Abstract
This work establishes the zebrafish embryo model for ionizing radiation (IR) modifier research and also evaluates the protective effect of l-alpha glycerylphosphorylcholine (GPC). Embryos were exposed to a single-fraction whole-body gamma irradiation (5, 10, 15, and 20 Gy) at different postfertilization time points and were serially assessed for viability and macro- and micromorphologic abnormalities. After toxicity evaluation, 194 μM of GPC was added for certain groups with 3-h incubation before the radiation. Nuclear factor kappa B (NF-κB) and interleukin-1β (IL-1β) expression changes were measured using quantitative real-time polymerase chain reaction. A higher sensitivity could be observed at earlier stages of the embryogenesis. The lethal dose (LD50) for 6 hours postfertilization (hpf) embryos was 15 Gy and for 24 hpf was 20 Gy on day 7, respectively. GPC administration resulted in a significant improvement in both the distortion rate and survival of the 24 hpf embryos. Qualitative evaluation of the histological changes confirmed the protective effect of GPC. IL-1β and NF-κB overexpression due to 10 Gy irradiation was also reduced by GPC. GPC exhibited promising radioprotective effects in our zebrafish embryo model, decreasing the irradiation-induced morphological damage and lethality with significant reduction of IR-caused pro-inflammatory activation.
Keywords: : zebrafish, radioprotection, radiobiology, NFκB, L-alpha glycerylphosphorylcholine
Introduction
Radiation therapy is one of the main pillars of complex cancer management. Radiation biological research has led to a significant improvement in effective cancer cell killing and the preservation of normal tissue function, resulting in an improvement in the therapeutic index. Cell cultures and small mammalian animals are well-established widely used models for preclinical investigations. In the era of rapid development of highly selective and combinative radiation technology, there is a need for a new in vivo model for studying the effects of different radiation qualities and radiation modifiers in a complex organism.1
Zebrafish (Danio rerio) embryos have recently been introduced as a novel vertebrate preclinical research model for various human diseases and treatments as their genome is remarkably similar to the human genome and they possess several other advantages.2 The zebrafish model represents an important step between in vitro cell culture experiments and small animal studies. These small Asian fishes can be rapidly bred in large numbers and are easy to maintain at a relatively low cost in the laboratory. They are vertebrate organisms, and therefore, complex biological processes can be investigated. Zebrafish are excellent tools for experimental human cancer research as many key genes involved in cell cycle, oncogenesis, tumor suppression, and DNA repair are conserved between the two species.3 Embryogenesis occurs outside of the body and genetic manipulation can be easily performed. Embryo development is extremely rapid during the first few days postfertilization while the embryos and larvae are transparent, giving the possibility to study the in vivo organ development and perturbations.4 These features, combined with easy handling in experimental conditions, enable the assessment of ionizing radiation (IR) effects on zebrafish development5 and for studying radiation response modifiers. The combination strategy, using radiation in conjunction with radiation modifiers, is designed to make the tumor cells more susceptible to radiation damage and/or to selectively protect the healthy tissues surrounding the tumor.
Phosphatidylcholine (PC), an essential lipid component of all kinds of cell membranes and blood proteins, has emerged as a promising tool for normal cell protection. PC serves in the central nervous system (CNS) as the main source of choline, an essential nutrient and precursor to the neurotransmitter acetylcholine, and has an enhanced role in mucosal cells, which maintain lung function and gastrointestinal health.6 GPC (l-alpha glycerylphosphorylcholine) is a water soluble deacylated PC intermediate, which has been developed and studied as a potent anti-inflammatory and neuroprotective agent. Research suggests that GPC positively enhances the inflammatory response and potentially reduces the tissue damage caused by IR.7 GPC can be hydrolyzed to choline and possibly used for the resynthesis of PC.8
Brownawell et al.9 investigated the toxicity of GPC in rodents by examining the acute, subacute, and late effects of different GPC doses from 100 mg/kg bw to 1000 mg/kg bw in rats. The acute lethal dose of intravenously administered GPC was 2000 mg/kg bw, and the intraperitoneal dosing of rats produced mortality starting at 1500 mg/kg bw, while oral administration resulted in mortality from 10,000 mg/kg bw. In subchronic or chronic studies and doses of 100 and 300 mg/kg bw, GPC did not alter the behavior, body weight, hematology, or clinical chemistry of rats and did not produce any signs of general toxicity. In hemorrhagic shock experiments (a prototype of systemic IR), concentrations of GPC significantly lower than the well tolerated dose caused rats to recover to baseline levels after 24 h.10
GPC has proved effective against the loss of the membrane function in CNS injuries11,12 and was previously tested as a centrally acting parasympathomimetic drug in dementia disorders and acute cerebrovascular diseases.13–15 GPC, after oral administration, has been shown to cross the blood–brain barrier and reach the CNS, where it is incorporated into the phospholipid fraction of the neuronal plasma membrane and microsomes.16 GPC was found to inhibit the transfer process of bifunctional phospholipid transfer protein.17 Furthermore, GPC has also been proven to protect membranes from oxidation and is able to improve membrane function after traumatic damage.12,18
GPC has exhibited protective activity in different experimental models against damage occurring due to inflammatory reaction.19 Experiments of Tőkés et al.20 and Ghyczy et al.21 have shown PC to be effective in stopping the production of reactive oxygen species both in vitro and in vivo and may exhibit anti-inflammatory properties.12,18 GPC may therefore be a possible future candidate for protection of the normal tissues exposed to incident IR during cancer radiotherapy. In a rat focal-brain irradiation model, GPC was proven to alleviate the radiation-induced decline in cognitive function and to decrease the microscopic brain damage.22,23
The primary aim of the research was to establish the zebrafish (D. rerio) embryo model for radiobiology research especially for the investigation of the effects of agents, which may modify (protect or sensitize) the IR. One of the emerging radioprotectors, the GPC, was tested in this work. Research performed included detection of survival, morphological, and histological changes and acute cytokine response. Radiation-induced damage at the membrane and DNA level can be modified either using sensitizer or protective agent. In the case of cancer therapy, both the selective tumor cell sensitizer and normal tissue radioprotective agent could enhance the efficacy of radiotherapy (i.e., the therapeutic index could be improved).
Materials and Methods
The experimental protocol was approved by the Ethics Committee for the Protection of Animals in Scientific Research at the University of Szeged (XXXII./1838.2015) and followed the National Institutes of Health (Bethesda, MD) guidelines on the care and use of laboratory animals.
Embryo harvesting and maintenance
Laboratory-bred strain (AB) of zebrafish (D. rerio) was purchased from the Department of Aquaculture, Szent István University, Gödöllő. Adult zebrafish were segregated by sex, fed twice a day with dry fish food supplemented with freshly hatched Artemia nauplii, and kept at 27.5°C on a 14-h light/10-h dark cycle. Wild-type adult fish were mated in embryo collection tanks in the afternoon, and the eggs were spawning the following morning. Viable embryos were washed and sorted by microscopic observation (1 embryo/well of standard 96-well polystyrene microplates in 250 μL embryo medium) at the one- to two-cell stages and maintained under normoxic conditions at 27.5°C. The embryo medium in each well was changed at 24, 48, 72, and 120 hours postfertilization (hpf).
Irradiation setup and initial testing
A Teragam K-01 (SKODA UJP) cobalt unit was used (average energy 1.25 MeV and source isocenter distance 80 cm) to irradiate embryos individually dispensed in 96-well plates. Irradiation time correction factors were used to compensate for the decay of the cobalt-60 source.
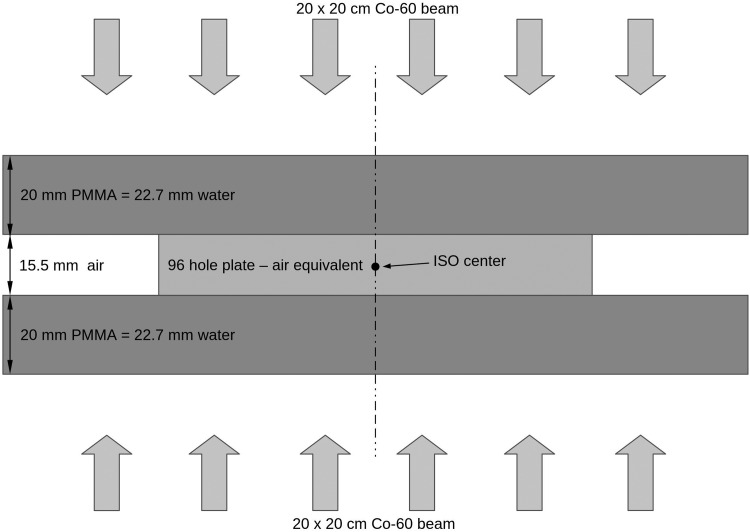
The plates were placed between two polymethyl methacrylate (PMMA) slabs (thickness 2 cm) to compensate for the built-up effect and to ensure the homogenous radiation exposure. The isocenter was positioned in the plates’ geometrical centers. Half of the planned dose were delivered by a 20 × 20 cm beam downward (gantry angle 0°), while the other half with an identical beam upward (gantry angle 180°) to maximize the dose homogeneity. This isocentric setup ensured that the dose uncertainty originating from the movements of the embryos could be eliminated (Fig. 1). The delivered dose was changed from 5 to 20 Gy in 5 Gy increments for each group of embryos at 6 hpf and at 24 hpf, respectively, to establish the age-related dose–response survival curves.
FIG. 1.
The radiation exposure experimental setup.
Thereafter, the 24 hpf embryos were selected for studying the radiation modifier effect at 20 Gy dose level, which proved to be the LD50. The highest nonlethal dose of 10 Gy was determined for the subsequent molecular investigations.
Toxicity assessment of GPC
Zebrafish embryos at 24 hpf were exposed to GPC (25 mg dissolved in 1 mL single distilled water; Lipoid GmbH). A toxicity test was conducted using different dilutions in the absence of radiation. GPC (194.0, 243.0, 324.0, 468.0, 972.0, and 1944 μM/L from 9720 μM/L stock solution) was added to embryos for 3 h followed by a change in the medium and then embryos were maintained at 28.5°C for 168 h to define survival and morphological changes.
Investigations of the effects of radiation on GPC pretreated embryos
The GPC at 194 μM/L dose level was incubated for 3 h and the embryos were exposed to 20 Gy at 24 hpf for survival analysis and to 10 Gy at 24 hpf for molecular examination, divided into four groups as follows: Control, GPC treated, irradiated, and GPC treated followed by irradiation.
Morphology and survival analysis
Individual embryo was continually assessed for survival in each experiment at 24-h intervals from the point of fertilization until 168 hpf. Embryos were observed without any manipulation in place in the microplates. Viability was analyzed with light transmission using a Nikon Eclipse TS100 inverted microscope (Nikon Americas, Inc.) at 10 × , 20 × magnification and representative images were acquired at 10 × magnification using a Nikon Coolpix 4500 (4.0 mega pixels 4 × zoom) camera. Survival was calculated as a percentage of viable embryos to the total number of embryos exposed in each treatment group over time. The reported results represent the mean of at least two replicated experiments. The criterion of embryonic survival was the presence of cardiac contractility. Similarly, morphology was assessed visually and photo documented. The size and shape of the embryo, skull, spine, and tail, the development of the different organs (eye, brain, and midline), yolk sac resorption, and pericardial edema were evaluated daily in the proportion of the living embryos.
Histopathology evaluation
For histological assessments, the groups of control and treated embryos were sacrificed after 7 days of observation, were placed in 1:100 dilution of 4 mg/mL tricaine methanesulfonate (Sigma-Aldrich®), then fixed by immersion (4% paraformaldehyde 3 days), and embedded in paraffin. Transverse whole-body sections (4 μm thickness) were taken and stained with hematoxylin and eosin.
Sections were analyzed using a Zeiss Axio Imager Z1 light microscope (Zeiss EC Plan Neofluar 5 × /0.16, 10 × /0.3, 20 × /0.5 M27) at 5 × to 20 × magnification, and photomicrographs were taken using an AxioCam MR5 camera equipment. At least 15 embryos from each treatment group were observed by two independent investigators.
RNA isolation, reverse transcription, and quantitative polymerase chain reaction
The inflammatory cytokine level was measured using three different 20 embryos/treatment group. The total RNA extraction was isolated by TRIzol (Invitrogen) using a homogenizer (IKA, T10 digital ULTRA-TURRAX). Chloroform (200 μL) was then added to the homogenized embryo tissue. RNA was precipitated by adding isopropanol (500 μL; Sigma-Aldrich), then the tubes were gently rotated, to ensure mixing, and then incubated for 10 min at room temperature. After centrifugation (12,000 g, 20 min, 4°C), the supernatant was removed and washed in 1 mL 75% ethanol (Reanal). The remaining material was dissolved in 22 μL RNAse-free water. RNA concentration was measured by the MaestroNano spectrophotometer. cDNA was synthetized from 1 μg total RNA with random hexamer primers using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer's instructions. Real-time polymerase chain reaction (PCR) was performed using a CFX96 Real Time System (Bio-Rad) to detect changes in mRNA expression, using 4 μL of diluted cDNA template mixed with 1 μL of each primer and 10 μL of SYBR Green PCR Master mix (Applied Biosystems) at a final volume of 20 μL with a thermal cycle (95°C 2 min) followed by 40 amplification cycles (95°C 10 s then 60°C 30 s).
Interleukin-1β (IL-1β) and nuclear factor kappa B (NF-κB) levels were measured using sequence-specific TaqMan gene expression assays (Life Technologies) with 18S rRNA used a preoptimized primer and probe assay (Applied Biosystems) for endogenous control. Each PCR reaction was repeated and the relative mRNA level was calculated by the 2−ΔΔCT method.24
Statistical analysis
All values are reported as standard error of mean (SEM). For survival curves, Kaplan–Meier analysis was used with SEM error bars. The averages of two groups were compared with one-way analysis of variance (ANOVA). Statistical significance was defined at p < 0.05.
Data assessment of the GPC radioprotection effects is performed using the Cox regression using the R statistical program language (R 3.2.2 for Windows).
For inflammatory cytokine measurement evaluation, data analysis was performed with a commercial statistical software package (SigmaStat for Windows; Jandel Scientific). Nonparametric methods were used, and the differences between groups were subjected to Kruskal–Wallis one-way ANOVA on ranks, followed by Dunn's method for pairwise multiple comparison. In Figures 6 and 7, median values (M), 75th percentiles (p75), and 25th percentiles (p25) are given. p-Values <0.05 were considered significant. °p < 0.05 relative to the control group, #p < 0.05 relative to the GPC-treated group, and *p < 0.05 relative to GPC and irradiated group.
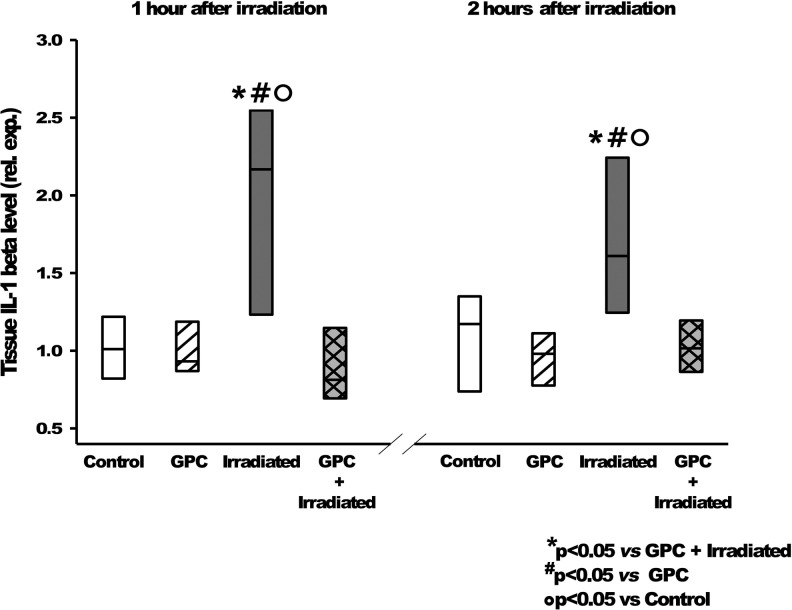
FIG. 6.
Tissue IL-1β level relative expression, 1 and 2 h after irradiation, shows the effects of the mock irradiation (white—control), GPC-treated mock irradiation (white hatching), irradiation (gray), and GPC treatment with irradiation (gray cross-hatching). *p < 0.05 relative to GPC-treated and irradiated group. #p < 0.05 relative to the GPC-treated group. °p < 0.05 relative to control group. IL-1β, interleukin-1β.
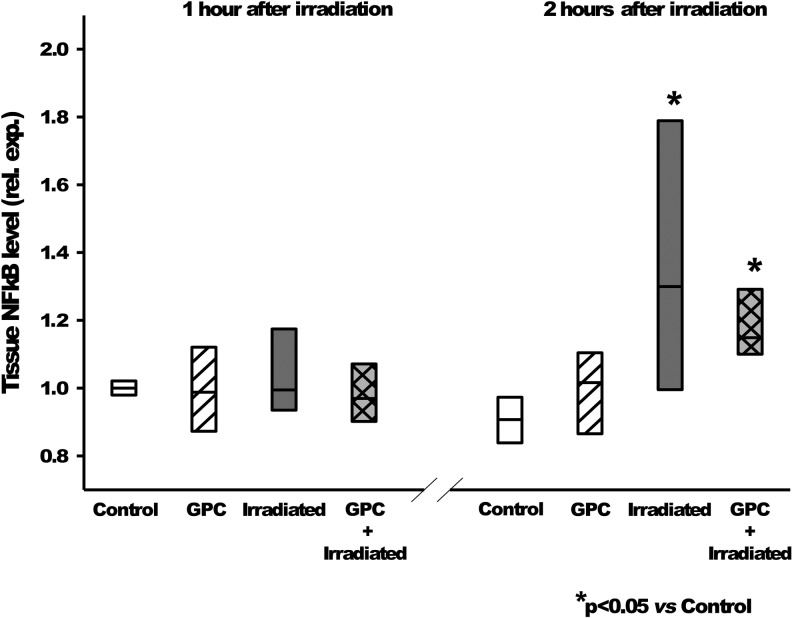
FIG. 7.
Tissue NF-κB level changes for the mock irradiation control group (white), the GPC-treated mock irradiated group (white hatching), the irradiated group (gray), and the GPC-treated irradiated group (gray cross-hatching). The tissue NF-κB level at 2 h after irradiation was significantly higher than the control group. *p < 0.05 relative to the control group. NF-κB, nuclear factor kappa B.
Results
Radiation dose–response relationship
Survival
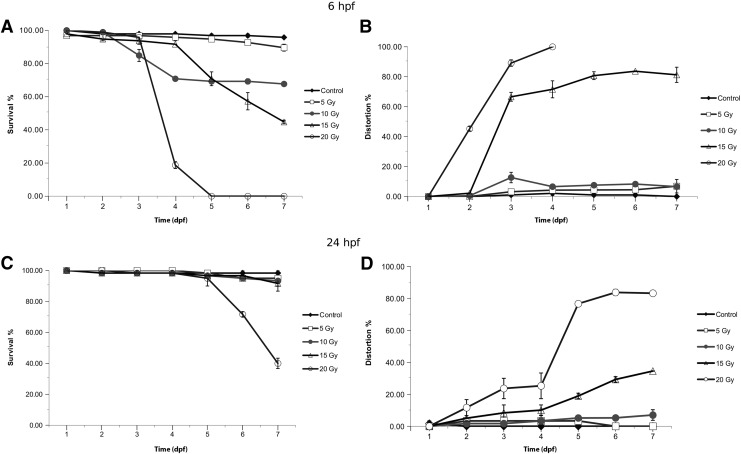
Daily assessment of embryo viability showed a strong inverse correlation with the radiation dose. During the 7-day observation period, no relevant mortality occurred at 5 Gy for embryos irradiated at 6 hpf, while more than 80% was already dead at 96 hpf at 20 Gy. From the beginning of day 4, a significant declination of the survival curve was seen at 10 and 15 Gy. At day 7, 70% of the embryos were alive in the 10 Gy group and 50% were alive in the 15 Gy irradiated group. Therefore, 15 Gy was established as the LD50 for embryos irradiated at 6 hpf. One hundred percent mortality occurred with 20 Gy irradiation at 6 hpf by day 5 and most died with severe morphologic abnormalities (Fig. 2A).
FIG. 2.
The effects of ionizing radiation on zebrafish development and survival. Zebrafish embryos at 6 or 24 hpf were exposed to 0, 5, 10, 15, or 20 Gy. (A, C) The proportion of viable to total zebrafish embryos at 6 and 24 hpf. 0 Gy (Control, ♦), 5 Gy (□), 10 Gy (●), 15 Gy (Δ), 20 Gy (◯), and survival were determined at 0, 24, 48, 72, 96, 120, 144, and 168 hpf. Morphology was also daily evaluated in each group (B, D). hpf, hours postfertilization.
Embryos at 24 hpf were less susceptible to radiation with 20 Gy shown to be LD50 at day 6–7. Lower doses (5–15 Gy) of irradiation caused no relevant mortality up to day 6. At day 7, 2%–7% of the embryos irradiated <20 Gy died with the remainder still viable (Fig. 2C).
Morphologic deterioration
Various distortions were detected after delivery of a dose ≥5 Gy, including a reduction in the body length, spine curvature, microcephaly, microphthalmia, micrognathia, pericardial edema, and the inhibition of yolk sac resorption.
The number of incidence of serious embryonic developmental defects was proportional to the radiation dose and age of the embryos similar to survival; major distortions occurred earlier if irradiation was performed at 6 hpf. On day 3, more than 60%–80% of the embryos, which had received 15–20 Gy, showed relevant developmental impairment. On day 4, the morbidity rate reached 100% at 20 Gy (Fig. 2B).
In contrast, at least 80% of the older embryos (24 hpf) had developed normally when exposed to IR. Relevant deterioration occurred during day 4 and increased thereafter at 15–20 Gy (Fig. 2D).
The effects of GPC on survival and distortion
GPC was added at different concentrations and incubation times to determine the GPC toxicity and tolerability. Zebrafish embryos at 24 hpf were exposed to increasing concentrations of GPC for 3–6 h, and the effects on survival and morphological changes were observed up to 7 days. Relevant toxicity was observed in the case of longer incubation time (>3 h) resulting in 3 h incubation being maintained throughout the study. The embryos treated with escalated doses of GPC during 3 h did not show any signs of toxicity; the viability and morphology were not affected.
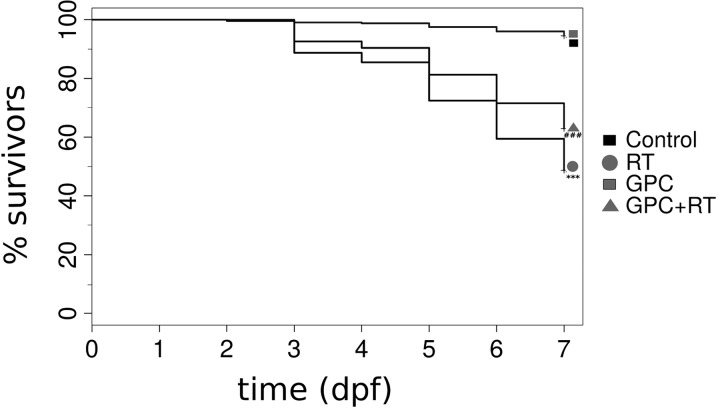
The pronounced protective effects were detected at each GPC dose level from 194 to 972 μM/L administered before IR treatment, but there were no perceptible differences between these concentrations. Both the GPC-treated and control groups were viable at day 7. Embryos irradiated with 20 Gy without GPC pretreatment started to die at day 3; in contrast, all embryos exposed to 20 Gy and treated with GPC were still alive. From day 4, survival decreased in both irradiated groups, but the mortality was more pronounced in the group without GPC pretreatment. On day 7, the difference in survival between the GPC pretreated and irradiated group and the irradiated control group was 20% and there were significant differences in both survival and morphologic alterations compared with the control samples. Figure 3 shows that 194 μM/L GPC with 3 h incubation before the radiation delivery has significant protective effects, and identical effect was detected with higher dose of GPC.
FIG. 3.
The effects of ionizing radiation on zebrafish survival in the presence of GPC (l-alpha glycerylphosphorylcholine). Zebrafish embryos at 24 hpf were mock irradiated (Control), mock irradiated in the presence of GPC (GPC), and irradiated with 20 Gy (RT) or 20 Gy in the presence of GPC (GPC+RT). Survival and morphology were evaluated at 24-h intervals up to 168 hpf. ***p < 0.001 relative to the control group. ###p < 0.001 relative to the irradiated group.
Histopathological and molecular evaluation
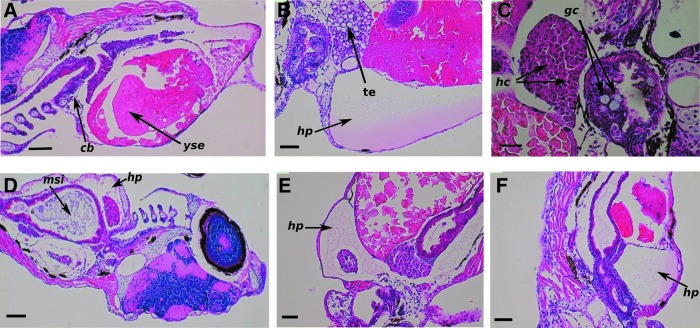
Microscopic examination of radiation-induced cell damage of the different organ systems revealed that the most severe deterioration occurred at higher dose levels and there was an important radioprotective effect of GPC. The histopathological assessment showed changes in the skin, such as the disappearance of mucous cells and development of subcutaneous edema in the groups irradiated with 10 Gy at 6 hpf or 20 Gy at 24 hpf. In the observed individuals, the nervous tissue was normal, but there were noticeable alterations in the ceratobranchials. Figure 4A shows that there was decrease in goblet cell numbers. In relation to the hepatopancreatic interstitial edema, hydropic and simple pathologic signs of hepatocytes were seen in individuals irradiated at 10 Gy dose level at 6 hpf (Fig. 4B). Pycnotic changes in the nuclei of the hepatocytes were also observed in the group irradiated with 20 Gy at 24 hpf (Fig. 4C).
FIG. 4.
The different histological changes in irradiated zebrafish embryos. In (A; HE. 90 × . Scale bar 280 μm), there is an observed reduction in the number of goblet cells in the multilayer epithelium of ceratobranchials (cb). Yolk sac edema (yse). Strength of vitellin was characterized by vacuoles. (B; HE. 180 × . Scale bar 100 μm) shows oppressive hydropericardium (hp), tissue edema (te), and hydropic and simple pathologic signs of hepatocytes. (C; HE. 200 × . Scale bar 40 μm) shows hepatocytes (hc) with pycnotic nuclei, mucous, and several goblet cells (gc) observed in the gastrointestinal mucous membrane in the intestinal lumen. The degree of hydropericardial disturbances and changes in small intestine. (D; HE. 90 × . Scale bar 200 μm) has large amounts of mucous in the small intestinal lumen (msi). Stand by this disorder slight degree of hydropericardium (hp) developed in (E; HE. 90 × . Scale bar 100 μm) shows severe, serous fibrinous hydropericardium (hp). Reduction of goblet cells. (F; HE. 90 × . Scale bar 200 μm) has severe hydropericardium (hp). Large amounts of mucus were noticeable in the intestinal lumen. HE, hematoxylin and eosin. Color images available online at www.liebertpub.com/zeb
The histological analysis revealed dose-dependent pericardial deterioration, such as slight hydropericardium (Fig. 4D) at the lowest dose level, and Figure 4E and F shows that this abnormality is more pronounced at higher doses.
In the course of microscopic monitoring, any irradiation-induced kidney damage was assessed, but disturbances were determined in the yolk sac and ascites in the groups irradiated with 10 or 20 Gy at 24 hpf. Furthermore, Figure 4 shows the stock of vitellin is characterized by vacuoles for the group exposed to 5 Gy at 6 hpf.
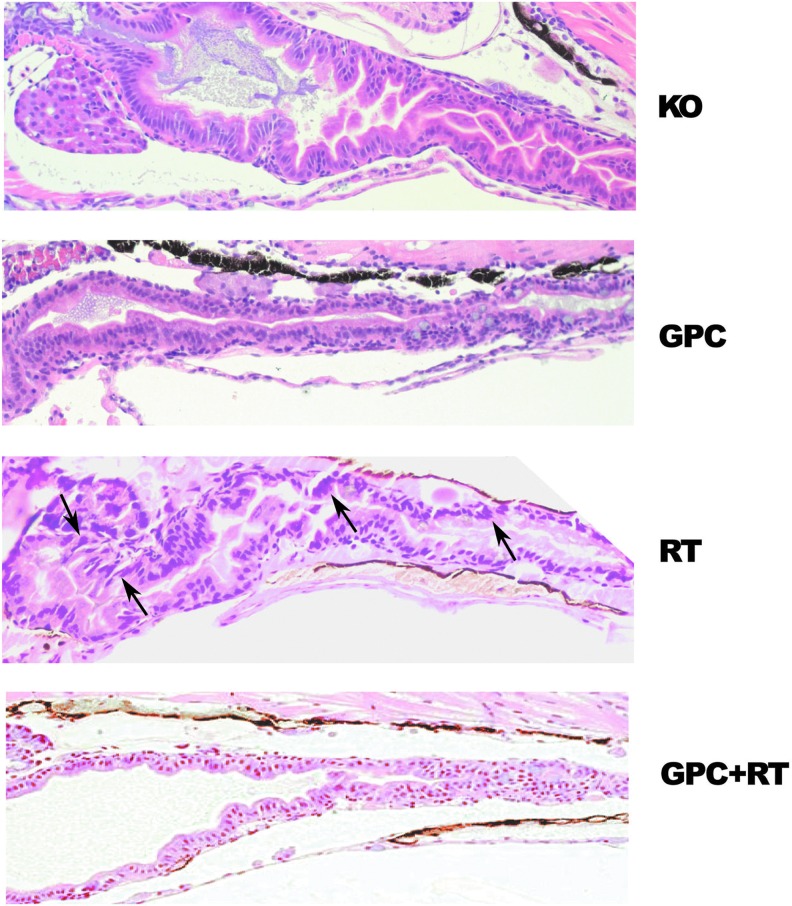
Finally, the histopathologic effects of radiation on the developing gastrointestinal system were determined. In each irradiated group, large amounts of mucous and catarrh were present in the intestinal flux, and goblet cells were found in the intestinal mucous membrane in the group irradiated with 20 Gy (Fig. 4C). In the 20 Gy irradiated group, cells with irregular shapes and a larger hyperchromatic nuclei were observed. These were characterized by pseudo-multilayer epithelia and moderate disorganization of the columnar cells, and the cytoplasm was wider in the intestinal lumen. The severe alterations induced by IR in the developing gastrointestinal system were reduced and partially restored by GPC pretreatment (Fig. 5).
FIG. 5.
The effects of GPC on radiation caused alterations of the gastrointestinal system. Representative histological sections of the intestine at 7 dpf; the arrows (→) indicate the disorganization of the columnar cells in the intestinal lumen. Embryos at 24 hpf were pretreated with 194 μM/L for 3 h before irradiation with 20 Gy. HE. 20 × . Scale bar 100 μm. dpf, days postfertilization. Color images available online at www.liebertpub.com/zeb
It was found while investigating the early phase of pro-inflammatory activation signal pathway that the IL-1β expression (Fig. 6) was reduced to the control level in the pretreated group, and thus, the induction of the NF-κB pathway activation (Fig. 7) had been prevented.
These results show that IL-1β and NF-κB are activated in response to injury at different times, when measured 1 and 2 h after 10 Gy irradiation. This activation level was reduced significantly in the GPC-treated groups.
Discussions
Our results are in line with other experiments,2 which found that the age and radiation dose were proportional to embryo malformation and lethality. McAleer et al.3 exposed embryos at 1, 2, 4, 8, and 24 hpf to different doses of IR (2–20 Gy). They found that embryos were much more sensitive (LD50 was 10 Gy for embryos irradiated at 4 hpf) in the gastrula stage (1–2 hpf) and blastula stage (4 hpf) than in the midblastula transition (8–24 hpf), and LD50 was 20 Gy for embryos exposed to IR at 24 hpf. Similarly, we defined LD50 to be 15 Gy for embryos exposed to IR at 6 hpf embryos, while half of the larvae irradiated with 20 Gy at 24 hpf were viable at day 7. Daroczi et al.25 defined LD50 for embryos irradiated at 24 hpf to be 20 Gy and, like this study, used this dose level for the investigation of potential radiation modifiers. These literature results are extremely consistent with those presented in this article despite different radiation sources being used for dose delivery. This strongly shows that the zebrafish embryo model is well suited for radiation biology experiments. In addition to survival, our malformation rate correlated to the dose up to the same degree as others had previously established.
Phospholipids play a major role in rearrangement and reduction of toxicity against agents damaging the cell membrane. However, little is known about the direct toxicity of administrated phospholipids at high concentrations. To study the GPC-related toxicity, we exposed embryos to increasing concentrations of GPC at 24 hpf. Administration of GPC in the range of 194 to 972 μM/L proved to be safe, because the viability or morphology was not adversely affected. Higher concentrations caused relevant distortions and there was almost 20% fatality at day 7 for the 1944 and 9720 μM/L samples.
GPC works by decreasing the inflammatory proteins and maintaining the membrane function. In our experiments, the reduction of the edema and the preserved viability demonstrated these protective effects and GPC provided protection against radiation-induced overall lethality and damage to multiple organ systems of the developing zebrafish. The postradiation surviving embryos were markedly malformed (shortened and curved bodies, pericardial edema, and malformations of the head and eye). These defects were also seen in embryos that survived irradiation in the presence of GPC, but appeared less severe and less pronounced.
Radiation causes significant functional damage to the gastrointestinal system and will lead to death by starvation within 10 days after conception.26 In our observations, morphologic alterations induced by IR in the developing gastrointestinal system were reduced and partially restored by pretreatment with GPC that may lead to the maintenance of nutritional capability while decreasing the mortality rate.
There is cumulative evidence that the activation of canonical NF-κB pathway is a major contributor to radiation-induced inflammatory changes in the normal tissues in the developing vertebrate organism. The hypothesis of radioprotection by inhibiting inflammatory modulators like NF-κB was confirmed in the zebrafish model, in which the NF-κB inhibitors ethyl-pyruvate and the synthetic triterpenoid CDDO-TFEA (RTA401) were given in combination with radiation. In other previous studies on zebrafish embryo radiation, investigators observed protective effect of several NF-κB pathway inhibitors.25 Therefore, the NF-κB and IL-1β gene expression was studied, assuming that the observed protective effect of GPC may follow from the inhibition of this pathway. IL-1β is one of the essential pro-inflammatory cytokine of interleukin-1 family members27 and is rapidly induced after irradiation, and this cytokine has also been implicated in edema shaping.28,29 Overexpression of IL-1β is the first sign of inflammatory activation after IR, which induces the activation of the NF-κB pathway.30
IL-1β and NF-κB overexpression was detected in the embryos, 2 h after IR. The addition of GPC reduced the IL-1β expression value down to the control level and prevented activation of the NF-κB pathway. The role of the NF-κB pathway activation in the early radiation response has already been clearly established.31 The in vitro experiments were mainly performed on tumor cell lines and there were relatively few investigations assessing the effect of NF-κB inhibitors on normal cells. The small animal studies with transgenic mouse models failed due to the high mortality of the mouse embryos with abnormal function of NF-κB pathway or were biased due to compensatory molecular process activity. The zebrafish embryos proved to be reliable model to study the effect of NF-κB pathway modulation on radiation response.25,32 Our observation of modulation of early pro-inflammatory activation by GPC at a nontoxic concentration could explain the pronounced radioprotective effect of this agent.
Zebrafish experiments provide a good basis to study the effects of IR on a large scale and proved to be a highly efficient model for investigations on potential radiation modifying agents. We have evaluated for the first time the protective effects of GPC against IR exposure in the zebrafish embryo model system. In this model, GPC exhibited promising protective effect against radiation-induced multiorgan morphological and histological impairment and overall lethality. Our results suggest that the inhibition of early radiation-induced activity of pro-inflammatory pathway activation could be a potential mode of action of GPC.
Acknowledgments
The authors are grateful to Nikolett Beretka, Boda Gyuláné, and Erika Szigeti for their valuable assistance and to Maria Kalinger for her excellent work. This work was funded by the ELI-ALPS project (GOP-1.1.1-12/B-2012-0001 and GINOP-2.3.6-15-2015-00001), which is supported by the European Union and cofinanced by the European Regional Development Fund.
Disclosure Statement
No competing financial interests exist.
References
- 1.Steel GG: Basic Clinical Radiobiology, Oxford University Press, USA, 1993 [Google Scholar]
- 2.Geiger GA, Parker SE, Beothy AP, Tucker JA, Mullins MC, Kao GD. Zebrafish as a “Biosensor”? Effects of ionizing radiation and amifostine on embryonic viability and development. Cancer Res 2006;66:8172–8181 [DOI] [PubMed] [Google Scholar]
- 3.McAleer MF, Davidson C, Davidson WR, Yentzer B, Farber SA, Rodeck U, et al. Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int J Radiat Oncol Biol Phys 2005;61:10–13 [DOI] [PubMed] [Google Scholar]
- 4.Bailey JM, Creamer BA, Hollingsworth MA. What a fish can learn from a mouse: Pprinciples and strategies for modeling human cancer in mice. Zebrafish 2009;6:329–337 [DOI] [PubMed] [Google Scholar]
- 5.Geiger GA, Fu W, Kao GD. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res 2008;68:3396–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treede I, Braun A, Jeliaskova P, Giese T, Füllekrung J, Griffiths G, et al. TNF-α-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidyalcholine in inetestinal epithelial cells. BMC Gastroenterol 2009;13:9–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tőkés T, Varga G, Garab D, Nagy Z, Fekete G, Tuboly E, et al. Peripheral inflammatory activation after hippocampus irradiation in the rat. Int J Radiat Biol 2014;90:1–6 [DOI] [PubMed] [Google Scholar]
- 8.Gallazzini M, Burg MB. What's new about osmotic regulation of glycerophosphocholine. Physiology (Bethesda) 2009;24:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownawell AM, Carmines EL, Montesano F. Safety assessment of AGPC as a food ingredient. Food Chem Toxicol 2011;49:1303–1315 [DOI] [PubMed] [Google Scholar]
- 10.Scribner DM, Witowski NE, Mulier KE, Lusczek ER, Wasiluk KR, Beilman GJ. Liver metabolomic changes identify biochemical pathways in hemorrhagic shock. J Surg Res 200;164:131–139 [DOI] [PubMed] [Google Scholar]
- 11.Amenta F, Liu A, Zeng YC, Zaccheo D. Muscarinic cholinergic receptors in the hippocampus of aged rats: influence of choline alphoscerate treatment. Mech Ageing Dev 1994;76:49–64 [DOI] [PubMed] [Google Scholar]
- 12.Onishchenko LS, Gaikova ON, Yanishevskii SN. Changes at the focus of experimental ischemic stroke treated with neuroprotective agents. Neurosci Behav Physiol 2008;38:49–54 [DOI] [PubMed] [Google Scholar]
- 13.Barbagallo SG, Barbagallo M, Giordano M, Meli M, Panzarasa R. alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks. An Italian multicenter clinical trial. Ann N Y Acad Sci 1994;30:253–269 [DOI] [PubMed] [Google Scholar]
- 14.De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther 2003;25:178–193 [DOI] [PubMed] [Google Scholar]
- 15.Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F: Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation? J Neurol Sci 2007;257:264–269 [DOI] [PubMed] [Google Scholar]
- 16.Tayebati SK, Tomassoni D, Di Stefano A, Sozio P, Cerasa LS, Amenta F. Effect of choline-containing phospholipids on brain cholinergic transporters in the rat. J Neurol Sci 2011;302:49–57 [DOI] [PubMed] [Google Scholar]
- 17.Komatsu H, Westerman J, Snoek GT, Taraschi TF, Janes N. L-alpha glycerylphosphorylcholine inhibits the transfer function of phosphatidylinositol transfer protein alpha. Biochim Biophys Acta 2003;1635:67–74 [DOI] [PubMed] [Google Scholar]
- 18.Kidd PM. Integrated brain restoration after ischemic stroke–medical management, risk factors, nutrients, and other interventions for managing inflammation and enhancing brain plasticity. Altern Med Rev 2009; 14:14–35 [PubMed] [Google Scholar]
- 19.Tőkés T, Tuboly E, Varga G, Major L, Ghyczy M, Kaszaki J, Boros M. Protective effects of L-alpha-glycerylphosphorylcholine on ischaemia-reperfusion-induced inflammatory reactions. Eur J Nutr 2015;54:109–118 [DOI] [PubMed] [Google Scholar]
- 20.Tőkés T, Erős G, Bebes A, Hartmann P, Várszegi S, Varga G, et al. Protective effects of a phosphatidylcholine-enriched diet in lipopolysaccharide-induced experimental neuroinflammation in the rat. Shock 2011;36:458–465 [DOI] [PubMed] [Google Scholar]
- 21.Ghyczy M, Torday C, Kaszaki J, Szabó A, Czóbel M, Boros M. Oral phosphatidylcholine pretreatment decreases ischemia-reperfusion-induced methane generation and the inflammatory response in the small intestine. Shock 2008;30:596–602 [DOI] [PubMed] [Google Scholar]
- 22.Plangár I, Szabó ER, Tőkés T, Mán I, Brinyiczki K, Fekete G, et al. Radio-neuroprotective effect of L-alpha glycerylphosphorylcholine (GPC) in an experimental rat model. J Neurooncol 2014;119:253–261 [DOI] [PubMed] [Google Scholar]
- 23.Drago F, Mauceri F, Nardo L, Valeris C, Lauria N, Rampello L, et al. Behavioral effects of L-alpha-glycerylphosphorylcholine: influence on cognitive mechanisms in the rat. Pharmacol Biochem Behav 1992;41:445–448 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Daroczi B, Kari G, Ren Q, Dicker AP, Rodeck U. Nuclear factor kB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther 2009;8:2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerfield M: The Zebrafish. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed. University of Oregon Press, Eugene, OR, 2000 [Google Scholar]
- 27.Vojtech LN, Scharping N, Woodson JC, Hansen JD. Roles of inflammatory caspases during processing of zebrafish interleukin-1 in Francisella noatunensis infection. Infect Immun 2012;8:2878–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaber MW, Sabek OM, Fukatsu K, Wilcox HG, Kiani MF, Merchant TE. Differences in ICAM-1 and TNF-alpha expression between large single fraction and fractionated irradiation in mouse brain. Int J Radiat Biol 2003;79:359–366 [DOI] [PubMed] [Google Scholar]
- 29.Mohanty S, Dey PK, Sharma HS, Singh S, Chansouria JP, Olsson Y. Role of histamine in traumatic brain edema. An experimental study in the rat. J Neurol Sci 1989;90:87–97 [DOI] [PubMed] [Google Scholar]
- 30.Ogryzko NV, Hoggett EE, Solaymani-Kohal S, Tazzyman S, Chico TJ, Renshaw SA, et al. Zebrafish tissue injury causes upregulation of interleukin-1 and caspase-dependent amplification of the inflammatory response. Dis Model Mech 2014;7:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Maggio FM, Minafra L, Forte GI, Cammarata FP, Lio D, Messa C, et al. Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond) 2015;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanisch UK, van Rossum D, Xie Y, Gast K, Misselwitz R, Auriola S, et al. The microglia-activating potential of thrombin: the protease is not involved in the induction of proinflammatory cytokines and chemokines. J Biol Chem 2004;279:51880–51887 [DOI] [PubMed] [Google Scholar]