Abstract
Significance: Alzheimer's disease is a neurodegenerative disorder that is projected to exceed more than 100 million cases worldwide by 2050. Aging is considered the primary risk factor for some 90% of Alzheimer's cases but a significant 10% of patients suffer from aggressive, early-onset forms of the disease. There is currently no effective Alzheimer's treatment and this, coupled with a growing aging population, highlights the necessity to understand the mechanism(s) of disease initiation and propagation. A major hallmark of Alzheimer's disease pathology is the accumulation of amyloid-β (Aβ) aggregates (an early marker of Alzheimer's disease), and neurofibrillary tangles, comprising the hyper-phosphorylated microtubule-associated protein Tau. Recent Advances: Protein oxidation is frequently invoked as a potential factor in the progression of Alzheimer's disease; however, whether it is a cause or a consequence of the pathology is still being debated. The Proteasome complex is a major regulator of intracellular protein quality control and an essential proteolytic enzyme for the processing of both Aβ and Tau. Recent studies have indicated that both protein oxidation and excessive phosphorylation may limit Proteasomal processing of Aβ and Tau in Alzheimer's disease. Critical Issues: Thus, the Proteasome may be a key factor in understanding the development of Alzheimer's disease pathology; however, its significance is still very much under investigation. Future Directions: Discovering how the proteasome is affected, regulated, or dysregulated in Alzheimer's disease could be a valuable tool in the efforts to understand and, ultimately, eradicate the disease. Antioxid. Redox Signal. 25, 886–901.
Keywords: : Alzheimer's disease, dementia, oxidative stress, protein oxidation, proteolysis, proteasome
Protein Degradation
Cellular homeostasis is maintained by the controlled balance between the synthesis and degradation of various proteins, which are necessary for proper cellular function. Evolution has provided cells with multiple mechanisms to regulate protein quality control, such as protein degradation by individual proteolytic enzymes (such as the Proteasome and the mitochondrial Lon protease), and autophagy within lysozomes, which removes damaged organelles and large protein aggregates (48, 69). The processing of damaged proteins is mediated mainly by the 20S and 26S Proteasomes, the Immunoproteasome (IP), and the mitochondrial Lon protease (14, 54, 155). However, age and disease can result in, or from, dysregulation of these cellular damage removal mechanisms (96, 155).
The proteasome and its regulators
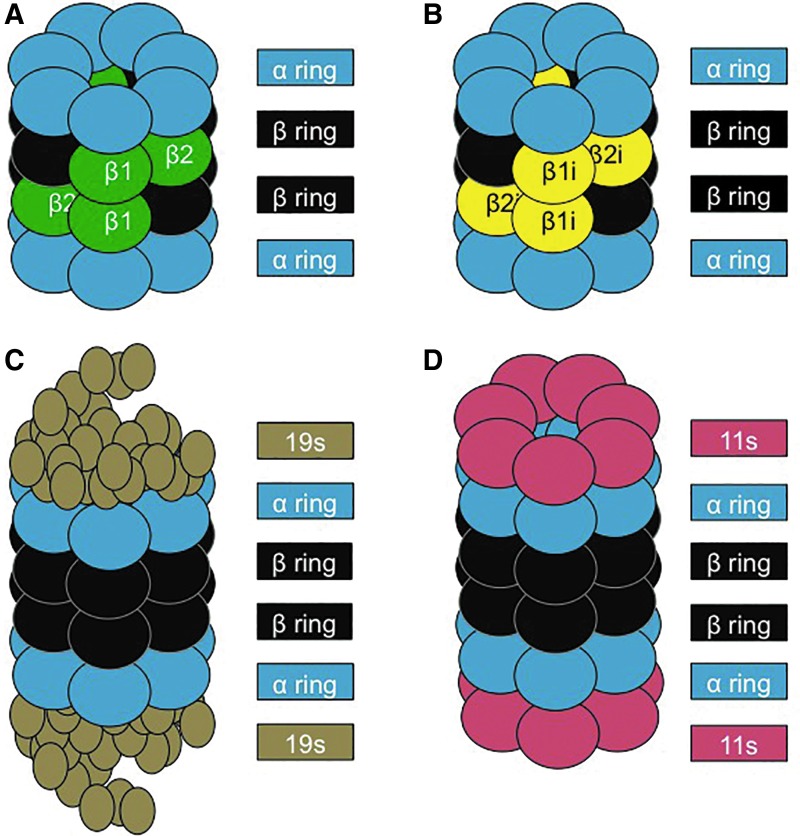
The 20S Proteasome is one of the cell's primary adenosine triphosphate (ATP)-independent mechanisms that is used to break down oxidatively damaged proteins (30). The 20S comprises four stacked rings. The two external rings are made of seven α subunits, whereas each ring of the core is made of seven β subunits, with three of them responsible for the proteolytic activity (13, 43). The β1 subunit has caspase-like activity, the β2 provides trypsin-like activity, and the β5 has chymotrypsin-like activity (10). In addition, various regulatory subunits can attach to the α rings at each end of the 20S Proteasome, including the 11S (Pa28) regulator (Fig. 1), which, during periods of oxidative stress, can expedite the degradation of oxidized proteins (173).
FIG. 1.
Structure of the proteasome and its regulatory subunits. (A) The 20S core proteasome is formed by four rings. The two outer rings contain seven α subunits (1–7), and the two inner rings consist of seven β subunits (1–7). Proteolysis is catalyzed within the β rings by three distinct β subunits: β1, β2, and β5. (B) The IP differs from the 20S proteasome by having three unique proteolytic sites: β1i, β2i, and β5i, which allow different substrate processing and larger peptide products that can be utilized in MHC class I antigen presentation. (C) The 26S Proteasome is formed on the addition of two 19S regulators (each consisting of multiple subunits) to the α rings of the 20S core Proteasome, allowing the 26S Proteasome to recognize and unfold (using ATP) poly-ubiquitin-tagged substrates for degradation. (D) 11S or Pa28 is a regulator that can bind to the α rings of both the 20S Proteasome and the IP, to enhance the degradation of oxidatively damaged protein substrates. ATP, adenosine triphosphate; IP, Immunoproteasome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Although the focus of this article is on oxidative stress, the IP was actually discovered, and is most known, as a specialized immune-activated form of the 20S catalytic core. Specifically the β1, β2, and β5 subunits of the 20S core, which are constitutively expressed, are substituted with the catalytic subunits β1i, β2i, and β5i of the IP (41). The IP has primarily been implicated in mediating an immune response. Various studies have shown that cells that are exposed to Interferon-γ trigger upregulation of the IP (112). In addition, cells of the immune system typically have constitutively high levels of IP, in contrast to other cell types (112) (Fig. 1).
The 26S Proteasome is formed from the addition of a 19S regulatory subunit to each of the α rings of the 20S Proteasome, in an ATP-dependent manner (10). During periods of homeostasis, the cell relies on the ubiquitin-Proteasome system, with the 26S Proteasome as the predominant form for protein degradation (13, 89) (Fig. 1). Proteins marked for degradation first undergo ubiquitinylation, involving three different enzymes: the ubiquitin-activating enzyme (E1), which binds a ubiquitin molecule in an ATP-dependent manner and transfers it to an ubiquitin-conjugating enzyme (E2), which binds multiple ubiquitin monomers into a poly-ubiquitin chain, which is then transferred to a specific ubiquitin-ligase enzyme (E3), which links the poly-ubiquitin to a target protein (43). This poly-ubiquitin chain acts as a targeting sequence or “tag” to direct proteins to the 26S Proteasome for degradation. The 19S regulatory subunits of 26S Proteasomes first remove the poly-ubiquitin “tags” of targeted proteins, then unfold those proteins, and finally deliver them into the 20S core of the 26S Proteasome for proteolysis (160).
During periods of oxidative stress, however, critical sulfhydryl groups in the 19S regulatory subunits are highly susceptible to oxidative damage and many lose their proteolytic capacity (156). Additionally, most of the remaining 26S Proteasomes are disassembled by Ecm29, with the 19S caps being sequestered by heat shock protein 70 (HSP70). This, in turn, provides additional free 20S core Proteasomes to quickly degrade oxidized proteins by interacting with their hydrophobic patches, thus ensuring their rapid removal before they can aggregate and cross-link. Approximately 3–5 h after the initial stress, the 26S Proteasome is reassembled in a process that is catalyzed by HSP70 (53, 137).
One of the primary pathways that triggers increased expression of the 20S Proteasome (and that is also negatively impacted by Alzheimer's disease) is through the activation of the cap-n-collar transcriptional activator, the nuclear factor erythroid 2-like factor 2 (Nrf2) (127). Nrf2 has been shown to activate numerous antioxidant response genes, which together provide a significant cellular defense to combat an oxidative insult (74, 179). Under homeostatic conditions, Nrf2 is inhibited from translocating into the nucleus by its negative regulator Keap1, which also targets Nrf2 for poly-ubiquitinylation and subsequent degradation by the 26S Proteasome, thus keeping intracellular levels quite low (180). However, during periods of stress, Keap1 is phosphorylated, causing its dissociation from Nrf2. Oxidant-induced dissociation of the 26S proteasome (mediated by Ecm29 and HSP70) prevents further Nrf2 degradation and increases the Nrf2 pool size (53, 179). After phosphorylation by AKT and/or PKCγ, Nrf2 migrates into the nucleus, where it binds to various antioxidant response elements (ARE), also called electrophile responsive elements (EpRE), within target genes, thereby increasing their transcription (179). During periods of high oxidative stress, when the cellular proteome is the most vulnerable to oxidative damage, it is critical that the cell has an available, functioning pool of 20S Proteasome. Interestingly, if proteasomal activity becomes inhibited, the Nrf2 signal transduction pathway is used to increase transcriptional activity (109).
Oxidative stress has been associated with Alzheimer's disease (159) and in the triple-transgenic murine model for Alzheimer's disease, young mice exhibit increased phosphorylation of Nrf2, whereas older triple-transgenic mice show a sex-dependent decrease in Nrf2 phosphorylation (109). These findings suggest that responses to the early stages of Alzheimer's disease include an attempt by the Nrf2 system to combat oxidative damage. As the disease progresses, however, Nrf2 phosphorylation decreases and it remains in the cytoplasm (133), allowing greater Nrf2 ubiquitinylation and consequent degradation by the 26S Proteasome. As a result, many Nrf2 antioxidant targets are not expressed in the highly oxidative environment that accompanies Alzheimer's disease development (109). In turn, decreased amounts of active Nrf2 greatly hinder the de novo pool of 20S Proteasome that could help compensate for the already diminishing proteasomal activity. Due to the importance of Nrf2 in the upregulation of various stress response genes, it has become a focal point for neurodegenerative therapies. Exposure to various Nrf2 inducers has been shown to successfully ameliorate the accumulation of Aβ plaques (176), possibly through increased activation of ARE/EpRE elements.
Structure and function of the mitochondrial Lon protease
Although the 20S Proteasome is the major protease dealing with oxidized protein substrates in the cell cytoplasm, nuclear and endoplasmic reticulum, intracellular organelles such as mitochondria and peroxisomes do not have Proteasomes and have developed specialized proteases to cope with protein damage. This is especially important, since mitochondria and peroxisomes are major sources of superoxide or hydrogen peroxide production (105). Multiple proteases have been uncovered in mitochondria, but perhaps none have been as well investigated as the ATP-dependent Lon P1 protease. Lon was initially discovered in Escherichia coli and was subsequently found to be conserved in all types of mammalian tissues, indicating its importance in mitochondrial protein quality control (49, 171).
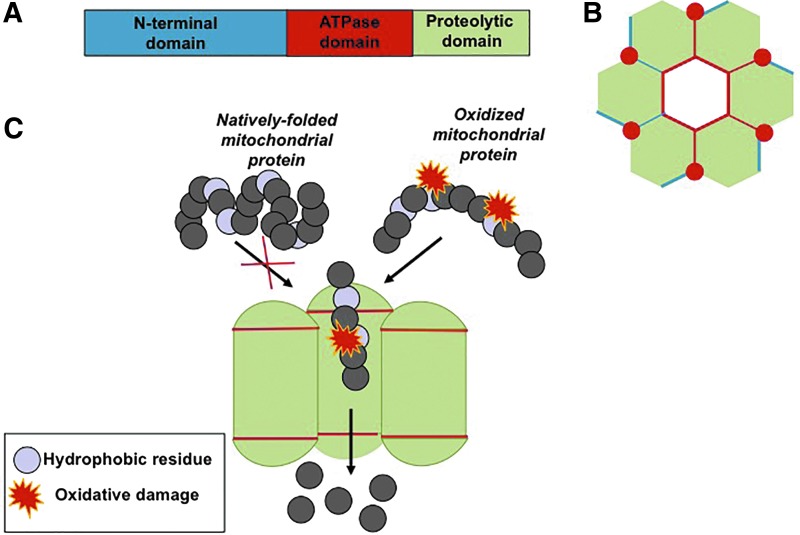
Lon P1 comprises six to seven monomeric subunits. Each subunit consists of three domains: an N-domain, which interacts with the hydrophobic regions of the substrate; an ATPase domain, which binds to ATP and facilitates the ring opening; and a serine proteolytic domain (115) (Fig. 2). Early studies showed Lon expression as highly controlled, with loss of this regulation being detrimental for the cell or organism. For example, in E. coli, continual overexpression of Lon is lethal (24), and in mammalian tissue, Lon P1 overexpression accelerates tumorigenesis (12, 68). In contrast, loss of Lon results in mitochondrial deficiency and cellular senescence (15, 114, 132). Lon P1 has been shown to be the primary mitochondrial protease that degrades mildly oxidatively damaged proteins, including aconitase, and other oxidized intramitochondrial proteins (14). During increased cellular stress, Lon is transiently induced to cope with elevated mitochondrial protein damage (67, 113). Peroxisomes, which generate significant amounts of H2O2 during metabolism of odd-chain fatty acids, have a different Lon variant called Lon P2 (129a).
FIG. 2.
Structure of the mitochondrial Lon protease. (A) The Lon protease consists of three domains: an N-terminal domain that recognizes oxidized substrates, an ATPase domain that hydrolyzes ATP to facilitate ring opening, and a proteolytic domain that degrades damaged proteins. (B) Top view of the Lon protease. (C) A side view of the Lon protease, showing the recognition and degradation of only oxidized proteins due to exposure of hydrophobic residues. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Alzheimer's Disease
Alzheimer's disease is the most common type of dementia that targets higher cognitive processes, resulting in memory impairment and behavioral disorders (141). In the United States alone, it is estimated that ∼14 million people will suffer from this disease by 2050 (7, 65). Current costs to treat individuals with Alzheimer's disease are estimated at $400 billion per year, and the economic burden will clearly only increase, unless a cure is found (6, 7).
The two major hallmarks of the disease are the formation of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs), comprising mainly hyperphosphorylated Tau protein. Both markers were originally detected by Alois Alzheimer, the disease's namesake and discoverer, in the early 20th century (3). Although the etiology of Alzheimer's disease remains elusive, scientists have developed several theories to explain its origins. Many fall within two scientific camps: those who believe Aβ accumulation is the central axis of the disease (“Baptists”) (59), and others who consider Tau to be the primary culprit (“Tauists”) (51).
Amyloid-β
The amyloid precursor protein (APP) functions as both a paracrine signaling peptide and a membrane-bound receptor protein. The secreted soluble APP fragment is critical in growth stimulation, cell adhesion, and blocking of various serine proteases of neighboring neurons (104, 148). APP is highly conserved, with homologs found in multiple species, including humans, mice, Drosophila melanogaster, and Caenorhabditis elegans (151). The importance of APP in neuronal health was explored in APP homozygous-deficient mice. These animals showed normal development, but they experienced increased reactive glial cell formation, coupled with decreased neuronal and muscular function in adulthood (182). Similarly, cultured neurons lacking APP exhibited decreased neurite outgrowth and decreased viability (122). Conversely, transgenic mice overexpressing APP show many of the pathological features of Alzheimer's disease, specifically the formation of Aβ plaques and a decline in neuronal function, highlighting the importance of a regulated amount of APP for proper neurological health with age (174).
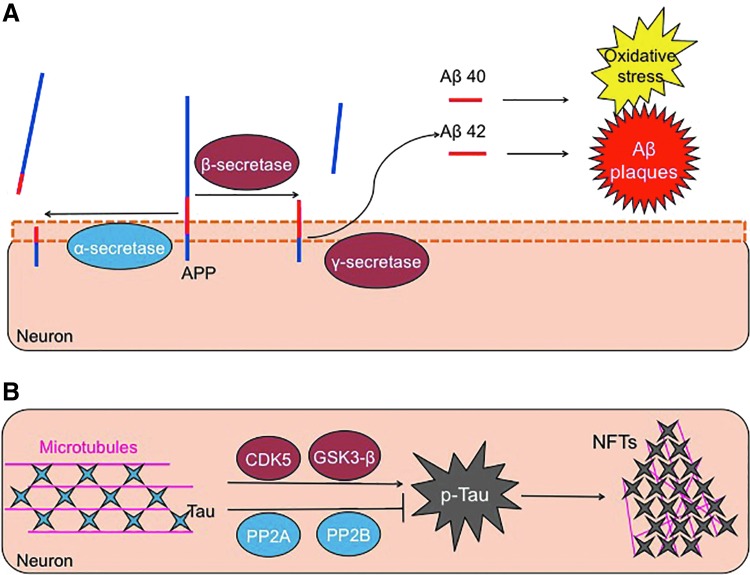
APP can undergo cleavage (Fig. 3) by an α-secretase enzyme, forming two fragments: a large 100 kD soluble fragment and an 11 kD membrane-associated protein (167). Importantly, α-secretase cleavage results in disruption (separation) of the Aβ region, between lysine 16 and leucine 17 (78, 167). Consequently, α-secretase cleavage does not produce Aβ peptides, and it does not have a pathological impact. Instead, Aβ is formed by the sequential proteolytic cleavage of the APP by a β-secretase enzyme, referred to as “β-site APP-cleaving enzyme (BACE-1),” followed by a γ-secretase enzyme or presenilin (72, 152). Two isoforms of Aβ are actually formed, with 40 amino acids, and with a second containing 42 residues. This slight cleavage variation allows for the inclusion of two additional hydrophobic residues in the Aβ42 peptide (75). As a result, Aβ42 aggregates much more readily compared with the more soluble Aβ40 form. Studies that focused on plaque formation in Alzheimer's disease brains found these deposits to mainly comprise Aβ42 (147). This indicates that the loss of regulated processing of the amyloid-β protein accelerates plaque formation, with this insoluble Aβ42 being much more toxic to neurons than is the soluble Aβ40 (178).
FIG. 3.
Aβ and p-Tau formation. (A) The APP is a transmembrane protein. APP can be cleaved in a nonpathological way through the action of an α-secretase, an enzyme that breaks the Aβ sequence. Conversely, APP can be cleaved in a pathological manner, involving a first cut by a β-secretase, followed by a second hydrolysis by a γ-secretase. These two cleavage steps form two slightly different Aβ peptides, which, in turn, can aggregate to form amyloid plaques and also cause much of the oxidative stress related to this disease. The two Aβ isoforms are 40 and 42 amino acids length (Aβ1-40 and Aβ1-42), with Aβ1-42 being, by far, the most toxic. (B) The Tau protein helps to maintain the structure of microtubules. The action of some kinases such as cyclin-dependent kinase 5 (CDK5) and GSK3β phosphorylates Tau. If Tau becomes excessively phosphorylated, or hyperphosphorylated, it tends to form aggregates that are known as NFTs. This process is also regulated by dephosphorylating enzymes, such as phosphatase 2A and 2B, that act to remove excessive phosphoryl groups and help Tau keep its proper structure and functionality. Aβ, amyloid-β; APP, amyloid precursor protein; GSK3β, glycogen synthase kinase 3β; NFTs, neurofibrillary tangles. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Interestingly, APP does not appear to be the preferred substrate of BACE-1, as APP lacks BACE-1's desired cleavage site. This indicates that the enzyme should serve a different, yet still unknown, role in the cell (91, 177). Studies in BACE-1 null mice showed decreased accumulation of amyloid-β and restoration of memory deficiencies that are common in the transgenic mouse model that overexpresses human-APP (118).
Aβ and mitochondria
The accumulation of Aβ not only hampers protein turnover but also contributes to the chronic elevation of oxidative damage, which is common in the Alzheimer's disease pathology. The brain is highly susceptible to oxidative damage due to its high lipid content and elevated energy demands (134). In turn, the high metabolic needs of the brain place a huge demand on the mitochondria, increasing their susceptibility to oxidative damage (19). In addition, postmortem studies of Alzheimer's disease brains show a decline in the enzymatic activity of both nuclear-derived and mitochondrial-encoded oxidative phosphorylation enzymes (46). In turn, the decline in ATP production may weaken the mitochondrial transmembrane electrochemical gradient, further contributing to oxidant formation (136). The close proximity of mitochondrial proteins to endogenously generated oxidants also makes them highly susceptible to damage.
To stave off the accumulation of oxidized proteins, mitochondria rely on various proteases to remove damaged proteins, with the most well studied being the Lon protease. Short-term exposure to various stresses (e.g., heat shock, serum starvation, hydrogen peroxide, peroxynitrite) has been shown to stimulate both Lon protein expression and proteolytic capacity (113, 162). However, long-term oxidant exposure or the aging process dampens Lon's ATP-dependent activity (16). Conversely, a decline in Lon expression has been associated with increased protein oxidation and decreased mitochondrial membrane potential (114).
Overall, mitochondrial dysfunction is a key feature of Alzheimer's disease pathology (22, 28, 135, 165), which is characterized by altered cellular metabolism. Recent studies have found that Aβ accumulation on mitochondrial membranes directly blocks the mitochondrial import of nuclear encoded proteins by the mitochondrial outer-membrane translocase (158). Additionally, Aβ has been shown to localize into the mitochondrial cristae, independent of the mitochondrial membrane potential (58). This poses two problems for mitochondria: (1) newly synthesized (and functioning) proteins are unable to enter; (2) localized Aβ aggregation hinders the electron transport chain and further increases free radical production (20, 21, 98, 169, 170). As Lon is the primary mitochondrial protease, the inability to import this nuclear-encoded protein could have dire consequences for mitochondrial function, and, in turn, further Alzheimer's disease progression (Fig. 4).
FIG. 4.
Aβ and the mitochondria. During homeostatic conditions, proteins that are encoded by nuclear genes and synthesized on cytoplasmic ribosomes are directed for import into mitochondria by their mitochondrial targeting sequences (MTS). In the presence of an electrochemical gradient, proteins are imported, first through the outer mitochondrial membrane by the outer mitochondrial transferase (TOM) and second, to the mitochondrial matrix by the inner mitochondrial transferase (TIM). To ensure a constant electrochemical gradient, mitochondria rely on the electron transport chain (ETC), which generates not only the cellular energy source in the form of ATP but also a small amount of oxidants that can damage proximal mitochondrial proteins. To prevent damage accumulation, the Lon protease quickly removes these oxidized proteins. However, during Alzheimer's disease, Aβ accumulates within the mitochondrial cristae (irrespective of the electrochemical gradient) and blocks the import of mitochondrial-targeted proteins. This, in turn, decreases the cell's ability to generate ATP. In an exaggeration of the dysregulation that can occur during aging, mitochondrial proteins may undergo increased oxidation in Alzheimer's mitochondria, which may surpass the degradation capacity of Lon and, ultimately, cause loss of mitochondrial function. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The importance of various mitochondrial metabolic enzymes (and their dysregulation) is readily evident in Alzheimer's disease pathology. Aconitase, a critical enzyme of the Krebs cycle, has been shown to become inactive during Alzheimer's disease (153). This is, in large part, due to the iron-sulfur cluster at its active site, which makes this enzyme highly susceptible to inactivation by oxidation (123). Under normal physiological conditions, mild oxidation of aconitase allows for rapid recognition and degradation by the Lon protease. However, in Alzheimer's disease pathology, which is characterized by a chronic elevation of oxidative stress, more severe aconitase oxidation occurs, making it a poor substrate for the Lon protease (14). The consequent accumulation and aggregation of inactive aconitase greatly decreases the metabolic production of NADH and FADH2 by the Krebs cycle (150). Both molecules are essential for the entry of reducing equivalents into the electron transport chain. Thus, a decline in Krebs cycle activity results in decreased activity of the respiratory chain, which further degrades the mitochondrial transmembrane potential and, ultimately, exacerbates the effects of the disease.
The Tau protein
Tau is a microtubule-associated protein that functions to stabilize microtubules, with the highest expression pattern found in the axons of neurons. It was originally characterized for its involvement and upregulation, along with tubulin, during neuronal differentiation (36). Due to Tau being critical for cellular morphology, its dynamic regulation is necessary to maintain neuronal structure and the migration of signaling vesicles (146). The six major isoforms of Tau, each varying in the number of microtubule-binding domains, are primarily regulated by phosphorylation, which is ideal due to the high amount of phosphorylation sites in Tau (45 serine, 35 threonines, and 4 tyrosines). Under Alzheimer's disease, phosphorylation of Tau is higher than in healthy people (85). Tau phosphorylation is also dependent on the developmental state, with peak levels occurring during fetal growth, and drastically tapering off in the adult brain (9, 102).
Tau activity is controlled by its phosphorylation state, which is modulated by two prominent kinases: cyclin-dependent kinase 5 and glycogen synthase kinase 3β (GSK3β), and phosphate removers: phosphatase 2A and 2B (17, 55). However, in the Alzheimer's brain, the highly orchestrated regulation of Tau is lost and Tau hyperphosphorylation occurs. Increased levels of unbound Tau (143), or declines in kinase activity (110, 121), may increase the likelihood of Tau misfolding. In turn, prevention of its normal turnover by the Proteasome leads to the disintegration of the microtubule skeleton and the formation of NFTs (25, 70).
The cause for Tau dysregulation is still unclear. Abnormal Tau phosphorylation may also be conducted by the phosphorylated protein itself, with hyperphosphorylated Tau sequestering away native Tau and resulting in microtubule disarrangement (70).
Linking Aβ and Tau
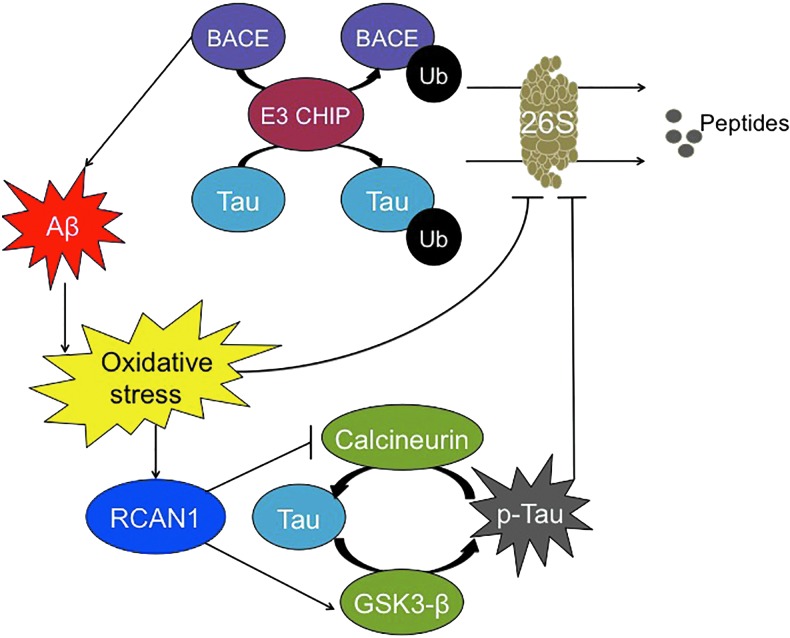
Though Tau hyperphosphorylation and Aβ aggregation have been linked to Alzheimer's disease development, the relationship between these two proteins remains unclear. The stress-inducible Regulator of Calcineurin gene (RCAN1) may be one of the bridges from one molecule to the other (93). Calcineurin is a serine/threonine phosphatase that is involved in p-Tau dephosphorylation. RCAN1 expression can result in synthesis of RCAN1-1L, RCAN1-1S, and/or RCAN1-4, proteins that inhibit calcineurin (44, 145). RCAN1 expression also causes an induction/activation of GSK3β, which is responsible for Tau phosphorylation (38). Thus, it can cause extensive Tau phosphorylation. During transient oxidative stress, RCAN1-4 is highly induced, causing beneficial short-term inhibition of calcineurin (29). However, chronic expression of the RCAN1-1L and RCAN1-1S isoforms, seen in Alzheimer's disease and Down syndrome, causes chronic inhibition of calcineurin and higher steady-state levels of phosphorylated Tau (97) (Fig. 5). This is further substantiated by studies conducted in RCAN1 knock-in mice, in which elevated levels of phosphorylated Tau are seen (39).
FIG. 5.
Relationship between Aβ and Tau. BACE-1, responsible for Aβ formation, and the Tau protein can be ubiquitinylated by the E3 CHIP ligase and degraded by the 26S Proteasome. However, Aβ aggregation inhibits 26S Proteasome activity indirectly, due to increased oxidative stress. Simultaneously, oxidative stress induces RCAN1 synthesis, which blocks calcineurin activity, causing decreased dephosphorylation of Tau, and it stimulates GSK3β activity, causing increased Tau phosphorylation: The net result is a large net increase in the steady-state phosphorylation of Tau, which then cannot be degraded by Proteasome. CHIP, C terminus of heat shock protein 70 interacting protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In parallel studies, Aβ42 has been shown to trigger the generation of oxidants in both cells and mitochondria, a characteristic of the Alzheimer's disease pathology (77). Specifically, Aβ42 can trigger the transcriptional increase of RCAN1 (60). In cultured rat neurons, the presence of Aβ42 resulted in the increase of steady-state levels of phosphorylated Tau, which was only reversed on silencing of RCAN1 (93). Hence, Aβ42, which is highly expressed in the Alzheimer's disease brain, triggers the chronic increase of RCAN1 and, therefore, the inhibition of calcineurin and activation of GSK3β, which accelerates the Tau hyperphosphorylation.
In humans, the apolipoprotein E (apoE) ɛ4 allele has been identified as a risk factor for developing sporadic Alzheimer's disease (128, 144). Potentially, this is because the apoE ɛ4 allele is not capable of exerting a neuroprotective effect, unlike the ɛ2 and ɛ3 alleles (64, 99). Individuals with the apoE ɛ4 allele have also been found to express higher levels of RCAN1 compared with noncarriers (8) and, consequently, more p-Tau (61, 93). Thus, this further cements a possible connection between Alzheimer's disease and RCAN1. Lloret and colleagues have also pointed out that various other molecules may represent potential links between Aβ and Tau, including Pin-1, p38 MAPK, or cyclin-dependent kinase 5-p25 (94).
Another possible link is the activation of the caspase pathway, which can be mediated by Aβ (143). In turn, this activates various kinases, such as MAP-kinase (110, 121) or GSK3β, all of which may contribute to Tau hyperphosphorylation (57).
Aβ, the Proteasome, and the IP
Under normal physiological conditions, Aβ is produced and secreted by neurons in response to synaptic activity. On secretion to the extracellular environment, Aβ is degraded by glial cells, which migrate toward amyloid deposits (76). Microglia can phagocytize and degrade Aβ (76), whereas astrocytes also degrade Aβ (175) and facilitate its clearance from the brain to the blood that is mediated by apoE (84, 131). Although the mechanism that causes the transition from normal physiological function to pathological Aβ accumulation is still unknown, several lines of evidence point to the involvement of a dysregulation of protein degradation (26, 73, 107, 168). The inability to maintain a homeostatic balance between amyloid production and degradation results in the damage from amyloid accumulation being twofold: direct inhibition of Proteasome activity (95, 117, 166), and the indirect elevation of oxidative stress (98, 159, 170) (Fig. 6), both of which contribute to protein malfunction. Aβ degradation by both the 20S Proteasome and the ATP-ubiquitin-dependent 26S Proteasome has been demonstrated in in vitro experiments (95, 100, 181), but there is insufficient evidence to tell which one may be more important in vivo.
FIG. 6.
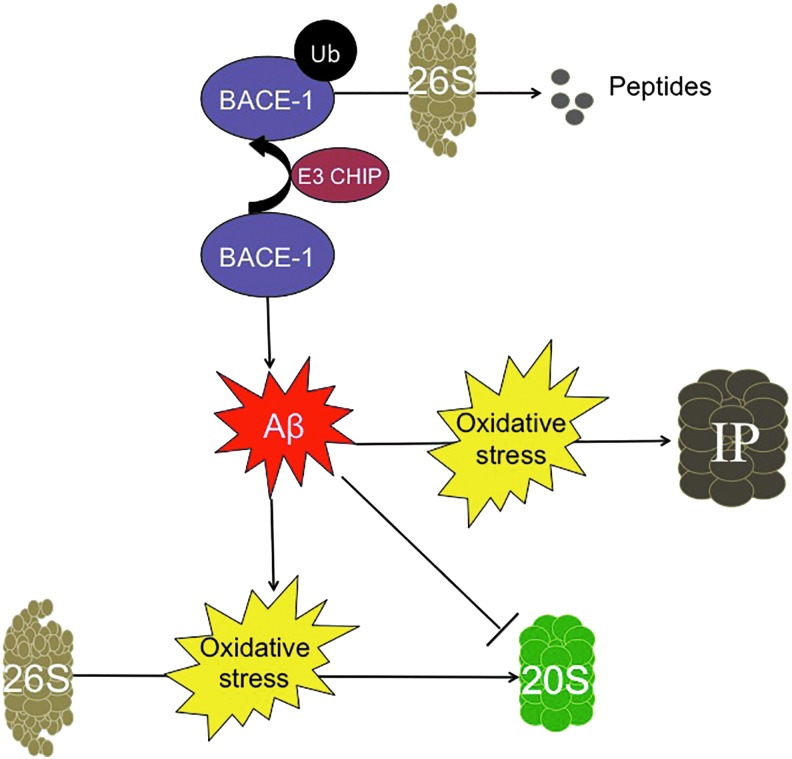
Aβ and the proteasome. Aβ plays a central role in Alzheimer's disease, and it has a well-established relationship with the proteasome. BACE-1, an enzyme that was fundamental in forming Aβ, is ubiquitinylated by the E3 CHIP ligase, for degradation by the ATP-dependent 26S proteasome, thus decreasing the amount of Aβ peptide generated. The 20S proteasome is directly inhibited by Aβ, thus blocking the effective removal of oxidized proteins. In response to the increased cellular damage caused by oxidative stress, the cell increases de novo synthesis of the IP. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Initial studies into the role of the 20S Proteasome in neurodegenerative diseases found that inhibition of Proteasome activity was enough to accelerate neuronal death. Early in vitro studies showed that direct application of Aβ to neuronal cells triggers Proteasome inhibition (140) (Fig. 6). Application of pharmacological inhibitors of the Proteasome was separately shown to induce neuronal toxicity and eventual death (130). Later studies demonstrated a substantial decrease in proteolytic activity when crude lysates of (postmortem) brain tissue from Alzheimer's disease patients were compared with age-matched disease-free controls. Specifically, 20S Proteasome activity was dramatically suppressed in the hippocampus, the superior and middle temporal gyri, and the inferior parietal lobule (81). The study also concluded that Proteasome activity most likely declined as a result of post-translational modifications, as there was little difference in the overall amount of the proteasomal α and β subunits between Alzheimer's disease subjects and controls. These findings were extended by a later study, which showed that purification of 20S Proteasomes from crude lysates of Alzheimer's disease brains resulted in a substantial increase in proteolytic capacity (44). These results indicate that Alzheimer-related decreases in 20S core Proteasome activity are likely due to accumulation of damaged, aggregated, and cross-linked cytosolic proteins (including Aβ) that act as Proteasome inhibitors (47). Additional studies showed that removal of Aβ by treatment with a γ-secretase inhibitor in the triple-transgenic Alzheimer's disease mouse model restores Proteasome degradation capacity (2).
Activity of the ATP-ubiquitin-dependent 26S Proteasome greatly diminishes in oxidizing environments, including the Alzheimer's disease brain (140). Various studies have shown the importance of protein ubiquitinylation in modulating multiple cellular processes, including the ubiquitin-dependent degradation of transcriptional activators such as Nrf2 and NF-κB, and a plethora of kinases (66, 101, 179). In turn, degradation by the 26S Proteasome provides the cell with a controlled mechanism to turn on and off key regulators of these various cellular response pathways.
Interestingly, the normal proteolytic turnover conducted by the ATP-ubiquitin-dependent 26S Proteasome is diminished in the Alzheimer's disease state. This is seen through the accumulation of ubiquitin in amyloid deposits and NFTs. Additionally, a mutant form of ubiquitin has been observed in the brains of Alzheimer's patients. This mutant form of ubiquitin (Ub+1) not only evades disassembly by deubiquitinating enzymes but is also a potent inhibitor of degradation by the 26S Proteasome (87). Importantly, loss of 26S Proteasome activity appears to accelerate the Alzheimer's disease phenotype. One study utilized a conditional knockout against psmc1, an ATPase subunit of the 26S Proteasome, and it selectively targeted neurons of the substancia nigra and forebrain. The impairment of ubiquitin-dependent proteolysis triggered the formation of inclusion bodies and neurodegeneration within the nigrostriatial pathway and forebrain regions (11).
Various proteins involved in the ATP-ubiquitin 26S Proteasome system appear to become dysfunctional during Alzheimer's disease development. One such protein is the C terminus of HSP70 interacting protein (CHIP), which is an E3 ligase that has been recently implicated in degrading BACE-1 (157). Hence, an inverse relationship exists between CHIP and BACE-1; by decreasing BACE-1 expression, CHIP prevents the aberrant processing of APP and decreases the accumulation of Aβ (Fig. 6). This relationship was further supported in studies by Oddo and colleagues, who found that Aβ accumulation inhibits CHIP expression (116), hence demonstrating a positive feedback loop that would only promote further amyloid accumulation.
Less well studied but, perhaps, no less important is the IP, which was originally characterized for its role in the immune response (4). Prior studies have shown that the IP works not only to degrade proteins for antigen presentation for the major histocompatibility complex but also to degrade oxidized proteins with rates and selectivity that are comparable to, or better than, those of the 20S Proteasome (126). This similarity in degradation rate may be partially attributed to the key roles that both proteasomes play in highly oxidant-rich environments: The immune response triggers an elevation in cellular oxidants and, in consequence, an increase in oxidized proteins (124). Therefore, it is perhaps unsurprising that in the highly oxidative environment of the human Alzheimer's disease brain, the IP is activated (106). More importantly, unlike the 20S and the 26S Proteasomes, which show decreased activity, both the expression and proteolytic activity of the IP are elevated in transgenic mice models of Alzheimer's disease (5, 119) (Fig. 6).
Other animal models of amyloidosis, such as the nematode C. elegans, have also studied the interaction of protein degradation systems with Aβ. Overexpression of proteasome subunits such as AIP-1 (63) {an ortholog of the human AIRAP component of the 19S regulatory subunit (161)}, or pbs-5 (23) {an ortholog of the human β5 subunit of the 20S core (10)}, has been shown to increase 26S and 20S Proteasome activities, respectively. In both cases, reduced Aβ toxicity and aggregation were observed in C. elegans. Promotion of the activity of protein degradation with polyphenols has also been successful in alleviating amyloidosis in these worms (138, 139). Compounds that act against Aβ aggregation help to recover protein homeostasis (1), through the action of transcription factors involved in the stress response, such as the heat shock factor 1 and SKIN-1 (homolog of human Nrf2).
Because of the critical role that amyloid accumulation has been believed to exert on the development of pathology, various therapies targeting Aβ removal are currently under development or evaluation. Those that have gained the most traction are Aβ immunotherapies. These treatments rely on antibodies that have high specificity toward a disease-related antigen (50). Bapineuzumab, which is a humanized IgG monoclonal antibody derived from the murine antibody, 3D6, nonselectively targets both soluble and fibrillary Aβ. This approach relies on the hope that nonspecific targeting of Aβ may decrease the total amount of Aβ. Unfortunately, phase three clinical trials have shown no difference in cognitive abilities of Alzheimer's disease patients treated with the antibody compared with controls (149). Other efforts utilize more stringent approaches by targeting soluble Aβ specifically, rather than its insoluble counterpart. However, the low penetrance of the antibody across the blood–brain barrier has made it difficult to show significant efficacy in patients in the early phases of the disease. Solanezumab, a humanized IgG1 monoclonal antibody derived from the murine monoclonal antibody 266, has high binding affinity only to soluble Aβ. However, phase three clinical trials have shown poor results (34, 40).
At the present time, it is fair to say that anti-amyloid therapies have so far not been useful in human Alzheimer's disease treatment. This may be because once overt disease symptoms appear, it is already too late to act against the pathology; this interpretation is invoked by those who argue for the need to find early and sensitive biomarkers. It is also possible, however, that Aβ is not the only factor(s) responsible for Alzheimer's disease, and that a multifactorial approach will be necessary, including Aβ, Tau, oxidative stress, and the regulation of proteostasis at the very least (31, 70, 86, 88). Clearly, we are still quite a long way from having any effective therapy to offer Alzheimer's disease patients and their families.
Tau and the Proteasome
Under homeostatic conditions, endogenous Tau is usually turned over by the Proteasome (42) (Fig. 7). However, pharmacological inhibition of the Proteasome by lactacystin results in Tau accumulation, regardless of its phosphorylation state (52, 92, 166). It is important to note that this finding has been contested, as other groups have shown that Proteasome inhibition decreases Tau accumulation, potentially through the activation of pathways that are secondary to the Proteasome, such as other proteases or apoptosis (42, 45). Importantly, in vitro aggregation of Tau blocks proteolysis by the 20S core Proteasome (79). This demonstrates that the Proteasome interacts with Tau, primarily during the disease state. Since the 20S Proteasome usually removes oxidatively damaged proteins, the large protein aggregates formed during Tau accumulation become difficult to degrade. In consequence, Tau continues to accumulate and the Proteasome is further inhibited (Fig. 7).
FIG. 7.
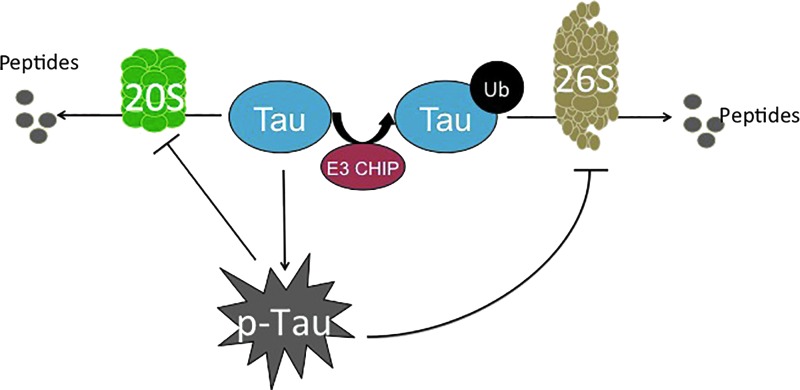
Tau and the proteasome. There are two pathways for degradation of phosphorylated tau: ATP-independent degradation by the 20S proteasome, or ubiquitinylation by the E3 CHIP ligase, and subsequent degradation by the ATP-dependent 26S Proteasome. Although a minimal amount of selective Tau phosphorylation activates degradation by the 26S Proteasome, excessive and unregulated Tau phosphorylation (hyperphosphorylation) inhibits the Proteasome, exacerbating the pathology and cellular damage. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Not only is the 20S core Proteasome's proteolytic capacity limited, but also the ATP-ubiquitin-dependent 26S Proteasome system appears to be inhibited by p-Tau. The relationship between the ATP-ubiquitin-dependent 26S proteasomal system and Alzheimer's disease pathology was initially evident on the identification of ubiquitin within NFTs (108). Further studies showed that the phosphorylation state of Tau dictates the addition of ubiquitin. Specifically, toxic Tau binds to Hsc70, a constitutively expressed protein of the heat-shock family that facilitates the ubiquitin tagging of Tau by the E3 ligase, CHIP (154). CHIP seems to be implicated, as with Aβ, in the processing of Tau, providing another link between the two of them, as suggested by Oddo and colleagues (116) (Fig. 5). Interestingly, Metcalfe et al. found that the pharmacological elevation of cyclic AMP (cAMP) levels provides neuronal protection by preventing caspase activation, which is shown to be increased due to tau hyperphosphorylation (103). In concordance with this study, Myeku et al. showed that activation of cAMP-protein kinase A is able to reverse part of the repression of proteolysis caused by p-Tau, thus promoting the reduction of Tau aggregation and diminishing the decline of cognitive abilities (111).
CHIP has been identified as a chaperon-like E3 ligase, due to its interaction not only with Hsc70 but also with Hsp70 and Hsp90, both of which are critical in protein folding (125). Apart from this, these heat-shock chaperones have been shown to directly interact with Tau, although its phosphorylation status was unknown (35). Thus, CHIP may act to selectively ubiquitin-tag misfolded proteins that cannot be correctly folded by various chaperone proteins (i.e., the heat-shock chaperones) and target them for degradation by the 26S Proteasome. However, in the advanced states of Alzheimer's disease pathology, which is already heavily plagued by an accumulation of highly insoluble Tau, the targeting of these protein aggregates for degradation may be futile. Large protein aggregates become too cumbersome for Proteasome degradation (they cannot enter into the proteasome cylinder for degradation), and they only continue to accumulate.
Various strategies are currently under development, which will attempt to combat loss of proteolytic activities in Alzheimer's disease. To combat the decline of Proteasome function in the Alzheimer's disease brain, a novel exogenous delivery method of Proteasome via nanoparticles has been successfully implemented in cell culture (56) but now awaits verification of efficacy in vivo.
Aging, the Proteasome, and Alzheimer's Disease
The exact relationship between Alzheimer's disease and aging is highly controversial, due to the difficulty of separating normal “brain” aging from disease development. Nevertheless, a strong predictor of disease development is age (90). Many older adults develop dementias, which, based on postmortem studies, would classify them as having suffered Alzheimer's disease, as evidenced by a high abundance of amyloid plaques and Tau tangles (163). Women are also more susceptible to the disease development than men (120, 169). Many others, however, either experience minimal dementia with aging or suffer from other forms of dementia entirely.
In “normal aging,” clinical studies have shown that certain processes of the brain decline, whereas others remain functional. For example, autobiographical memory and semantic knowledge typically remain untouched, compared with working memory and processing, which appear to diminish (164). These findings have been further supported by neuroimaging studies that found atrophy in the prefrontal cortex, indicative of a decline in executive function, whereas the hippocampus shows no significant shrinkage (142).
In contrast, Alzheimer's disease pathology shows a much greater decline in cognitive functions and more marked molecular changes. Not only there is a physical atrophy of the white matter, but also there is shrinkage within the hippocampus. This results in a decline in CA1 hippocampal neurons that does not occur during normal aging (172). These neurons are critical in the output pathway from the hippocampus, which is involved in the formation of long-term memory and spatially oriented tasks (172). Also, postmortem studies show a high accumulation of NFTs and Aβ plaques. However, it should be noted that simply the presence of these plaques and tangles is no longer considered a definitive diagnosis of Alzheimer's disease, as postmortem brains of healthy individuals also show this morphology (80). Brain histology from cognitively healthy individuals 85 years and older typically shows at least some amount of plaque and tangle formation (129). Therefore, many clinicians believe that cognitive decline in Alzheimer's disease occurs much sooner than normal age-related dementia. Thus, early-age onset of dementia-like symptoms places these individuals at a high risk for developing the disease (37).
Both Alzheimer's disease and normal brain aging show a decline in protein turnover (62). This has been partially attributed to the increased oxidative stress that arises in the brain with age, due to the brain's already high metabolic rate, and the formation of potentially toxic byproducts from neurotransmitter signaling (18, 83). Concurrently, Proteasome activity declines, with mild inhibition causing up to 5% of total cellular proteins to aggregate, with a small portion showing heavy amounts of cross-linking (32, 82). This indicates that low levels of Proteasome inhibition are capable of triggering many of the phenotypes of neuronal aging, but can be rescued, on increased expression of various proteasomal subunits (33). In the Alzheimer's disease state, Proteasome inhibition is more pronounced, potentially due to the rise in protein oxidation, and Proteasome activity declines significantly below the age-related norms. Clearly, future work will be critical in deciphering whether the differences in Proteasome dysfunction during normal aging versus Alzheimer's disease are outcomes or contributors to the decline of neuronal function.
Conclusions
Alzheimer's disease is one of the most common neurodegenerative diseases whose prevalence is projected to continue increase significantly due to the world's aging population. As current treatments are able to do little to alleviate the disease or slow its progression, researchers must explore alternative approaches beyond those that target the disease characteristics: Aβ accumulation, Tau hyperphosphorylation, and oxidative stress. Since protein homeostasis is critical in the disease progression, this pathway offers a new avenue for exploration. Much work is still necessary to understand how the normal neuronal circuitry loses regulation of proteostasis.
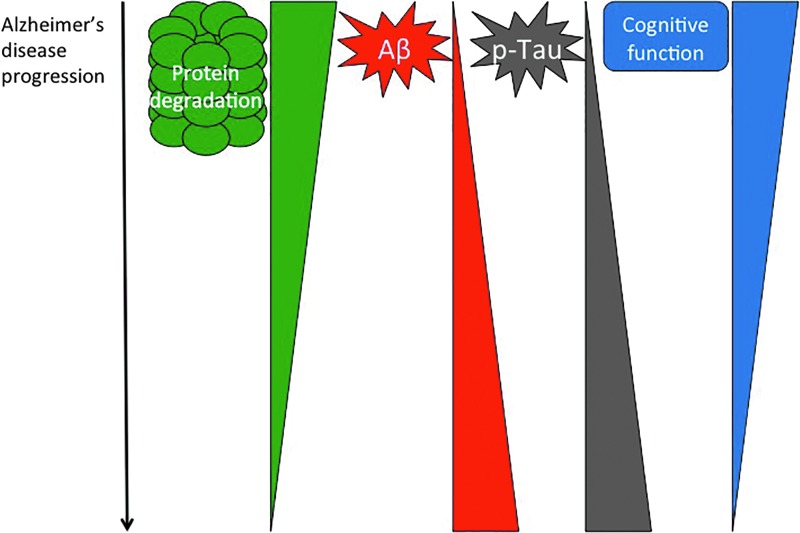
In many ways, Proteasome activity now appears to be at the tipping point for disease development. Regulated proteostasis is critical for proper cellular function, and loss of Proteasome activity is a feature of Alzheimer's disease pathology. Both amyloid accumulation and Tau hyperphosphorylation appear to negatively impact Proteasome function, placing it at a key point in disease progression (Fig. 8). Much prior work has focused on characterizing the dysregulation of the Proteasome in the disease state. However, we are only just beginning to unravel the underlying mechanism(s) behind Proteasome dysfunction. Such mechanistic understanding will be critical in unraveling Alzheimer's disease initiation and development, and in the targeted exploration of disease prevention or effective therapies.
FIG. 8.
Protein degradation and Alzheimer's disease. There is an inverted correlation between protein degradation mechanisms for protein quality control (or proteostasis) and Alzheimer's disease progression. Although Aβ and Tau increase their pathology, proteasome decreases its activity, perhaps allowing formation of the plaques and NFTs that are characteristic of this neurological disorder. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- Aβ
amyloid-β
- apoE
apolipoprotein E
- APP
amyloid precursor protein
- ARE
antioxidant response element
- ATP
adenosine triphosphate
- BACE-1
β-site APP-cleaving enzyme
- cAMP
cyclic AMP
- CHIP
C terminus of heat shock protein 70 interacting protein
- E1
ubiquitin-activating enzyme
- E2
ubiquitin-conjugating enzyme
- E3
ubiquitin ligase enzyme
- EpRE
electrophile responsive element
- ETC
electron transport chain
- GSK3β
glycogen synthase kinase 3β
- HSP
heat shock protein
- IP
immunoproteasome
- MTS
mitochondrial targeting sequences
- NFTs
neurofibrillary tangles
- Nrf2
nuclear factor erythroid 2-like factor 2
- RCAN1
regulator of Calcineurin gene
- TIM
mitochondrial transferase
- TOM
outer mitochondrial transferase
Acknowledgments
The authors were supported by Grant No. ES003598 from the National Institute of Environmental Health Sciences, of the U.S. National Institutes of Health, to K.J.A.D.
References
- 1.Alavez S, Vantipalli MC, Zucker DJ, Klang IM, and Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472: 226–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida CG, Takahashi RH, and Gouras GK. Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci 26: 4277–4288, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer A. Über eine eigenartige erkrankung der hirnrinde. Allgemeine Zeitschrift für Psychiatrie und psychiatrisch-gerichtliche Medizin 64: 146–148, 1907 [Google Scholar]
- 4.Angeles A, Fung G, and Luo H. Immune and non-immune functions of the immunoproteasome. Front Biosci 17: 1904–1916, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Aso E, Lomoio S, Lopez-Gonzalez I, Joda L, Carmona M, Fernandez-Yague N, Moreno J, Juves S, Pujol A, Pamplona R, Portero-Otin M, Martin V, Diaz M, and Ferrer I. Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer's disease. Brain Pathol 22: 636–653, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 9: 208–245, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimers Dement 10: e47–e92, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Badia MC, Lloret A, Giraldo E, Dasi F, Olaso G, Alonso MD, and Vina J. Lymphocytes from young healthy persons carrying the ApoE4 allele overexpress stress-related proteins involved in the pathophysiology of Alzheimer's disease. J Alzheimers Dis 33: 77–83, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Ballatore C, Lee VMY, and Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8: 663–672, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Baumeister W, Walz J, Zühl F, and Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92: 367–380, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Layfield R, Sheppard PW, Ebendal T, Usoskin D, Lowe J, and Mayer RJ. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci 28: 8189–8198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, Morse KM, Metcalfe HM, Skalska J, and Andreeff M. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood 119: 3321–3329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharyya S, Yu H, Mim C, and Matouschek A. Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol 15: 122–133, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bota DA. and Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Bota DA, Ngo JK, and Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic Biol Med 38: 665–677, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Bota DA, Van Remmen H, and Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett 532: 103–106, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, and Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33: 95–130, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Butterfield DA. and Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev 122: 945–962, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Cadenas E. and Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Cardoso SM, Santos S, Swerdlow RH, and Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J 15: 1439–1441, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, and Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J 19: 2040–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, and Smith MA. Role of mitochondrial dysfunction in Alzheimer's disease. J Neurosci Res 70: 357–360, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, and Gonos ES. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J 29: 611–622, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen SK, Maenhaut-Michel G, Mine N, Gottesman S, Gerdes K, and Van Melderen L. Overproduction of the Lon protease triggers inhibition of translation in Escherichia coli: involvement of the yefM-yoeB toxin-antitoxin system. Mol Microbiol 51: 1705–1717, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Chun W. and Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci 12: 733–756, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ciechanover A. and Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40: 427–446, 2003 [DOI] [PubMed] [Google Scholar]
- 27.This reference has been deleted.
- 28.Coskun PE, Beal MF, and Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A 101: 10726–10731, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford DR, Leahy KP, Abramova N, Lan L, Wang Y, and Davies KJ. Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch Biochem Biophys 342: 6–12, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie 83: 301–310, 2001 [DOI] [PubMed] [Google Scholar]
- 31.de la Torre JC. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med 370: 1459–1460, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Ding Q, Dimayuga E, Martin S, Bruce-Keller AJ, Nukala V, Cuervo AM, and Keller JN. Characterization of chronic low-level proteasome inhibition on neural homeostasis. J Neurochem 86: 489–497, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, and Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett 546: 228–232, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Doody RS, Farlow M, Aisen PS; Alzheimer's Disease Cooperative Study Data Analysis and Publication Committee. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med 370: 1460, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, and Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci 100: 721–726, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drubin DG. and Kirschner MW. Tau protein function in living cells. J Cell Biol 103: 2739–2746, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, and Galasko D. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 9: 1118–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Ermak G, Harris CD, Battocchio D, and Davies KJ. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta. FEBS J 273: 2100–2109, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ermak G, Pritchard MA, Dronjak S, Niu B, and Davies KJ. Do RCAN1 proteins link chronic stress with neurodegeneration? FASEB J 25: 3306–3311, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, and Sethuraman G. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement 8: 261–271, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Ferrington DA. and Gregerson DS. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci 109: 75–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feuillette S, Blard O, Lecourtois M, Frébourg T, Campion D, and Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res 80: 400–405, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78: 477–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, and de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet 9: 1681–1690, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, and Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 100: 10032–10037, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson GE, Sheu KFR, and Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105: 855–870, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Gillardon F, Kloß A, Berg M, Neumann M, Mechtler K, Hengerer B, and Dahlmann B. The 20S proteasome isolated from Alzheimer's disease brain shows post-translational modifications but unchanged proteolytic activity. J Neurochem 101: 1483–1490, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Gottesman S. and Maurizi MR. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev 56: 592–621, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goure WF, Krafft GA, Jerecic J, and Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer's disease immunotherapeutics. Alzheimers Res Ther 6: 42, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, and Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261: 6084–6089, 1986 [PubMed] [Google Scholar]
- 52.Grune T, Botzen D, Engels M, Voss P, Kaiser B, Jung T, Grimm S, Ermak G, and Davies KJ. Tau protein degradation is catalyzed by the ATP/ubiquitin-independent 20S proteasome under normal cell conditions. Arch Biochem Biophys 500: 181–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, and Davies KJ. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med 51: 1355–1364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grune T, Merker K, Sandig G, and Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun 305: 709–718, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Hamdane M, Sambo AV, Delobel P, Begard S, Violleau A, Delacourte A, Bertrand P, Benavides J, and Buee L. Mitotic-like tau phosphorylation by p25-Cdk5 kinase complex. J Biol Chem 278: 34026–34034, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Han DH, Na HK, Choi WH, Lee JH, Kim YK, Won C, Lee SH, Kim KP, Kuret J, Min DH, and Lee MJ. Direct cellular delivery of human proteasomes to delay tau aggregation. Nat Commun 5: 5633, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Hanger DP, Anderton BH, and Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15: 112–119, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, and Ankarcrona M. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci 105: 13145–13150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardy JA. and Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 256: 184–185, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Harris C, Ermak G, and Davies K. Multiple roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product calcipressin 1 (or RCAN1) in disease. Cell Mol Life Sci CMLS 62: 2477–2486, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris CD, Ermak G, and Davies KJ. RCAN1-1L is overexpressed in neurons of Alzheimer's disease patients. FEBS J 274: 1715–1724, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto M, Rockenstein E, Crews L, and Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med 4: 21–35, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Hassan WM, Merin DA, Fonte V, and Link CD. AIP-1 ameliorates beta-amyloid peptide toxicity in a Caenorhabditis elegans Alzheimer's disease model. Hum Mol Genet 18: 2739–2747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayashi H, Campenot RB, Vance DE, and Vance JE. Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cgamma1 and inhibition of calcineurin. J Biol Chem 284: 29605–29613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hebert LE, Weuve J, Scherr PA, and Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80: 1778–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet 30: 405–439, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, and Ron D. Transmission of cell stress from endoplasmic reticulum to mitochondria enhanced expression of Lon protease. J Cell Biol 157: 1151–1160, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu J, Bianchi F, Ferguson M, Cesario A, Margaritora S, Granone P, Goldstraw P, Tetlow M, Ratcliffe C, Nicholson AG, Harris A, Gatter K, and Pezzella F. Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene 24: 1212–1219, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Hubbard VM, Valdor R, Macian F, and Cuervo AM. Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 13: 21–35, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Iqbal K, Liu F, Gong CX, Alonso Adel C, and Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118: 53–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.This reference has been deleted.
- 72.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, and Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13: 159–170, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Jansen AH, Reits EA, and Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci 7: 73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaramillo MC. and Zhang DD. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev 27: 2179–2191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarrett JT. and Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73: 1055–1058, 1993 [DOI] [PubMed] [Google Scholar]
- 76.Kalaria RN. Microglia and Alzheimer's disease. Curr Opin Hematol 6: 15, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Kanamaru T, Kamimura N, Yokota T, Iuchi K, Nishimaki K, Takami S, Akashiba H, Shitaka Y, Katsura K, and Kimura K. Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer's disease. Neurosci Lett 587: 126–131, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, and Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736, 1987 [DOI] [PubMed] [Google Scholar]
- 79.Keck S, Nitsch R, Grune T, and Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem 85: 115–122, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, and Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol 36: 2376–2391, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Keller JN, Hanni KB, and Markesbery WR. Impaired proteasome function in Alzheimer's disease. J Neurochem 75: 436–439, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Keller JN, Hanni KB, and Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev 113: 61–70, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Keller JN. and Mattson MP. Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci 9: 105–116, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, and Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10: 719–726, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Köpke E, Tung Y-C, Shaikh S, Alonso AdC, Iqbal K, and Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268: 24374–24384, 1993 [PubMed] [Google Scholar]
- 86.Kozauer N. and Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med 368: 1169–1171, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, Mayer RJ, and Layfield R. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc Natl Acad Sci 97: 9902–9906, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laske C. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med 370: 1459, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Lilienbaum A. Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol 4: 1–26, 2013 [PMC free article] [PubMed] [Google Scholar]
- 90.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, and McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol 156: 445–453, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Liu K, Doms RW, and Lee VMY. Glu11 site cleavage and N-terminally truncated Aβ production upon BACE overexpression. Biochemistry 41: 3128–3136, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Liu YH, Wei W, Yin J, Liu GP, Wang Q, Cao FY, and Wang JZ. Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol Aging 30: 1949–1961, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Lloret A, Badia MC, Giraldo E, Ermak G, Alonso MD, Pallardo FV, Davies KJ, and Vina J. Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer's disease. J Alzheimers Dis 27: 701–709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lloret A, Fuchsberger T, Giraldo E, and Vina J. Molecular mechanisms linking amyloid beta toxicity and Tau hyperphosphorylation in Alzheimers disease. Free Radic Biol Med 83: 186–191, 2015 [DOI] [PubMed] [Google Scholar]
- 95.Lopez Salon M, Pasquini L, Besio Moreno M, Pasquini JM, and Soto E. Relationship between beta-amyloid degradation and the 26S proteasome in neural cells. Exp Neurol 180: 131–143, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lovestone S. and Reynolds C. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience 78: 309–324, 1997 [DOI] [PubMed] [Google Scholar]
- 98.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, and Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 304: 448–452, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Maezawa I, Jin LW, Woltjer RL, Maeda N, Martin GM, Montine TJ, and Montine KS. Apolipoprotein E isoforms and apolipoprotein AI protect from amyloid precursor protein carboxy terminal fragment-associated cytotoxicity. J Neurochem 91: 1312–1321, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Marambaud P, Zhao H, and Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem 280: 37377–37382, 2005 [DOI] [PubMed] [Google Scholar]
- 101.May MJ. and Ghosh S. Signal transduction through NF-κB. Immunol Today 19: 80–88, 1998 [DOI] [PubMed] [Google Scholar]
- 102.Mazanetz MP. and Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6: 464–479, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Metcalfe MJ, Huang Q, and Figueiredo-Pereira ME. Coordination between proteasome impairment and caspase activation leading to TAU pathology: neuroprotection by cAMP. Cell Death Dis 3: e326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meziane H, Dodart J-C, Mathis C, Little S, Clemens J, Paul S, and Ungerer A. Memory-enhancing effects of secreted forms of the β-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci 95: 12683–12688, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miquel J, Economos A, Fleming J, and Johnson J. Mitochondrial role in cell aging. Exp Gerontol 15: 575–591, 1980 [DOI] [PubMed] [Google Scholar]
- 106.Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, and Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer's disease brains. Neurobiol Aging 27: 54–66, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Morawe T, Hiebel C, Kern A, and Behl C. Protein homeostasis, aging and Alzheimer's disease. Mol Neurobiol 46: 41–54, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mori H, Kondo J, and Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 235: 1641–1644, 1987 [DOI] [PubMed] [Google Scholar]
- 109.Mota SI, Costa RO, Ferreira IL, Santana I, Caldeira GL, Padovano C, Fonseca AC, Baldeiras I, Cunha C, Letra L, Oliveira CR, Pereira CMF, and Rego AC. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer's disease. Biochim Biophys Acta 1852: 1428–1441, 2015 [DOI] [PubMed] [Google Scholar]
- 110.Munoz L. and Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer's disease. Neuropharmacology 58: 561–568, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Myeku N, Clelland CL, Emrani S, Kukushkin NV, Yu WH, Goldberg AL, and Duff KE. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 22: 46–53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nandi D, Jiang H, and Monaco JJ. Identification of MECL-1 (LMP-10) as the third IFN-gamma-inducible proteasome subunit. J Immunol 156: 2361–2364, 1996 [PubMed] [Google Scholar]
- 113.Ngo JK. and Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med 46: 1042–1048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ngo JK, Pomatto LC, Bota DA, Koop AL, and Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J Gerontol A Biol Sci Med Sci 66: 1178–1185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ngo JK, Pomatto LC, and Davies KJ. Upregulation of the mitochondrial Lon protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol 1: 258–264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, and LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J Neurosci 28: 12163–12175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh S, Hong HS, Hwang E, Sim HJ, Lee W, Shin SJ, and Mook-Jung I. Amyloid peptide attenuates the proteasome activity in neuronal cells. Mech Ageing Dev 126: 1292–1299, 2005 [DOI] [PubMed] [Google Scholar]
- 118.Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, and Disterhoft JF. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron 41: 27–33, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Orre M, Kamphuis W, Dooves S, Kooijman L, Chan ET, Kirk CJ, Dimayuga Smith V, Koot S, Mamber C, Jansen AH, Ovaa H, and Hol EM. Reactive glia show increased immunoproteasome activity in Alzheimer's disease. Brain 136: 1415–1431, 2013 [DOI] [PubMed] [Google Scholar]
- 120.Paganini-Hill A. and Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 140: 256–261, 1994 [DOI] [PubMed] [Google Scholar]
- 121.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, and Cowburn RF. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis 3: 41–48, 2001 [DOI] [PubMed] [Google Scholar]
- 122.Perez RG, Zheng H, Van der Ploeg LH, and Koo EH. The β-amyloid precursor protein of Alzheimer's disease enhances neuron viability and modulates neuronal polarity. J Neurosci 17: 9407–9414, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R, and Butterfield DA. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics Clin Appl 3: 682–693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pétrilli V, Dostert C, Muruve DA, and Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19: 615–622, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, and Prihar G. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13: 703–714, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Pickering A, Koop A, Teoh C, Ermak G, Grune T, and Davies K. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J 432: 585–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pickering AM, Linder RA, Zhang H, Forman HJ, and Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem 287: 10021–10031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinisto L, Halonen P, and Kontula K. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med 333: 1242–1247, 1995 [DOI] [PubMed] [Google Scholar]
- 129.Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinistö L, Notkola IL, Verkkoniemi A, Viramo P, Juva K, and Haltia M. Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology 26: 76–82, 2006 [DOI] [PubMed] [Google Scholar]
- 129a.Pomatto L.C-D, Raynes R, and Davies KJA. The peroxisomal Lon protease LonP2 in aging and disease: functions and comparisons with mitochondrial Lon protease LonP1. Biol Rev Camb Philos Soc 2016. [Epub ahead of print]; DOI: 10.1111/brv.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiu JH, Asai A, Chi S, Saito N, Hamada H, and Kirino T. Proteasome inhibitors induce cytochrome c–caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J Neurosci 20: 259–265, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Querfurth HW. and LaFerla FM. Alzheimer's disease. N Engl J Med 362: 329–344, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Quirós PM, Español Y, Acín-Pérez R, Rodríguez F, Bárcena C, Watanabe K, Calvo E, Loureiro M, Fernández-García MS, and Fueyo A. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep 8: 542–556, 2014 [DOI] [PubMed] [Google Scholar]
- 133.Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, and Jordan-Sciutto KL. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66: 75–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer's disease. J Neurochem 96: 1–13, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Reddy PH. and Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev 49: 618–632, 2005 [DOI] [PubMed] [Google Scholar]
- 136.Reddy PH. and Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14: 45–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reeg S, Jung T, Castro JP, Davies KJA, Henze A, and Grune T. The molecular chaperone Hsp70 promotes the selective proteolytic removal of oxidatively damaged proteins by the proteasome. Free Redic Biol Med 99: 153–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Regitz C, Dussling LM, and Wenzel U. Amyloid-beta (Abeta(1)(-)(4)(2))-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol Nutr Food Res 58: 1931–1940, 2014 [DOI] [PubMed] [Google Scholar]
- 139.Regitz C, Fitzenberger E, Mahn FL, Dussling LM, and Wenzel U. Resveratrol reduces amyloid-beta (Abeta)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur J Nutr 55: 741–747, 2016 [DOI] [PubMed] [Google Scholar]
- 140.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, and Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J 335 (Pt 3): 637–642, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reisberg B, Borenstein J, Salob SP, and Ferris SH. Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry 48: 9–15, 1987 [PubMed] [Google Scholar]
- 142.Resnick SM, Pham DL, Kraut MA, Zonderman AB, and Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 23: 3295–3301, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, and Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114: 121–130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med 47: 387–400, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, and Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem 275: 8719–8725, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Roy S, Zhang B, Lee VY, and Trojanowski J. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol 109: 5–13, 2005 [DOI] [PubMed] [Google Scholar]
- 147.Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, and Kawashima S. Dominant and differential deposition of distinct β-amyloid peptide species, Aβ N3 (pE), in senile plaques. Neuron 14: 457–466, 1995 [DOI] [PubMed] [Google Scholar]
- 148.Saitoh T, Sundsmo M, Roch J-M, Kimura N, Cole G, Schubert D, Oltersdorf T, and Schenk DB. Secreted form of amyloid β protein precursor is involved in the growth regulation of fibroblasts. Cell 58: 615–622, 1989 [DOI] [PubMed] [Google Scholar]
- 149.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, and Brashear HR. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med 370: 322–333, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salminen A, Haapasalo A, Kauppinen A, Kaarniranta K, Soininen H, and Hiltunen M. Impaired mitochondrial energy metabolism in Alzheimer's disease: impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Prog Neurobiol 131: 1–20, 2015 [DOI] [PubMed] [Google Scholar]
- 151.Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol 8: 447–453, 1998 [DOI] [PubMed] [Google Scholar]
- 152.Selkoe DJ. and Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Tox 43: 545–584, 2003 [DOI] [PubMed] [Google Scholar]
- 153.Shen L, Chen C, Yang A, Chen Y, Liu Q, and Ni J. Redox proteomics identification of specifically carbonylated proteins in the hippocampi of triple transgenic Alzheimer's disease mice at its earliest pathological stage. J Proteomics 123: 101–113, 2015 [DOI] [PubMed] [Google Scholar]
- 154.Shimura H, Schwartz D, Gygi SP, and Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 279: 4869–4876, 2004 [DOI] [PubMed] [Google Scholar]
- 155.Shringarpure R. and Davies KJ. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med 32: 1084–1089, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Shringarpure R, Grune T, and Davies K. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci 58: 1442–1450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh AK. and Pati U. CHIP stabilizes amyloid precursor protein via proteasomal degradation and p53-mediated trans-repression of beta-secretase. Aging Cell 14: 595–604, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, and Mills LR. Chronic exposure to sub-lethal beta-amyloid (Aβ) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells. J Neurochem 103: 1989–2003, 2007 [DOI] [PubMed] [Google Scholar]
- 159.Smith MA, Rottkamp CA, Nunomura A, Raina AK, and Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta 1502: 139–144, 2000 [DOI] [PubMed] [Google Scholar]
- 160.Stadtmueller BM. and Hill CP. Proteasome activators. Mol Cell 41: 8–19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stanhill A, Haynes CM, Zhang Y, Min G, Steele MC, Kalinina J, Martinez E, Pickart CM, Kong XP, and Ron D. An arsenite-inducible 19S regulatory particle-associated protein adapts proteasomes to proteotoxicity. Mol Cell 23: 875–885, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Stanyer L, Jorgensen W, Hori O, Clark JB, and Heales SJ. Inactivation of brain mitochondrial Lon protease by peroxynitrite precedes electron transport chain dysfunction. Neurochem Int 53: 95–101, 2008 [DOI] [PubMed] [Google Scholar]
- 163.Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging 28: 1465–1480, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta 1812: 1630–1639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tatsuta T. and Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27: 306–314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tseng BP, Green KN, Chan JL, Blurton-Jones M, and LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging 29: 1607–1618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turner PR, O'Connor K, Tate WP, and Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70: 1–32, 2003 [DOI] [PubMed] [Google Scholar]
- 168.Vilchez D, Saez I, and Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5: 5659, 2014 [DOI] [PubMed] [Google Scholar]
- 169.Viña J. and Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis 20 Suppl 2: S527–S533, 2010 [DOI] [PubMed] [Google Scholar]
- 170.Viña J, Lloret A, Valles SL, Borras C, Badia MC, Pallardo FV, Sastre J, and Alonso MD. Mitochondrial oxidant signalling in Alzheimer's disease. J Alzheimers Dis 11: 175–181, 2007 [DOI] [PubMed] [Google Scholar]
- 171.Wang N, Gottesman S, Willingham MC, Gottesman MM, and Maurizi MR. A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proc Natl Acad Sci 90: 11247–11251, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.West MJ, Coleman PD, Flood DG, and Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344: 769–772, 1994 [DOI] [PubMed] [Google Scholar]
- 173.Whitby FG, Masters EI, Kramer L, Knowlton JR, Yao Y, Wang CC, and Hill CP. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature 408: 115–120, 2000 [DOI] [PubMed] [Google Scholar]
- 174.Wong PC, Cai H, Borchelt DR, and Price DL. Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci 5: 633–639, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, and Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9: 453–457, 2003 [DOI] [PubMed] [Google Scholar]
- 176.Yamazaki H, Tanji K, Wakabayashi K, Matsuura S, and Itoh K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol Int 65: 210–219, 2015 [DOI] [PubMed] [Google Scholar]
- 177.Yang L-B, Lindholm K, Yan R, Citron M, Xia W, Yang X-L, Beach T, Sue L, Wong P, and Price D. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 9: 3–4, 2003 [DOI] [PubMed] [Google Scholar]
- 178.Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, and Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science 245: 417–420, 1989 [DOI] [PubMed] [Google Scholar]
- 179.Zhang H, Davies KJ, and Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88: 314–336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, and Biswal S. Loss of Kelch-Like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther 9: 336–346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao X. and Yang J. Amyloid-beta peptide is a substrate of the human 20S proteasome. ACS Chem Neurosci 1: 655–660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, and Conner MW. β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81: 525–531, 1995 [DOI] [PubMed] [Google Scholar]