Abstract
Objective:
To examine the effect of using different b-values on the utility of diffusion-weighted (DW) MRI in differentiating acute infectious spondylitis from Modic type 1 and the discriminative accuracy of related apparent diffusion coefficient (ADC), claw-sign and amorphous increased signal.
Methods:
43 patients with equivocal diagnosis of acute infectious spondylitis/Modic type 1 by using MR images were prospectively studied. The discriminative accuracy of DW MRI using three b-values of 50, 400, 800 s mm−2, ADC, claw sign and amorphous increased signal was examined.
Results:
DW MRI differentiated infectious spondylitis from Modic type 1 change most accurately when a b-value of 800 s mm−2 was chosen [sensitivity, 91.7%; specificity, 96.8%; positive-predictive value (PPV), 91.7%; negative-predictive value (NPV), 96.8%; and accuracy, 95.3%]. The optimal cut-off ADC value was 1.52 × 10−3 mm2 s−1 (sensitivity, 91.7%; specificity, 100%; PPV, 100%; NPV, 96.9%; and accuracy, 97.7%). Best visualized at a b-value of 50 s mm−2, claw sign (for degeneration) and amorphous increased signal (for infection) were 100% accurate.
Conclusion:
Should DW MRI be used in differentiating acute infectious spondylitis from degeneration, large b-values are required. With low b-values, however, claw sign and amorphous increased signal are very accurate in this regard.
Advances in knowledge:
DW MRI using large b-values could be used in differentiating acute infectious spondylitis from Modic type I.
INTRODUCTION
The documentation of decreased signal intensity on T1 weighted (T1W) MRI coupled with increased signal intensity on T2 weighted (T2W) sequences in the marrow adjacent to the end plates indicates the replacement of the bone marrow elements with fibrovascular tissue, a condition known as degenerative Modic type 1 change.1–3 In addition to degeneration, such signal changes may exist in infection, rendering conventional MRI non-specific for diagnostic purposes,4 particularly when clinical clues are also inconclusive.5 This diagnostic dilemma could increase cost and lead to mismanagement of patients.6
At the moment, spinal infections are encountered more often than before, possibly due to increased elderly and immunocompromised populations, conducting more spinal surgeries and improved imaging techniques.7
Initially aimed to investigate brain abnormalities, diffusion-weighted (DW) MRI and related diffusion values such as the apparent diffusion coefficient (ADC) are now being used frequently in evaluating a wide range of spinal pathologies such as benign and malignant compression fractures,8–10 osteoporosis11 and infection.8–10 DW MRI works on the basis of signal attenuation in biological tissues caused by the Brownian water motion of the spins,12 and the ADC combines the effects of diffusion and perfusion in the extracellular extravascular space.13
An important parameter that has consequential influences on the quality of DW imaging (DWI) is the chosen b-value.14 It has been shown that T2 effect can interfere with a correct interpretation of DW images, a phenomenon that is known as “T2-shine-through” effect. To overcome this flaw, choosing diffusion gradients with high b-values (i.e. >150 s mm−2) has been suggested.15 Despite this widely accepted notion that DWI with high b-values enhances the sensitivity,13 to the best of the authors' knowledge, the role of b-values has not been investigated in relation to the discriminative accuracy of DW MRI in patients with equivocal changes of the end plates on conventional MR images. A factual answer to this ambiguity is clinically important, because modern MR devices often provide instructions that include several suggested b-values for imaging of a particular region in the body that may cause uncertainty in making optimal decisions by physicians and imaging technologists.
Apart from signal intensity and ADC values, it has been recently proposed that some specific findings on DW sequences namely “claw sign” and “amorphous increased signal” could be used with high precision in distinguishing between infection and degeneration in the lumbar vertebral end plates.16–20 The relevant works, however, are scarce, and the results need to be confirmed in further studies.20
Thus, the present study is designed to examine the utility of DW MRI in differentiating acute infectious spondylitis from Modic type 1 in patients with low back pain and equivocal findings by conventional MRI and, at the same time, to assess the discriminative accuracy of related ADC values, claw sign and amorphous increased signal in this regard.
METHODS AND MATERIALS
Study design and patients
From April 2010 through to June 2014, 48 consecutive patients with end plate changes on T1W and T2W MR images of the lumbar spine suggesting both acute spondylodiscitis and Modic type I change were prospectively enrolled in this study. The patients were identified from a total of 1806 lumbar MR images in subjects with low back pain acquired during this time at our imaging centre.
The initial inclusion criterion was the presence of acute lumbar symptoms accompanied with bone marrow changes adjacent to the end plates, detectable as hypointensity on T1W images and hyperintensity on T2W images. A senior radiologist specialized in MRI of the spine decided on the inclusion of patients into this study after reviewing the initial lumbar MR images. This person had no other role in conducting the present study.
An institutional ethics committee approved this work and informed written consents were obtained from the participants. This study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The exclusion criteria were a history of a previous spinal surgery, intervertebral disc herniation or vertebral fracture (n = 3); documentation of a recent antibiotic/anti-inflammatory therapy (n = 1); the presence of advanced infectious spondylitis with abscess formation detectable on MR images or disseminated bone marrow disease such as a metastatic condition (n = 1); and pregnancy. On this basis, 5 patients were excluded, leaving 43 patients for the final analysis.
Standard of reference
The diagnosis of infectious spondylitis (n, 12) was established with CT-guided bone biopsy in 7 patients and on the basis of clinical and laboratory findings and imaging follow-ups21 in 5 patients. The supporting laboratory findings were an elevated erythrocyte sedimentation rate, elevated levels of C-reactive protein, leukocytosis and positive blood cultures.21 The final diagnosis was brucellosis in 10 patients and tuberculosis in 2 patients. The remaining 31 patients with no clinical or laboratory findings suggesting infection were assigned to the Modic type-1 degeneration group.22 All patients were followed up clinically and by MRI for at least 2 years,18,22 and the initial diagnosis was established in all cases. On the follow up in degenerative group, Modic type 1 in 7 patients partially converted Modic type 2, and 17 patients fully converted Modic type 2. No change was documented in seven patients.
Imaging techniques
A standard lumbar MRI protocol was used in this study. Sagittal and axial T1W and T2W non-contrast MR images were obtained from the lumbosacral spine using a 1.5-T scanner (MAGNETOM Avanto 1.5-T MRI system; Siemens, Erlangen, Germany) with gradient echo-planar capabilities and a standard phased-array surface receiver coil for imaging the spine.
Axial and sagittal T1W turbo spin-echo sequences [repetition time (TR)/echo time (TE), 580/11; turbo factor, 22; turbo acquisition time, 2 min 29 s], axial and sagittal T2W turbo spin-echo sequences [TR/TE, 4000/120; turbo factor, 4; turbo acquisition time, 2 min 5 s (axial views); turbo acquisition time, 2 min 9 s (sagittal views)] and sagittal DW sequences (three-scan trace) were acquired. The resulting DW images were non-isotropic.
An echo-planar two-dimensional diffusion sequence (TR/TE, 5900/84 ms; field of view 300 × 70 mm; matrix 156 × 192) was used for sagittal spinal DWI using three b-values of 50, 400 and 800 s mm−2 in the same plane and orientation as used in routine sequences. These three b-values were suggested by the manufacturer of the imaging machine, and the present study aimed to compare them to find the strongest diffusion effects. Of note, some previous studies have suggested that using a high diffusion gradient (b-value) may enhance the sensitivity of DWI compared with lower b-values.13,23 The acquisition time for echo-planar two-dimensional diffusion was about 2 min.
Image analysis
The signal intensity of the intervertebral disc and body of the abnormal vertebra was obtained adjacent to the end plates and reported as hypointense, isointense or hyperintense (Figures 1 and 2) relative to the presumed normal marrow1 in both T1W and T2W sequences by the senior radiologist who selected patients for inclusion in the study, and in DW MR images by two other radiologists who were unaware of clinical and laboratory findings.
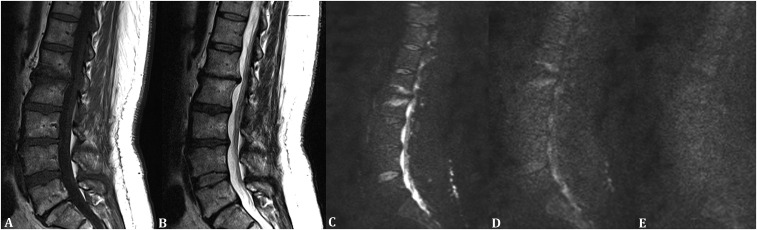
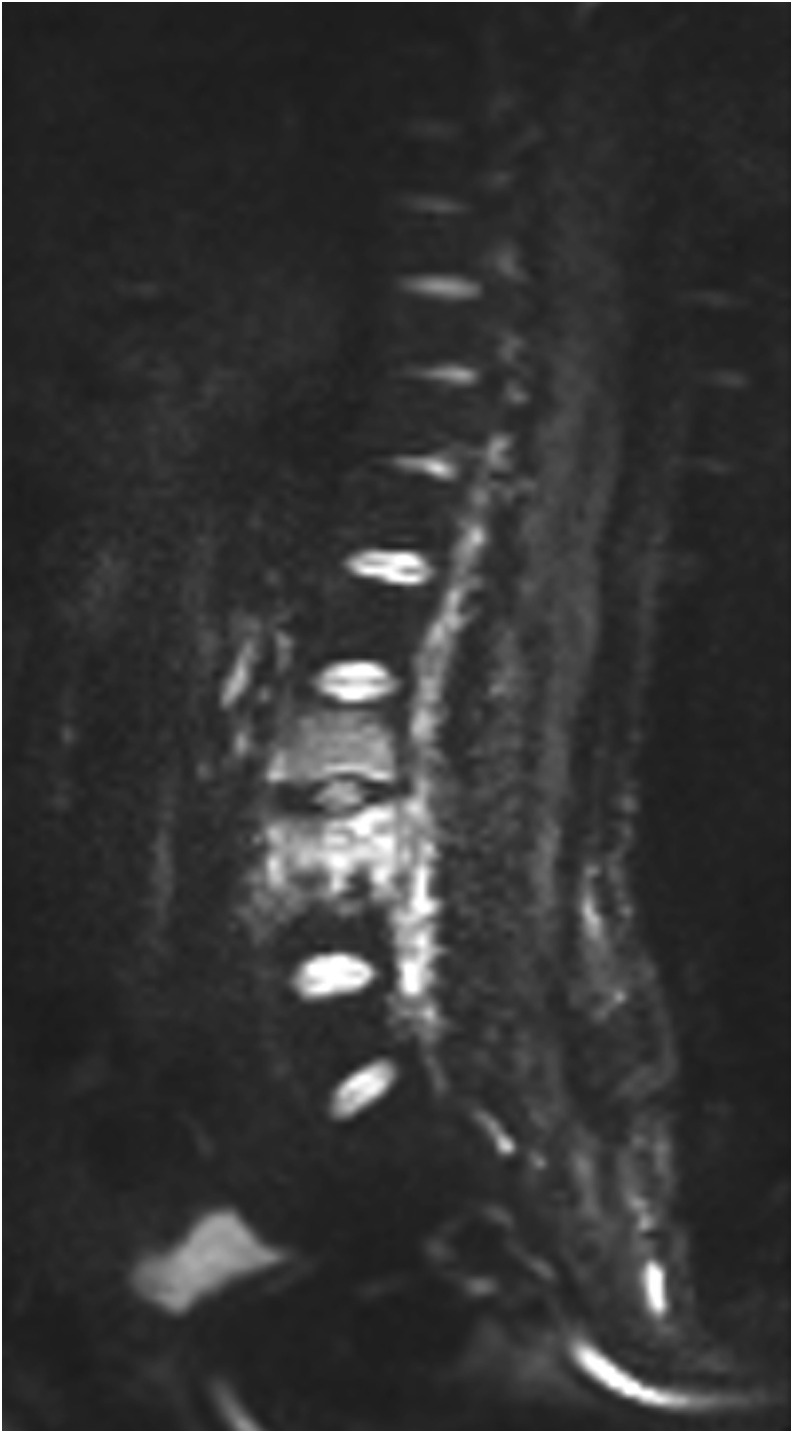
Figure 1.
A 42-year-old female with Modic type 1 degenerative change in the lumbar region. (a) Sagittal T1 weighted MR image showing hypointensity at the involved bone marrow and intervertebral level. (b) Sagittal T2 weighted MR image showing hyperintensity at the involved bone marrow and intervertebral level. (c) Sagittal diffusion-weighted MR image with a b-value of 50 s mm−2 showing hyperintensity at the involved bone marrow and intervertebral level. (d) Sagittal diffusion-weighted MR image with a b-value of 400 s mm−2 showing hyperintensity at the involved bone marrow and intervertebral level. (e) Sagittal diffusion-weighted MR image with a b-value of 800 s mm−2 showing isointensity at the involved bone marrow and intervertebral level.
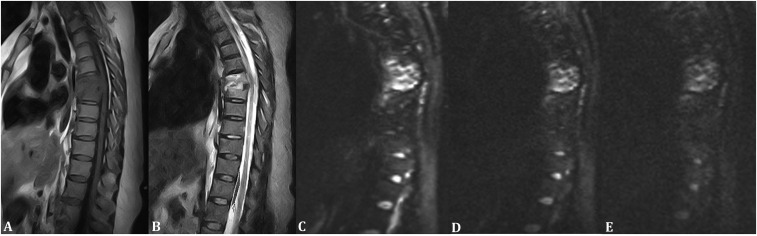
Figure 2.
A 51-year-old female with infectious spondylitis in the lumbar region. (a) Sagittal T1 weighted MR image showing hypointensity at the involved bone marrow and intervertebral level. (b) Sagittal T2 weighted MR image showing hyperintensity at the involved bone marrow and intervertebral level. (c) Sagittal diffusion-weighted MR image with a b-value of 50 s mm−2 showing hyperintensity at the involved bone marrow and intervertebral level. (d) Sagittal diffusion-weighted MR image with a b-value of 400 s mm−2 showing hyperintensity at the involved bone marrow and intervertebral level. (e) Sagittal diffusion-weighted MR image with a b-value of 800 s mm−2 showing hyperintensity at the involved bone marrow and intervertebral level.
An excellent interrater agreement24 was present in reporting signal intensities in DW MR images (Cohen's kappa = 91%). In case of any discrepancy, a third radiologist was arbitrated.
ADC mapping was performed after DWI data were transferred to a workstation (Leonardo Console v. 2.0; Siemens AG Medical Solutions, Forchheim, Germany). We used all the three b-values (i.e. 50, 400 and 800 s mm−2) for ADC calculation. Briefly, on sagittal ADC maps, three circular regions of interest (ROIs) in sizes proportionate to the vertebral lesion were placed on three different locations in the region with abnormal signals (Figure 3) by a medical imaging technologist accompanied with a radiologist. The ROIs that included regions with normal signals or vertebral margins were excluded. The related mean ADC value was generated automatically by the dedicated software provided by the manufacturer of the imaging machine syngo MR B15 (Siemens Healthcare, Erlangen, Germany). For each lesion, the three ADC values were averaged and reported.
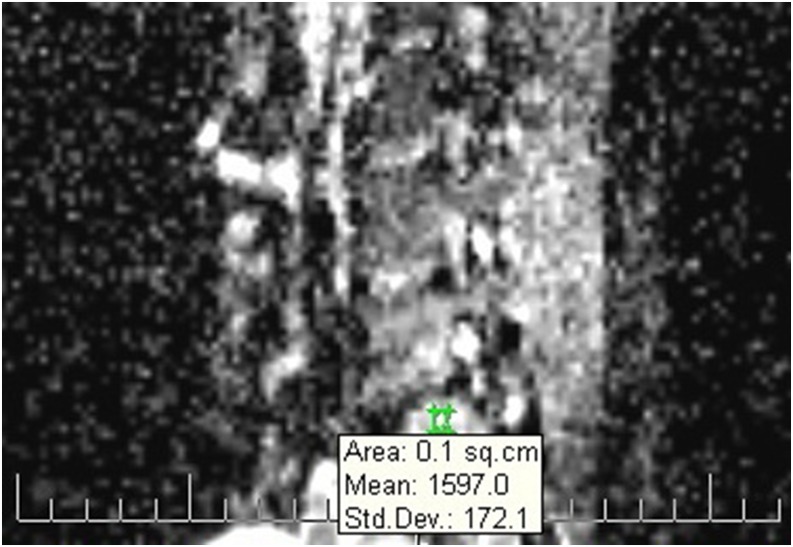
Figure 3.
A sample image illustrating the use of a circular region of interest in a patient with Modic type I change. The related mean apparent diffusion coefficient value was generated automatically (1597.0 in this case). Std. Dev., standard deviation; sq.cm, square centimeter.
To prevent image distortion by susceptibility artefacts, distortion correction filters available on the imaging system were used.
Finally, the same two radiologists who reported signal intensities on DW images were asked to report the presence or absence of “claw sign” (Figure 4)17–19 and/or “amorphous increased signal” (Figure 5)16 on DW MR images acquired using all the three mentioned b-values. Complete (100%) interrater agreements were present in this regard.
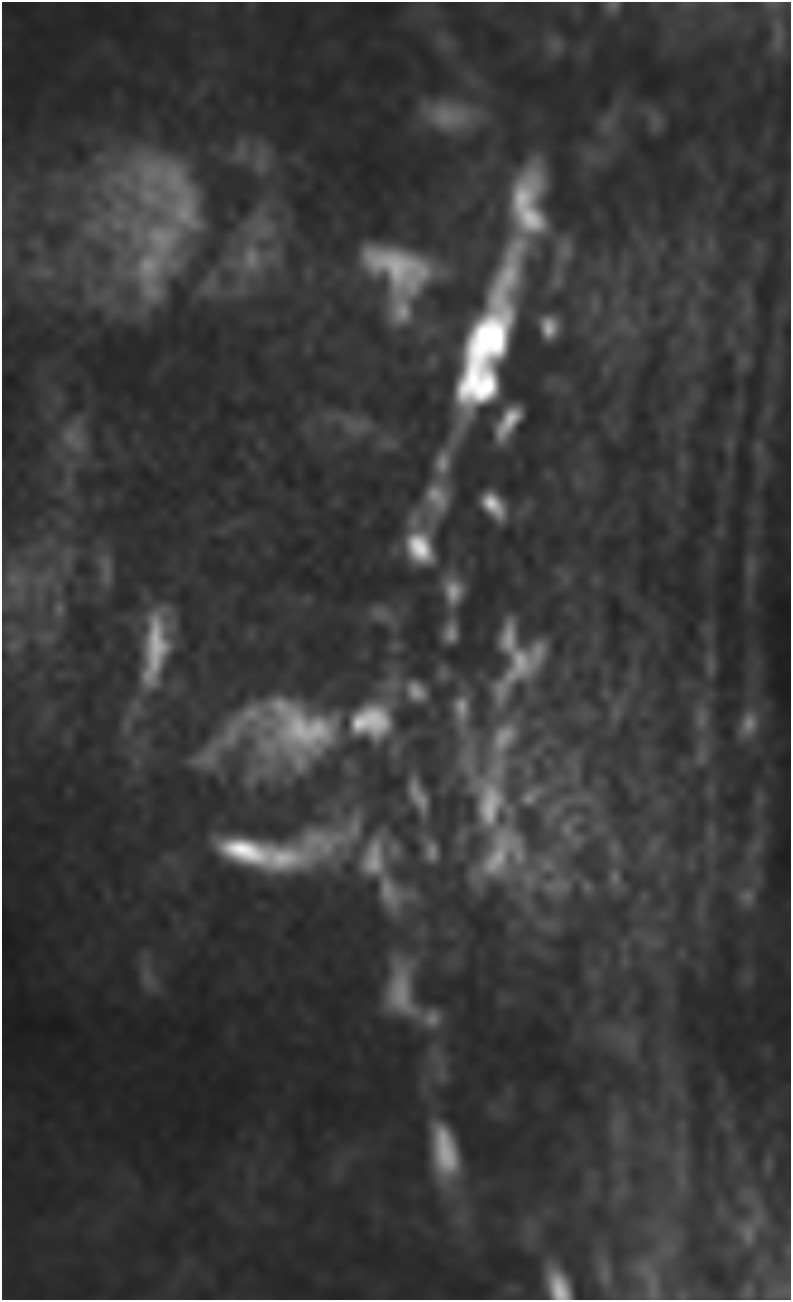
Figure 4.
Sagittal diffusion-weighted MR image with a b-value of 50 s mm−2 in a 68-year-old male with Modic type 1 change and typical claw-sign.
Figure 5.
Sagittal diffusion-weighted MR image with a b-value of 50 s mm−2 in a 39-year-old male with infectious spondylitis and amorphous increased signal.
Statistical analysis
The SPSS® software v. 19.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used for statistical analysis. The distribution of quantitative data was tested using the Kolmogorov–Smirnov analysis. Fisher's exact test, independent samples t-test and independent samples Mann–Whitney U test were used, where appropriate. The receiver operator characteristics curve was used in reporting the optimal cut-off value. Sensitivity, specificity, PPV, NPV and accuracy were also calculated.25 The paired inter- and intraobserver comparisons were analyzed using Cohen's kappa coefficient. A significance level of p-value ≤0.05 was used.
RESULTS
The infectious spondylitis group comprised seven males (58.3%) and five females (41.7%), with the mean age of 52.33 ± 16.00 years (range: 32–79 years). In the group with Modic type 1 change, there were 9 males (29%) and 22 females (71%), with the mean age of 44.32 ± 11.30 years (range: 23–74 years). The two groups were comparable in terms of sex (Fisher's exact test p = 0.09) and age (independent samples t-test p = 0.08).
All the lesions in both groups were reported as hypointense on T1W images and as hyperintense on T2W images (Figures 1(a,b) and 2(a,b)).
Findings on DW MR images using the three different b-values are summarized in Table 1. By using a b-value of 50 s mm−2, all the lesions were reported as hyperintense (Figures 1c and 2c). By using a b-value of 400 s mm−2, all the lesions in the infectious spondylitis group were reported as hyperintense, whereas in the Modic type 1 change group, there were 27 images (87.1%) reported as hyperintense and 4 images (12.9%) reported as isointense (Fisher's exact test p = 0.56) (Figures 1d and 2d). When a b-value of 800 s mm−2 was selected, reported hyperintense lesions were significantly more frequent in the infectious spondylitis group than in the Modic type 1 change group (91.7% vs 3.2%; Fisher's exact test p < 0.001) (Figures 1e and 2e).
Table 1.
Signal intensity of the end plates with infectious spondylitis or Modic type 1 changes compared with normal bone marrow on diffusion-weighted MR sequences using three different b-values
| b-value (s mm−2) | Group | Signal intensity |
p-value | ||
|---|---|---|---|---|---|
| Hyperintense | Isointense | Hypointense | |||
| 50 | Infectious spondylitis | 12 (100) | 0 (0) | 0 (0) | – |
| Modic type 1 | 31 (100) | 0 (0) | 0 (0) | ||
| 400 | Infectious spondylitis | 12 (100) | 0 (0) | 0 (0) | 0.56 |
| Modic type 1 | 27 (87.1) | 4 (12.9) | 0 (0) | ||
| 800 | Infectious spondylitis | 11 (91.7) | 1 (8.3) | 0 (0) | <0.001a |
| Modic type 1 | 1 (3.2) | 26 (83.9) | 4 (12.9) | ||
Values in parentheses indicate percentage.
p ≤ 0.05 is statistically significant.
The mean ADC value in patients with infectious spondylitis was significantly lower than that in patients with Modic type 1 change (1.31 ± 0.12 × 10−3 mm2 s−1; median, 1.30; range, 1.16–1.61 vs 1.80 ± 0.12 × 10−3 mm2 s−1; median, 1.76; range, 1.61–1.97; independent samples Mann–Whitney U test p < 0.001).
Using the receiver operator characteristics curve analysis, the optimal cut-off ADC value in differentiating infectious spondylitis from Modic type 1 change was set at 1.52 × 10−3 mm2 s−1 with an area under the curve of 0.99 (95% confidence interval: 0.98–1.00; p < 0.001), a sensitivity of 91.7% and a specificity of 100%.
Claw sign (Figure 4) was present on all DW MR images of the patients with Modic type 1 change, whereas no case with infectious spondylitis showed this sign (Fisher's exact test p < 0.001). Amorphous increased signal (Figure 5), by contrast, was found only on DW MR images of the patients with infectious spondylitis (100% vs 0%; Fisher's exact test p < 0.001).
Indicators of the diagnostic performance of the studied variables in differentiating infectious spondylitis from Modic type 1 change are set out in Table 2.
Table 2.
Sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV) and accuracy of different study variables on diffusion-weighted MR sequences in differentiating infectious spondylitis from Modic type 1 change
| Parameter | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| Signal intensity | |||||
| b-value: 50 s mm−2 | 100 | 0 | 27.9 | 0 | 27.9 |
| b-value: 400 s mm−2 | 100 | 12.9 | 30.8 | 100 | 37.2 |
| b-value: 800 s mm−2 | 91.7 | 96.8 | 91.7 | 96.8 | 95.3 |
| ADC (>1.52 × 10−3 mm2 s−1) | 91.7 | 100 | 100 | 96.9 | 97.7 |
| Claw-sign | 100 | 100 | 100 | 100 | 100 |
| Amorphous increased signal | 100 | 100 | 100 | 100 | 100 |
ADC, apparent diffusion coefficient.
Figures indicate percentage.
DISCUSSION
End plate changes frequently spotted on spinal images are usually associated with degeneration. Sometimes, however, it is difficult to distinguish between degenerative changes and infection relying only on conventional imaging findings, clinical presentations and laboratory derangements.26 This is a clinically important issue, because infectious spondylitis is far more serious than degeneration in terms of morbidity.22
Although various imaging techniques such as plain radiography, CT and radionuclide studies have been tried to diagnose spinal infections, they are generally inaccurate in acute cases.21,27 MRI has been found the most sensitive (94%) and specific (93–97%) imaging modality for early detection of spinal infection.27 When a conventional sequence is used, however, a repeat study in the following 2 weeks is usually necessary in highly suspicious cases with equivocal initial findings.21
In line with previous reports,1,19,28 signals of abnormal vertebrae with degeneration or infection were similar in the present work, namely hypointense on T1W images and hyperintense on T2W images. In healthy adults, the bone marrow contains 20–70% fatty tissue with nearly 7% increase per decade of life. In the case of infection, replacement and depletion of fatty bone marrow occurs, leading to hypointense signal on T1W images and hyperintense signal on T2W images.26 Because of this similarity between degeneration and infection in routine MR sequences, differential diagnosis mandates employment of more sensitive surrogate imaging methods.19
In contrast to conventional MRI, DW sequences provide microscopic and dynamic information.29 The latter could be used for biological tissue characterization through exploiting random motion of water protons in that tissue.30 Few studies, hitherto, have examined usefulness of DW MRI in differentiating Modic type 1 change from infectious spondylitis. Buyn28 examined 10 patients with pyogenic spondylitis and 50 patients with erosive osteochondritis. Using a b-value of 165 s mm−2, all patients with infection had hyperintense signals on DW images. Park et al19 analyzed spin-echo and DW MR images of 8 patients with Modic type 1 change and 14 cases with infectious spondylitis. On DW images with a b-value of 165 s mm−2, 11 infectious cases were hyperintense and the 3 others were hypointense. All cases with degenerative changes, however, were hypointense. Balliu et al8 examined 14 patients with acute spondylodiscitis using DWI with a b-value of 500 s mm−2. Again, hyperintensity was reported in all patients. In a retrospective investigation, Oztekin et al1 reviewed radiology records of 27 patients with Modic type 1 change and 18 patients with spondylodiscitis. On DW MR images with a chosen b-value of 150 s mm−2, all patients with spondylodiscitis were hyperintense, and the remaining cases with Modic type 1 change were hypointense. Eguchi et al21 studied 16 patients with end plate abnormalities apparent on MR images of the lumbar spine, including 5 patients with infectious spondylitis and 4 patients with Modic type 1 change. Using a b-value of 1000 s mm−2, DWI revealed hyperintensity in the group with infectious spondylitis and isointensity/hypointensity in cases with degeneration.
DW images are generated by using bipolar, large gradient, magnetic fields. A b-value denotes the amount of diffusion weighting and using larger b-values leads to higher sensitivity in differentiating between regions of high and low diffusion.31,32 Choosing a correct b-value is pivotal for obtaining a proper DW MR image because it determines assessment of perfusion and cellular density in a selected region of a biological tissue.33,34 All the mentioned studies examined the usefulness of DWI in differentiating between infectious and degenerative end plate changes employing different b-values. It is not clear whether using different b-values could influence the accuracy of DWI for this purpose. Using lower b-values (50 and 400 s mm−2) in the present study proved not to be sufficiently discriminative (Figures 1(c,d) and 2(c,d)). On DW MR images with a larger b-value (400 s mm−2), however, all cases with acute infectious spondylitis showed hyperintensity, whereas in the group with Modic type 1 change only isointense/hypointense signals were observed (Figures 1e and 2e).
Accuracy of DW sequence could be compromised by T2-shine-through effect.35 The fact that majority of our cases showed hyperintensity on both T2W and DW images with low b-values reflects the presence of this effect. Suppose a pair of DW images is obtained at b0 = 0 and b1 > 0. The ADC value can be calculated as
where S0 and S1 are signal intensities of the images obtained at b0 and b1, respectively.36 Signal attenuation on DW images at a given b-value is influenced by both diffusion and perfusion. Since the signal attenuation of the perfusion component on DW images takes place mostly with low b-values, when a larger gradient factor (b-value) is chosen, signals from the perfusion component could be eliminated, leaving a DW image that largely reflects the diffusion component. Therefore, using higher b-values attenuates interference from T2 at the expense of reducing signal-to-noise ratio.33,34 Such reduction, however, did not negatively affect the principal objective of the present work (Table 2). Nevertheless, a possible solution to wrinkle out T2-shine-through effect without changing signal-to-noise ratio is using quantitative analysis of DWI, such as calculating ADC.15,33
The mean/median ADC value in infectious spondylitis ranged between 0.79 and 1.16 × 10−3 mm2 s−1 in previous reports.9,37,38 It was 1.80 ± 0.12 × 10−3 mm2 s−1 in our patients with degeneration, significantly higher than that in the other group with infection (1.31 ± 0.12 × 10−3 mm2 s−1; p < 0.001). In a similar study performed by Dallaudière et al,38 ADC values were compared between patients with spondylarthritis axial active inflammatory lesions (n, 27) and those with Modic type 1 changes (n, 22). They found significantly higher ADC values for axial active inflammatory lesions than for Modic type 1 changes (median: 0.79 × 10−3 mm2 s−1 vs 0.59 × 10−3 mm2 s−1). In conformity with our results, they concluded that DWI is a sensitive and fast sequence that could help to discriminate between the two conditions. By contrast, Eguchi et al21 reported a lower mean ADC value in cases with degeneration (Modic type 1, type 2 and type 3) than in patients with infection (0.62 ± 0.32 × 10−3 mm2 s−1 vs 1.07 ± 0.12 × 10−3 mm2 s−1).
It should be borne in mind that larger ADC values indicate an increased diffusion of water in a sampled region on the ADC map (or the ROI) by the examiner. Conversely, lower ADC values correspond to ROIs with lower diffusion.39 In Modic type 1 change, the water content of a depleted bone marrow increases. This condition, in turn, leads to an increase in the extracellular volume fraction, a raised ADC value, and development of low signal intensity on DW MR images. Conversely, in acute infectious spondylitis densely infiltrated inflammatory cells reduces the extracellular volume and results in low ADC values and documentation of high signal intensity on DW MR sequences.1,9,28 Therefore, the discrepancy in the study of Eguchi et al21 was that despite documentation of a significantly higher rate of hyperintensity on DW images of patients with infectious spondylitis than those with degenerative changes, the mean ADC value, at the same time, was significantly higher in the same group of patients.
It has been shown that when spinal infection is not acute or when antimicrobial therapy was already started before DW study, ADC values are expected to be less than that in cases with acute, untreated infection; possibly due to the regression of the inflammatory activity and resolution of local oedema by the time of treatment.31 To the best of our knowledge, the present study is the first one that took this critical factor into consideration.
Our optimal cut-off value of ADC for distinguishing lumbar spine degeneration from infection was 1.52 (sensitivity, 100%; specificity, 91.7%). This figure is similar to that (1.3 × 10−3 mm2 s−1) calculated by Pui et al.9 Using this cut-off point, the sensitivity, specificity, PPV, NPV and accuracy of ADC for characterizing between Modic type 1 changes and infectious spondylitis were 91.7%, 100%, 100%, 96.9% and 97.7%, respectively. Comparing these figures with those resulted from DWI with a b-value of 800 s mm−2 (91.7%, 96.8%, 91.7%, 96.8% and 95.3%, respectively) indicates superiority of ADC value over DWI in differentiating between degeneration and infection in the end plates.
On DW images, a restricted diffusion at the boundary of Modic type 1 changes and the normal marrow has been reported and called as claw sign.16 The presence of this well-defined, high signal claw and diffusion restriction at the advancing border of the proliferative process has been reported to be consistent with degenerative disease rather than infection in the end plates with a very high PPV and NPV.17–20 By contrast, the presence of amorphous increased signal on DW images has been suggested as a good indicator of osteomyelitis and infection.16 In conformity with these reports, the claw sign was present on all our DW images obtained with lower b-values (more conspicuous with 50 s mm−2) in patients with Modic type 1 changes, whereas no case with infectious spondylitis demonstrated this sign on related DW images. Amorphous increased signal, by contrast, was only evident on DW images obtained with lower b-values in patients who suffered from infectious spondylitis. Interestingly, the sensitivity, specificity, PPV, NPV and accuracy of both signs for characterizing between Modic type 1 changes and infectious spondylitis were all 100%. This is the first study in the literature that compares the utility of DWI (using different b-values), ADC value, the claw sign and amorphous increased signal simultaneously in differentiating lumbar spine acute infection from degeneration.
This work bears some limitations that need to be acknowledged here. Bone biopsy is known as the gold standard in differentiating infectious spondylitis from degenerative changes in the end plates.1,21 For obvious reasons, it was not possible to obtain biopsy samples in patients with degeneration in the present study. However, it has been reported that clinical and radiological follow-up can be relied on in such cases with equivocal imaging findings at presentation.1,21 Long follow-ups of patients in the present study (at least 2 years) is an important point of strength that almost fully compensates the mentioned limitation.
Although not routinely used in clinical setting,38 dynamic contrast-enhanced MRI has been suggested as an optimal imaging technique in quantifying spondyloarthritic inflammatory changes, especially during the posttherapy period.40 Comparing the results of dynamic contrast-enhanced MRI to DWI/ADC findings in patients with equivocal diagnosis of lumbar vertebral infection/degeneration might be interesting in future studies.
A rather small sample size of patients with infectious spondylitis due to its rarity is another potential limitation of the present prospective study. Although this shortcoming could potentially decrease the power of the ADC cut-off value in differentiating infection from degeneration, the relevant results in the present work were striking, indicating that the effect of this limitation was, if any, minimal.
Last but not least, it should be noted that infectious spondylitis and Modic type 1 changes might share some physiopathological features that may interfere with discriminative capability of DWI. For example, the inflammatory nature of Modic type 1 changes has been suggested previously.5 In addition, a concomitant presence of pyogenic spondylitis has been reported in 4% of patients with Modic type 1 changes with no external manifestation of infection.41 In some recent studies, even the possibility of an infectious aetiology has been raised in patients with Modic type 1 changes.2,42 These possibilities need to be elucidated in future studies.
CONCLUSIONS
This study showed that DWI is an accurate method in distinguishing Modic type 1 changes from acute infectious spondylitis only if large b-values (800 × 10−3 mm2 s−1 in the present work) are used. When lower b-values are chosen, the claw sign and amorphous increased signal are very helpful in this regard. The discriminative accuracy of quantitative analysis (ADC value) lies between those of DWI with large b-values and in using claw sign/amorphous increased signal.
FUNDING
The author Mohammad Hossein Daghighi is the main funder of this work.
Contributor Information
Mohammad Hossein Daghighi, Email: medicorelax3@yahoo.com.
Masoud Poureisa, Email: lily_rasouli2005@yahoo.com.
Mohsen Safarpour, Email: mohsensfr@yahoo.com.
Razieh Behzadmehr, Email: a.fakhrjou2009@yahoo.com.
Daniel F Fouladi, Email: Fouladi.daniel@yahoo.com.
Ali Meshkini, Email: medicorelax@gmail.com.
Mojtaba Varshochi, Email: dougan.pierre@yahoo.com.
Ali Kiani Nazarlou, Email: dr.rafikhah@yahoo.com.
REFERENCES
- 1.Oztekin O, Calli C, Kitis O, Adibelli ZH, Eren CS, Apaydin M, et al. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol 2010; 44: 97–102. doi: 10.2478/v10019-010-0006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedderkopp N, Thomsen K, Manniche C, Kolmos HJ, Secher Jensen T, Leboeuf Yde C. No evidence for presence of bacteria in modic type I changes. Acta Radiol 2009; 50: 65–70. doi: 10.1080/02841850802524485 [DOI] [PubMed] [Google Scholar]
- 3.Rahme R, Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol 2008; 29: 838–42. doi: 10.3174/ajnr.A0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoda K, Ida M, Murakami Y, Harada J, Tada S. MR imaging of degenerative lumbar disc disease emphasizing on signal intensity changes in vertebral body. [In Japanese.] Nihon Igaku Hoshasen Gakkai Zasshi 1992; 52: 1611–9. [PubMed] [Google Scholar]
- 5.Fayad F, Lefevre-Colau MM, Rannou F, Quintero N, Nys A, Macé Y, et al. Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J 2007; 16: 925–31. doi: 10.1007/s00586-006-0301-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar JA, Sandoe JA, Rao AS, Crimmins DW, Baig W, Rankine JJ. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol 2010; 65: 974–81. doi: 10.1016/j.crad.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 7.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009; 39: 10–7. doi: 10.1016/j.semarthrit.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Balliu E, Vilanova JC, Peláez I, Puig J, Remollo S, Barceló C, et al. Diagnostic value of apparent diffusion coefficients to differentiate benign from malignant vertebral bone marrow lesions. Eur J Radiol 2009; 69: 560–6. doi: 10.1016/j.ejrad.2007.11.037 [DOI] [PubMed] [Google Scholar]
- 9.Pui MH, Mitha A, Rae WI, Corr P. Diffusion-weighted magnetic resonance imaging of spinal infection and malignancy. J Neuroimaging 2005; 15: 164–70. doi: 10.1111/j.1552-6569.2005.tb00302.x [DOI] [PubMed] [Google Scholar]
- 10.Chan JH, Peh WC, Tsui EY, Chau LF, Cheung KK, Chan KB, et al. Acute vertebral body compression fractures: discrimination between benign and malignant causes using apparent diffusion coefficients. Br J Radiol 2002; 75: 207–14. doi: 10.1259/bjr.75.891.750207 [DOI] [PubMed] [Google Scholar]
- 11.Hatipoglu HG, Selvi A, Ciliz D, Yuksel E. Quantitative and diffusion MR imaging as a new method to assess osteoporosis. AJNR Am J Neuroradiol 2007; 28: 1934–7. doi: 10.3174/ajnr.A0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raya JG, Dietrich O, Reiser MF, Baur-Melnyk A. Methods and applications of diffusion imaging of vertebral bone marrow. J Magn Reson Imaging 2006; 24: 1207–20. doi: 10.1002/jmri.20748 [DOI] [PubMed] [Google Scholar]
- 13.Bozgeyik Z, Ozgocmen S, Kocakoc E. Role of diffusion-weighted MRI in the detection of early active sacroiliitis. AJR Am J Roentgenol 2008; 191: 980–6. doi: 10.2214/AJR.07.3865 [DOI] [PubMed] [Google Scholar]
- 14.Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 2006; 26(Suppl. 1): S205–23. doi: 10.1148/rg.26si065510 [DOI] [PubMed] [Google Scholar]
- 15.Conturo TE, McKinstry RC, Aronovitz JA, Neil JJ. Diffusion MRI: precision, accuracy and flow effects. NMR Biomed 1995; 8: 307–32. doi: 10.1002/nbm.1940080706 [DOI] [PubMed] [Google Scholar]
- 16.Tanenbaum LN. Diffusion imaging in the spine. Appl Radiol 2011; 40: 9–11. [Google Scholar]
- 17.Poplawski MM, Pawha P, Naidich TP, Tanenbaum LN, eds. Diffusion-weighted MRI (DWI) “claw sign” is useful in differentiation of infectious from degenerative Modic I signal changes of the spine. Proceedings of the American Society of Neuroradiology 50th Annual Meeting; 2012; New York, NY. [DOI] [PMC free article] [PubMed]
- 18.Kwon JW, Yoon YC, Choi SH, Jung JY, Choe BK, Yoon C. MR imaging for the differentiation of early infectious spondylitis and modic Type I change in the lumbar spine. J Korean Soc Radiol 2010; 62: 563–70. doi: 10.3348/jksr.2010.62.6.563 [DOI] [Google Scholar]
- 19.Park WK, Byun WM, Choi JH. The usefulness of diffusion-weighted MR imaging for differentiation between degenerative spines and infectious spondylitis. J Korean Soc Magn Reson Med 2002; 6: 152–57. [Google Scholar]
- 20.Patel KB, Poplawski MM, Pawha PS, Naidich TP, Tanenbaum LN. Diffusion-weighted MRI “claw sign” improves differentiation of infectious from degenerative modic type 1 signal changes of the spine. AJNR Am J Neuroradiol 2014; 35: 1647–52. doi: 10.3174/ajnr.A3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eguchi Y, Ohtori S, Yamashita M, Yamauchi K, Suzuki M, Orita S, et al. Diffusion magnetic resonance imaging to differentiate degenerative from infectious endplate abnormalities in the lumbar spine. Spine (Phila Pa 1976) 2011; 36: E198–202. doi: 10.1097/BRS.0b013e3181d5ff05 [DOI] [PubMed] [Google Scholar]
- 22.Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, Von Schulthess GK. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol 2002; 179: 1151–7. doi: 10.2214/ajr.179.5.1791151 [DOI] [PubMed] [Google Scholar]
- 23.Meurin A, Cernicanu A, Molinier S, Menegon P, Barreau X, Berge J, et al. Diffusion-weighted MR imaging of the spine and cord. [In French.] J Radiol 2010; 91(3 Pt 2): 352–66; quiz 67–8. [DOI] [PubMed] [Google Scholar]
- 24.Fleiss JL, Levin B, Paik MC, Fleiss J. Statistical methods for rates and proportions. 3rd edn. NJ: Wiley-Interscience; 2003. [Google Scholar]
- 25.Akobeng AK. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr 2007; 96: 338–41. doi: 10.1111/j.1651-2227.2006.00180.x [DOI] [PubMed] [Google Scholar]
- 26.Herneth AM, Dominkus M, Kurtaran A, Lang S, Rand T, Kainberger F. Bone metastases: new trends in diagnostic imaging. [In German.] Wien Med Wochenschr Suppl 2002; 92–4. [PubMed] [Google Scholar]
- 27.Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985; 157: 157–66. doi: 10.1148/radiology.157.1.3875878 [DOI] [PubMed] [Google Scholar]
- 28.Buyn WM. Diffusion-weighted MR imaging of vertebral bone marrow: differentiation of degenerative spines and spondylitis involving bone marrow adjacent to end plates. Proc Int Soc Magn Reson Med 2001; 9: 1626. [Google Scholar]
- 29.Baur A, Dietrich O, Reiser M. Diffusion-weighted imaging of bone marrow: current status. Eur Radiol 2003; 13: 1699–708. doi: 10.1007/s00330-003-1873-0 [DOI] [PubMed] [Google Scholar]
- 30.Boraschi P, Donati F, Gigoni R, Salemi S, Bartolozzi C, Falaschi F. Diffusion-weighted MRI in the characterization of cystic pancreatic lesions: usefulness of ADC values. Magn Reson Imaging 2010; 28: 1447–55. doi: 10.1016/j.mri.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 31.Bozgeyik Z, Onur MR, Poyraz AK. The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant Imaging Med Surg 2013; 3: 269–78. doi: 10.3978/j.issn.2223-4292.2013.10.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007; 188: 1622–35. doi: 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol 2007; 188: 409–14. doi: 10.2214/AJR.05.1918 [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol 2006; 187: 1521–30. doi: 10.2214/AJR.05.0778 [DOI] [PubMed] [Google Scholar]
- 35.Castillo M, Arbelaez A, Smith JK, Fisher LL. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol 2000; 21: 948–53. [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505. doi: 10.1148/radiology.168.2.3393671 [DOI] [PubMed] [Google Scholar]
- 37.Fawzy F, Tantawy HI, Ragheb A, Hashem SA. Diagnostic value of apparent diffusion coefficient to differentiate benign from malignant vertebral bone marrow lesions. Eur J Radiol 2013; 44: 265–71. doi: 10.1016/j.ejrnm.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 38.Dallaudière B, Dautry R, Preux PM, Perozziello A, Lincot J, Schouman-Claeys E, et al. Comparison of apparent diffusion coefficient in spondylarthritis axial active inflammatory lesions and type 1 modic changes. Eur J Radiol 2014; 83: 366–70. doi: 10.1016/j.ejrad.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 39.Beattie PF, Morgan PS, Peters D. Diffusion-weighted magnetic resonance imaging of normal and degenerative lumbar intervertebral discs: a new method to potentially quantify the physiologic effect of physical therapy intervention. J Orthop Sports Phys Ther 2008; 38: 42–9. doi: 10.2519/jospt.2008.2631 [DOI] [PubMed] [Google Scholar]
- 40.Mandl P, Navarro-Compán V, Terslev L, Aegerter P, van der Heijde D, D'Agostino MA, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74: 1327–39. doi: 10.1136/annrheumdis-2014-206971 [DOI] [PubMed] [Google Scholar]
- 41.Ohtori S, Koshi T, Yamashita M, Yamauchi K, Inoue G, Suzuki M, et al. Existence of pyogenic spondylitis in Modic type 1 change without other signs of infection: 2-year follow-up. Eur Spine J 2010; 19: 1200–5. doi: 10.1007/s00586-010-1358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 2013; 22: 690–6. doi: 10.1007/s00586-013-2674-z [DOI] [PMC free article] [PubMed] [Google Scholar]