Abstract
Background
We hypothesized that factors associated with an institution’s residual risk unaccounted for by population-based models may be identifiable and used to enhance the value of population-based risk scores for quality improvement.
Methods
From January 2000 to January 2010, 4,971 patients underwent aortic valve replacement (AVR), either isolated (n= 2,660) or with concomitant coronary artery bypass grafting (AVR+CABG; n = 2,311). Operative mortality and major morbidity and mortality predicted by The Society of Thoracic Surgeons (STS) risk models were compared with observed values. After adjusting for patients’ STS score, additional and refined risk factors were sought to explain residual risk. Differences between STS model coefficients (risk-factor strength) and those specific to our institution were calculated.
Results
Observed operative mortality was less than predicted for AVR (1.6% [42 of 2,660] vs 2.8%, p < 0.0001) and AVR+CABG (2.6% [59 of 2,311] vs 4.9%, p < 0.0001). Observed major morbidity and mortality was also lower than predicted for isolated AVR (14.6% [389 of 2,660] vs 17.5%, p < 0.0001) and AVR+CABG (20.0% [462 of 2,311] vs 25.8%, p < 0.0001). Shorter height, higher bilirubin, and lower albumin were identified as additional institution-specific risk factors, and body surface area, creatinine, glomerular filtration rate, blood urea nitrogen, and heart failure across all levels of functional class were identified as refined risk-factor variables associated with residual risk. In many instances, risk-factor strength differed substantially from that of STS models.
Conclusions
Scores derived from population-based models can be enhanced for institutional level use by adjusting for institution-specific additional and refined risk factors. Identifying these and measuring differences in institution-specific versus population-based risk-factor strength can identify areas to target for quality improvement initiatives.
Population-based risk models, such as those from The Society of Thoracic Surgeons (STS), are used for nationwide clinical practice benchmarking, patient counseling, clinical decision making, and patient selection for experimental therapies and clinical trials [1]. However, an individual institution’s actual risk likely differs from the national average [2]. The difference could be due to institution-specific risk factors not accounted for in the population models or to differences in the strength of the association of some risk factors with adverse events [2–4]. However, no single institution has a sufficient volume of cases or adverse events—the effective sample size—to develop a suite of variable-rich institutional models matching the magnitude of STS models.
What we hypothesize instead is that factors associated with an institution’s residual risk—positive or negative—unaccounted for by STS models may be identifiable and useful. We tested this hypothesis for outcomes after aortic valve replacement (AVR). For this, we (1) calculated the difference between our institution’s observed vs predicted risk (residual risk) after AVR, (2) identified factors associated with these differences for institution-specific risk prediction and quality improvement initiatives, and (3) measured the difference in strength of risk factors between those of STS models, representing the national average, and those of our institution to identify areas for quality improvement.
Patients and Methods
Patients
From January 2000 to January 2010, 4,971 patients underwent isolated AVR (n = 2,660) or AVR and concomitant coronary artery bypass grafting (AVR+CABG, n = 2,311) at Cleveland Clinic. Mean age was 70 ± 12 years (range, 18 to 95 years), and 67% were men (Table E1).
Patients were identified and preoperative, operative, and postoperative variables retrieved from the prospective Cardiovascular Information Registry of the Heart and Vascular Institute. The Cleveland Clinic Institutional Review Board has approved this database for research, with patient consent waived.
End Points
End points were (1) operative mortality and (2) major morbidity and mortality, defined as for the STS national database (available at http://www.sts.org/sites/default/files/documents/word/STSAdultCVDataSpecifications V2_73%20with%20correction.pdf).
Data Analysis
RESIDUAL RISK
For the two procedure groups, isolated AVR and AVR+CABG, we created variables corresponding to STS outcomes models [5, 6]. These models were solved for each patient; the sum of the resulting probabilities yielded the total predicted number of events. Total observed operative mortality was compared with predicted mortality, and total observed major morbidity and mortality was compared with predicted major morbidity and mortality using the χ2 test of goodness of fit and observed/predicted (O/P) ratios.
In addition, the logit of all patient-specific STS risk scores was entered into logistic regression outcomes models. The interpretation of such models [7, 8] is that when the intercept is less than 0, predicted scores are higher than observed, and when greater than 0, lower than observed. Similarly, when the slope exceeds 1, predicted scores are in the right general direction but vary less than the national average. When the slope is between 0 and 1, predicted scores are still in the right direction, but vary more than the national average.
FACTORS ASSOCIATED WITH RESIDUAL RISK
To investigate whether residual risk after adjusting for the STS model can be partly explained by institution-specific additional and refined risk factors, for each patient we identified factors associated with operative mortality and major morbidity and mortality in the presence of the STS score using preoperative variables (Appendix E1) and multivariable logistic regression. In addition to patient characteristics used in STS models, we included laboratory variables representing noncardiac comorbidity, valve pathophysiology, disease etiology, and detailed left-heart morphology and function (Appendix E1) as candidates in the multivariable analysis (details given in Appendix E2).
INSTITUTION-SPECIFIC DIFFERENCE IN RISK-FACTOR STRENGTH
Using STS variables in a logistic regression model, we examined differences between our institution-specific risk-factor coefficients and published STS coefficients (SAS PROC LOGISTIC; SAS Institute Inc, Cary, NC). Results are presented as standardized differences: Standardized difference = CC coefficient – STS coefficient/SE(CC coefficient), where CC is Cleveland Clinic and SE is the standard error. This standardized measure can be interpreted as the number of standard deviations by which the Cleveland Clinic coefficient deviates from that of the STS national average. Cleveland Clinic coefficients within 0.5 SE of STS coefficients were considered to be similar in magnitude, those below 0.5 SE smaller, and those exceeding 0.5 SE larger.
Results
Deviation of Our Patient Characteristics From Those of the STS Population
Characteristics of Cleveland Clinic patients undergoing isolated AVR differed in many respects from those of the STS population as a whole. More of our patients were male (63% vs 58%), had less chronic lung disease (14% vs 20%), and more previous cardiovascular operations (27% vs 16%) and urgent operations (28% vs 23%); fewer had a history of congestive heart failure (24% vs 38%; Table E1).
Compared with the STS population, our AVR+CABG patients had more hypertension (84% vs 79%), less chronic lung disease (15% vs 23%), more cerebral vascular disease (28% vs 19%), more previous cardiovascular operations (26% vs 12%), less 3-system coronary artery disease (15% vs 43%), and more remote myocardial infarctions (31% vs 14%); fewer had a history of congestive heart failure (28% vs 36%).
Residual Risk
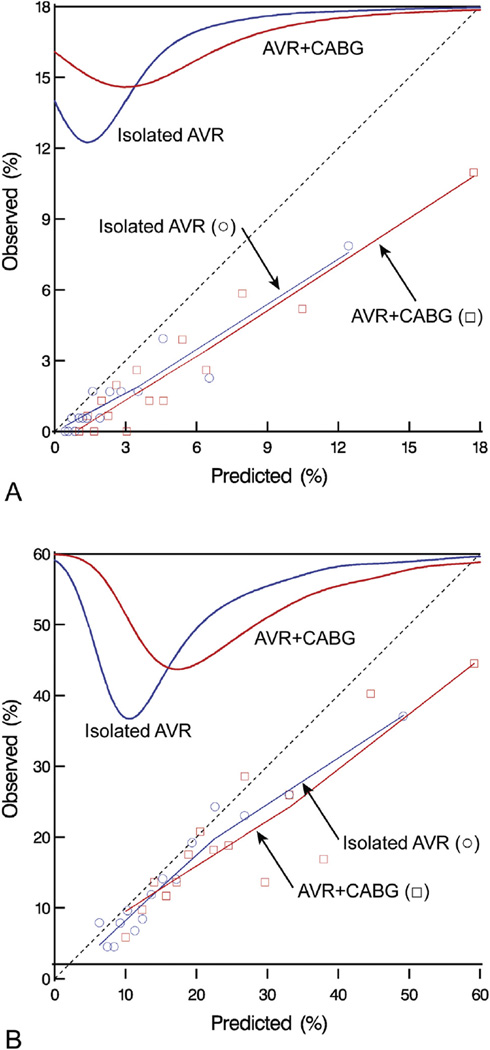
Observed operative mortality was lower than predicted for isolated AVR (1.6% vs 2.8%, p = .0006; O/P ratio, 0.57; 68% confidence limits [CL], 0.48, 0.65) and AVR+CABG (2.6% vs 4.9%, p < 0.0001; O/P ratio, 0.52; 68% CL, 0.45, 0.59). This was observed over the entire spectrum of risk and over the entire 10-year study period (Fig 1A and Fig E1A). Thus, the intercept of an unadjusted model of the STS risk score for operative mortality was negative, indicating that observed risk was lower than predicted for isolated AVR and AVR+CABG. Slopes for operative mortality were close to 1, indicating that direction and variability of mortality were consistent with STS predictions (Table 1).
Fig 1.
Observed vs predicted operative mortality and major morbidity and mortality over different predicted risk spectrum in patients undergoing isolated aortic valve replacement (AVR, blue lines) and AVR and coronary artery bypass grafting (AVR+CABG, red lines).Top smoothed curves depict density of predicted probability of operative mortality. These curves show the distribution of predicted mortality in our cohort. Symbols (isolated AVR, circles; AVR+CABG, squares) are data points obtained by averaging observed and predicted mortality over different predicted risk groups. The thin lines are locally weighted scatterplot smoothing (LOWESS) nonparametric smoothed lines obtained by using the symbols. (A) Operative mortality. (B) Major morbidity and mortality.
Table 1.
Additional or Refined Patient Variables Associated With Higher Likelihood of Operative Mortalitya
| Factor | Coefficient ± SE | p Value | Reliability (%)b |
|---|---|---|---|
| Isolated AVR | |||
| Unadjusted model | |||
| STS risk scorec | 1.01 ± 0.16 | <0.0001 | … |
| Intercept | −0.56 ± 0.50 | 0.3 | … |
| Adjusted model | |||
| STS risk scorec | 0.92 ± 0.16 | <0.0001 | … |
| Shorter heightd | 1.9 ± 0.66 | 0.005 | 46 |
| Higher creatininee | 0.022 ± 0.010 | 0.04 | 61 |
| Intercept | −3.2 ± 1.01 | 0.002 | … |
| AVR+CABG | |||
| Unadjusted model | |||
| STS risk scorec | 1.2 ± 0.16 | <0.0001 | … |
| Intercept | −0.21 ± 0.42 | 0.6 | … |
| Adjusted model | |||
| STS risk scorec | 0.79 ± 0.19 | <0.0001 | … |
| Lower BSAf | 4.04 ± 1.8 | 0.03 | 54 |
| Higher NYHA Functional Classg | 1.1 ± 0.50 | 0.009 | 63 |
| Higher bilirubinh | 0.15 ± 0.046 | 0.002 | 91 |
| Lower albumini | −0.105 ± 0.039 | 0.006 | 49 |
| Intercept | −2.2 ± 1.1 | 0.04 | … |
In addition to usual patient characteristics used in the STS model, we have included detailed laboratory variables (noncardiac comorbidity), detailed valve pathology (aortic, mitral, and tricuspid valves), aortic valve etiology, size, and pressure, and detailed left ventricular variables (see Appendix E1).
Percent of times factor appeared in 500 bootstrap models.
Logit of STS risk score.
(180/height)2, inverse squared transformation.
(Creatinine)2, squared transformation.
(1/BSA)2, inverse squared transformation.
Log(NYHA), logarithmic transformation.
Exp(bilirubin), exponential transformation.
(Albumin)2, squared transformation.
AVR = aortic valve replacement; BSA = body surface area; CABG = coronary artery bypass grafting; NYHA = New York Heart Association; SE = standard error; STS = The Society of Thoracic Surgeons.
Observed major morbidity and mortality was lower than predicted for isolated AVR (14.6% vs 17.5%, p < 0.0001; O/P ratio, 0.83; 68% CL, 0.79, 0.87) and AVR+CABG (20.0% vs 25.8%, p < 0.0001; O/P ratio, 0.77; 68% CL, 0.74, 0.81). This was observed over the entire spectrum of risk (Fig 1B) and over the entire 10-year study period (Fig E1B). Consistent with the findings for operative mortality, the intercepts for major morbidity and mortality were less than 0, indicating that the observed risk was lower than predicted for isolated AVR and AVR+CABG (Table 2). However, unlike mortality, slopes for the STS risk score for major morbidity and mortality were between 0 and 1, indicating that although the direction of variability in scores was generally consistent with the STS, it varied more than the national average.
Table 2.
Additional or Refined Patient Variables Associated with Higher Likelihood of Major Morbidity or Mortalitya
| Variable | Coefficient ± SE | p Value | Reliability (%)b |
|---|---|---|---|
| Isolated AVR | |||
| Unadjusted model | |||
| STS risk scorec | 0.92 ± 0.073 | <0.0001 | … |
| Intercept | −0.35 ± 0.12 | 0.003 | … |
| Adjusted model | |||
| STS risk scorec | 0.66 ± 0.097 | <0.0001 | 100 |
| Larger LV massd | 0.16 ± 0.058 | 0.005 | 87 |
| Lower albumine | 4.9 ± 1.6 | 0.002 | 77 |
| Shorter heightf | 1.1 ± 0.34 | 0.002 | 86 |
| Lower GFRg | −0.32 ± 0.15 | 0.04 | 92 |
| Intercept | −2.0 ± 0.82 | 0.01 | … |
| AVR + CABG | |||
| Unadjusted model | |||
| STS risk scorec | 0.83 ± 0.076 | <0.0001 | … |
| Intercept | −0.52 ± 0.089 | <0.0001 | … |
| Adjusted model | |||
| STS risk scorec | 0.42 ± 0.098 | <0.0001 | 100 |
| Lower albuminh | −2.6 ± 0.43 | <0.0001 | 99 |
| Higher BUN | 0.014 ± 0.0050 | 0.005 | 56 |
| Higher bilirubin | 0.36 ± 0.15 | 0.01 | 63 |
| Insulin-dependent diabetes | 0.41 ± 0.16 | 0.01 | 42 |
| More recent date of operationi | 0.29 ± 0.066 | <0.0001 | 90 |
| Intercept | 1.6 ± 0.60 | 0.008 |
In addition to usual patient characteristics used in the STS model, we have included detailed laboratory variables (noncardiac comorbidity) and detailed valve pathology.
Percent of times factor appeared in 500 bootstrap models.
Logit of STS risk score.
(LV mass/275)2, squared transformation.
(1/albumin), inverse transformation.
(180/height)2, inverse squared transformation.
Log(GFR), logarithmic transformation.
Log(albumin), logarithmic transformation.
Log(date of operation [years since 1/1/2000]), logarithmic transformation.
AVR = aortic valve replacement; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting; GFR = glomerular filtration rate; LV = left ventricular; SE = standard error; STS = The Society of Thoracic Surgeons.
Similar investigations were made for other STS-benchmarked outcomes (Table E2).
Factors Associated with Residual Risk
After adjusting for the STS risk score, residual risk of operative mortality and major morbidity and mortality was partly explained by additional risk factors and refined risk factor variables for some of the factors present in STS models.
OPERATIVE MORTALITY
For isolated AVR, an additional risk factor for operative mortality (Table 1) was shorter height (particularly below ~154 cm; Fig E2A). Creatinine (particularly below or above 0.8 to 1.3 g/dL; Fig E2B) as a continuous nonlinear variable was identified as a refined risk factor variable. Together, these decreased the residual risk of operative mortality after isolated AVR (C statistic, 0.78 to 0.80; Brier score, 0.0153 to 0.0151).
For AVR+CABG, institution-specific additional risk factors for operative mortality (Table 2) were higher bilirubin (particularly above ~1.25 g/dL; Fig E2C) and lower albumin (particularly below ~3 g/dL; Fig E2D). Body surface area as a continuous variable (Fig E2E) rather than as a series of categories and heart failure across all levels of New York Heart Association Functional Classification rather than just class IV vs lower classes were identified as refined risk factor variables. Together, these decreased the residual risk of operative mortality after AVR+CABG (C statistic, 0.77 to 0.82; Brier score, 0.0238 to 0.0228).
MAJOR MORBIDITY AND MORTALITY
For isolated AVR, additional risk factors for major morbidity and mortality (Table 2) were shorter height (particularly below ~155 cm; Fig E3A), large left ventricular mass (particularly above ~400 g; Fig E3B), and lower albumin (particularly below ~3.4 g/dL; Fig E3C). Lower glomerular filtration rate (Fig E3D) was identified as a refined risk factor variable. Together, these decreased the residual risk of major morbidity and mortality after isolated AVR (C statistic, 0.70 to 0.71; Brier score, 0.12 to 0.11).
For AVR+CABG, institution-specific additional risk factors for major morbidity and mortality (Table 2) were lower albumin (particularly below ~3.7 g/dL; Fig E3E) and higher bilirubin (particularly above ~0.5 g/dL; Fig E3F). Higher blood urea nitrogen (Fig E3G) and insulin-dependent diabetes were identified as refined risk factor variables. Together, these decreased the residual risk of major morbidity and mortality after AVR+CABG (C statistic, 0.66 to 0.70; Brier score, 0.15 to 0.145).
Institution-Specific Difference in Risk-Factor Strength
OPERATIVE MORTALITY FOR ISOLATED AVR
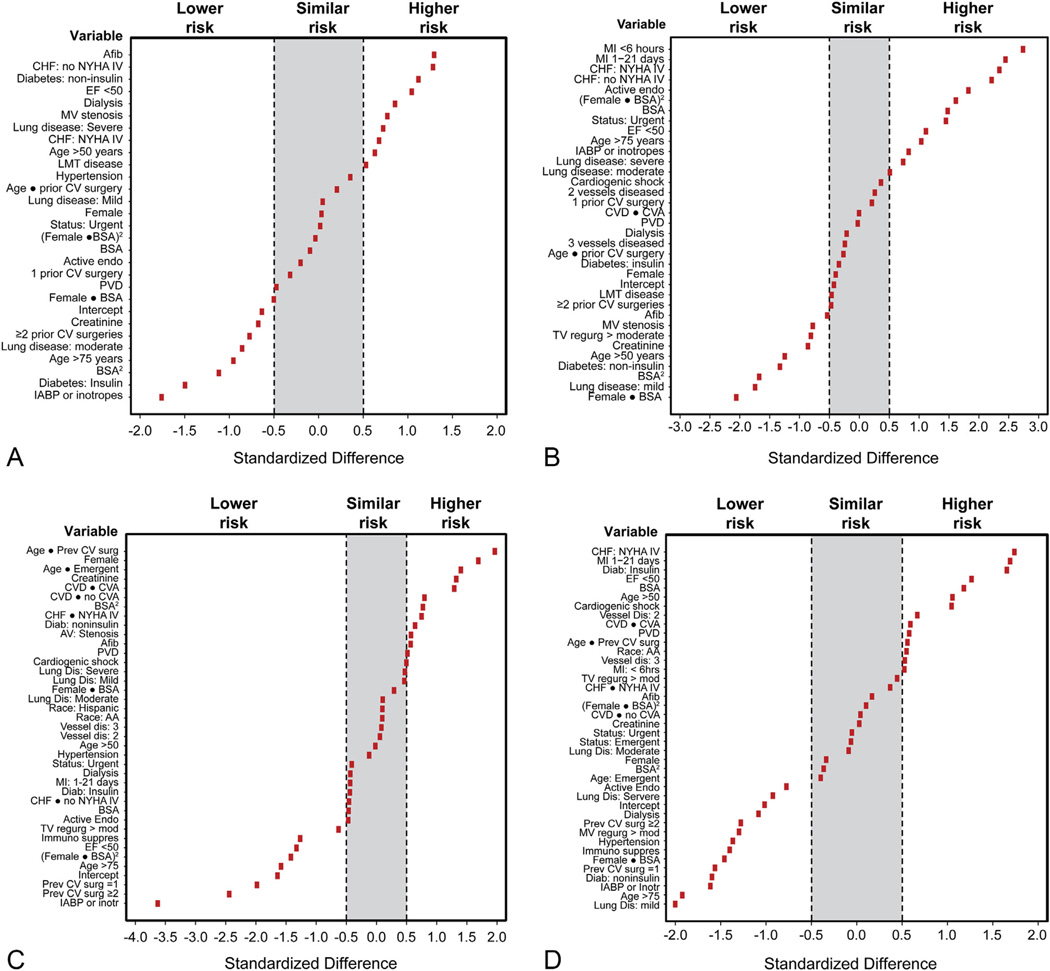
Coefficients for individual STS-defined variables demonstrated similarities and some differences from coefficients of the STS models. Coefficients exceeding 0.5 SE (higher risk) were observed for patients with atrial fibrillation, symptomatic congestive heart failure, non–insulin-dependent diabetes, severe chronic lung disease, ejection fraction of less than 0.50, those on dialysis, and those with nonbypassed left main trunk disease (Fig 2A). Coefficients below 0.5 SE (lower risk) were observed for patients with insulin-dependent diabetes, patients on preoperative intraaortic balloon pump or inotropic support, patients older than 75 years, patients with moderate chronic lung disease, and patients with two or more prior cardiovascular operations.
Fig 2.
Standardized differences by which Cleveland Clinic coefficients deviate from The Society of Thoracic Surgeons (STS) coefficients, demonstrating institution-specific lower, similar, and higher risk variables using the STS operative mortality and major morbidity and mortality models. (A) Operative mortality after isolated aortic valve replacement (AVR). (B) Operative mortality after AVR and coronary artery bypass grafting (AVR+CABG). (C) Major morbidity and mortality after isolated AVR. (D) Major morbidity and mortality after AVR+CABG. (Afib = atrial fibrillation; BSA = body surface area; CHF = congestive heart failure; CV = cardiovascular; CVA = cerebrovascular accident; CVD = cerebrovascular disease; EF = ejection fraction; endo = endocarditis; IABP = intraaortic balloon pump; LMT = left main trunk; MI = myocardial infarction; MV = mitral valve; NYHA = New York Heart Association; PVD = peripheral vascular disease; TV = tricuspid valve.)
OPERATIVE MORTALITY FOR AVR+CABG
Coefficients exceeding 0.5 SE were seen for patients with myocardial infarction, symptomatic congestive heart failure, ejection fraction of less than 0.50, active endocarditis, patients undergoing nonelective operations, patients older than 75 years, patients on preoperative intraaortic balloon pump or inotropic support, and those with severe chronic lung disease (Fig 2B). Coefficients below 0.5 SE were seen for patients with mild lung disease, non–insulin-dependent diabetes, moderate or severe tricuspid regurgitation, mitral valve stenosis, and higher creatinine.
MAJOR MORBIDITY AND MORTALITY FOR ISOLATED AVR
Coefficients exceeding 0.5 SE were seen for women, older patients with previous cardiovascular operations, older patients undergoing emergency operations, patients with cardiovascular disease, with or without a cerebrovascular accident, and patients with higher creatinine, symptomatic congestive heart failure, and non–insulin-dependent diabetes (Fig 2C). Coefficients of less than 0.5 SE were seen for patients on preoperative intraaortic balloon pump or inotropic support, those with previous cardiovascular operations, patients older than 75 years, patients with low ejection fraction, and patients on immunosuppressive therapy.
MAJOR MORBIDITY AND MORTALITY FOR AVR+CABG
Coefficients exceeding 0.5 SE were seen for patients with myocardial infarction, low ejection fraction, insulin-dependent diabetes, age older than 50 years, cardiogenic shock, and 2-vessel coronary artery disease (Fig 2D). Coefficients of less than 0.5 SE were seen for non–insulin-dependent diabetes, patients on preoperative intraaortic balloon pump or inotropic support, patients with lung disease, previous cardiovascular operations, moderate or severe mitral regurgitation, hypertension, patients older than 75 years, and patients on immunosuppressive therapy and dialysis.
Comment
Principal Findings
The risk of operative mortality and major morbidity and mortality predicted by population-based models for isolated AVR and AVR+CABG was different from the observed risk at our institution. Population-based models may be enhanced for use at the institutional level by institution-specific additional risk factors and refined risk factors. Moreover, in many instances, institution-specific risk-factor strength differed from that of STS models.
Findings in Context
STS models serve as benchmarks for clinical centers and individual surgeon performance, quality improvement, public reporting, “star ranking,” pay for performance, value-based purchasing, and penalties by insurers. These scores are used at the national level as a common metric for assessing center-to-center performance. They are encouraged to be used at individual institutions for patient counseling, clinical decision making, and to help identify patients for experimental therapies and clinical trials [1]. However, institutional performance may be above or below these benchmarks, either generally or for specific patient profiles. Disclosure of such departures would seem to be prudent to incorporate into truly informed patient consent. The same seems true for recruitment of patients into clinical trials.
Institution-specific risk prediction is important to supplement these purposes. For example, if we use only national averages to determine patient eligibility for experimental therapies, patients with lower actual institutional risk may have a risk-to-benefit ratio lower than national averages, and this may affect surgeon equipoise and patient recruitment [9].
Identifying additional risk factors unique to a particular institution [3] could point out areas to be targeted for quality improvement initiatives. We found higher bilirubin and lower albumin to be additional institution-specific risk factors for patients undergoing AVR, pointing out areas to be targeted for quality improvement initiatives at the institutional level. Higher bilirubin may indicate hemolysis, underlying liver disease, or bile duct obstruction and is an independent risk factor for increased operative mortality after cardiac operations [10]. Hypoalbuminemia is also a marker of increased operative morbidity and mortality [11]. Identifying underlying pathology and managing it appropriately may improve the outcomes of patients undergoing AVR at our institution.
By refining certain risk-factor variables, better use can be made of available data that may enhance the value of population-based scores for use at the institutional level. Examples include creatinine as a continuous nonlinear variable rather than as a linear variable, body surface area as a continuous variable rather than as a series of categories, and heart failure across all levels of the New York Heart Association Functional Classification rather than just class IV vs lower classes.
By measuring differences in institution-specific vs population-based risk-factor strength, an institution may identity risk factors with higher or lower influence on an end point. The former could highlight areas that should be targeted for quality improvement initiatives at the institutional level. The latter indicate neutralization of risk and may point to management strategies that have worked and that may be shared with others.
Based on these different kinds of departures of observed from expected risk based on STS models, we have initiated a number of quality improvement projects. Foremost among these are investigations of blood product use [12, 13]. We studied the effect of transfusion and reoperation for bleeding on outcomes [12, 13] and developed and implemented a reoperation for bleeding checklist that resulted in a reduction in reoperations for bleeding [14]. We evaluated the frequency of laboratory testing in patients undergoing cardiac operations and proposed strategies to reduce phlebotomy volumes for preventing hospital-acquired anemia [15]. We also studied the prevalence, outcomes, and health care implications of hospital-acquired anemia [16], adopted less invasive approaches to reduce prolonged ventilation [17, 18], and studied our infection outcomes to identify risk factors for infections after cardiac operations [19–21].
Applicability of Methodology
The specific findings are institution specific, but the concepts and methodology used to enhance the value of population-based risk models and scores would be valuable for centers with sufficient volume to perform a similar study. To investigate residual risk, 10 events may be needed. To identify factors associated with institution-specific difference in risk, 20 to 50 events may be needed. However, more end points are required to measure the difference in strength of risk factors between those of STS models and those of an institution, and only institutions with larger volume would be able to do this. However, institutions with lesser volume may use a composite STS end point such as major morbidity and mortality.
It is important to bear in mind that this methodology should be used only for enhancing the value of population-based risk scores for use at the institutional level and not for center-to-center comparisons. A common national benchmark is vital as a benchmark for center-to-center comparisons.
Limitations
This was purposely a single-center study to understand, examine, and use the difference in risk predicted by population-based scores and observed risk. However, the effective sample size of our study is the number of end points, which not only affects statistical power but also may preclude identifying all but a few variables as additional risk factors. We purposely avoided calibrating risk scores using STS calibration factors because our intent was not to simply calibrate the STS model to Cleveland Clinic results. Rather, we wanted to expose differences from national benchmarks that would provide clues that may be valuable for improving our local results. We used model coefficients from STS version 2.61 to estimate predicted probability. This model is based on patients from January 2002 to January 2007, whereas our study population is from January 2000 to January 2010. Therefore, our study population consists of patients from before and after the time frame of the STS model. STS uses an O/P ratio method to calibrate risk scores, a method that raises some mathematical concerns [22].
Conclusions
Risk predicted by population-based models for an individual institution may differ from the actual risk at that institution. Scores derived from population-based models can be enhanced for use at the institutional level by adjusting for institution-specific additional and refined risk factors. Identifying these and measuring differences in institution-specific vs population-based risk-factor strength can assist in patient counseling, clinical decision making, quality improvement, and patient selection for experimental therapies or clinical trials.
Supplementary Material
Acknowledgments
This study was partly funded by the Cleveland Clinic Department of Thoracic and Cardiovascular Surgery, the Gus P. Karos Registry Fund, the Sheikh Hamdan bin Rashid Al Maktoum Distinguished Chair in Thoracic and Cardiovascular Surgery (held by J.F.S.), and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (held by E.H.B.).
Footnotes
Presented at the American Heart Association 2012 Scientific Sessions, Los Angeles, CA, November 3–7.
Dr Sabik discloses a financial relationship with Edwards Lifesciences and Medtronic, and Dr Svensson and Dr Blackstone with Edwards Lifesciences.
The Supplemental Figures, Tables, and Appendices can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2015.12.055] on http://www.annalsthoracicsurgery.org.
References
- 1.Shahian DM, Blackstone EH, Edwards FH, et al. Cardiac surgery risk models: a position article. Ann Thorac Surg. 2004;78:1868–1877. doi: 10.1016/j.athoracsur.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 2.Nowicki ER. What is the future of mortality prediction models in heart valve surgery? Ann Thorac Surg. 2005;80:396–398. doi: 10.1016/j.athoracsur.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Sergeant P, Blackstone E, Meyns B. Can the outcome of coronary bypass grafting be predicted reliably? Eur J Cardiothorac Surg. 1997;11:2–9. doi: 10.1016/s1010-7940(96)01032-9. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov J, Tu JV, Naylor CD. Ready-made, recalibrated, or remodeled? Issues in the use of risk indexes for assessing mortality after coronary artery bypass graft surgery. Circulation. 1999;99:2098–2104. doi: 10.1161/01.cir.99.16.2098. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3—valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Cox DR. Two further applications of a model for binary regression. Biometrika. 1958;45:562–565. [Google Scholar]
- 8.Miller ME, Langefeld CD, Tierney WM, Hui SL, McDonald CJ. Validation of probabilistic predictions. Med Decis Making. 1993;13:49–58. doi: 10.1177/0272989X9301300107. [DOI] [PubMed] [Google Scholar]
- 9.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Lin CH, Hsu RB. Cardiac surgery in patients with liver cirrhosis: risk factors for predicting mortality. World J Gastroenterol. 2014;20:12608–12614. doi: 10.3748/wjg.v20.i35.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 12.Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146:1480 e6–1487 e6. doi: 10.1016/j.jtcvs.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Vivacqua A, Koch CG, Yousuf AM, et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg. 2011;91:1780–1790. doi: 10.1016/j.athoracsur.2011.03.105. [DOI] [PubMed] [Google Scholar]
- 14.Loor G, Vivacqua A, Sabik JF, 3rd, et al. Process improvement in cardiac surgery: development and implementation of a reoperation for bleeding checklist. J Thorac Cardiovasc Surg. 2013;146:1028–1032. doi: 10.1016/j.jtcvs.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Koch CG, Reineks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg. 2015;99:779–784. doi: 10.1016/j.athoracsur.2014.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8:506–512. doi: 10.1002/jhm.2061. [DOI] [PubMed] [Google Scholar]
- 17.Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg. 2014;147:355 e5–361 e5. doi: 10.1016/j.jtcvs.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg. 2012;144:852 e3–858 e3. doi: 10.1016/j.jtcvs.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kelava M, Robich M, Houghtaling PL, et al. Hospitalization before surgery increases risk for postoperative infections. J Thorac Cardiovasc Surg. 2014;148:1615–1621. doi: 10.1016/j.jtcvs.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Keshavamurthy S, Koch CG, Fraser TG, et al. Clostridium difficile infection after cardiac surgery: prevalence, morbidity, mortality, and resource utilization. J Thorac Cardiovasc Surg. 2014;148:3157–3165. e1–e5. doi: 10.1016/j.jtcvs.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Raza S, Sabik JF, 3rd, Masabni K, Ainkaran P, Lytle BW, Blackstone EH. Surgical revascularization techniques that minimize surgical risk and maximize late survival after coronary artery bypass grafting in patients with diabetes mellitus. J Thorac Cardiovasc Surg. 2014;148:1257–1264. doi: 10.1016/j.jtcvs.2014.06.058. discussion 1264–6. [DOI] [PubMed] [Google Scholar]
- 22.Jin R, Furnary AP, Fine SC, Blackstone EH, Grunkemeier GL. Using Society of Thoracic Surgeons risk models for risk-adjusting cardiac surgery results. Ann Thorac Surg. 2010;89:677–682. doi: 10.1016/j.athoracsur.2009.10.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.