurethane (PU), and 3-hydroxybutyrate-co-4hydroxybutyrate [P(3HB-co-4HB)] are examples of these scaffold biomaterials. The use of these patches has shown increased vascularization and improvement in cardiac function. They are also an improvement over cellular cardiomyoplasty, which is limited because in this technique cell survival involves enzymatic cell dissociation before injection and because of the poor vascularization in the infarct site.116 Cardiac patches could overcome these limitations by retaining viable transplanted stem cells in a scaffold that would facilitate cell migration and cell adhesion toward the infarcted area.48 In addition, these transplanted cells could stimulate formation of vasculature and cardiomyocytes. As with most of the cell-bearing scaffolds, optimal properties of these include high porosity, high surface area to volume ratio, microenvironment similar to natural ECM, good mechanical properties, biodegradability, and biocompatibility.47,117 These parameters can be modulated with the choice of materials used and the synthesis conditions chosen for its fabrication.
Collagen
Collagen matrices loaded with MSCs have proven to be a good alternative biomaterial for cardiac patches. Simpson et al. used collagen matrices seeded with human MSCs (hMSCs) to treat MI in the male CDF rat model.118 The results showed that the application of this patch promoted myocardial regeneration and induced the expression of α-SMA marker, which was associated with an increase in myofibroblasts within the infarct region. Likewise, Maureira et al. developed a cardiac patch based on collagen and autologous MSCs, which was implanted in an MI rat model.119 The results of this study showed reverse remodeling of the infarcted area, improvement of perfusion, a reduced infarction, an increase of ventricular wall thickness, enhanced angiogenesis, and formation of myofibroblast-like tissue.
Similarly, the effectiveness of collagen seeded with adipose tissue-derived stem cell epicardial patches to preserve LV function, decrease fibrosis, and increase vessels in the infarct area was confirmed in reported studies.116 Furthermore, studies have evaluated the response of collagen matrices seeded with hMSCs and human ESC-derived mesenchymal cells (hESC-MC) to repair the infarcted myocardium of athymic nude rats.105 This in vitro study showed similar responses with regard to stem cell potency, viability, and cell proliferation for both cell types, while the results from the in vivo study demonstrated maintenance of the diastolic function and attenuated adverse LV remodeling. Interestingly, even with the observed differences in response to secreted paracrine factors, local angiogenic effects were seen in both cell types. Shi et al. demonstrated that collagen scaffolds covalently conjugated with the Sca-1 antibody (present in adult murine hematopoietic stem cells120) can attract native autologous stem cells to encourage cardiomyocyte regeneration after MI.121 In another study, collagen I patches seeded with human bone marrow CD133+ cells were used on cryoinjured rat hearts to assess their in vitro and in vivo cardiomyocyte differentiation potential.122 Results showed a capacity to induce angiogenesis, but not to induce cell differentiation. In a similar type of study, a collagen type I patch seeded with human umbilical cord blood mononuclear cells was used in MI mouse model to assess their cardiomyocyte differentiation potential.123 These patches showed improvements in vascularization and cardiac function, as measured by an increase in ejection fraction. The same group also conducted the Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM) phase I clinical trial using the same autologous cell-seeded patches.124 Ten patients presenting LV postischemic myocardial scars that were undergoing CABG had the seeded patches fixed onto the ischemic tissue. Results showed that the patch increased the LV wall with viable tissue and helped normalize cardiac wall stress in injured regions, improving diastolic function.
Fibrin
Fibrin is a hydrogel that contains adhesion molecules and has been shown to be biocompatible. However, fibrin-based scaffolds must be stabilized with other materials to compensate for their rapid reabsorption.125,126 Studies demonstrate the feasibility of applying injectable fibrin gels into the infarcted myocardium.127,128 Recently, Bago et al. observed cardiomyogenic differentiation of adipose tissue-derived progenitor cells using fibrin scaffolds in a mouse model.129 This system promoted endothelial lineage, increased vessel density, improved cardiac function, and diminished scar size after MI. Wendel et al. reported infarct size reduction, elimination of LV wall thinning, vascularization, and the restoration of cardiac function in rats 4 weeks after transplantation of the cardiac patch, which consisted of fibrin scaffolds seeded with neonatal rat cardiac cells.48 Geuss et al. showed PEGylated fibrin gels as an alternative to promote proliferation and cardiomyogenic differentiation.126 However, by comparing two-dimensional (2D) versus 3D cell cultures, contractile activity was maintained when cells were cultured on layers of PEGylated fibrin and significantly reduced when cultured as aggregates. The authors argued that the lack of contractility was related to low cardiomyocyte proliferation and not to the influence of mechanical properties (i.e., elastic modulus).
To develop cardiac patches that more closely mimic ECM, current tissue engineering approaches to myocardial regeneration are implementing the use of therapeutic genes to enhance paracrine action. An example, insulin-like growth factor-1 (IGF-1), which is a hormone that has been shown to enhance cardiac differentiation, reduces apoptosis and enhances neovascularization.79,130,131 Recently, Li et al. published the first study showing cardiac repair in a large animal model using fibrin patch seeded with IGF-1-modified MSCs.79 The results of this study were interesting because, despite the absence of cardiomyogenic differentiation in vivo, it promoted cardiac repair through other pathways. Similarly, Ye and colleagues used three cell types: human iPSC (hiPSC)-derived cardiomyocytes, endothelial cells, and smooth muscle cells in combination with fibrin patches seeded with IGF-1-encapsulated microspheres to treat MI in a porcine model.132 The results demonstrated cardiac repair without developing ventricular arrhythmias. In addition, Xiong et al. seeded fibrin patches with hESC-derived endothelial and smooth muscle cells and implanted on a porcine MI model.133 Results after 7 days showed a significant improvement in LV ejection fraction, which was maintained for over 4 weeks.
ECM scaffolds
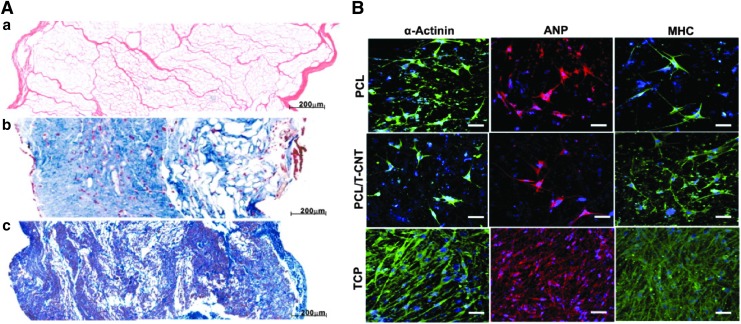
An innovative alternative that has proven very effective is the use of natural matrices as scaffolds. Wang et al. demonstrated that a cardiac patch consisting of decellularized porcine myocardium seeded with porcine bone marrow MSCs can support cardiomyogenic differentiation and angiogenesis in vitro (Fig. 4A).134 In addition, decellularized human myocardium was seeded with human mesenchymal progenitor cells suspended in a fibrin hydrogel and fixed on the MI tissue in a nude mice model.104 Results showed a significant improvement in cardiac function and vascularization. French et al. indicated that CPCs seeded on naturally derived cardiac ECM show enhanced adhesion, maturation, proliferation, and survival compared with a collagen matrix.135 Singelyn et al. used porcine myocardial tissue as an injectable myocardial matrix into rat myocardium.91 The results demonstrated that this matrix not only allowed the formation of nanofibrous structures containing glycosaminoglycans but also the migration of endothelial and smooth muscle cells. The feasibility of this approach was demonstrated in a large animal model through a transendocardial catheter injection.136
FIG. 4.
(A) Decellularized porcine myocardium can support cardiomyogenic and angiogenic differentiation. (a) Large pores evenly distributed across the 2 mm thick acellular scaffold (H&E). (b) Edge to edge view of thorough recellularization at 2 weeks in which cells were found to infiltrate and distribute within the myocardial scaffold. (c) Tissue remodeling was observed in the 4-week recellularization tissue construct; cells were still observed, and cell density was found higher than of the 2 week's construct. Reprinted with permission from Wang et al.134 (B) Immunofluorescence images showing the expression of cardiomyocyte markers, α-actinin, ANP, and MHC in CPCs that have been induced to differentiate 21 days after seeding onto PCL or PCL–TCNT meshes, or TCP. Scale bar, 50 μm. Reprinted with permission from Wickham et al.150 ANP, atrial natriuretic peptide; CPC, cardiac progenitor cell; MHC, myosin heavy chain; TCP, tissue culture plastic. Color images available online at www.liebertpub.com/teb
Extracellular matrices derived from porcine UBM can also serve as an inductive scaffold for myocardial repair. Potapova et al. found formation of myocytes and improved mechanical function in canine heart using UBM seeded with partially differentiated hMSCs.137 Shah et al. also probed the ability of this natural matrix to support and promote the growth of mature cardiomyocytes.138 In addition, Wei et al. used decellularized bovine pericardial tissue as scaffolds seeded with multilayered MSCs on a male syngeneic Lewis rats MI model.139 The advantage of this study was the development of vasculogenesis, angiogenesis, and differentiation of MSCs toward myofibroblast, although no mature cardiomyocytes were found within the patch.
Poly (glycerol sebacate)
In 2002, Langer and coworkers reported the synthesis of PGS, a biodegradable elastomer composed of glycerol and sebacic acid with desirable chemical and physical properties for tissue engineering applications.140 In addition, this polyester is considered a good material for cardiac patches because of its biodegradability, excellent mechanical properties, and low cost.141–143 Chen et al. used PGS patches to deliver differentiated cardiomyocytes from hESCs to treat MI in adult male Sprague Dawley rats.144 The results showed that this system not only maintained active cardiomyocytes beating for an extended time but it also supported cells during in vivo implantation. In addition, the PGS/fibrinogen cardiac patch has demonstrated excellent mechanical properties, good cell–scaffold interactions, and capacity to stimulate the expression of cardiac-specific markers (i.e., α-actinin, troponin, ß-myosin heavy chain, and connexin 43).145 In addition, PGS/fibrinogen/VEGF scaffolds loaded with MCSs have been shown to promote both the expression of cardiac and endothelial cell markers while preventing negative ventricular remodeling in vivo in a porcine MI model.117 Likewise, it was demonstrated that PGS/collagen patches can provide a good local microenvironment similar to the natural interactions existing between cells and the native ECM, stimulating cardiogenic differentiation of MSCs.146
Polycaprolactone
PCL is a FDA-approved biodegradable synthetic polymer used as a scaffold material for cardiac tissue regeneration due to its mechanical properties and biodegradability. Nevertheless, PCL must be combined with other polymers (e.g., dextran, poly-vinylalcohol, and poly-ethylene oxide) to improve its hydrophobicity through the presence of ester and keto groups in its structure, which inhibit cell binding.147 Therefore, electrospun nanofibrous scaffolds fabricated from this material can simulate the ECM microenvironment, allowing diffusion of nutrients, cell adhesion, cell growth, migration, proliferation, and differentiation.148 Studies have reported that the use of a PCL/oligomer hydrogel can simulate ECM.149 The incorporation of thiophene-conjugated carbon nanotubes into PCL scaffolds enhances the mechanical properties, cell adhesion, proliferation, and differentiation of CPCs (Fig. 4B).150 Similarly, in vivo studies in rats have demonstrated that PCL/gelatin scaffolds loaded with MSCs can restrain LV remodeling, improve cardiac function, and promote both angiogenesis and cardiomyogenesis in the infarcted area.47 In addition, scaffolds of the PCL derivative poly(glycolide-co-caprolactone) were seeded with bone marrow mononuclear cells and fixed in a rat MI model.151 Results after 4 weeks showed significant improvements in ejection fraction, ventricular diameter, and hemodynamics.
Others
Research in the development of cardiac patches has also explored other polymeric materials. For instance, a commercial biological matrix (cell-PuraMatrix™) formed of self-assembled peptides can serve as a biological scaffold to evaluate the feasibility of different types of cells involved in myocardial repair in mice [(i.e., clonal stem cell antigen-1-positive cardiac progenitors (cSca-1), bone marrow mononuclear cells, skeletal myoblasts, and adipose tissue-derived MSCs)]. Results indicate that cSca-1 is better in preventing cardiac remodeling and systolic dysfunction compared to the other cell types.152 In vitro and in vivo studies have demonstrated that [P(3HB-co-4HB)] and PU can support stem cell growth,153 while a cardiac patch consisting of hMSCs encapsulated in alginate and seeded in polyethylene glycol hydrogels stimulates healing after a heart attack.154 In addition, Miyagi et al. developed a hybrid scaffold using a gelatin scaffold (i.e., Gelfoam, Merck, Co.) coated with PCL (for reinforcement) and seeded with bone marrow MSCs and the cytokine stem cell factor and stromal cell-derived factor-1alpha.155 Results in a small animal ventriculotomy model showed a significant improvement in cardiac function when compared to controls (i.e., animals and unmodified gelatin sponge). This improvement was observed in all groups with the scaffold alone, with or without cytokines or cells.
Advanced Culture Systems
Appropriate cell function and tissue integration depend on cell–cell and ECM interactions. In the myocardium, electrical stimuli are propagated through interconnected cardiomyocytes highlighting the importance of gap junctions and cell polarization for appropriate function. Intercellular communication between cardiomyocytes is essential for the coordinated and synchronized contraction of the cardiac muscle. This communication occurs through gap junctions, which are membrane channels formed by the connexin family proteins which allow the propagation of rapid anisotropic impulses. These connexins have been shown to play a crucial role in determining impulse conduction and the heart morphogenesis, as well as in several cardiomyopathies, such as myocardial ischemia.156 In vitro, cell polarization and cell–cell interconnectivity can be achieved by modulating cell adhesion to the culture surface. In this section, we discussed current engineered techniques used to develop optimal ECM architecture and improve cardiac cell–cell adhesion and function.
Electrospinning nanofibers
Electrospinning is a technique that uses an electrical charge to produce nanofibers from polymer solutions. This technique has gained a lot of popularity for tissue engineering applications due to its ability to produce a network of interconnected nanofibers with similar fibrous architecture to natural ECM found in soft tissues. Several biocompatible and biodegradable polymers have been used to develop nanofiber networks using the electrospinning technique for cardiac tissue regeneration applications. Zong et al. used electrospinning to fabricate biodegradable nonwoven poly(lactide)- and poly(glycolide)-based (PLGA) scaffolds.157 In these polymeric fibers, primary cardiomyocytes developed mature contractile machinery (sarcomeres) and showed electrical activity using voltage-sensitive dyes over 7 days. Similar cardiomyocyte contractility and survival have been observed with other biodegradable polymers used in electrospinning, such as polyurethane,158 poly(lactic-co-glycolic) acid (PLGA),159 and PCL.160 However, these in vitro studies were carried out in the absence of fibroblasts, which can modulate cardiomyocyte function and structure161 and thereby impact long-term survival and tissue integration. Hussain's group addressed the fibroblast gap in 3D cardiac tissue constructs by fabricating a 3D chitosan nanofiber scaffold using an electrospinning technique to maintain cardiomyocyte function with fibroblast cocultures.162 A mat of chitosan fibers (∼150 μm thick) was coated with fibronectin to enhance cell adhesion of neonatal cardiomyocytes isolated from rats in coculture with 3t3-J2 fibroblasts. Cardiomyocyte cocultures maintained cell polarization and expressed high levels of Conexin43, a gap junction protein required to propagate electrical stimuli, over 3 weeks. Image analysis of intracellular calcium ion staining showed that cardiomyocytes maintained an elongated morphology and beating frequency of 17 ± 3 contractions per minute only in 3D cocultures, but not in 2D or 3D monocultures highlighting the importance of fibroblasts for long-term function in cardiac tissue constructs. Overall electrospinning techniques have shown great potential for heart tissue scaffold. However, in vivo studies are needed to evaluate heart function and recovery over time following MI.
Engineered cell sheets
Cells sheets have improved cell survival compared to single cells injected in tissues163 and have been used clinically for the repair of several tissues such as eye cornea,164 cartilage,165 and heart.166 Cell sheets are formed by detachment of 2D cell monolayers using surface detachable polymers or magnetic particles. Okano and colleagues develop a method to harvest cell sheets by lowering the culture temperature, using the temperature-responsive polymer poly(N-isopropylacrylamide) (PIPAAm).167 When covalently grafted on the surface of culture dishes, cells adhere via serum and cell membrane proteins, due to PIPAAm hydrophobicity at the standard culture temperature of 37°C. Below its critical temperature of 32°C, PIPAAm becomes very hydrophilic and protein nonadhesive; thus, at lower temperatures, cell sheets detach without damaging cell–cell junctions or ECM proteins underneath the cell sheets. Using this method, Okano and colleagues fabricated a 3D sheet of pulsatile neonatal rat cardiomyocytes.167 The multilayer cardiac construct was macroscopically observed to pulse spontaneously and showed a diffuse pattern of Connexin 43 gap junctions. Long-term survival was observed up to 1 year, and the cell sheet construct showed well-differentiated sarcomeres and abundant mitochondria.168 Usually thick cell sheets (>300 μm) are preferred for tissue transplant, but limited due to lack of sufficient vascularization. To overcome this limitation, Okano's research group explored the possibility of adding endothelial cells to the 3D contractile constructs.169 Cell sheets were stacked and transplanted into the dorsal subcutaneous tissue of rats. One week after transplantation, fluorescence-labeled endothelial cells in the stack formed continuous tubular blood vessel networks in the construct and integrated with the underlying host tissues. The myocardial endothelial tissue grafts showed improved vascularization compared to myocardial cell sheets that can potentially overcome the limits of mass transport to create functional integrated tissues. However, these studies were carried out in subcutaneous tissue rather than in damaged heart tissue; thus, results may not fully reflect their potential for myocardial repair.
Sawa's group evaluated the use of myoblast sheets for treatment of MI. Two weeks after MI, cell sheets were directly implanted over the scar area and heart function was monitored. Myoblast sheet MI-treated group demonstrated uniform repair in the anterior wall and improved function probably due to remodeling of the geometry of the LV chamber. In addition, myoblast sheets showed higher secretion of proangiogenic factors HGF and VEGF compared to single cell injection and controls.168 In another study, clinical implantation of autologous myoblast sheets in a patient who had been supported with a LV assist system for dilated cardiomyopathy showed significant improvement.170
Another strategy termed magnetite force-based tissue engineering has been successfully used to generate cell sheets for tissue engineering applications.171,172 This method uses a magnetic field to generate sheets of magnetized cells. Liposomes are loaded with magnetite to display a positive charge that electrostatically fuses with the cell membrane. Cells are grouped to form sheets by placing a magnet at the bottom of a cell culture well. Ishii et al. used this technique to implant a multilayered sheet of adipose-derived regenerative cells (ADRC) in the infarcted myocardium.173 After 28 days of implantation, echocardiographic measurements revealed that the decreases in LVFS and LVEF following MI had significantly improved in ADRC sheets compared to collagen gel and ADRC controls. One of the main advantages of this cell sheet technique is the improvement in angiogenesis more effectively than direct injection of cell suspensions, which can explain the observed significant decrease in cardiac fibrosis and increased myocardial capillaries, compared with the control groups. One major drawback in this study is that ADRC sheets were placed on the surface on the infarcted myocardium after 1 min of coronary occlusion, which could have contributed to the decrease of adverse remodeling as necrotic tissue is minimal. Cell death can occur as quickly as 20 min after coronary artery occlusion, which is within the time frame of MI, and can negatively impact the regenerative potential of the tissue. Thus, additional studies with various times of sheet implantation are required to determine the potential of these cell sheets for cardiac regeneration.
Multicellular constructs and advanced culture systems
As our understanding of the architecture and composition of myocardial tissue increases, it becomes apparent that a single cell type-based tissue engineering approach may not be sufficient to repair the myocardium. Two culture and triculture systems might be required to provide the appropriate combination able to improve cell coupling with the host's cells and ultimately improve function. This will also require a new 3D culture system to recapitulate the ultrastructural composition and orientation of the myocardium while allowing for proper diffusion of nutrients and cell survival. The only cell sources that can reproducibly provide functional cardiomyocytes in vitro are pluripotent stem cells (ESCs and iPSCs). Although these cells provide functional myocytes, when assembled into 3D tissues an optimal ratio of supporting cells (such as fibroblast) is needed for the formation of myocardial-like tissue.174 As shown in previous sections, the infiltration of these supporting cells is a requirement for a viable scaffold. For instance, the combination of cardiomyocytes with endothelial cells and mesenchymal cells has been shown to improve contractility in vitro, further supporting the role of multiple cells during the formation of engineered tissues.175 Another level of complexity that will need to be added is the incorporation of biophysical stimulation. This has been reviewed elsewhere,176,177 but will be needed to improve the function and maturity of cardiac constructs in vitro.
Another interesting approach has been to decellularize whole organs to provide the best 3D environment for cells to attach and grow in their appropriate in vivo compartments.178,179 The work by Wang et al. is a good example, in which a 2-mm section of porcine myocardium was reseeded with differentiated bone marrow mononuclear cells.134 This scaffold maintained the cardiomyocyte-like phenotype of the seeded cells and showed angiogenesis potential and recovery of the native tissue's mechanical properties after remodeling. Knowing the extent of recapitulation of the native structure or the correct combination of cells needed to provide physiologically relevant bioengineered tissues still remains to be determined. It is clear that although some cardiomyocyte 2D culture systems have proved adequate for gathering cardiac physiological data in applications such as drug discovery,180 these are not adequate for tissue regeneration applications, where the in vivo tissue complexity needs to be recapitulated. Future culture systems should advance toward 3D cultures providing appropriate biophysical conditions and oxygen tension to support native-like bioengineered cardiac tissues for implantation.
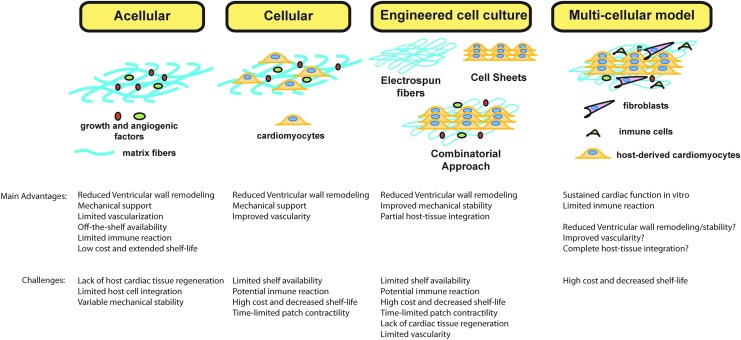
In addition to creating tissues in the laboratory, it is also important to test such constructs under physiologically relevant conditions. The traditional approach has been to test cardiac patches in animal models (often in small animal models). This approach provides information regarding survival of the cardiac repair cells, but remains a poor model when testing human-derived cells given the physiological differences between rodents and humans and the need for immune-compromised animals. The development of in vitro culture systems that use relevant cells during the host tissue response such as macrophages to test bioengineered cardiac tissues has been proposed.9–11 As explained in previous sections, after an MI there is a dynamic response driven by different subtypes of macrophage (proinflammatory vs. prohealing), which play a major role during the remodeling process. However, the interactions between inflammatory cells and engineered cardiac patches have been widely ignored during in vitro screenings. A recent study by Pallotta et al. showed that there is potential cross talk between the macrophage subtype and cardiomyocytes derived from human ESCs further supporting the need to understand these interactions.181 Advanced culture systems that incorporate the host tissue response and repair cells will allow for the manufacture and screening of bioengineered cardiac tissues that can be more readily translated to clinical use. The main advantages and challenges of the different approaches to cardiac tissue regeneration are summarized in Figure 5.
FIG. 5.
Summary of engineered approaches to cardiac tissue regeneration. Extracellular matrix materials such as collagen, alginate, hyaluronic acid, and chitosan are used alone or combined as a cardiac patch to provide mechanical strength and stabilize the ventricular wall (acellular). Addition of cells to extracellular matrix constructs (cellular) or engineered matrices improves cardiac function and vascularization compared to acellular models. The question mark (?) implies it remains to be determined in future studies. Color images available online at www.liebertpub.com/teb
Conclusions and Future Directions
Engineering approaches toward the repair of myocardial tissue have shown promise in the past, but full restoration of myocardial function remains elusive. As our understanding of the heart's physiology and function grows, tissue engineering principles can be applied toward the design of an engineered cardiac patch that ultimately restores function to the heart and prevents myocardial wall remodeling. Among the potential approaches are (1) acellular scaffolds that provide biological activity and biophysical support to the heart and (2) cellular scaffolds that provide the minimum combination of cells and biomaterial composition needed for increased biological activity and biophysical support. It is also clear that advanced culture systems (i.e., triculture systems) will be needed to create more advance engineered cardiac tissues and to create better screening tools to test bioengineered cardiac patches in vitro.
Acknowledgments
This work was supported by NIH-NCI 1K01 CA188167, NYSTEM C026721A Empire State Stem Cell Scholars: Fellow-to-Faculty Award, Oak Foundation, and NYSCF. The authors would also like to thank Dr. Isabella Pallotta for her careful evaluation and help during the preparation of this review.
Disclosure Statement
No competing financial interests exist.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Judd S.E., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Mackey R.H., Magid D.J., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Mussolino M.E., Neumar R.W., Nichol G., Pandey D.K., Paynter N.P., Reeves M.J., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics, C., and Stroke Statistics S. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129, e28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins S.L., Kumar V., and Cotran R.S. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders/Elsevier, 2010 [Google Scholar]
- 3.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R.H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T.R., Libby P., Weissleder R., and Pittet M.J. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L., Libby P., Weissleder R., and Pittet M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204, 3037, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahrendorf M., and Swirski F.K. Monocyte and macrophage heterogeneity in the heart. Circ Res 112, 1624, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski F.K., and Nahrendorf M. Macrophage-stem cell crosstalk after myocardial infarction. J Am Coll Cardiol 62, 1902, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis N.G. The immune system and cardiac repair. Pharmacol Res 58, 88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11, 255, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freytes D.O., Kang J.W., Marcos-Campos I., and Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem 114, 220, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Freytes D.O., Santambrogio L., and Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs 195, 171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller K.L., Freytes D.O., andVunjak-Novakovic G. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann Biomed Eng 43, 616, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon R.J., Quagliarello B., and Lowy F.D. Ventricular assist device-related infections. Lancet Infect Dis 6, 426, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Danchin N., Coste P., Ferrieres J., Steg P.G., Cottin Y., Blanchard D., Belle L., Ritz B., Kirkorian G., Angioi M., Sans P., Charbonnier B., Eltchaninoff H., Gueret P., Khalife K., Asseman P., Puel J., Goldstein P., Cambou J.P., Simon T., and Investigators F.-M. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the french registry on acute ST-elevation myocardial infarction (FAST-MI). Circulation 118, 268, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Aversano T., Aversano L.T., Passamani E., Knatterud G.L., Terrin M.L., Williams D.O., Forman S.A., and Atlantic Cardiovascular Patient Outcomes Research T. Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial. JAMA 287, 1943, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Grines C.L., Browne K.F., Marco J., Rothbaum D., Stone G.W., O'Keefe J., Overlie P., Donohue B., Chelliah N., Timmis G.C., et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 328, 673, 1993 [DOI] [PubMed] [Google Scholar]
- 16.de Boer M.J., Ottervanger J.P., van 't Hof A.W., Hoorntje J.C., Suryapranata H., Zijlstra F., and Zwolle Myocardial Infarction Study G. Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy. J Am Coll Cardiol 39, 1723, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Verma S., Fedak P.W., Weisel R.D., Butany J., Rao V., Maitland A., Li R.K., Dhillon B., and Yau T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 105, 2332, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B., Heusch G., Ovize M., andVan de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65, 1454, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Gaudron P., Eilles C., Ertl G., and Kochsiek K. Compensatory and noncompensatory left ventricular dilatation after myocardial infarction: time course and hemodynamic consequences at rest and during exercise. Am Heart J 123, 377, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Di Donato M., Sabatier M., Dor V., Toso A., Maioli M., and Fantini F. Akinetic versus dyskinetic postinfarction scar: relation to surgical outcome in patients undergoing endoventricular circular patch plasty repair. J Am Coll Cardiol 29, 1569, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Cooley D.A., Collins H.A., Morris G.C., Jr., and Chapman D.W. Ventricular aneurysm after myocardial infarction; surgical excision with use of temporary cardiopulmonary bypass. J Am Med Assoc 167, 557, 1958 [DOI] [PubMed] [Google Scholar]
- 22.Dor V., Saab M., Coste P., Kornaszewska M., and Montiglio F. Left ventricular aneurysm: a new surgical approach. Thorac Cardiovasc Surg 37, 11, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Mickleborough L.L., Carson S., andIvanov J. Repair of dyskinetic or akinetic left ventricular aneurysm: results obtained with a modified linear closure. J Thorac Cardiovasc Surg 121, 675, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Stoney W.S., Alford W.C., Jr., Burrus G.R., and Thomas C.S., Jr. Repair of anteroseptal ventricular aneurysm. Ann Thorac Surg 15, 394, 1973 [DOI] [PubMed] [Google Scholar]
- 25.Athanasuleas C.L., Stanley A.W., Jr., and Buckberg G.D. Restoration of contractile function in the enlarged left ventricle by exclusion of remodeled akinetic anterior segment: surgical strategy, myocardial protection, and angiographic results. J Card Surg 13, 418, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Jones R.H., Velazquez E.J., Michler R.E., Sopko G., Oh J.K., O'Connor C.M., Hill J.A., Menicanti L., Sadowski Z., Desvigne-Nickens P., Rouleau J.L., Lee K.L., and Investigators S.H. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 360, 1705, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murry C.E., Field L.J., and Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation 112, 3174, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Wang J.S., Shum-Tim D., Galipeau J., Chedrawy E., Eliopoulos N., and Chiu R.C. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg 120, 999, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Hutcheson K.A., Atkins B.Z., Hueman M.T., Hopkins M.B., Glower D.D., and Taylor D.A. Comparison of benefits on myocardial performance of cellular cardiomyoplasty with skeletal myoblasts and fibroblasts. Cell Transplant 9, 359, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H., Hata J., Umezawa A., and Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103, 697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangi A.A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J.S., and Dzau V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9, 1195, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Nygren J.M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B.K., and Jacobsen S.E. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 10, 494, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Fraidenraich D., Stillwell E., Romero E., Wilkes D., Manova K., Basson C.T., and Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science 306, 247, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., Mu H., Noiseux N., Zhang L., Pratt R.E., Ingwall J.S., and Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11, 367, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Murry C.E., Soonpaa M.H., Reinecke H., Nakajima H., Nakajima H.O., Rubart M., Pasumarthi K.B., Virag J.I., Bartelmez S.H., Poppa V., Bradford G., Dowell J.D., Williams D.A., and Field L.J. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kajstura J., Rota M., Whang B., Cascapera S., Hosoda T., Bearzi C., Nurzynska D., Kasahara H., Zias E., Bonafe M., Nadal-Ginard B., Torella D., Nascimbene A., Quaini F., Urbanek K., Leri A., and Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res 96, 127, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Noiseux N., Gnecchi M., Lopez-Ilasaca M., Zhang L., Solomon S.D., Deb A., Dzau V.J., and Pratt R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 14, 840, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Hatzistergos K.E., Quevedo H., Oskouei B.N., Hu Q., Feigenbaum G.S., Margitich I.S., Mazhari R., Boyle A.J., Zambrano J.P., Rodriguez J.E., Dulce R., Pattany P.M., Valdes D., Revilla C., Heldman A.W., McNiece I., and Hare J.M. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 107, 913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chachques J.C., Acar C., Herreros J., Trainini J.C., Prosper F., D'Attellis N., Fabiani J.N., and Carpentier A.F. Cellular cardiomyoplasty: clinical application. Ann Thorac Surg 77, 1121, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Teng C.J., Luo J., Chiu R.C., and Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg 132, 628, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Terrovitis J.V., Smith R.R., and Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res 106, 479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng K., Li T.S., Malliaras K., Davis D.R., Zhang Y., and Marban E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res 106, 1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robey T.E., Saiget M.K., Reinecke H., and Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 45, 567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L., Haider H., Guo C., and Sim E.K. Cell-based VEGF delivery prevents donor cell apoptosis after transplantation. Ann Thorac Surg 83, 1233, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., and Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33, 907, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Terrovitis J., Lautamaki R., Bonios M., Fox J., Engles J.M., Yu J., Leppo M.K., Pomper M.G., Wahl R.L., Seidel J., Tsui B.M., Bengel F.M., Abraham M.R., and Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 54, 1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kai D., Wang Q.L., Wang H.J., Prabhakaran M.P., Zhang Y., Tan Y.Z., and Ramakrishna S. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater 10, 2727, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Wendel J.S., Ye L., Zhang P., Tranquillo R.T., and Zhang J.J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A 20, 1325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansaloni L., Cambrini P., Catena F., Di Saverio S., Gagliardi S., Gazzotti F., Hodde J.P., Metzger D.W., D'Alessandro L., and Pinna A.D. Immune response to small intestinal submucosa (surgisis) implant in humans: preliminary observations. J Invest Surg 20, 237, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Cheng A., and Saint-Cyr M. Comparison of different ADM materials in breast surgery. Clin Plast Surg 39, 167, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Wallace D.G., and Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev 55, 1631, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Van Vlierberghe S., Dubruel P., and Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12, 1387, 2011 [DOI] [PubMed] [Google Scholar]
- 53.de Souza R.R. Aging of myocardial collagen. Biogerontology 3, 325, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Gaballa M.A., Sunkomat J.N., Thai H., Morkin E., Ewy G., and Goldman S. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant 25, 946, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Yang Y.L., Leone L.M., and Kaufman L.J. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys J 97, 2051, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serpooshan V., Zhao M.M., Metzler S.A., Wei K., Shah P.B., Wang A., Mahmoudi M., Malkovskiy A.V., Rajadas J., Butte M.J., Bernstein D., and Ruiz-Lozano P. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 34, 9048, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng C., Zhang P., Vulesevic B., Kuraitis D., Li F., Yang A.F., Griffith M., Ruel M., and Suuronen E.J. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng Part A 16, 3099, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Chiu L.L., and Radisic M. Controlled release of thymosin beta4 using collagen-chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. J Control Release 155, 376, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Peattie R.A., Nayate A.P., Firpo M.A., Shelby J., Fisher R.J., and Prestwich G.D. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials 25, 2789, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Oksala O., Salo T., Tammi R., Hakkinen L., Jalkanen M., Inki P., and Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem 43, 125, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Yoon S.J., Fang Y.H., Lim C.H., Kim B.S., Son H.S., Park Y., and Sun K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater 91, 163, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Abdalla S., Makhoul G., Duong M., Chiu R.C.J., and Cecere R. Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction. Interact Cardiovasc Thorac Surg 17, 767, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon S.J., Hong S., Fang Y.H., Song M., Son K.H., Son H.S., Kim S.K., Sun K., and Park Y. Differential regeneration of myocardial infarction depending on the progression of disease and the composition of biomimetic hydrogel. J Biosci Bioeng 118, 461, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Ifkovits J.L., Tous E., Minakawa M., Morita M., Robb J.D., Koomalsingh K.J., Gorman J.H., 3rd, Gorman R.C., and Burdick J.A. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A 107, 11507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Augst A.D., Kong H.J., and Mooney D.J. Alginate hydrogels as biomaterials. Macromol Biosci 6, 623, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Yu J., Gu Y., Du K.T., Mihardja S., Sievers R.E., and Lee R.J. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 30, 751, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Tsur-Gang O., Ruvinov E., Landa N., Holbova R., Feinberg M.S., Leor J., and Cohen S. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials 30, 189, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Landa N., Miller L., Feinberg M.S., Holbova R., Shachar M., Freeman I., Cohen S., and Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117, 1388, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Leor J., Tuvia S., Guetta V., Manczur F., Castel D., Willenz U., Petnehazy O., Landa N., Feinberg M.S., Konen E., Goitein O., Tsur-Gang O., Shaul M., Klapper L., and Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol 54, 1014, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Sabbah H.N., Wang M., Gupta R.C., Rastogi S., Ilsar I., Sabbah M.S., Kohli S., Helgerson S., and Lee R.J. Augmentation of left ventricular wall thickness with alginate hydrogel implants improves left ventricular function and prevents progressive remodeling in dogs with chronic heart failure. JACC Heart Fail 1, 252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabbah H.N., Shimoyama H., Kono T., Gupta R.C., Sharov V.G., Scicli G., Levine T.B., and Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 89, 2852, 1994 [DOI] [PubMed] [Google Scholar]
- 72.Azad A.K., Sermsintham N., Chandrkrachang S., and Stevens W.F. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater 69, 216, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Dai T., Tanaka M., Huang Y.Y., and Hamblin M.R. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther 9, 857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhattarai N., Gunn J., and Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62, 83, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Dhandayuthapani B., Krishnan U.M., and Sethuraman S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J Biomed Mater Res B Appl Biomater 94, 264, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Madihally S.V., and Matthew H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 20, 1133, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Pok S., Myers J.D., Madihally S.V., and Jacot J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater 9, 5630, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmadi A., Vulesevic B., Blackburn N.J.R., Ruel J., and Suuronen E.J. A Collagen-chitosan injectable hydrogel improves cardiac remodeling in a mouse model of myocardial infarction. J Biomater Tissue Eng 4, 886, 2014 [Google Scholar]
- 79.Li Y., Meng H., Liu Y., and Lee B.P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci World J 2015, 685690, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W., Wu B., Asakura S., Kohno I., and Matsuda M. Soluble fibrin augments spreading of fibroblasts by providing RGD sequences of fibrinogen in soluble fibrin. Thromb Res 114, 293, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Christman K.L., Fok H.H., Sievers R.E., Fang Q.H., and Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10, 403, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Christman K.L., Vardanian A.J., Fang Q., Sievers R.E., Fok H.H., and Lee R.J. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44, 654, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Ye Q., Zünd G., Benedikt P., Jockenhoevel S., Hoerstrup S.P., Sakyama S., Hubbell J.A., and Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardio Thorac Surg 17, 587, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Badylak S.F., Freytes D.O., and Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 5, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Robinson K.A., Li J., Mathison M., Redkar A., Cui J., Chronos N.A., Matheny R.G., and Badylak S.F. Extracellular matrix scaffold for cardiac repair. Circulation 112, I135, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Badylak S.F., Kochupura P.V., Cohen I.S., Doronin S.V., Saltman A.E., Gilbert T.W., Kelly D.J., Ignotz R.A., and Gaudette G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transpl 15 Suppl 1, S29, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Kochupura P.V., Azeloglu E.U., Kelly D.J., Doronin S.V., Badylak S.F., Krukenkamp I.B., Cohen I.S., and Gaudette G.R. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation 112, I144, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Zhao Z.Q., Puskas J.D., Xu D., Wang N.P., Mosunjac M., Guyton R.A., Vinten-Johansen J., and Matheny R. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. J Am Coll Cardiol 55, 1250, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Ravi S., Caves J.M., Martinez A.W., Xiao J., Wen J., Haller C.A., Davis M.E., and Chaikof E.L. Effect of bone marrow-derived extracellular matrix on cardiac function after ischemic injury. Biomaterials 33, 7736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan M.Y., Zhi W., Wei R.Q., Huang Y.C., Zhou K.P., Tan B., Deng L., Luo J.C., Li X.Q., Xie H.Q., and Yang Z.M. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials 30, 3234, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., and Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 30, 5409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu J., Christman K.L., Chin E., Sievers R.E., Saeed M., and Lee R.J. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg 137, 180, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Singelyn J.M., and Christman K.L. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res 3, 478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujimoto K.L., Ma Z., Nelson D.M., Hashizume R., Guan J., Tobita K., and Wagner W.R. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials 30, 4357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang T., Wu D.-Q., Jiang X.-J., Zhang X.-Z., Li X.-Y., Zhang J.-F., Zheng Z.-B., Zhuo R., Jiang H., and Huang C. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail 11, 14, 2009 [DOI] [PubMed] [Google Scholar]
- 96.Kelley S.T., Malekan R., Gorman J.H., 3rd, Jackson B.M., Gorman R.C., Suzuki Y., Plappert T., Bogen D.K., Sutton M.G., and Edmunds L.H., Jr. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation 99, 135, 1999 [DOI] [PubMed] [Google Scholar]
- 97.Bowen F.W., Jones S.C., Narula N., St John Sutton M.G., Plappert T., Edmunds L.H., Jr., and Dixon I.M. Restraining acute infarct expansion decreases collagenase activity in borderzone myocardium. Ann Thorac Surg 72, 1950, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Chaudhry P.A., Mishima T., Sharov V.G., Hawkins J., Alferness C., Paone G., and Sabbah H.N. Passive epicardial containment prevents ventricular remodeling in heart failure. Ann Thorac Surg 70, 1275, 2000 [DOI] [PubMed] [Google Scholar]
- 99.Konertz W.F., Shapland J.E., Hotz H., Dushe S., Braun J.P., Stantke K., and Kleber F.X. Passive containment and reverse remodeling by a novel textile cardiac support device. Circulation 104, I270, 2001 [DOI] [PubMed] [Google Scholar]
- 100.Franco-Cereceda A., Lockowandt U., Olsson A., Bredin F., Forssell G., Owall A., Runsio M., and Liska J. Early results with cardiac support device implant in patients with ischemic and non-ischemic cardiomyopathy. Scand Cardiovasc J 38, 159, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Wang T., Jiang X.-J., Tang Q.-Z., Li X.-Y., Lin T., Wu D.-Q., Zhang X.-Z., and Okello E. Bone marrow stem cells implantation with α-cyclodextrin/MPEG–PCL–MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater 5, 2939, 2009 [DOI] [PubMed] [Google Scholar]
- 102.Kutschka I., Chen I.Y., Kofidis T., Arai T., von Degenfeld G., Sheikh A.Y., Hendry S.L., Pearl J., Hoyt G., Sista R., Yang P.C., Blau H.M., Gambhir S.S., and Robbins R.C. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 114, I, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Wang H., Zhang X., Li Y., Ma Y., Zhang Y., Liu Z., Zhou J., Lin Q., Wang Y., Duan C., and Wang C. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive Chitosan hydrogel. J Heart Lung Transpl 29, 881, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Godier-Furnemont A.F., Martens T.P., Koeckert M.S., Wan L., Parks J., Arai K., Zhang G., Hudson B., Homma S., and Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci USA 108, 7974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simpson D.L., Boyd N.L., Kaushal S., Stice S.L., and Dudley S.C., Jr. Use of human embryonic stem cell derived-mesenchymal cells for cardiac repair. Biotechnol Bioeng 109, 274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li R.K., Jia Z.Q., Weisel R.D., Mickle D.A., Choi A., and Yau T.M. Survival and function of bioengineered cardiac grafts. Circulation 100, II63, 1999 [DOI] [PubMed] [Google Scholar]
- 107.Leor J., Aboulafia-Etzion S., Dar A., Shapiro L., Barbash I.M., Battler A., Granot Y., and Cohen S. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation 102, III56, 2000 [DOI] [PubMed] [Google Scholar]
- 108.Xiang Z., Liao R., Kelly M.S., and Spector M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue Eng 12, 2467, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Nadal-Ginard B., Kajstura J., Leri A., and Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92, 139, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Beltrami A.P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., Leri A., Kajstura J., Nadal-Ginard B., and Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Laflamme M.A., and Murry C.E. Heart regeneration. Nature 473, 326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ben-Mordechai T., Palevski D., Glucksam-Galnoy Y., Elron-Gross I., Margalit R., and Leor J. Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther 20, 36, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Mu X., Bellayr I., Walters T., and Li Y. Mediators leading to fibrosis - how to measure and control them in tissue engineering. Oper Tech Orthop 20, 110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aurora A.B., Porrello E.R., Tan W., Mahmoud A.I., Hill J.A., Bassel-Duby R., Sadek H.A., and Olson E.N. Macrophages are required for neonatal heart regeneration. J Clin Invest 124, 1382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polo-Corrales L., Latorre-Esteves M., and Ramirez-Vick J.E. Scaffold design for bone regeneration. J Nanosci Nanotech 14, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamdi H., Planat-Benard V., Bel A., Neamatalla H., Saccenti L., Calderon D., Bellamy V., Bon M., Perrier M.C., Mandet C., Bruneval P., Casteilla L., Hagège A.A., Pucéat M., Agbulut O., and Menasché P. Long-term functional benefits of Epicardial patches as cell carriers. Cell Transpl 23, 87, 2014 [DOI] [PubMed] [Google Scholar]
- 117.Ravichandran R., Venugopal J.R., Mukherjee S., Sundarrajan S., and Ramakrishna S. Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Eng Part A 21, 1288, 2015 [DOI] [PubMed] [Google Scholar]
- 118.Simpson D., Liu H., Fan T.H., Nerem R., and Dudley S.C., Jr. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 25, 2350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maureira P., Marie P.Y., Yu F., Poussier S., Liu Y., Groubatch F., Falanga A., and Tran N. Repairing chronic myocardial infarction with autologous mesenchymal stem cells engineered tissue in rat promotes angiogenesis and limits ventricular remodeling. J Biomed Sci 19, 93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holmes C., and Stanford W.L. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25, 1339, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Shi C., Li Q., Zhao Y., Chen W., Chen B., Xiao Z., Lin H., Nie L., Wang D., and Dai J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials 32, 2508, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Pozzobon M., Bollini S., Iop L., De Gaspari P., Chiavegato A., Rossi C.A., Giuliani S., Fascetti Leon F., Elvassore N., Sartore S., and De Coppi P. Human bone marrow-derived CD133(+) cells delivered to a collagen patch on cryoinjured rat heart promote angiogenesis and arteriogenesis. Cell Transpl 19, 1247, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Cortes-Morichetti M., Frati G., Schussler O., Duong Van Huyen J.P., Lauret E., Genovese J.A., Carpentier A.F., and Chachques J.C. Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng 13, 2681, 2007 [DOI] [PubMed] [Google Scholar]
- 124.Chachques J.C., Trainini J.C., Lago N., Cortes-Morichetti M., Schussler O., and Carpentier A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg 85, 901, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Liu H., Collins S.F., and Suggs L.J. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials 27, 6004, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Geuss L.R., Allen A.C., Ramamoorthy D., and Suggs L.J. Maintenance of HL-1 cardiomyocyte functional activity in PEGylated fibrin gels. Biotechnol Bioeng 112, 1446–1456, 2015 [DOI] [PubMed] [Google Scholar]
- 127.Zhang X., Wang H., Ma X., Adila A., Wang B., Liu F., Chen B., Wang C., and Ma Y. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med 235, 1505, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Ryu J.H., Kim I.K., Cho S.W., Cho M.C., Hwang K.K., Piao H., Piao S., Lim S.H., Hong Y.S., Choi C.Y., Yoo K.J., and Kim B.S. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials 26, 319, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Bago J.R., Soler-Botija C., Casani L., Aguilar E., Alieva M., Rubio N., Bayes-Genis A., and Blanco J. Bioluminescence imaging of cardiomyogenic and vascular differentiation of cardiac and subcutaneous adipose tissue-derived progenitor cells in fibrin patches in a myocardium infarct model. Int J Cardiol 169, 288, 2013 [DOI] [PubMed] [Google Scholar]
- 130.Hahn J.Y., Cho H.J., Kang H.J., Kim T.S., Kim M.H., Chung J.H., Bae J.W., Oh B.H., Park Y.B., and Kim H.S. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol 51, 933, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Chavakis E., Koyanagi M., and Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation 121, 325, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Wendel J.S., Ye L., Zhang P., Tranquillo R.T., and Zhang J.J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A 20, 1325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xiong Q., Hill K.L., Li Q., Suntharalingam P., Mansoor A., Wang X., Jameel M.N., Zhang P., Swingen C., Kaufman D.S., and Zhang J. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 29, 367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang B., Borazjani A., Tahai M., Curry A.L., Simionescu D.T., Guan J., To F., Elder S.H., and Liao J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A 94, 1100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.French K.M., Boopathy A.V., DeQuach J.A., Chingozha L., Lu H., Christman K.L., and Davis M.E. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater 8, 4357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singelyn J.M., Sundaramurthy P., Johnson T.D., Schup-Magoffin P.J., Hu D.P., Faulk D.M., Wang J., Mayle K.M., Bartels K., Salvatore M., Kinsey A.M., Demaria A.N., Dib N., and Christman K.L. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol 59, 751, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Potapova I.A., Doronin S.V., Kelly D.J., Rosen A.B., Schuldt A.J., Lu Z., Kochupura P.V., Robinson R.B., Rosen M.R., Brink P.R., Gaudette G.R., and Cohen I.S. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol 295, H2257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shah U., Bien H., and Entcheva E. Microtopographical effects of natural scaffolding on cardiomyocyte function and arrhythmogenesis. Acta Biomater 6, 3029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei H.J., Chen C.H., Lee W.Y., Chiu I., Hwang S.M., Lin W.W., Huang C.C., Yeh Y.C., Chang Y., and Sung H.W. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials 29, 3547, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Ameer G.A., Sheppard B.J., and Langer R. A tough biodegradable elastomer. Nat Biotechnol 20, 602, 2002 [DOI] [PubMed] [Google Scholar]
- 141.Rai R., Tallawi M., Barbani N., Frati C., Madeddu D., Cavalli S., Graiani G., Quaini F., Roether J.A., Schubert D.W., Rosellini E., and Boccaccini A.R. Biomimetic poly(glycerol sebacate) (PGS) membranes for cardiac patch application. Mater Sci Eng C Mater Biol Appl 33, 3677, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Kemppainen J.M., and Hollister S.J. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res Part A 94, 9, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Venugopal J.R., Prabhakaran M.P., Mukherjee S., Ravichandran R., Dan K., and Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface 9, 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Q.Z., Ishii H., Thouas G.A., Lyon A.R., Wright J.S., Blaker J.J., Chrzanowski W., Boccaccini A.R., Ali N.N., Knowles J.C., and Harding S.E. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials 31, 3885, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Ravichandran R., Venugopal J.R., Sundarrajan S., Mukherjee S., Sridhar R., and Ramakrishna S. Expression of cardiac proteins in neonatal cardiomyocytes on PGS/fibrinogen core/shell substrate for Cardiac tissue engineering. Int J Cardiol 167, 1461, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Ravichandran R., Venugopal J.R., Sundarrajan S., Mukherjee S., and Ramakrishna S. Cardiogenic differentiation of mesenchymal stem cells on elastomeric poly (glycerol sebacate)/collagen core/shell fibers. World J Cardiol 5, 28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ciardelli G., Chiono V., Vozzi G., Pracella M., Ahluwalia A., Barbani N., Cristallini C., and Giusti P. Blends of poly-(epsilon-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules 6, 1961, 2005 [DOI] [PubMed] [Google Scholar]
- 148.Ho Y.C., Huang F.M., and Chang Y.C. Cytotoxicity of formaldehyde on human osteoblastic cells is related to intracellular glutathione levels. J Biomed Mater Res Part B Appl Biomater 83, 340, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Reddy C.S., Venugopal J.R., Ramakrishna S., and Zussman E. Polycaprolactone/oligomer compound scaffolds for cardiac tissue engineering. J Biomed Mater Res Part A 102, 3713, 2014 [DOI] [PubMed] [Google Scholar]
- 150.Wickham A.M., Islam M.M., Mondal D., Phopase J., Sadhu V., Tamas E., Polisetti N., Richter-Dahlfors A., Liedberg B., and Griffith M. Polycaprolactone-thiophene-conjugated carbon nanotube meshes as scaffolds for cardiac progenitor cells. J Biomed Mater Res B Appl Biomater 102, 1553, 2014 [DOI] [PubMed] [Google Scholar]
- 151.Piao H., Kwon J.S., Piao S., Sohn J.H., Lee Y.S., Bae J.W., Hwang K.K., Kim D.W., Jeon O., Kim B.S., Park Y.B., and Cho M.C. Effects of cardiac patches engineered with bone marrow-derived mononuclear cells and PGCL scaffolds in a rat myocardial infarction model. Biomaterials 28, 641, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Tokunaga M., Liu M.L., Nagai T., Iwanaga K., Matsuura K., Takahashi T., Kanda M., Kondo N., Wang P., Naito A.T., and Komuro I. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. J Mol Cell Cardiol 49, 972, 2010 [DOI] [PubMed] [Google Scholar]
- 153.Niu H., Mu J., Zhang J., Hu P., Bo P., and Wang Y. Comparative study of three types of polymer materials co-cultured with bone marrow mesenchymal stem cells for use as a myocardial patch in cardiomyocyte regeneration. J Mater Sci Mater Med 24, 1535, 2013 [DOI] [PubMed] [Google Scholar]
- 154.Levit R.D., Landazuri N., Phelps E.A., Brown M.E., Garcia A.J., Davis M.E., Joseph G., Long R., Safley S.A., Suever J.D., Lyle A.N., Weber C.J., and Taylor W.R. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc 2, e000367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miyagi Y., Zeng F., Huang X.P., Foltz W.D., Wu J., Mihic A., Yau T.M., Weisel R.D., and Li R.K. Surgical ventricular restoration with a cell- and cytokine-seeded biodegradable scaffold. Biomaterials 31, 7684, 2010 [DOI] [PubMed] [Google Scholar]
- 156.Tirziu D., Giordano F.J., and Simons M. Cell communications in the heart. Circulation 122, 928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zong X., Bien H., Chung C.Y., Yin L., Fang D., Hsiao B.S., Chu B., and Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 26, 5330, 2005 [DOI] [PubMed] [Google Scholar]
- 158.Rockwood D.N., Akins R.E., Jr., Parrag I.C., Woodhouse K.A., and Rabolt J.F. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials 29, 4783, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Senel Ayaz H.G., Perets A., Ayaz H., Gilroy K.D., Govindaraj M., Brookstein D., and Lelkes P.I. Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials 35, 8540, 2014 [DOI] [PubMed] [Google Scholar]
- 160.Shin M., Ishii O., Sueda T., and Vacanti J.P. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials 25, 3717, 2004 [DOI] [PubMed] [Google Scholar]
- 161.LaFramboise W.A., Scalise D., Stoodley P., Graner S.R., Guthrie R.D., Magovern J.A., and Becich M.J. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol 292, C1799, 2007 [DOI] [PubMed] [Google Scholar]
- 162.Hussain A., Collins G., Yip D., and Cho C.H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol Bioeng 110, 637, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Sekine H., Shimizu T., Dobashi I., Matsuura K., Hagiwara N., Takahashi M., Kobayashi E., Yamato M., and Okano T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A 17, 2973, 2011 [DOI] [PubMed] [Google Scholar]
- 164.Nishida K., Yamato M., Hayashida Y., Watanabe K., Maeda N., Watanabe H., Yamamoto K., Nagai S., Kikuchi A., Tano Y., and Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77, 379, 2004 [DOI] [PubMed] [Google Scholar]
- 165.Sato M., Yamato M., Hamahashi K., Okano T., and Mochida J. Articular cartilage regeneration using cell sheet technology. Anat Rec (Hoboken) 297, 36, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A., Shimizu T., and Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today 42, 181, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., Kikuchi A., Umezu M., and Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90, e40, 2002 [DOI] [PubMed] [Google Scholar]
- 168.Shimizu T., Sekine H., Isoi Y., Yamato M., Kikuchi A., and Okano T. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. Tissue Eng 12, 499, 2006 [DOI] [PubMed] [Google Scholar]
- 169.Sekiya S., Shimizu T., Yamato M., Kikuchi A., and Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun 341, 573, 2006 [DOI] [PubMed] [Google Scholar]
- 170.Menasche P., Hagege A.A., Scorsin M., Pouzet B., Desnos M., Duboc D., Schwartz K., Vilquin J.T., and Marolleau J.P. Myoblast transplantation for heart failure. Lancet 357, 279, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Ito A., Hayashida M., Honda H., Hata K., Kagami H., Ueda M., and Kobayashi T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng 10, 873, 2004 [DOI] [PubMed] [Google Scholar]
- 172.Shimizu K., Ito A., Arinobe M., Murase Y., Iwata Y., Narita Y., Kagami H., Ueda M., and Honda H. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J Biosci Bioeng 103, 472, 2007 [DOI] [PubMed] [Google Scholar]
- 173.Ishii M., Shibata R., Shimizu Y., Yamamoto T., Kondo K., Inoue Y., Ouchi N., Tanigawa T., Kanemura N., Ito A., Honda H., and Murohara T. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int J Cardiol 175, 545, 2014 [DOI] [PubMed] [Google Scholar]
- 174.Thavandiran N., Dubois N., Mikryukov A., Masse S., Beca B., Simmons C.A., Deshpande V.S., McGarry J.P., Chen C.S., Nanthakumar K., Keller G.M., Radisic M., and Zandstra P.W. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A 110, E4698, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burridge P.W., Metzler S.A., Nakayama K.H., Abilez O.J., Simmons C.S., Bruce M.A., Matsuura Y., Kim P., Wu J.C., Butte M., Huang N.F., and Yang P.C. Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am J Transl Res 6, 724, 2014 [PMC free article] [PubMed] [Google Scholar]
- 176.Vunjak Novakovic G., Eschenhagen T., and Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harb Perspect Med 4, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Burdick J.A., and Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 15, 205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Badylak S.F., Taylor D., and Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13, 27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Crapo P.M., Gilbert T.W., and Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Caspi O., Itzhaki I., Kehat I., Gepstein A., Arbel G., Huber I., Satin J., and Gepstein L. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev 18, 161, 2009 [DOI] [PubMed] [Google Scholar]
- 181.Pallotta I., Sun B., Wrona E.A., and Freytes D.O. BMP protein-mediated crosstalk between inflammatory cells and human pluripotent stem cell-derived cardiomyocytes. J Tissue Eng Regen Med 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]