Abstract
Particle therapy of moving targets is still a great challenge. The motion of organs situated in the thorax and abdomen strongly affects the precision of proton and carbon ion radiotherapy. The motion is responsible for not only the dislocation of the tumour but also the alterations in the internal density along the beam path, which influence the range of particle beams. Furthermore, in case of pencil beam scanning, there is an interference between the target movement and dynamic beam delivery. This review presents the strategies for tumour motion monitoring and moving target irradiation in the context of hadron therapy. Methods enabling the direct determination of tumour position (fluoroscopic imaging of implanted radio-opaque fiducial markers, electromagnetic detection of inserted transponders and ultrasonic tumour localization systems) are presented. Attention is also drawn to the techniques which use external surrogate motion for an indirect estimation of target displacement during irradiation. The role of respiratory-correlated CT [four-dimensional CT (4DCT)] in the determination of motion pattern prior to the particle treatment is also considered. An essential part of the article is the review of the main approaches to moving target irradiation in hadron therapy: gating, rescanning (repainting), gated rescanning and tumour tracking. The advantages, drawbacks and development trends of these methods are discussed. The new accelerators, called “cyclinacs”, are presented, because their application to particle therapy will allow making a breakthrough in the 4D spot scanning treatment of moving organs.
INTRODUCTION
Hadron beams are predestined to be used for highly conformal cancer treatment owing to their unique properties. The main advantages of protons and heavier ions compared with conventional radiation such as X-rays or γ-rays are finite range and different depth dose distribution with a small entrance dose and an evident maximum Bragg peak, whose location at a specific depth in the tissue is determined by the particle energy.1–4 Thanks to these features, it is possible to treat deep-seated tumours with maximal protection of the surrounding healthy tissues.4,5 In case of megavoltage X-ray beams used in conventional teletherapy, after a short build-up region, the energy deposition decreases exponentially with increasing depth in tissue, so that the dose is higher near the surface than at the depth of the tumour. In order to increase the dose delivered to the target and minimize the dose received by surrounding tissues, few beams overlapping in target volume must be used.6 Hadron beams are well localized in three dimensions (3D), so the region of maximum energy deposition can be positioned within the target for each beam direction, which gives the opportunity to obtain very good 3D dose conformation.7 In comparison with photon stereotactic body radiotherapy, the application of proton stereotactic body radiotherapy contributes to a significant reduction of the dose received by the chest wall, lung and critical structures and simultaneously allows maintaining the same dose coverage of the planning target volume (PTV).8 Carbon ions, owing to the higher linear energy transfer and greater relative biological effectiveness than photons and protons, are intended for the treatment of tumours that are radioresistant and have greater ability to repair sublethal damage.9–11 Unfortunately, the precision of irradiation is strongly affected by interfractional and intrafractional motions of the clinical target volume (CTV). Also, setup errors have a negative impact on treatment quality. It has been proven that dose distributions for carbon ions are more sensitive to setup errors than those obtained for photon intensity-modulated radiotherapy.12 Moreover, the expected setup uncertainties are different for various tumour sites.12,13 One should therefore remember that only great exactness of patient positioning and high accuracy of tumour targeting allow to take advantage of the benefits of particle beams. The degree of influence of organ and tumour motion on the dose distribution depends on the applied irradiation method. In general, in hadron therapy, one can distinguish two different beam delivery systems: passive beam-shaping technique and active scanning. In the first-mentioned method, a beam with constant energy and intensity is spread over the longitudinal direction by ridge filters or modulator wheels, creating a spread-out Bragg peak and broadened laterally by a scattering system to obtain a uniform, flat transversal profile.10,14 Finally, the broad beam is shaped by patient-specific passive devices like collimators and compensators to accurately irradiate the irregular tumour volume.1,9 It is worth noting that the passive technique has several disadvantages, such as: the impossibility of tailoring the dose to the proximal end of the target, the requirement for expensive custom-made patient beam-modifying accessories and beam contamination with light nuclear fragments and neutrons produced as a result of the nuclear interaction between the particles and the material of beam-modifying devices.1,3,13,15,16 These drawbacks do not occur in fully active scanning techniques, in which the narrow pencil beam irradiates successively the small, spatial tumour elements.3,14,16,17 Voxels located at a given depth within an isoenergy layer are filled with an appropriate dose by the beam that is moved in horizontal and vertical directions, thanks to dipole magnets. When the slice is fully scanned, the energy of the particles is changed and the irradiation is repeated for the next tumour cross-section.3,4,13,18 Synchrotrons enable varying energy from pulse to pulse, but in case of cyclotrons, the modification of beam energy is performed by absorbers of variable thickness.14,19,20 It is important to remember that the dose is delivered to voxels by not only the stopping particles, but also the particles going to the deeper layers, and this pre-irradiation has to be taken into account during the process of treatment planning.14,20
Although pencil beam scanning offers a highly conformal treatment of irregular tumours situated in regions which can be externally immobilized (e.g. head, neck and spinal cord areas), its application to the moving targets is very problematic.17 The sensitivity of active system to the organ movement is higher compared with the passive scattering technique because of the possible inconsistency between beam scanning and target motion.13,15,19,21,22 Local underdosage of the CTV or overdosage of neighbouring tissues caused by this interference effect can be unacceptable.18,21 Apart from the dislocation of the tumour, patient respiration also triggers off the change in the anatomical structure configuration, which leads to the alterations in the internal density along the beam path and consequently influences the range of particle beams.18,23,24 For example: thick tissue can be temporarily replaced with low-density lung. Change in the length of the radiological path entails unplanned stopping position of charged particles and thus the dose shift from target to surrounding tissue or inhomogeneous dose with hot spots and cold spots within the planned volume.21,25,26 Therefore, it is absolutely necessary to minimize the negative influence of target motion on radiotherapy precision by the proper use of motion compensation techniques. The application of such techniques allows diminishing the CTV–PTV margin and thus improves the quality of treatment.
ORGAN MOTION
The problem of target motion is particularly important during the radiotherapy of tumours located in the thorax and abdomen. In general, one should consider interfractional and intrafractional organ motions. Interfractional motion is related to the degree of bladder and intestine filling on a given day.27 Since it takes place in timescales from minutes to hours, an action is required only before the delivery of each fraction.21 In contrast, the intrafractional motion, which is understood as a change in the organ position from seconds to minutes, causes the real problem in all kinds of external-beam radiotherapy. Breathing and cardiac pulsation considerably influence the treatment of lung, liver and pancreas cancers.28,29 Motion patterns are elaborate with amplitudes even 20 times higher than the uncertainty in static conditions.3,30 Regular changes due to respiration and heartbeat might be considered as systematic intrafraction motions as opposed to stochastic movement, which is random in time and direction and cannot be efficiently predicted.31 Therefore, it is worth looking at the motion problem in the context of particular organs. The intrafractional motion of the prostate is neither static nor stationary and some evidence suggests that it can be considered as a random walk.32 Although the maximal absolute values of intrafractional prostate displacement can be as high as 7.9 mm in craniocaudal (CC), 2.1 mm in left–right (LR) and 11.5 mm in anteroposterior (AP) directions, in 95% of cases, the displacement values do not exceed 5 mm.33 When considering the interfractional prostate motion in patients who are immobilized, the smallest movement occurs in the LR direction and the maximal displacement of >10 mm is observed only in the superior–inferior (SI) direction.34 The variation in pancreas position during quiet breathing is closely linearly correlated with external abdominal movement.31 Even though intrafractional pancreatic motion, which is induced principally by respiration, has the highest amplitude (<20.6 mm) in the CC direction, the AP (<10.1 mm) and LR (<6.7 mm) movements are also important.35 The situation is analogous in case of liver motion, which is most dominant in the CC direction (16.5 ± 5.7 mm), followed by the AP direction (5.3 ± 3.1 mm) and the LR direction (2.8 ± 1.6 mm).36 However, one can remember that the motion amplitude and pattern is conditioned by the site. The kinetics of the liver is influenced by the shape of patient ribcage.27 Tumours situated in the upper liver region nearby the diaphragm are especially susceptible to the motion that can cause the temporary replacement of liver tissue by lung tissue and thus alter the beam range.22 The diaphragm also drives the movements of lung tumours, especially those located in the lower part of the lungs.37 In such an instance, it is possible to correlate the displacement of the tumour with the external motion of the abdomen. The situation is more complicated in the case of cancers situated in the middle and upper lobes. Although their motion is weaker, it is not directly coupled to the chest or abdomen.31 The rotation of the tumour may occur at the periphery of the lung.3 In general, 3D motion pattern is very complex and determined by both patient factors (e.g. respiration regularity and possible alterations caused by the loss of pulmonary function) and tumour features (e.g. site and dimensions).38 Also, heartbeat can influence the lesion position.31 The amplitudes of motion during quiet normal breathing extend from <1 cm (central, superior regions) to 3 cm (lower outer parts).14,38 A retrospective analysis of 166 lung tumours (diverse in terms of location, size and morphology) indicated that the motion magnitude for the 95% of them was <1.34 cm, 0.40 cm and 0.59 cm along the SI, lateral and AP directions, respectively.38 It is worth noting that SI type of lung tumour motion has been already modelled as an asymmetric sine function with a peak-to-peak amplitude of between 10 and 30 mm.
MOTION MONITORING
The precise radiotherapy of moving targets requires monitoring of their location, preferably during each irradiation. Effective motion compensation can be performed only when the tumour site is exactly known. In the currently existing particle treatment centres, daily internal target position is usually verified radiographically, but real-time tracking still presents a challenge. Organ displacement might be directly measured by means of fluoroscopy and internal radio-opaque fiducial markers, electromagnetic detection and radiofrequency transponders implanted in the patient, or in some cases with the help of ultrasonography.21,28,39 It can also be indirectly estimated using correlation models grounded on the external surrogate (e.g. thoracoabdominal surface) movement registered by non-invasive optical devices.28,29,40 Another solution is the prediction of the motion pattern based on respiratory-correlated CT [four-dimensional CT (4DCT)]. It is worth looking at the individual motion-monitoring techniques in the context of their usefulness in hadron therapy.
X-ray systems/fluoroscopy
X-ray imaging systems are normally installed in the treatment rooms of hadron therapy facilities. Aside from the verification of patient positioning, they allow recording time-resolved two-dimensional image sequences.21 One can quote the example of the beam-eye-view fluoroscopy device, which acquires X-ray images synchronized with proton irradiation.41 Another case is the orthogonal (vertical and horizontal directions) X-ray imaging system with moving flat-panel detectors located within the irradiation port for carbon ion scanning therapy.16 The image acquisition might be triggered off by the respiratory sensor and thus superimposed on the breath waveform.37 However, the repeated fluoroscopic imaging performed throughout the treatment has two important drawbacks: additional non-therapeutic imaging dose delivered to the patient and a difficulty with the visualization of soft-tissue organs.21,40,42–45 It should be emphasized that owing to the poor contrast of tumours, the direct observation of the target position is very difficult. The partial solution of the problem of non-therapeutic dose seems to be the reduction of X-ray imaging frequency and the prediction of tumour location in between. The adaptive prediction algorithm based on the autoregressive moving average model can successfully predict even irregular respiratory motions, reduce the tumour localization error and improve the precision of radiotherapy.43 It is important that the system proposed by Ren et al43 gives accurate results after collecting only 20 initial position data points and is able to carry out real-time estimation and update of model parameters.
The better visualization of soft-tissue structures seems to be achieved with the help of implanted fiducial markers. X-ray systems for patient positioning and continuous imaging of moving targets have already been installed in several proton therapy centres, for e.g., at Paul Scherrer Institute (PSI) in Switzerland, at Hokkaido University in Japan and at the Massachusetts General Hospital in USA.41,46,47 Furthermore, fluoroscopy is used in the carbon ion treatment facility at the National Institute of Radiological Sciences in Japan and the application of this technique is also planned at the Kanagawa Cancer Center in Japan.16
Internal fiducial markers
Radio-opaque fiducial markers implanted near the tumour are used to facilitate the fluoroscopic tracking of the movement of low-contrast lesions.28,48 Although commercially available markers are popular in photon radiotherapy, the possibility of their direct implementation in hadron therapy is still tested. As an example, one can mention the PROMETHEUS trial carried out at the Heidelberg Ion Beam Therapy (HIT) Center, where different markers were evaluated for suitability for the treatment of hepatocellular carcinoma using scanned ion beams.49
Clips can be made of gold, platinum, carbon-coated zirconium oxide and alternative materials, which are clearly visible in X-ray imaging.48,49 Markers allow directly characterizing both interfractional and intrafractional motions of tumours located in the lung, pancreas or liver. Such markers are usually implanted via bronchoscopy, bronchial fiberscopy or sometimes percutaneously by means of the special needle.21,48,50,51 A number of applied markers typically range from 3 to 6.35,36,52 Their 3D trajectory can be traced by two fluoroscopy units with a frequency of 30 Hz, which is sufficient to observe both the cardiac and respiratory motions.51–53 In case of real-time tumour-tracking radiotherapy systems, recorded images are compared automatically and the displacement of markers is measured by the pattern-recognition algorithm.51,52 The advantage is that the fully automated process works fast and enables eliminating the interobserver measurement variation.
Although the application of fiducial markers can noticeable increase the treatment accuracy, internal clips also have many disadvantages. The process of marker implantation is invasive, so there is an additional risk of complications such as pneumothorax or haemorrhage.21,28,53 The second drawback is the possibility of unforeseen marker displacement, which might be even >5 mm. One can distinguish different reasons for this dislocation; e.g. marker migration from the implanted position, tumour regression or deformation and swelling of surrounding tissues.52,53 In addition, the loss of synchronization between the marker and organ motion may occur and cause inaccurate target localization during the irradiation process. Therefore, it is necessary to avoid or predict such situations. The insertion of multiple radio-opaque clips around the tumour enables to determine their position by checking the intermarker distance.48 Owing to the fact that gold marker migration decreases with time as a result of fibroblastic changes, it is recommended to start radiotherapy treatment at least 5 days after clips' implantation when they are fixed in the pulmonary parenchyma.50 Another concern is that fiducial markers made of high-Z materials cause unfavourable artefacts in conventional CT scans.39 The inaccurate representation of the electron density and thus Hounsfield units near the inserted clips may result in improper dose calculation.49 Furthermore, metal markers can interact with particle beams (particularly scanned ion beams) and have a considerable impact on the therapy.21 The degree of their influence on the dose distribution, fluence and range of ions depends on the material, thickness and location in the treatment field.49 Only thin markers (<0.5 mm) or those made of relatively low-Z materials, e.g. carbon-coated zirconium oxide clips, may be considered for use in hadron therapy.49 However, before the application, it is necessary to prove their long-term safety and harmlessness to tissues, e.g. lung parenchyma.
Fiducial electromagnetic transponders
Electromagnetic localization of internal transponders is considered as an alternative method of motion detection.39 Such a technique is able to improve the patient positioning and measure a tumour position in real time during treatment. One can cite the example of the tumour location (TULOC) system, which was developed and successfully tested at PSI for the proton treatment of moving tumours.30 It uses an alternating magnetic field (12 kHz) to exchange information between a field generator (six differential coils) and a small implantable sensor (a miniaturized induction coil coated with a thin layer of synthetic material) located near the moving cancer lesion.30
Laboratory tests have shown that the spatial accuracy of the TULOC system is 1–2 mm and the angular accuracy is 0.5–1°, depending on the sensor orientation.30
Another localization system takes advantage of glass-encapsulated, wireless electromagnetic transponders, whose positions in the patient body are monitored continuously at a rate of 10 Hz.54 In this case, the way of operation is somewhat different. Magnetic source (4 coils) generates an oscillating field. After the induction of a resonance in the transponder, the excitation field is turned off. The response signal emitted by the beacon during relaxation is detected by 32 receiver coils localized in the same array as transmitting coils. Array position is followed in real time by an optical tracking device to combine the location of implanted transponders with the isocentre. The submillimetre precision (position error <0.2 mm) of the array transponder measurements has been proven, but the combined accuracy of the whole system (containing magnetic and optical subsystems) in a clinical environment must be experimentally determined.54
Although the above-mentioned electromagnetic tracking systems improved the radiotherapy precision in trial irradiations, work is under way on their implementation to clinical use at particle treatment centres. The fact remains that the process of patient sensor insertion by means of a catheter or a biopsy needle is invasive and can cause side effects.
External surrogate
The situation is totally different in the case of motion monitoring by means of an external surrogate. In this method, the organ or tumour displacement during the treatment is indirectly determined on the basis of the movement of the body surface, e.g. the chest or abdominal wall. It is possible to monitor the whole thoracoabdominal surface, but more often, the optical system trails only the position of the markers placed on the patient skin.28,40 Various non-invasive optical tracking devices are used to localize the markers and reconstruct their trajectory. The most popular option is the system which consists of infrared light-emitting diodes attached to patient body and a position-sensitive semiconductor detector camera.16,18,37 Such a system has already been implemented for motion monitoring during respiratory-gated carbon ion therapy in Japan (at the National Institute of Radiological Sciences) and used as a back-up solution in the ion beam-tracking system developed at Helmholtz Centre for Heavy Ion Research (GSI) in Germany.16,18 In the modified version of the real-time position management respiratory-gating system, the chest wall kinetics is observed during daily treatment with the help of the marker box with two passive infrared reflecting markers. The motion of the box that is situated on the patient chest is tracked with reference to the third stationary marker by an infrared-sensitive video camera.55
Commercially available strain gauge and belt-type pickup sensors or even the industrial laser distance sensors that use triangulation have also been tested for motion management during heavy ion irradiation.18,37 The successive positions of the marker, which are recorded with the acquisition frequency characteristic of the applied system (e.g. 7.9, 30 or 100 Hz), serve as the basis for the respiration waveform curve.16,28,29,40 It is necessary to find the correlation between the surrogate and internal tumour motion. At the first stage of this process, X-ray fluoroscopic images of internal target position and optically measured displacement of the external surface markers are simultaneously collected as a training data set.28,31 The coaching procedure enables establishing the correlation function on the basis of the training information and optimizing the model parameters. Diverse models can be used to infer the internal target position in real time, one can mention a state-space model, an artificial neural network model, fuzzy logic or probabilistic models and different linear or non-linear mathematical correlation models.21,40 The signal-processing algorithms (adaptive filters) are also used for the prediction of future movements based on the past motion.31 It is important to overcome the latency between motion monitoring and data application, because the slow data acquisition, processing speed and reaction time might be responsible for the gating delays during radiotherapy.40,42 Obviously, external/internal correlation has to be verified by episodic, stereoscopic X-ray imaging of the target in order to perceive the modifications of respiratory patterns and reduce uncertainties.28,31,40 It is the potential inconsistency between the surrogate position and actual target location that is the major disadvantage of this method. Patient respiration is not always stationary and the breathing irregularities can result in a phase shift, which is critical to external/internal correlation model accuracy.16,21,40 Another problem can be caused by inadequate immobilization of the surface markers and relaxation or flexing of patient muscles.29 In addition, commercially available strain gauge sensors, which are taped to the skin, can generate instable signals depending on the tape tension.37 The belt-type pickup sensors are not easy to adjust during treatment.37 Despite the imperfection of the system, it is often used for acquiring respiratory traces during both radiotherapy treatment and 4DCT imaging.38
CT motion prediction
4DCT is often used for the assessment of organ motion before proton and carbon ion treatment.25,27,40 The displacement of internal structure is visualized as a function of time. For this purpose, CT images are acquired at multiple breathing phases and matched with the respiratory signal, which is recorded simultaneously by means of external surrogate motion-monitoring system. Tagged CT images are retrospectively sorted and coherent 3D volumes are reconstructed for each breathing phase.27 Consecutive volumes form together 4DCT data for the whole respiratory cycle. Breathing motion is periodic, so its pattern, assessed on the basis of CT examination, can be used for the prediction of future target position at a given time during a radiotherapy session.17 However, one should remember that irregularities in breathing, which frequently occur in real patients, are responsible for geometrical uncertainties and misrepresentation of tumour trajectory.22,56 Owing to the possible interfraction and intrafraction variations in amplitude and phase, the internal motion pattern created on the basis of 4DCT should be updated before each treatment session with reference to the data from in-room radiography and surface imaging systems.28 There is also another option of avoiding irregular breathing. During 4DCT examination and treatment sessions, patients can be instructed how to regulate their breathing by means of audio coaching or special video goggles that display breathing traces in real time.38,55 In general, 4DCT examinations are carried out under free-breathing conditions using multislice CT scanners, collaborating with external respiratory-sensing systems.26,57 Although the best available 4DCT detector is based on 320-multislice CT, which enables acquiring a 13-cm scan range in a single rotation, most treatment centres have less technically advanced scanners with longer acquisition times.26 Therefore, the planning respiratory cycle is reconstructed by averaging data from multiple breathing periods.
It is worth considering what the benefits of 4DCT for the particle therapy of moving targets are. Pre-treatment time-resolved CT allows visualizing the target displacement caused by breathing and also evaluating the chest wall invasion by checking whether the tumour moves independently of the thorax.8 Images recorded for different breathing phases (e.g. end-inspiration or end-expiration) are useful for dose distribution calculation and simulations.23 4DCT can also be used to determine the mid-ventilation scan, which represents the time-weighted mean tumour position. Treatment planning based on this scan allows reducing the required CTV-to-PTV margin in comparison with those assessed with the help of 3DCT data.56
Another form of CT examination, which can be performed before patient treatment, is gated CT. In this case, images are recorded at one motion phase, as a rule at the end of expiration, when the target position is expected to be most stable and reproducible.25 CT scanners work in synchronization with the respiration waveform and the acquisition is triggered off by the gate signal. As for the technical point of view, it can be performed thanks to the possibility of starting X-ray emission at any angular position of the source, which rotates continuously around the patient.37 One should remember that CT imaging increases the radiation dose delivered to the body.57
Novel radiographic techniques
At the leading hadron therapy centres, such as HIT Center, work is going on to develop novel devices that use particle beams for planar or volumetric imaging. Heavy charged particles slow down in the patient body because of energy loss in the Coulomb inelastic interaction with the electrons. Transmission imaging systems utilize protons or carbon ions, which have sufficiently high energy to completely traverse the patient.58 Therefore, accelerator has to be able to deliver particles with an initial energy of greater value than that used in a standard treatment. In the prototype appliance in HIT, the detector comprises a stack of 61 large-area parallel-plate ionization chambers interleaved with removable polymethyl methacrylate absorber plates and readout electronics.2 The channel with the maximum ionization chamber current enables estimating the Bragg peak position, which is necessary for imaging.2 The assets of particle transmission imaging are worth mentioning. Proton or carbon ion CT has a higher density resolution in comparison with standard photon tomography, so images with higher contrast might be obtained using lower doses of radiation.45,58 Not only can this method improve the soft-tissue distinction but also provide information about the stopping properties of the patient body, which is useful for treatment planning and indirect beam range verification.58 Up till now, particle range monitoring has been performed in clinical practice by means of positron emission tomography. β+ emitters are generated in the tissue owing to the collisions between 1H or 12C ions from the beam and atomic nuclei in the patient body.59 In the case of radiotherapy of intrafractionally moving targets, during the assessment of the β+ activity distribution, one should consider the motion-dependent displacement of the positron emitters and upgrade positron emission tomography data simulations using time-resolved (4D) algorithms.60 Proton or carbon ion transmission volumetric imaging may become in future an alternative method of beam range monitoring in the presence of target motion. However, before clinical applications, particle tomography requires a lot of research and development.45
Ultrasonic tumour localization
Among the non-invasive and non-ionizing abdominal organ motion-monitoring systems, ultrasound-based devices certainly deserve attention. Nowadays, they are mainly used for the precise positioning and assessment of the interfractional movements of the prostate.21,39,61 However, research is ongoing on their implementation to track liver cancers in the presence of respiratory motion.44 Real-time volumetric imaging of soft-tissue targets and surrounding structures can be performed during radiotherapy treatment by means of a remotely operated ultrasound system. Such a device, integrated with a medical linear accelerator, has been already built and successfully tested.39 High-quality prostate images were obtained thanks to the telerobotic manipulator, which was able to maintain a constant probe force and suitable pitch angle.39 The commercially available ultrasound-based system for organ localization and repositioning called B-mode acquisition and targeting (BAT) is another example of a technical solution designed to deal with variations of patient position and day-to-day prostate mobility caused by organ motion.61 During patient alignment, two suprapubic ultrasound images are acquired by means of the probe that is mounted on a robotic arm. Afterwards, they are overlaid with patient contours imported from planning CT scans in order to check the displacement of internal structures and calculate the corresponding couch shift necessary to compensate this dislocation. Unfortunately, the interuser differences in the contour alignment process can result in different couch shifts and thus lower the precision of the system.61 Although the vast majority of ultrasound localization systems operate in collaboration with linear accelerators for photon therapy, the usefulness of ultrasound imaging of moving targets in particle therapy has also been proved. A phantom-based experiment has shown that real-time ultrasound motion detection and beam tracking enable considerably reducing the interplay effects in scanned ion beam radiotherapy.62 Apart from the ultrasound beam former and conventional diagnostic probe, the tested device consisted of a special tracking probe with two 5.5-MHz, 64-element phased-array ultrasound transducers encompassing two perpendicular image planes.62 The tracking frame rate of 6.7 Hz (150 ms per frame) of the ultrasound probe was influenced by several factors such as the finite speed of sound and the time required for image processing.63 The time delay due to position reconstruction and data communication (200 ms) was compensated by using an artificial neural network prediction in order to minimize a position error.62,63
Ultrasound techniques are cheap, non-invasive and have the potential to be used in particle therapy for the real-time motion monitoring of the targets located in the abdominal region. The tissue-mimicking phantom studies have shown that the accuracy of the 4D ultrasonic tracking system with the curvilinear probe (bandwidth 4–7 MHz) can be better than 1 mm for 3D sinusoidal motion sequences.44 Unfortunately, such a system is unsuitable for lungs owing to their low density.17 Therefore, ultrasound methods are geared towards tracking of moving targets situated in the abdominal region.
IRRADIATION OF MOVING TARGETS
The optimal choice of motion-monitoring method is the first step in the process of radiotherapy of tumours located in the thoracic or abdominal regions. The second issue is the accurate irradiation of moving targets. The total time of irradiation depends on the applied beam delivery system and many factors; e.g. in case of active scanning, the variation in time results from different total number of spots (typical irradiation time: 1–20 ms per raster point) and varying spill intensities.24,64 Diverse techniques are used for moving target irradiation. On can distinguish such methods as: beam gating, rescanning and beam tracking. Other strategies such as standard PTV or breath-hold techniques were previously considered, but their usefulness in particle therapy is insufficient. Also, abdominal compression by means of a special plate is sometimes performed in patients with liver tumours in order to suppress their motion.36,65 Breath-hold method demands of patients that they stop their breathing several times during the treatment session, which is necessary to deliver the whole prescribed fractional dose.37 These artificial pauses allow arresting the motion and irradiating a static target. However, the reproducibility in target position between different breath stops is uncertain.24 Moreover, the numerous pauses considerably prolong the treatment time.31
Standard PTV techniques, which deal with intrafractional tumour motions by adding margins around the CTV, are often used in conventional radiotherapy.38 In patients with complex motion patterns, the precise prediction of internal margins is improbable; one can only try to assess them with reasonable accuracy on the basis of the previously preformed measurements of maximal target displacement.25 4DCT is helpful in determining the motion amplitudes in each direction throughout the entire respiratory cycle.65 The PTVs have to be expanded to such a degree that they completely cover the volume of the moving target at any time during the irradiation.3 The most obvious drawback is that the normal healthy tissue, which surrounds the tumour, is exposed to the excessive dose and in order to reduce this undesirable effect, the dose might be limited, resulting in a lower tumour control probability.31,56 In the case of scanned particle beams, the treatment of intrafractionally moving tumours using only the standard PTV technique is currently not performed, because this method cannot mitigate the interplay effects between the target motion and dynamic beam delivery.3,21,24,66 However, certain margins are needed when the problem of irradiation of moving targets is solved by means of such techniques as gating or rescanning.21
Gating the irradiation
Gating is commonly used in the particle therapy of intrafractionally moving targets.21,25 The essence of this method is to deliver the dose to the target only when it is localized in the predetermined position (within a gating window).29,52 Therefore, the irradiation is performed in the respiratory phase, which is most reproducible, stable and is characterized by a relatively low organ motion; for example, at the end of exhalation.14,21,37 This means that the real-time monitoring of the respiration cycle is vital to choose the right moment of beam generation. The gate-on period is set by specifying the respiration waveform threshold in consideration of the factors affecting the uncertainty of the target position; e.g. the phase difference between the sensor signal and organ motion.37 Repeated beam-on/beam-off cycles have to be ensured by the accelerator and energy selection system.21 Cyclotrons practically generate a continuous beam, which can be delivered constantly throughout the respiratory gate-on period.67 In case of the synchrotron beam, extraction from the main ring occurs periodically in pulses (discrete “spills”) related to magnet excitation cycles. A single excitation cycle comprises three different phases: acceleration, flat top and deceleration.68 Particles are delivered to the target only during the flat-top period, which is synchronized with the gate-on state.67 Therefore, a high correlation between the magnet excitation cycle and tumour motion pattern is crucial for the effectiveness of respiratory-gated radiotherapy.68 Nowadays, the radiofrequency knockout slow extraction method is commonly used for ion beam extraction from the synchrotron in response to the gate signal.69 Work on the development of a dynamic beam intensity control system with radiofrequency knockout slow extraction has been carried out at the Heavy Ion Medical Accelerator in Chiba synchrotron in order to enable a quick (within several 10 ms) start or stop of beam delivery in the response to a trigger synchronized with patient respiration even in the presence of breathing irregularities.70–72 In the situation when the dose monitor installed on the beam port detects that the particles are delivered beyond the gate, the beam is automatically halted at the time shorter than 100 ms, thanks to the interlock system.37
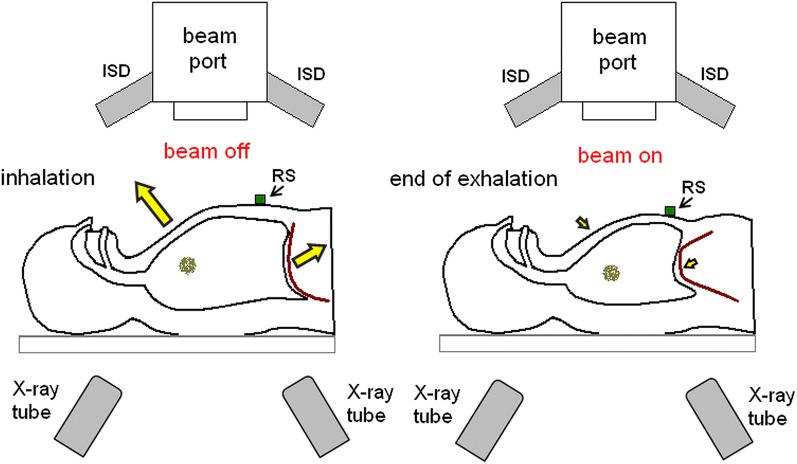
The gating of irradiation carried out with simultaneous monitoring of tumour position (as shown in Figure 1) can significantly improve the precision of therapy of moving targets. Gated spot-scanning proton beam therapy system, which uses real-time imaging of internal fiducial markers implanted in the tumour neighbourhood, works at the Hokkaido University. Real-time imaging is carried out by means of two orthogonal X-ray fluoroscopes installed in the gantry and can be performed simultaneously with proton beam irradiation.46 Another example is the PSI experiment, in which gating of the proton beam performed according to the guidance from the real-time TULOC system allowed obtaining the sharp dose distribution in contrast to noticeable inhomogeneities observed without gating.30 On the other hand, the effectiveness of gating is limited by residual motion within the gating window.29 For scanned beams, this movement may still be responsible for interplay effects.3 The mitigation of residual motion in the gated carbon ion therapy of large liver tumours can be attained by increasing the beam spot size (beam full width at half maximum).73 For the passively scattered technique, inaccurate irradiation at the end of the expiration phase occurs more likely for longer (>4 s) synchrotron magnet excitation cycles.67 The shortening of the gate-on phase improves the conformity, but extends the duration of dose delivery.37 In general, gating strategy considerably prolongs the treatment time because of frequent pauses in irradiation.21,29 For synchrotron-based proton beam that is delivered at 30% respiratory-gated duty cycle using the passive scattering method, the total irradiation time is on an average 2–5 times longer in comparison with non-gated situation.68 Long interruptions in irradiation are not recommended, especially for beam scanning where the exposure time is long.14 An increase in duty cycle of respiratory-gated treatment might be achieved via trailing strategy, which combines image-guided radiation delivery with robust motion-adaptive treatment planning, but this method has been tested only for photon intensity-modulated radiotherapy.29 The next problem is the reproducibility of the positions of internal anatomical structures in consecutive gating periods and a correlation between the target motion and the monitored respiratory waveform.24,25 The gating window during irradiation may differ from that determined based on the average respiratory curve obtained from planning 4DCT. Therefore, even if the breathing pattern is known from 4DCT, it is advisable to check it in real time with the help of a motion-monitoring device. Despite the fact that many treatment centres perform gating based on external surrogate motion monitoring, one should remember that the movement of patient surface does not directly reflect the target displacement.58 Furthermore, the delays of the motion-monitoring system may cause an alteration in the gating window and thus slightly disturb the dose distribution.42 The best option to solve this problem is to predict and correct the gating delays. Such online adaptation of the gating window is advisable for scanned particle beams, where increased margins do not minimize the interplay effect.42
Figure 1.
The gating of irradiation carried out with simultaneous monitoring of tumour position. The optical system trails the motion of the respiratory sensor (RS) placed on the patient chest and tumour displacement is additionally verified by the X-ray system consisting of X-ray tubes and imaging detectors (ISD).
Rescanning
Rescanning (also called repainting) is applied to the scanned beam in order to deliver the dose to intrafractionally moving targets and minimize the negative influence of interplay effects.24 The method utilizes the fact that repeated irradiations of the target lead to statistical dose averaging. Multiple scanning is expected to eradicate the overdosage or underdosage regions and thus provide a homogeneous dose distribution within the CTV.21 The intensity during a single scan is equivalent to the total intensity divided by the number of repetitions.24 Owing to the reduced particle fluence for each field, the proper dose is deposited within the target only when all fields are irradiated together.3 One can distinguish rescanning of successive isoenergy layers from full volumetric rescanning, which is applied to the entire target in 3D.19,21 The time needed to alter the energy and thus shift the Bragg peak position in depth (usually 1–5 s) is longer than the duration of scanning across a single slice (100–300 ms).66 The repainting in depth requires faster changes of beam energy. Unfortunately, it is impossible to obtain such variations using standard synchrotrons.21 The delays occur for cyclotron sources also, because of the need for the insertion of variable-thickness degraders and the adjustment of the beamline tune settings.66 Nowadays, cyclotrons are designed especially for cancer therapy with ultrafast pencil beam scanning. One can cite the example of the compact superconducting isochronous cyclotron C400, which is able to deliver protons (265 MeV/u), carbon and helium ions (400 MeV/u) and offers very good beam intensity control.74 Owing to the fact that such machines still need an energy selection system to alter the beam energy, it is proposed to utilize fast dynamic degraders supported by a range shifter in the nozzle of the beam line.19
A more detailed classification marks out five diverse repainting methods: uniform, random, level, time delay and breath sampling repainting strategies.66 In uniform rescanning, consecutive layers are painted with 1/N of the prescribed dose in the sequence from the deepest to the shallowest slice. This cycle is repeated N-times to deposit the whole dose within the target volume. In random repainting, the cross-sections are irradiated in a random order. The level repainting reduces the treatment time by performing all planned rescans of a single layer before moving on to the next tier. However, in order to reach a clinically acceptable dose error one has to carry out many (>15) paintings, so time saving is not significant.66 The time-delay repainting strategy uses the same irradiation sequence, but additionally introduces a random delay between successive rescans. When the paintings are performed at regular intervals equal to the breathing period divided by the number of rescans, one considers the breath sampling repainting. Evidence suggests that among the previously mentioned techniques, breath sampling repainting is most effective in minimizing dose errors with only a slight increase in treatment time.66 In standard rescanning, there is a necessity for expanded margins in order to reach the planned dose distribution within the target volume.24 Nevertheless, the significant loss of sharp dose gradients at the field borders is the main drawback of this method.17,21 It is not the beam profile but the target motion amplitude that specifies the field gradients for rescanning.3 The solution, which is aimed at improving the precision of rescanning by providing the homogeneity of target coverage with maximal sparing of surrounding tissues, seems to be gated rescanning. A repainting raster-scanning method with respiration-gated irradiation has been already introduced at the new treatment facility at the Heavy Ion Medical Accelerator in Chiba in Japan.69 The technique, called phase-controlled rescanning (PCR), carries out repainting of all spots of a single layer within the gating window.57,75 When one slice is fully irradiated by the pencil beam (with Bragg peak spread-out of 3 mm by the mini ridge filter), the energy is changed and the rescanning is performed for the next layer.26,57 The process is repeated (11 synchrotron energy steps are available) until all target cross-sections obtain the prescribed dose.57 The PCR utilizes advanced technologies such as high-speed scanning magnet (100 mm ms−1 in horizontal and 50 mm ms−1 in vertical directions), extended flat-top operation of the synchrotron and the spill control system designed to deliver an intensity-modulated beam.75 The simulation study showed that in case of liver tumours, gated rescanning is able to ensure good dose conformity and maximal sparing of healthy tissues on the condition that an odd number of at least seven PCRs are performed.22 Another research demonstrated that multiple PCRs with amplitude-based gating allows providing clinically acceptable dose distributions for the lung and liver moving tumours, even in the presence of irregular breathing.57 Nevertheless, the residual interplay effect characteristic of uneven respiration is responsible for small hot spots, which still remain.57 In standard PCR method, the dose rate for each slice is calculated in advance and cannot be adjusted during irradiation. The situation, in which the rescanning of the isoenergy layer is not finished within a gate window, can happen in patients with breathing pattern variations. In such cases, the repainting is completed at the beginning of the following window, which leads to the desynchronization of PCR and deterioration in dose conformity.26 In the response to this problem, the extended phase-correlated rescanning technique was created. Extended PCRs allow maintaining the constant number of rescans in each gating window. If multiple gating windows are required to irradiate the target cross-section, the dose rate is adapted by dividing its initial value by the number of windows.76 In comparison with standard PCR, the extended version of this method provides better dose homogeneity and is suitable for machines with a low maximum dose rate.76 However, the issue of real-time adaptation of irradiation patterns to the variations in the internal target position caused by unplanned changes in patient breathing still needs to be resolved.
Tumour tracking
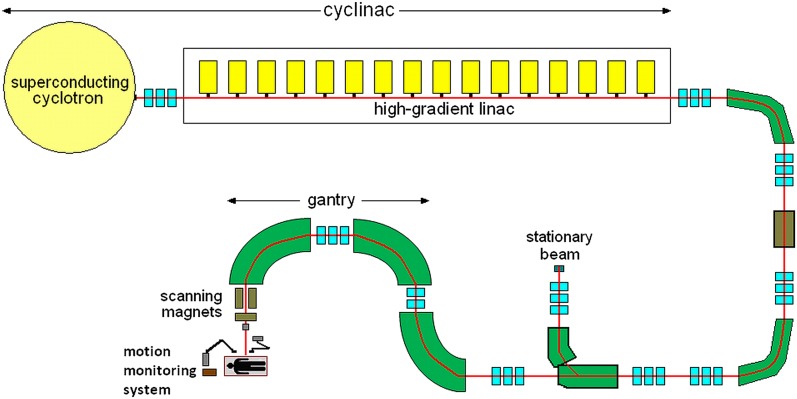
Real-time tumour tracking is the most advanced strategy proposed for the irradiation of intrafractionally moving lesions. The main idea of this method is to dynamically steer the beam and follow the moving target along its 3D trajectory while the patient is breathing naturally.37,40 The irradiation synchronized with the motion requires a real-time measurement of tumour position, a continuous adjustment of the delivery sequence and an adaptation of dosimetry to changing configurations of the target and critical structures.29,31 The unavoidable system latency related to the time necessary for signal processing, communication between devices, hardware response etc. has to be compensated on the basis of the prediction of the movement occurring in this finite time delay.43 The lag of milliseconds is short compared with a typical organ movement period (seconds), so the target displacement occurring during the delay might be predicted based on present motion by means of linear extrapolation method.18 One should remember that the conditions of real-time tracking are fulfilled only then the system response time is considerably shorter than the timescale of clinically relevant movement.31 During the irradiation process, the beam has to be adjusted in 3D. In the tumour-tracking system developed at GSI, the lateral correction of pencil carbon ion beam is performed using scanning magnets by applying appropriate displacements to the initial raster points.18,40 However, it is the fast range adaptation by shifting the Bragg peak correctly in depth that really causes the problem. Sufficiently quick energy variations cannot be achieved directly using a traditional synchrotron.3,17 The alteration of the beam energy by synchrotron requires about 3 s, which is more than 1–2 s needed for the irradiation of about 100–1000 raster points in a single isoenergy layer.24 Therefore, the longitudinal compensation is performed by means of a dedicated range shifter, which consists of two kits of polymethyl methacrylate wedges installed on fast servo linear motors. The change in the mutual wedges' position (the degree of their overlap) alters the amount of wedge material traversed by the ion beam and thus regulates the particle range.18,40 Although the time needed for the range adaptation should be similar to the duration of raster point irradiation, the application of the mechanically operated range shifter limits the tracking speed.18 The implementation of new types of accelerators seems to be the alternative to standard devices assisted by passive absorbers. Cyclinac is an example of a prototype technical solution, which is able to deliver intensity- and energy-modulated proton and carbon ion beams for the 4D spot scanning treatment of moving organs. Such a device consists of a pulsed ion source, a superconducting isochronous cyclotron and a high-gradient linear accelerator. Owing to the fact that the cyclinac beam dedicated for tumour tracking must have special characteristics with very short pulses of enough current (about 2·105 C6+ ions in 1.5 μs pulse), novel compact superconducting electron beam ion sources will be used instead of the standard electron cyclotron resonance sources. The detailed requirements, which have to be met by proton and carbon ion sources for cyclinacs, can be found in the study by Garonna et al.77 Cyclotrons accelerate particles to a fixed initial energy that is afterwards boosted by the linear accelerator powered by many separately controlled klystrons. Switching off a definite number of klystrons and changing of the phase and amplitude of the drive signal sent to the last active unit enable altering the beam energy in a few milliseconds.77 Technical solutions of accelerating structures designed for the cyclinac have already been created and patented. Linac booster (LIBO) is a 3-GHz modular linear accelerator. The prototype device, which is composed of 9 coupled modules (1 module comprises 4 tanks, each of them consist of 13 accelerating cells), is able to increase the energy of protons extracted from a cyclotron from 62 MeV/u up to 200 MeV/u.78,79 It is important that the energy of protons entering LIBO cannot be lower than 30 MeV/u, in order to avoid the decrease in the shunt impedance. However, the use of 15-MeV proton beams produced by small commercial cyclotrons will also be possible, thanks to the 1.5-GHz CLUSTER (Coupled-cavity Linac Using Transverse Electric Radial field).80 This accelerating structure can boost the proton energy up to the value appropriate for injection into LIBO. Regarding carbon ion therapy, the high-frequency, fast-cycling linear accelerator called Carbon Booster for Therapy in Oncology (CABOTO) has been designed for the treatment of moving organs with rapid tumour scanning in depth. This machine will enable boosting the particle energy from 150 to 410 MeV/u and vary it in steps of minimal value of 2 MeV/u in 1–2 ms without using any passive absorbers.81 The optimal input energy of CABOTO was established to be 150 MeV/u, but owing to the lack of cyclotron delivering carbon ions of such energy, during the CABOTO test, a SCENT300 superconducting cyclotron (300 MeV/u) was used as an injector.81 The linear accelerators do not require complicated injection and extraction systems; so, the beams produced by cyclinac are more flexible than those extracted from cyclotrons and synchrotrons.82 It should be stressed that rapid (1–2 ms) correction of the energy and thus the range of particle beams, which will enable performing 4D hadron therapy of moving targets, is the main beneficial aspect of cyclinac introduction.83 An additional advantage of the cyclinacs seems to be the possibility of using beams from 30-MeV cyclotrons for alternative medical purposes; e.g. the production of radioisotopes and radiopharmaceuticals.83,84 A schematic representation of the treatment facility equipped with cyclinac and rotating gantry with fast beam scanning magnets is shown in Figure 2. It is worth noting that cyclinac is less expensive than synchrotron, but the total cost varies, depending on the parameters of the device; e.g. the size of the cyclotron magnet and length of the linear accelerator. Higher output energy of the cyclotron is associated with bigger cyclotron magnet and lower flexibility in altering the beam energy by linear accelerator; but, on the other hand, this allows reducing the length of the linear accelerator and total power consumption.77 Because of the large number of expensive 5.7-GHz modulator/klystron systems, the price of the carbon ion cyclinac with CABOTO booster is more in comparison with the 3-GHz proton cyclinac.81 Despite the difference in price, both systems are planned to be implemented in treatment facilities. The first use of the cyclinac for proton therapy is planned in Italy at the Institute for Diagnostics and Radiotherapy in Biella.84 There is also an idea of installing the CABOTO accelerating structure for carbon ions in Catania at Cannizzaro Hospital.82
Figure 2.
A schematic representation of the treatment facility equipped with a cyclinac (superconducting cyclotron combined with a high-gradient linear accelerator), rotating gantry with fast beam-scanning magnets and motion-monitoring system.
Providing of a highly conformal treatment using scanned ion beam tracking is still pretty hard to achieve. Hence, new 4D treatment plan optimizations, which consider all motion types including rotation and deformation, are being developed and tested. An exemplary approach to the problem is to divide the treated volume into sectors (one sector corresponds to each motion phase of 4DCT) and target them by dedicated raster fields. This method requires a synchronization of irradiation with online tumour motion detection and therefore relies on a precise motion-monitoring and dedicated 4D therapy control system.85 In contrast to previous 3D optimized beam-tracking approaches, where the pencil beam follows the target motion and delivers identical particle fluence for each motion state, in 4D optimized tracking, the fluence is varied depending on a state of motion. The particle fluence is decreased when the target is closest to critical organs and increased under conditions where the target is furthest from the organ at risk.86 Computational tests and film phantom study have shown that the implementation of 4D optimization can result in improved target dose coverage of thoracic CTV while reducing the dose in nearby avoidance volumes.86 Multigating is another proposal for 4D optimized extension of a standard beam-tracking strategy. In this method, beam spots are allocated to particular motion phases at the planning stage of treatment and the synchronization of scanning with tumour motion is assisted by fast gating.87 However, this concept needs further investigation. Work on the development of tracking techniques and 4D treatment plans can be facilitated by specially designed phantoms. An example is an artificial thorax phantom with ribs and an internal robotic-driven detector head, which mimics a lung tumour. The programmable device performs target movement in six dimensions (3D translation, 3D rotation) independently of thorax breathing motion and thus enables studying the dosimetric effects of irregular breathing and incongruity between internal and external motions.88 Also, new algorithms implemented in research treatment-planning systems are needed to model the impact of various deviations from perfect tracking on target dose coverage. The simulations performed without using setup or motion margins have shown that beam tracking of moving targets in both water phantoms and patients with lung cancer is sensitive to a number of motion uncertainties such as changes in the breathing period and the phase of respiration at the start of treatment.89 Nevertheless, studies on tumour tracking are very promising, because it is expected that this method will improve the efficiency of the therapy and reduce the treatment time by maintaining a 100% beam duty cycle.28,31,40,52 One should not forget that the ideal continuous real-time tumour-tracking system would allow compensating the motion of CTV with high precision and thus minimizing the CTV–PTV margin as much as possible.21,24,31 Unfortunately, such a perfect tracking system has not been invented and clinically implemented yet.86
CONCLUSION
The mitigation of the negative influence of intrafractional target motion on radiotherapy precision requires advanced techniques of movement monitoring and tumour irradiation. Motion pattern can be predicted based on respiratory-correlated CT (4DCT) examination performed prior to the treatment. However, the possible variations in patient breathing necessitate the real-time monitoring of tumour position during irradiation. It can be performed by fluoroscopic imaging of implanted radio-opaque fiducial markers or by electromagnetic detection of transponders (beacons) inserted in the target neighbourhood. The monitoring of external surrogate (thoracoabdominal surface) movement allows estimating the target motion; but, in this case, it is necessary to establish the external/internal correlation and verify it by episodic X-ray imaging. Moreover, novel devices that use particle beams for planar or volumetric imaging are under development. The problem of irradiation of moving targets is tried to be solved using such approaches as: gating, rescanning (repainting), gated rescanning or tumour tracking. The methods are still imperfect and there is a lot of work to be carried out on their improvement. The future implementation of new accelerators called “cyclinacs” promises a breakthrough in 4D spot scanning treatment of moving organs.
REFERENCES
- 1.Krämer M, Jäkel O, Haberer T, Kraft G, Schardt D, Weber U. Treatment planning for heavy-ion radiotherapy: physical beam model and dose optimization. Phys Med Biol 2000; 45: 3299–317. doi: 10.1088/0031-9155/45/11/313 [DOI] [PubMed] [Google Scholar]
- 2.Rinaldi I, Brons S, Gordon J, Panse R, Voss B, Jäkel O, et al. Experimental characterization of a prototype detector system for carbon ion radiography and tomography. Phys Med Biol 2013; 58: 413–27. doi: 10.1088/0031-9155/58/3/413 [DOI] [PubMed] [Google Scholar]
- 3.Schardt D, Elsässer T, Schulz-Ertner D. Heavy-ion tumor therapy: physical and radiobiological benefits. Rev Mod Phys 2010; 82: 383–425. doi: 10.1103/RevModPhys.82.383 [DOI] [Google Scholar]
- 4.Giordanengo S, Donetti M, Marchetto F, Ansarinejad A, Attili A, Bourhaleb F, et al. Performances of the scanning system for the CNAO center of oncological hadron therapy. Nucl Instr Meth Phys Res A 2010; 613: 317–22. doi: 10.1016/j.nima.2009.11.068 [DOI] [Google Scholar]
- 5.Dosanjh M, Hoffmann HF, Magrin G. Status of hadron therapy in Europe and the role of ENLIGHT. Nucl Instr Meth Phys Res A 2007; 571: 191–4. doi: 10.1016/j.nima.2006.10.060 [DOI] [Google Scholar]
- 6.Schlegel W. New technologies in 3D conformal radiation therapy: introduction and overview. In: Schlegel W, Bortfeld T, Grosu AL, eds. New Technologies in Radiation Oncology. Berlin, Germany: Springer; 2006. pp. 1–6. [Google Scholar]
- 7.Paganetti H, Bortfeld T. Proton therapy. In: Schlegel W, Bortfeld T, Grosu AL, eds. New technologies in radiation oncology. Berlin, Germany: Springer; 2006. pp. 345–63. [Google Scholar]
- 8.Welsh J, Amini A, Ciura K, Nguyen N, Palmer M, Soh H, et al. Evaluating proton stereotactic body radiotherapy to reduce chest wall dose in the treatment of lung cancer. Med Dosim 2013; 38: 442–7. doi: 10.1016/j.meddos.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaldi U, Kraft G. Recent applications of synchrotrons in cancer therapy with carbon ions. Europhys News 2005; 36: 114–8. doi: 10.1051/epn:2005402 [DOI] [Google Scholar]
- 10.Gueulette J, Wambersie A. Comparison of the methods of specifying carbon ion doses at NIRS and GSI. J Radiat Res 2007; (48 Suppl. A): A97–102. [DOI] [PubMed] [Google Scholar]
- 11.Schlaff CD, Krauze A, Belard A, O'Connell JJ, Camphausen KA. Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol 2014; 9: 88. doi: 10.1186/1748-717X-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karger CP, Schulz-Ertner D, Didinger BH, Debus J, Jäkel O. Influence of setup errors on spinal cord dose and treatment plan quality for cervical spine tumours: a phantom study for photon IMRT and heavy charged particle radiotherapy. Phys Med Biol 2003; 48: 3171–89. doi: 10.1088/0031-9155/48/19/006 [DOI] [PubMed] [Google Scholar]
- 13.Jäkel O, Schulz-Ertner D, Debus J. Specifying carbon ion doses for radiotherapy: the heidelberg approach. J Radiat Res 2007; (48 Suppl. A): A87–95. [DOI] [PubMed] [Google Scholar]
- 14.Kraft G. Tumor therapy with heavy charged particles. Prog Part Nucl Phys 2000; 45: 473–544. doi: 10.1016/S0146-6410(00)00112-5 [DOI] [Google Scholar]
- 15.Matsufuji N, Kanai T, Kanematsu N, Miyamoto T, Baba M, Kamada T, et al. Specification of carbon ion dose at the National Institute of Radiological Sciences (NIRS). J Radiat Res 2007; (48 Suppl. A): A81–6. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Shirai T, Takei Y, Furukawa T, Inaniwa T, Matsuzaki Y, et al. Patient handling system for carbon ion beam scanning therapy. J Appl Clin Med Phys 2012; 13: 226–40. doi: 10.1120/jacmp.v13i6.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft G, Kraft SD. Research needed for improving heavy-ion therapy. New J Phys 2009; 1: 025001. [Google Scholar]
- 18.Saito N, Bert C, Chaudhri N, Gemmel A, Schardt D, Durante M, et al. Speed and accuracy of a beam tracking system for treatment of moving targets with scanned ion beams. Phys Med Biol 2009; 54: 4849–62. doi: 10.1088/0031-9155/54/16/001 [DOI] [PubMed] [Google Scholar]
- 19.Pedroni E, Bearpark R, Böhringer T, Coray A, Duppich J, Forss S, et al. The PSI Gantry 2: a second generation proton scanning gantry. Z Med Phys 2004; 14: 25–34. [DOI] [PubMed] [Google Scholar]
- 20.Kubiak T. Beam delivery systems in hadron therapy for cancer treatment. PiJ 2014; 1: 67–73. [Google Scholar]
- 21.Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol 2011; 56: 113–44. doi: 10.1088/0031-9155/56/16/R01 [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Zenklusen S, Inaniwa T, Furukawa T, Imada H, Shirai T, et al. Conformity and robustness of gated rescanned carbon ion pencil beam scanning of liver tumors at NIRS. Radiother Oncol 2014; 111: 431–6. doi: 10.1016/j.radonc.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Liu W, Gillin M, Gomez DR, Komaki R, Cox JD, et al. Assessing the robustness of passive scattering proton therapy with regard to local recurrence in stage III non-small cell lung cancer: a secondary analysis of a phase II trial. Radiat Oncol 2014; 9: 108. doi: 10.1186/1748-717X-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grözinger SO, Rietzel E, Li Q, Bert C, Haberer T, Kraft G. Simulations to design an online motion compensation system for scanned particle beams. Phys Med Biol 2006; 51: 3517–31. doi: 10.1088/0031-9155/51/14/016 [DOI] [PubMed] [Google Scholar]
- 25.Tashiro M, Ishii T, Koya J, Okada R, Kurosawa Y, Arai K, et al. Technical approach to individualized respiratory-gated carbon-ion therapy for mobile organs. Radiol Phys Technol 2013; 6: 356–66. doi: 10.1007/s12194-013-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori S, Inaniwa T, Furukawa T, Zenklusen S, Shirai T, Noda K. Effects of a difference in respiratory cycle between treatment planning and irradiation for phase-controlled rescanning and carbon pencil beam scanning. Br J Radiol 2013; 86: 20130163. doi: 10.1259/bjr.20130163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rietzel E, Rosenthal SJ, Gierga DP, Willet CG, Chen GT. Moving targets: detection and tracking of internal organ motion for treatment planning and patient set-up. Radiother Oncol 2004; (73 Suppl 2): S68–72. [DOI] [PubMed] [Google Scholar]
- 28.Fassi A, Schaerer J, Fernandes M, Riboldi M, Sarrut D, Baroni G. Tumor tracking method based on a deformable 4D CT breathing motion model driven by an external surface surrogate. Int J Radiat Oncol Biol Phys 2014; 88: 182–8. doi: 10.1016/j.ijrobp.2013.09.026 [DOI] [PubMed] [Google Scholar]
- 29.Trofimov A, Vrancic C, Chan TC, Sharp GC, Bortfeld T. Tumor trailing strategy for intensity-modulated radiation therapy of moving targets. Med Phys 2008; 35: 1718–33. doi: 10.1118/1.2900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiler PG, Blattmann H, Kirsch S, Muench RK, Schilling C. A novel tracking technique for the continuous precise measurement of tumour positions in conformal radiotherapy. Phys Med Biol 2000; 45: 103–10. doi: 10.1088/0031-9155/45/9/402 [DOI] [PubMed] [Google Scholar]
- 31.Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol 2004; 14: 91–100. doi: 10.1053/j.semradonc.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 32.Ballhausen H, Li M, Hegemann NS, Ganswindt U, Belka C. Intra-fraction motion of the prostate is a random walk. Phys Med Biol 2015; 60: 549–63. doi: 10.1088/0031-9155/60/2/549 [DOI] [PubMed] [Google Scholar]
- 33.Hamamoto Y, Inata H, Sodeoka N, Nakayama S, Tsuruoka S, Takeda H. Observation of intrafraction prostate displacement through the course of conventionally fractionated radiotherapy for prostate cancer. Jpn J Radiol 2015; 33: 187–93. doi: 10.1007/s11604-015-0396-3 [DOI] [PubMed] [Google Scholar]
- 34.Ikeda I, Mizowaki T, Sawada Y, Nakata M, Norihisa Y, Ogura M, et al. Assessment of interfractional prostate motion in patients immobilized in the prone position using a thermoplastic shell. J Radiat Res 2014; 55: 168–74. doi: 10.1093/jrr/rrt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield G, Jain P, Green M, Watkins G, Henry A, Stratford J, et al. Quantifying motion for pancreatic radiotherapy margin calculation. Radiother Oncol 2012; 103, 360–6. doi: 10.1016/j.radonc.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 36.Park JC, Park SH, Kim JH, Yoon SM, Song SY, Liu Z, et al. Liver motion during cone beam computed tomography guided stereotactic body radiation therapy. Med Phys 2012; 39: 6431–42. doi: 10.1118/1.4754658 [DOI] [PubMed] [Google Scholar]
- 37.Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 1097–103. doi: 10.1016/S0360-3016(00)00524-1 [DOI] [PubMed] [Google Scholar]
- 38.Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2007; 68: 531–40. doi: 10.1016/j.ijrobp.2006.12.066 [DOI] [PubMed] [Google Scholar]
- 39.Schlosser J, Salisbury K, Hristov D. Telerobotic system concept for real-time soft-tissue imaging during radiotherapy beam delivery. Med Phys 2010; 37: 6357–67. doi: 10.1118/1.3515457 [DOI] [PubMed] [Google Scholar]
- 40.Seregni M, Kaderka R, Fattori G, Riboldi M, Pella A, Constantinescu A, et al. Tumor tracking based on correlation models in scanned ion beam therapy: an experimental study. Phys Med Biol 2013; 58: 4659–78. doi: 10.1088/0031-9155/58/13/4659 [DOI] [PubMed] [Google Scholar]
- 41.Safai S, Bula C, Meer D, Pedroni E. Improving the precision and performance of proton pencil beam scanning. Transl Cancer Res 2012; 1: 196–206. [Google Scholar]
- 42.Steidl P, Haberer T, Durante M, Bert C. Gating delays for two respiratory motion sensors in scanned particle radiation therapy. Phys Med Biol 2013; 58: N295–302. doi: 10.1088/0031-9155/58/21/N295 [DOI] [PubMed] [Google Scholar]
- 43.Ren Q, Nishioka S, Shirato H, Berbeco RI. Adaptive prediction of respiratory motion for motion compensation radiotherapy. Phys Med Biol 2007; 52: 6651–61. doi: 10.1088/0031-9155/52/22/007 [DOI] [PubMed] [Google Scholar]
- 44.Harris EJ, Miller NR, Bamber JC, Symonds-Tayler JR, Evans PM. Speckle tracking in a phantom and feature-based tracking in liver in the presence of respiratory motion using 4D ultrasound. Phys Med Biol 2010; 55: 3363–80. doi: 10.1088/0031-9155/55/12/007 [DOI] [PubMed] [Google Scholar]
- 45.Bert C, Durante M. Particle radiosurgery: a new frontier of physics in medicine. Phys Med 2014; 30: 535–8. doi: 10.1016/j.ejmp.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 46.Shimizu S, Miyamoto N, Matsuura T, Fujii Y, Umezawa M, Umegaki K, et al. A proton beam therapy system dedicated to spot-scanning increases accuracy with moving tumors by real-time imaging and gating and reduces equipment size. PLoS One 2014; 9: e94971. doi: 10.1371/journal.pone.0094971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharp GC, Lu HM, Trofimov A, Tang X, Jiang SB, Turcotte J, et al. Assessing residual motion for gated proton-beam radiotherapy. J Radiat Res 2007; (48 Suppl A): A55–9. [DOI] [PubMed] [Google Scholar]
- 48.Van der Voort van Zyp NC, Hoogeman MS, van de Water S, Levendag PC, van der Holt B, Heijmen BJ, et al. Stability of markers used for real-time tumor tracking after percutaneous intrapulmonary placement. Int J Radiat Oncol Biol Phys 2011; 81: e75–81. [DOI] [PubMed] [Google Scholar]
- 49.Habermehl D, Henkner K, Ecker S, Jäkel O, Debus J, Combs SE. Evaluation of different fiducial markers for image-guided radiotherapy and particle therapy. J Radiat Res 2013; 54: i61–8. doi: 10.1093/jrr/rrt071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imura M, Yamazaki K, Kubota KC, Itoh T, Onimaru R, Cho Y, et al. Histopathologic consideration of fiducial gold markers inserted for real-time tumor-tracking radiotherapy against lung cancer. Int J Radiat Oncol Biol Phys 2008; 70: 382–4. doi: 10.1016/j.ijrobp.2007.06.064 [DOI] [PubMed] [Google Scholar]
- 51.Onimaru R, Shirato H, Fujino M, Suzuki K, Yamazaki K, Nishimura M, et al. The effect of tumor location and respiratory function on tumor movement estimated by real-time tracking radiotherapy (RTRT) system. Int J Radiat Oncol Biol Phys 2005; 63: 164–9. doi: 10.1016/j.ijrobp.2005.01.025 [DOI] [PubMed] [Google Scholar]
- 52.Shirato H, Suzuki K, Sharp GC, Fujita K, Onimaru R, Fujino M, et al. Speed and amplitude of lung tumor motion precisely detected in four-dimensional setup and in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2006; 64: 1229–36. doi: 10.1016/j.ijrobp.2005.11.016 [DOI] [PubMed] [Google Scholar]
- 53.Shirato H, Shimizu S, Kunieda T, Kitamura K, van Herk M, Kagei K, et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys 2000; 48: 1187–95. doi: 10.1016/S0360-3016(00)00748-3 [DOI] [PubMed] [Google Scholar]
- 54.Balter JM, Wright JN, Newell LJ, Friemel B, Dimmer S, Cheng Y, et al. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys 2005; 61: 933–7. doi: 10.1016/j.ijrobp.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 55.Juhler Nøttrup T, Korreman SS, Pedersen AN, Aarup LR, Nyström H, Olsen M, et al. Intra- and interfraction breathing variations during curative radiotherapy for lung cancer. Radiother Oncol 2007; 84: 40–8. doi: 10.1016/j.radonc.2007.05.026 [DOI] [PubMed] [Google Scholar]
- 56.Aznar MC, Persson GF, Kofoed IM, Nygaard DE, Korreman SS. Irregular breathing during 4DCT scanning of lung cancer patients: is the midventilation approach robust? Phys Med 2014; 30: 69–75. doi: 10.1016/j.ejmp.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 57.Mori S, Inaniwa T, Furukawa T, Takahashi W, Nakajima M, Shirai T, et al. Amplitude-based gated phase-controlled rescanning in carbon-ion scanning beam treatment planning under irregular breathing conditions using lung and liver 4DCTs. J Radiat Res 2014; 55: 948–58. doi: 10.1093/jrr/rru032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parodi K. Heavy ion radiography and tomography. Phys Med 2014; 30: 539–43. doi: 10.1016/j.ejmp.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 59.Enghardt W, Crespo P, Fiedler F, Hinz R, Parodi K, Pawelke J, et al. Charged hadron tumour therapy monitoring by means of PET. Nucl Instr Meth Phys Res A 2004; 525: 284–8. doi: 10.1016/j.nima.2004.03.128 [DOI] [Google Scholar]
- 60.Laube K, Menkel S, Bert C, Enghardt W, Helmbrecht S, Saito N, et al. 4D particle therapy PET simulation for moving targets irradiated with scanned ion beams. Phys Med Biol 2013; 58: 513–33. doi: 10.1088/0031-9155/58/3/513 [DOI] [PubMed] [Google Scholar]
- 61.Langen KM, Pouliot J, Anezinos C, Aubin M, Gottschalk AR, Hsu IC, et al. Evaluation of ultrasound-based prostate localization for image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 635–44. doi: 10.1016/S0360-3016(03)00633-3 [DOI] [PubMed] [Google Scholar]
- 62.Prall M, Kaderka R, Saito N, Graeff C, Bert C, Durante M, et al. Ion beam tracking using ultrasound motion detection. Med Phys 2014; 41: 041708. doi: 10.1118/1.4868459 [DOI] [PubMed] [Google Scholar]
- 63.Schwaab J, Prall M, Sarti C, Kaderka R, Bert C, Kurz C, et al. Ultrasound tracking for intra-fractional motion compensation in radiation therapy. Phys Med 2014; 30: 578–82. doi: 10.1016/j.ejmp.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 64.Ammazzalorso F, Graef S, Weber U, Wittig A, Engenhart-Cabillic R, Jelen U. Dosimetric consequences of intrafraction prostate motion in scanned ion beam radiotherapy. Radiother Oncol 2014; 112: 100–5. doi: 10.1016/j.radonc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 65.Richter D, Saito N, Chaudhri N, Härtig M, Ellerbrock M, Jäkel O, et al. Four-dimensional patient dose reconstruction for scanned ion beam therapy of moving liver tumors. Int J Radiat Oncol Biol Phys 2014; 89: 175–81. doi: 10.1016/j.ijrobp.2014.01.043 [DOI] [PubMed] [Google Scholar]
- 66.Seco J, Robertson D, Trofimov A, Paganetti H. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol 2009; 54: N283–94. doi: 10.1088/0031-9155/54/14/N01 [DOI] [PubMed] [Google Scholar]
- 67.Tsunashima Y, Vedam S, Dong L, Umezawa M, Balter P, Mohan R. The precision of respiratory-gated delivery of synchrotron-based pulsed beam proton therapy. Phys Med Biol 2010; 55: 7633–47. doi: 10.1088/0031-9155/55/24/016 [DOI] [PubMed] [Google Scholar]
- 68.Tsunashima Y, Vedam S, Dong L, Umezawa M, Sakae T, Bues M, et al. Efficiency of respiratory-gated delivery of synchrotron-based pulsed proton irradiation. Phys Med Biol 2008; 53: 1947–59. doi: 10.1088/0031-9155/53/7/010 [DOI] [PubMed] [Google Scholar]
- 69.Noda K, Furukawa T, Fujisawa T, Iwata Y, Kanai T, Kanazawa M, et al. New accelerator facility for carbon-ion cancer-therapy. J Radiat Res 2007; (48 Suppl. A): A43–54. [DOI] [PubMed] [Google Scholar]
- 70.Noda K, Kanazawa M, Itano M, Takada E, Torikoshi M, Araki N, et al. Slow beam extraction, by a transverse RF filed with AM and FM. Nucl Instr Meth Phys Res A 1996; 374: 269–77. doi: 10.1016/0168-9002(96)00096-4 [DOI] [Google Scholar]
- 71.Furukawa T, Noda K, Uesugi TH, Naruse T, Shibuya S. Intensity control in RF-knockout extraction for scanning irradiation. Nucl Instr Meth Phys Res B 2005; 240: 32–5. doi: 10.1016/j.nimb.2005.06.083 [DOI] [Google Scholar]
- 72.Sato S, Furukawa T, Noda K. Dynamic intensity control system with RF-knockout slow-extraction in the HIMAC synchrotron. Nucl Instr Meth Phys Res A 2007; 574: 226–31. doi: 10.1016/j.nima.2007.01.174 [DOI] [Google Scholar]
- 73.Richter D, Graeff C, Jäkel O, Combs SE, Durante M, Bert C. Residual motion mitigation in scanned carbon ion beam therapy of liver tumors using enlarged pencil beam overlap. Radiother Oncol 2014; 113: 290–5. doi: 10.1016/j.radonc.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 74.Jongen Y, Abs M, Blondin A, Kleeven W, Zaremba S, Vandeplassche D, et al. Compact superconducting cyclotron C400 for hadron therapy. Nucl Instr Meth Phys Res A 2010; 624: 47–53. doi: 10.1016/j.nima.2010.09.028 [DOI] [Google Scholar]
- 75.Noda K, Furukawa T, Fujimoto T, Hara Y, Inaniwa T, Iwata Y, et al. Recent progress of HIMAC for sophisticated heavy-ion cancer radiotherapy. Nucl Instr Meth Phys Res B 2014; 331: 6–9. doi: 10.1016/j.nimb.2013.12.036 [DOI] [Google Scholar]
- 76.Ogata R, Mori S, Yasuda S. Extended phase-correlated rescanning irradiation to improve dose homogeneity in carbon-ion beam liver treatment. Phys Med Biol 2014; 59: 5091–9. doi: 10.1088/0031-9155/59/17/5091 [DOI] [PubMed] [Google Scholar]
- 77.Garonna A, Amaldi U, Bonomi R, Campo D, Degiovanni A, Garlasché M, et al. Cyclinac medical accelerators using pulsed C6+/H2+ ion sources. J Instrum 2010; 5: C09004. doi: 10.1088/1748-0221/5/09/C09004 [DOI] [Google Scholar]
- 78.De Martinis C, Giove D, Amaldi U, Berra P, Crandall K, Mauri M, et al. Acceleration tests of a 3 GHz proton linear accelerator (LIBO) for hadrontherapy. Nucl Instr Meth Phys Res A 2012; 681: 10–15. doi: 10.1016/j.nima.2012.04.017 [DOI] [Google Scholar]
- 79.Amaldi U, Berra P, Crandall K, Toet D, Weiss M, Zennaro R, et al. LIBO—a linac-booster for protontherapy: construction and tests of a prototype. Nucl Instr Meth Phys Res A 2004; 521: 512–29. doi: 10.1016/j.nima.2003.07.062 [DOI] [Google Scholar]
- 80.Amaldi U, Citterio A, Crescenti M, Giuliacci A, Tronci C, Zennaro R. CLUSTER: a high-frequency H-mode coupled cavity linac for low and medium energies. Nucl Instr Meth Phys Res A 2007; 579: 924–36. doi: 10.1016/j.nima.2007.05.208 [DOI] [Google Scholar]
- 81.Verdú-Andrés S, Amaldi U, Faus-Golfe Á. CABOTO, a high-gradient linac for hadrontherapy. J Radiat Res 2013; (54 Suppl. 1): i155–61. doi: 10.1093/jrr/rrt053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amaldi U. Cyclinacs: novel fast-cycling accelerators for hadron therapy. Proceedings of the 18th International Conference on Cyclotrons and their Applications; 1–5 October 2007; Italy, Giardini Naxos; 2007. pp. 166–68. [Google Scholar]
- 83.Amaldi U, Bonomi R, Braccini S, Crescenti M, Degiovanni A, Garlasché M, et al. Accelerators for hadron therapy: from Lawrence cyclotrons to linacs. Nucl Instr Meth Phys Res A 2010; 620: 563–77. doi: 10.1016/j.nima.2010.03.130 [DOI] [Google Scholar]
- 84.Holzscheiter MH, Bassler N. Future particle accelerator developments for radiation therapy. In: Gómez-Tejedor GG, Fuss MC, eds. Radiation damage in biomolecular systems. Dordrecht, Netherlands: Springer; 2012. pp. 491–506. [Google Scholar]
- 85.Graeff C, Lüchtenborg R, Eley JG, Durante M, Bert C. A 4D-optimization concept for scanned ion beam therapy. Radiother Oncol 2013; 109: 419–24. doi: 10.1016/j.radonc.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 86.Eley JG, Newhauser WD, Lüchtenborg R, Graeff C, Bert C. 4D optimization of scanned ion beam tracking therapy for moving tumors. Phys Med Biol 2014; 59: 3431–52. doi: 10.1088/0031-9155/59/13/3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graeff C, Constantinescu A, Lüchtenborg R, Durante M, Bert C. Multigating, a 4D optimized beam tracking in scanned ion beam therapy. Technol Cancer Res Treat 2014; 13: 497–504. doi: 10.7785/tcrtexpress.2013.600277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steidl P, Richter D, Schuy C, Schubert E, Haberer T, Durante M, et al. A breathing thorax phantom with independently programmable 6D tumour motion for dosimetric measurements in radiation therapy. Phys Med Biol 2012; 57: 2235–50. doi: 10.1088/0031-9155/57/8/2235 [DOI] [PubMed] [Google Scholar]
- 89.Eley JG, Newhauser WD, Richter D, Lüchtenborg R, Saito N, Bert C. Robustness of target dose coverage to motion uncertainties for scanned carbon ion beam tracking therapy of moving tumors. Phys Med Biol 2015; 60: 1717–40. doi: 10.1088/0031-9155/60/4/1717 [DOI] [PMC free article] [PubMed] [Google Scholar]