Abstract
The identification of characteristic genetic alteration in gynaecological malignancies has opened the door for molecular targeted therapy. The purpose of this review is to provide a primer for the radiologist on these agents with emphasis on the role of imaging in treatment response assessment and drug toxicities. The use of targeted therapy in gynaecological malignancies will likely increase in the future and make the role of the radiologist critical in response assessment and detection of toxicities.
INTRODUCTION
Gynaecological malignancies account for 11.7% of all malignancies; the estimated annual incidence of gynaecological malignancies is 94,990 with approximately 28,790 deaths in the USA.1 Management of gynaecological malignancies is challenging because of striking heterogeneity in the prognosis.
In the past decade, there have been dramatic advances in our understanding of the pathological and molecular basis of gynaecological malignancies, leading to availability of a spectrum of molecular targeted therapeutic options. Just between November 2014 and February 2015, two molecular targeted agents were approved by the US Food and Drug Administration (FDA) in a subset of bevacizumab for platinum-resistant epithelial ovarian cancer and olaparib for breast cancer (BRCA)-mutation-associated ovarian cancer. In addition, olaparib is recommended under the National Institute for Health and Care Excellence guidelines for the maintenance treatment of BRCA1 or BRCA2 mutated, relapsed, platinum-sensitive ovarian, fallopian tube and peritoneal cancer in people whose relapsed disease has responded to platinum-based chemotherapy.2 Novel targeted therapy has been actively tested and is now an important option for gynaecological malignancies, including epithelial ovarian malignancies, endometrial malignancies, vulvar malignancies, uterine sarcomas and lymphomas involving gynaecological organs.3–21
With these advances, the role of imaging and that of the radiologist has evolved. With the increasing use of targeted therapy, imaging plays a critical role in assessing the response to these novel drugs and in detection of drug- and class-specific toxicities that are often first detected on imaging studies. The aim of this review is to discuss the evolving role of imaging, particularly as it relates to emerging molecular targeted therapies and the commonly encountered potential toxicities of which radiologists should be aware of.
RECENT ADVANCES IN MOLECULAR TARGETED THERAPY OF EPITHELIAL MALIGNANCIES
Ovarian malignancy
Ovarian cancer is the second most common gynaecological malignancy and the leading cause of death of gynaecological malignancies.1 About 90% of ovarian malignancies are derived from epithelial cells. Primary treatment of presumed ovarian malignancies consists of cytoreductive surgery followed by systemic chemotherapy.22 Most but not all patients with epithelial ovarian malignancies receive adjuvant chemotherapy. Recommended chemotherapy regimen is the combination of intravenous paclitaxel plus cisplatin.4 Despite marginal improvement in 5-year overall survival during the past 15 years, most patients relapse after primary treatment and lead to disease progression. Several therapeutic options are available for treatment of recurrent ovarian malignancies,4 including molecular targeted therapies such as anti-angiogenic agents, inhibitors of the enzyme poly adenosine diphosphate (ADP) ribose polymerase (PARP inhibitors) and hormonal agents.4,6,12,16,21
Anti-angiogenic agents
Angiogenesis plays an important role not only in normal ovarian physiology but also in the pathogenesis of epithelial ovarian malignancies and their progression through ascites formation, and metastatic spread.23–25 Therefore, angiogenesis has been a key target in clinical cancer research. Bevacizumab is a recombinant humanized monoclonal IgG1 antivascular endothelial growth factor (VEGF) antibody which prevents VEGF from binding to its receptor. Bevacizumab blocks growth and maintenance of tumour-associated blood vessels. Recently, several Phase III randomized trials4,19 have assessed combination therapy with bevacizumab for recurrent ovarian malignancies (Figure 1). In a study of 361 patients with platinum-resistant recurrent ovarian cancer, Pujade-Lauraine et al19 demonstrated that adding bevacizumab to chemotherapy significantly improved progression-free survival and objective response rate, although the overall survival was not significantly different. Based on these trials, bevacizumab plus chemotherapy for platinum-resistant epithelial ovarian cancer was approved by the FDA in November 2014. Several other anti-angiogenic agents, including aflibercept, nintedanib, trebananib, pazopanib, sunitinib, sorafenib and cediranib, also have been investigated in epithelial ovarian malignancies but not approved by FDA yet.
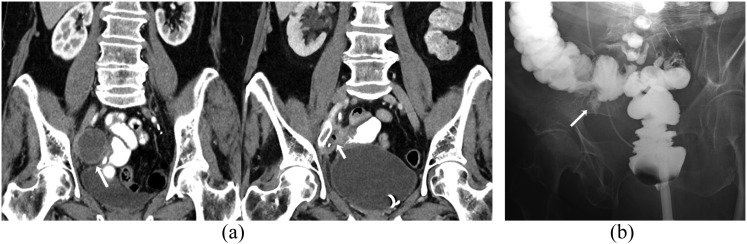
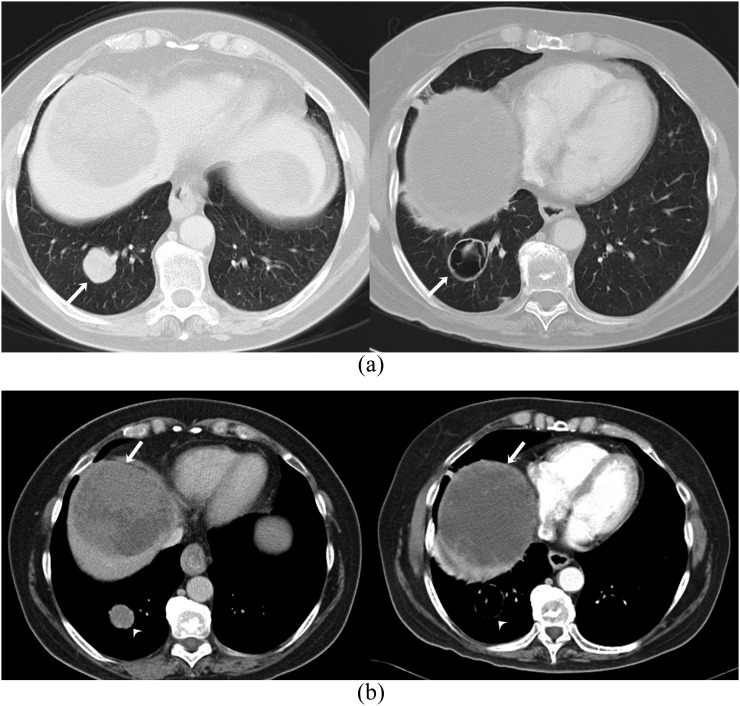
Figure 1.
A 76-year-old female with metastatic ovarian carcinoma on treatment with bevacizumab complicated by tumour–bowel fistula. (a) Coronal contrast-enhanced CT scan obtained at baseline (left) shows a right pelvic side-wall metastatic tumour (arrow). Coronal contrast-enhanced CT scan obtained 1 month after the start of the treatment (right) demonstrates small focus of air in the tumour (arrow). (b) Enema study with water-soluble contrast demonstrates the fistulous connection between the caecum and the right pelvic side-wall tumour (arrow).
Poly ADP ribose polymerase inhibitors
PARP inhibitors work synergistically with the deficiencies of DNA repair seen in cancer occurring in patients with BRCA1 or BRCA2 mutations.26 In addition, PARP inhibitors also show activity in high-grade serous ovarian malignancy occurring in patients who do not harbour a BRCA1 or BRCA2 mutation, likely due to the loss of BRCA function from deletion, somatic mutations or methylation.27 Olaparib (AZD2281) is an oral PARP inhibitor that has undergone the most extensive investigation in ovarian malignancies. Olaparib is active in BRCA1- and BRCA2-mutated patients with chemotherapy-resistant ovarian malignancies, especially those with platinum-sensitive disease (Figure 2).6,12,14,16 Based on these studies, olaparib for BRCA-mutation-associated ovarian cancer received regulatory approval from FDA in February 2015.
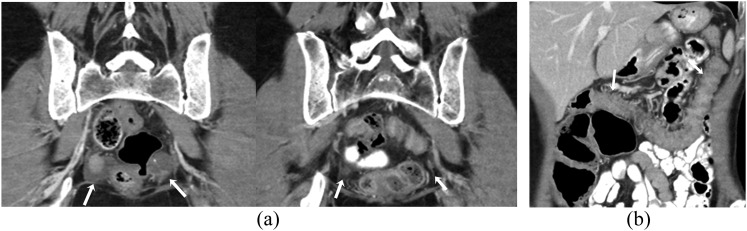
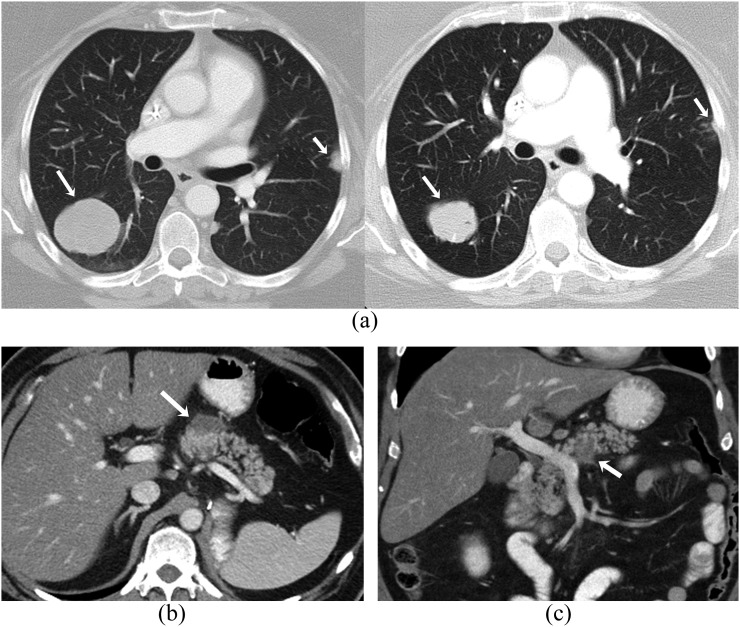
Figure 2.
A 55-year-old female with recurrent ovarian cancer on treatment with olaparib complicated by colitis. (a) Coronal contrast-enhanced CT images before (left) and after (right) treatment show interval decrease in the size of metastatic deep-pelvic soft-tissue masses (arrows). (b) Coronal contrast-enhanced CT image shows diffuse wall thickening involving transverse colon with pericolic stranding consistent with colitis (arrows).
Hormonal agents
Since endometrioid ovarian cancer is often oestrogen receptor (ER)/progesterone receptor (PR) positive, hormonal treatment also can be a therapeutic option for recurrent endometrioid type of epithelial ovarian cancer.28 For females with radiological evidence of disease progression but with little or no symptoms associated with recurrent ovarian epithelial malignancies, hormonal treatment can be an option.21
Endometrial malignancy
Endometrial cancer is the most common gynaecological malignancy and the second most common cause of death of gynaecological malignancies.1 Adenocarcinomas of the endometrium are the most common histological type of endometrial cancer. Total hysterectomy and bilateral salpingo-oophorectomy is usually curative for females who present with low-risk disease. Females with intermediate- or high-risk disease may benefit from adjuvant therapy.29
Hormonal agents
Hormonal treatment is suggested as an initial treatment option for patients in whom secondary cytoreduction or cytotoxic chemotherapy is not planned.29 The role of hormonal treatment in recurrent or metastatic cancer has been primarily evaluated in patients with endometrioid histology.29 Hormonal treatment agents include progestational agents, tamoxifen, aromatase inhibitors (e.g. anastrozole) (Figure 3) and megestrol/tamoxifen (alternating).30–41 Progestational agents are mainly used for metastatic disease.32,33,36,39 For asymptomatic or low-grade disseminated metastases, progestational agents have shown good responses, particularly in patients with ER/PR-positive disease.32,33,36,39 Tamoxifen with alternating megestrol34,35,38,41 and aromatase inhibitors are also being used (Figure 3).30,31 Tamoxifen has a 20% response rate in those who do not respond to progesterone agents.37,40
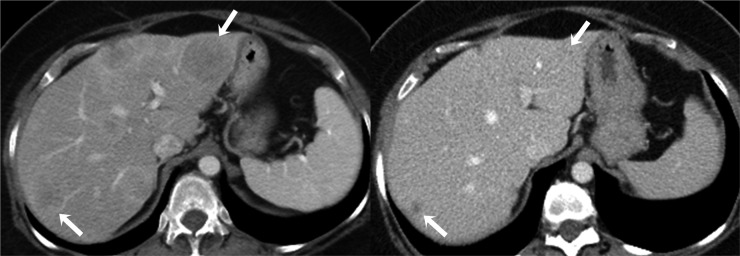
Figure 3.
A 59-year-old female with metastatic endometrial adenocarcinoma on treatment with anastrozole. Axial contrast-enhanced CT image before the start of chemotherapy (left) shows multiple ill-defined low-attenuation hepatic metastatic lesions (arrows). Axial contrast-enhanced CT image after 2 months of chemotherapy (right) shows significant interval decrease in size of hepatic metastatic lesions (arrows).
Vulvar malignancy
Vulvar cancer is the fourth common gynaecological malignancy and accounts for 5% of malignancies of the female genital tract.1 Approximately 90% of vulvar cancer is squamous-cell carcinoma. Treatment of choice for early stage vulvar malignancies includes radical local excision with or without lymphadenectomy,42 whereas for locally advanced diseases, adjuvant/neoadjuvant chemotherapy and radiation are often employed.43 In spite of optimal management, there is still a high rate of recurrence (37.3%).44
Epidermal growth factor receptor inhibitors
Several studies have demonstrated high levels of epidermal growth factor receptor (EGFR) protein expression in vulvar squamous-cell carcinomas and EGFR protein expression correlated with advanced stage, lymph node metastases and survival.45–47 Targeting EGFR with novel inhibitors including erlotinib or gefitinib has shown modest clinical response (Figure 4).48 In a study of 41 patients with squamous-cell carcinoma of the vulva treated with EGFR inhibitor, Horowitz et al49 found an overall clinical benefit rate of 67.5% with 11 (27.5%) partial responses, 16 (40.0%) stable disease and 7 (17.5%) progressive disease.
Figure 4.
A 57-year-old female with metastatic vulvar carcinoma on treatment with erlotinib. Coronal contrast-enhanced CT images before (left) and after (right) treatment show interval decrease in size of bilateral metastatic adrenal lesions (arrows). Also note the interval decrease in left-sided pleural effusion.
Vaginal melanoma
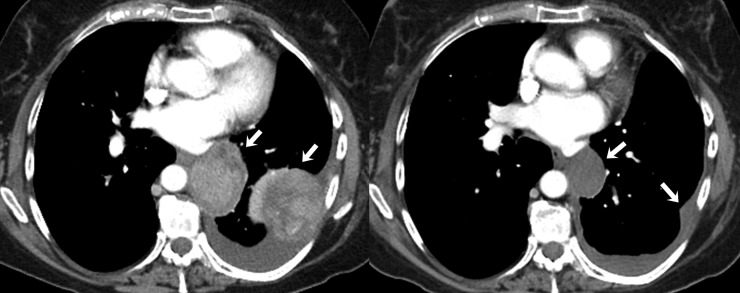
Primary malignant vaginal melanoma is an extremely rare, highly aggressive tumour and accounts for <5% of all vaginal malignancies and 0.2–0.8% of all malignant melanomas.50–52 Surgical excision is the primary treatment for vaginal melanoma. The therapeutic landscape for metastatic vaginal melanoma has rapidly changed in the past decade with availability of agents with better efficacy than conventional chemotherapy.53–55 Ipilimumab is a monoclonal antibody directed to cytotoxic T-lymphocyte antigen-4 and received FDA approval for metastatic melanoma treatment in 2011.55 Vemurafenib, FDA-approved for treatment of metastatic or unresectable melanoma with BRAF mutation, is a specific inhibitor of the intracellular signalling by mutated BRAF which is found in approximately half of patients with metastatic melanoma.56 C-kit mutations may be present in mucosal and acral subtypes of melanoma,57 for which imatinib has demonstrated a 23% overall response rate (Figure 5).53
Figure 5.
A 63-year-old female with metastatic vaginal melanoma on treatment with imatinib. (a) Axial contrast-enhanced lung window CT images before (left) and after (right) treatment with imatinib show interval appearance of cavitation within the right lower lobe pulmonary nodule (arrows) consistent with treatment response. (b) Axial contrast-enhanced CT images at the same level in soft-tissue window before (left) and after (right) treatment show mildly increased size of metastatic liver lesion but with decreased homogeneous low density consistent with response to therapy (arrows). Interval appearance of cavitation within the right lower lobe pulmonary nodule (arrowheads) again demonstrated.
ROLE OF IMAGING: EPITHELIAL MALIGNANCIES
Treatment response assessment
The Response Evaluation Criteria in Solid Tumours (RECIST 1.0 and 1.1) has become the most widely accepted criteria for response evaluation for clinical trials and practice in most solid tumours, including gynaecological malignancies.58,59 The RECIST, which is size-based response criteria, works well in treatment with conventional cytotoxic chemotherapy such as paclitaxel- or cisplatin-based regimens.4 However, in patients treated with new therapeutic agents including molecular targeted agents, morphologic assessment of treatment response is needed because of the mechanism of action of these therapeutic agents.
For example, the anti-angiogenic agents often show response with a decrease in tumour attenuation and enhancement with stable-to-mild decrease in tumour size.60 Occasionally, response with these agents may mimic tumour progression (pseudoprogression) (Figure 5). These atypical response patterns include an increase in size with decrease in density due to cystic change and an increase in size with increase in density due to haemorrhagic change.61
Toxicities
Radiologists should be aware of the toxicities of anti-angiogenic agents: increased risk of gastrointestinal complications including pneumatosis, perforation and tumour-bowel fistula (Figure 1), which may come to attention for the first time on imaging.19,62–66 CT findings of pneumatosis include subserosal and submucosal gas-filled cysts in gastrointestinal tract with or without pneumoperitoneum. Pneumatosis can represent an incidentally detected harmless finding or signal a more serious underlying condition including bowel ischaemia.66 Bevacizumab is known to cause intestinal perforation or fistulae in 1.5–4% of patients.63,64,67 Imaging findings include frank pneumoperitoneum, localized extraluminal locules of air or localized fluid collections adjacent to the perforation. Although the exact mechanism of bevacizumab-associated intestinal perforation is unknown, the proposed mechanisms include drug-specific anti-VEGF effects compromising bowel wall integrity, intestinal wall disruption because of necrosis of the serosal tumour deposits, impaired healing of pathological or surgical bowel injury, and ischaemia associated with mesenteric thrombosis.65 In terms of tumour-bowel fistula, as the mass necroses, a fistula may form from the bowel lumen into the tumour's necrotic centre (Figure 1).68
A common adverse effect of PARP inhibitors is chronic colitis (Figure 2b);6,69 this can be seen on imaging as a spectrum of findings ranging from fluid-filled colon-to-bowel wall thickening with or without surrounding fat stranding (Figure 2b). Hormonal agents, in particular megestrol, are known to cause venous thromboembolism.34
The immune-related adverse events have been reported in patients undergoing treatment with ipilimumab, including enterocolitis, hypophysitis, hepatitis and pancreatitis.69 In a study of 16 patients diagnosed with ipilimumab-associated colitis, Kim et al70 demonstrated that the common CT findings were mesenteric vessel engorgement, bowel wall thickening and fluid-filled colonic distension. They also reported two distinct CT patterns of ipilimumab-associated colitis: the diffuse colitis pattern and the segmental colitis associated with diverticulosis pattern. The presence of these immune-related adverse events has been shown to be predictive of better clinical responses and outcomes.70
RECENT ADVANCES IN MOLECULAR TARGETED THERAPY OF MESENCHYMAL MALIGNANCIES
Uterine sarcomas are rare neoplasms and account for 5% of uterine malignancies.1,71 These tumours are broadly classified into three groups by composition, including tumours with both smooth muscle and epithelial components, smooth muscle tumours and endometrial stromal tumours.71 Perivascular epithelioid cell tumour (PEComa) has received increased attention in recent years.72
Leiomyosarcoma
Leiomyosarcomas are the second most common subtype of uterine sarcoma comprising nearly 40% of cases. The treatment of choice is hysterectomy, often with ovarian preservation, which can be considered in young females with organ-confined disease.73 For advanced or recurrent disease, chemotherapeutic regimens generally involve a combination of doxorubicin and ifosfamide.
Trabectedin (ET-743)
Trabectedin (ET-743), a tetrahydroisoquinoline alkaloid, binds to and inhibits DNA transcription preventing the progression of the cell cycle beyond the G2/M phase.74 It has shown efficacy in Phase II trials in the treatment of leiomyosarcomas and myxoid liposarcomas and is approved for use in Europe.7,13 However, trabectedin is not currently available outside of a clinical trial in the USA.
Pazopanib
Pazopanib is a multitargeted tyrosine kinase inhibitor with VEGF inhibition. Pazopanib was approved by the FDA for the advanced leiomyosarcoma that fail to respond to first-line chemotherapy agents (Figures 6 and 7). Uterine leiomyosarcoma exhibits 25–60% of ER and 35–60% of PR expression.75 Therefore, early trials are also being conducted in the use of hormonal agents, including megestrol, medroxyprogesterone and aromatase inhibitors, as targeted therapy depending on receptor status.76 In addition, depending on receptor status, molecular targeted therapy with mammalian target of rapamycin (mTOR) inhibitors (Figure 8), cyclo-oxygenase inhibitors and bevacizumab have been investigated.56
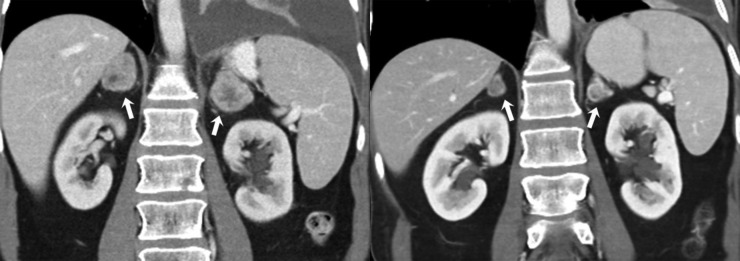
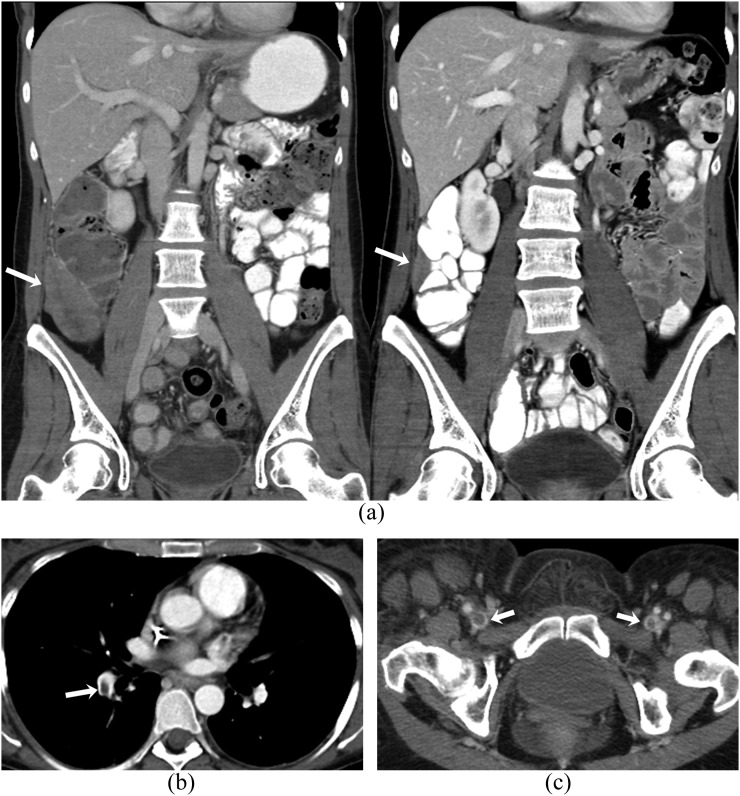
Figure 6.
A 43-year-old female with metastatic uterine leiomyosarcoma on treatment with pazopanib complicated by pancreatitis. (a) Axial contrast-enhanced lung window CT images before (left) and after (right) treatment with pazopanib show decrease in size of pulmonary metastases (arrows). Axial (b) and coronal (c) contrast-enhanced CT images of the abdomen show an ill-defined hypodense fluid collection adjacent to proximal body of pancreas (arrows) consistent with acute pancreatitis.
Figure 7.
A 46-year-old female with metastatic uterine leiomyosarcoma on treatment with pazopanib complicated by pulmonary embolism and venous thromboembolism. (a) Coronal contrast-enhanced CT images before (left) and after (right) treatment show interval decrease in the size of metastatic soft-tissue mass in the right paracolic region (arrows). (b, c) Axial contrast-enhanced CT images show filling defect (arrows) in the right lower lobe pulmonary artery and in both common femoral veins.
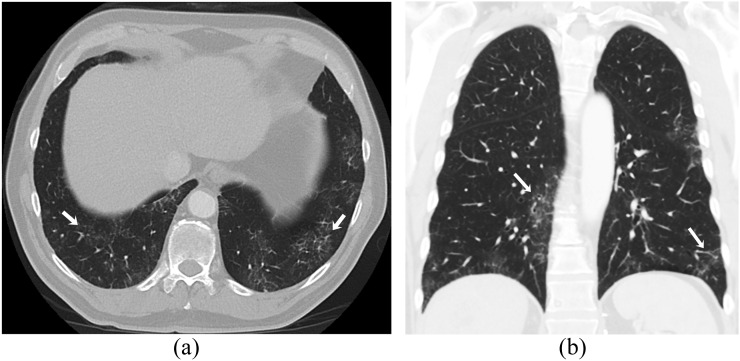
Figure 8.
A 49-year-old female with metastatic uterine leiomyosarcoma on treatment with sirolimus. Axial (a) and coronal (b) contrast-enhanced lung window CT images show patchy ground-glass opacities in both lungs with peripheral subpleural distribution suggestive of drug-associated pneumonitis (arrows).
Endometrial stromal sarcoma
Endometrial stromal sarcomas account for 10–15% of uterine mesenchymal tumours. As with other uterine sarcomas, the treatment of choice involves hysterectomy and bilateral salpingo-oophorectomy. Post-operative hormonal therapy is recommended for Stages I–IV, recurred or unresectable endometrial stromal sarcomas.18
Hormonal agents
Hormonal treatment using megestrol, medroxyprogesterone, aromatase inhibitors or gonadotropin-releasing hormone analogues has been shown to be effective in endometrial stromal sarcomas as adjuvant therapy after the surgery or in advanced disease.3,5,18
Perivascular epithelioid cell tumour
PEComa is a new entity composed of distinct HMB-45 or actin-positive epithelioid cells in a perivascular distribution. Subsets of PEComa have aggressive biological behaviour and labelled as malignant PEComas based on large size, high grade and mitotic rate; necrosis; and vascular invasion. Kidneys, retroperitoneum and uterus are the most commons sites of malignant PEComa. Treatment of malignant PEComa is surgical resection because they are usually resistant to chemotherapy and radiotherapy.20
Mammalian target of rapamycin inhibitors
PEComa is increasingly recognized as a prototype for mTOR-driven sarcomas, and treatment options are being developed to target this pathway. The mTOR serine/threonine kinase is an integral part of the PI3K pathway that is activated in several mTOR-driven malignancies in regulatory genes.77 mTOR inhibitors, including sirolimus, temsirolimus, ridaforolimus and everolimus, are a group of drugs that inhibit the mTOR cascade by binding to the mTOR Complex 1 protein78 These agents act by directly inhibiting the mTOR Complex 1 protein, which is upregulated in other tumours of the same family, including angiomyolipoma and lymphangiomyomatosis, that are associated with tuberous sclerosis complex.20 In a study of three PEComas patients treated with sirolimus, Wagner et al20 found significant clinical response in all the patients (Figure 9).
Figure 9.
A 55-year-old female with metastatic uterine perivascular epithelioid cell tumour on treatment with sirolimus. Axial contrast-enhanced CT images before (left) and after (right) treatment show significant interval decrease in size and enhancement of pleural-based lesions on the left side (arrows).
ROLE OF IMAGING: MESENCHYMAL MALIGNANCIES
Treatment response assessment
Currently, RECIST is the most commonly used criteria for response evaluation for clinical trials and practice in uterine sarcomas. However, response to molecular targeted therapy in these sarcomas may be seen as changes in density with or without changes in size. Therefore, morphological tumour response criteria such as the Choi criteria (originally suggested for gastrointestinal stromal tumour);79 the size and attenuation CT criteria; and morphology, attenuation, size and structure criteria (originally for metastatic renal cell carcinoma)80,81 may be useful in assessing treatment response in high-grade soft-tissue sarcoma.82 For trabectedin, treatment assessment is indicated by a decrease in tumour density with or without tumour shrinkage.74
Toxicities
Trabectedin has been associated with capillary leak syndrome which appears as a generalized oedema, and pulmonary oedema may be seen in the setting of dyspnoea.7,13 CT findings show smooth interlobular septal thickening and ground-glass opacities, consistent with pulmonary oedema which can occur as a unilateral process.83
Pazopanib have been reported to cause hepatic steatosis, hepatitis, pancreatitis and cholecystitis.66 Hepatic steatosis may be suggested sonographically if the hepatic echogenicity exceeds that of the renal cortex, if the ultrasound wave is significantly attenuated or if there is poor delineation of the intrahepatic architecture.84 Imaging findings of hepatitis include alteration in hepatic echogenicity or attenuation, wall thickening of the gall bladder, ascites and periportal low attenuation. Pancreatitis was diagnosed on the basis of CT imaging of an oedematous pancreas with peripancreatic inflammation and extremely elevated amylase and lipase levels (Figure 6b,c).66 In a study of 15 patients receiving molecular targeted therapy, Tirumani et al85 demonstrated that molecular targeted therapy-associated pancreatitis was usually mild, focal and managed conservatively with discontinuation of the agents. Imaging findings of cholecystitis include gall bladder wall thickening, wall oedema or hyperaemia, and pericholecystic fluid. In addition, pazopanib is commonly implicated in vascular thrombosis (Figure 7b,c).86 CT findings include partial or complete filling defect of the lumen of a vein or artery.
For mTOR inhibitors, immunosuppression predisposing to infections, pulmonary toxicity (Figure 8) and gastrointestinal side effects (diarrhoea, enteritis) has been reported as the adverse effect.87 Everolimus is rarely associated with acute cholecystitis probably due to ischaemia related to endothelial injury.8 mTOR inhibitors are also related to dose-dependent non-infectious pneumonitis in 2–36% patients, and CT images show interlobar septal thickening and ground-glass opacities with basilar and peripheral distribution (Figure 8).83,88 Less commonly, diffuse alveolar haemorrhage, alveolar proteinosis and desquamative interstitial pneumonia may be seen. Gastrointestinal toxicities of mTOR inhibitors include enteritis and bowel perforation.20,66 In a study of 46 patients treated with mTOR inhibitors for metastatic renal cell carcinoma, Dabydeen et al88 suggested that pneumonitis may be a marker of stable disease by RECIST and, thus, a marker of therapeutic benefit. Therefore, careful patient assessment should be undertaken before the drug is discontinued.
RECENT ADVANCES IN MOLECULAR TARGETED THERAPY OF HAEMATOLOGICAL MALIGNANCIES
Primary lymphomas of the gynaecological organs are exceedingly rare. Most of these tumours are non-Hodgkin's lymphoma, and the most common cell type is diffuse large B-cell lymphoma (DLBCL).89 The prevalence of primary lymphomas of the gynaecological organs has been reported to range from 0.2% to 1.1%. By contrast, prevalence of secondary lymphomas of the gynaecological organs has been reported to be 7–30%.90 The ovary is most commonly affected by lymphoma (Figure 10), followed by the cervix, uterine corpus and vagina.90
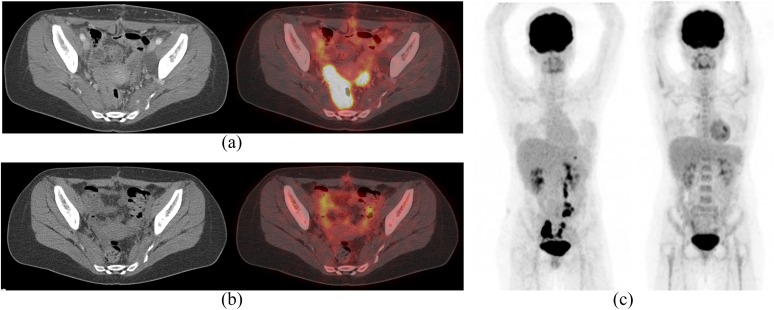
Figure 10.
A 28-year-old female with diffuse large B-cell lymphoma involving the bilateral ovaries and retroperitoneal lymph nodes treated with one cycle of CODOX, followed by five cycles of dose-adjusted R-EPOCH with a complete remission. (a) Axial contrast-enhanced CT (left) and fused positron emission tomography (PET)/CT (right) axial images show intensely fluorine-18 fludeoxyglucose (18F-FDG) avid in both ovaries. (b) Axial contrast-enhanced CT (left) and fused PET/CT (right) axial images after five cycles of chemotherapy show significant decrease in 18F-FDG uptake in ovaries. (c) Coronal maximum intensity projection 18F-FDG PET images before (left) and after (right) treatment show significant decrease in 18F-FDG-avid lesions suggestive of response to therapy. CODOX, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin.
Diffuse large B-cell lymphoma
DLBCL is the most common lymphoid neoplasm in adults comprising approximately 30% of non-Hodgkin's lymphoma diagnosed annually.1 During the past several decades, therapeutic options for DLBCL have evolved from the use of conventional chemotherapy to adding molecular targeted therapy. The advent of immunotherapeutic agents such as monoclonal antibodies that target cell surface antigens (CD20, CD30, CD52) have led to the development of new and effective mechanisms of action.8,9,11,15,17 A large number of clinical trials are currently ongoing to evaluate novel investigational agents that target specific pathways of B-cell malignancies.
Rituximab
Limited stage DLBCL (usually Ann Arbor Stage I or II) is primarily treated with combined modality therapy, including systemic chemotherapy (combination of cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab; anti-CD20 monoclonal antibody; and involved field radiation therapy. Advanced stage DLBCL (usually Ann Arbor Stage III or IV) is primarily treated with systemic chemotherapy plus rituximab.29 Rituximab is also recommended for DLBCL at Ann Arbor Stages II–IV under the National Institute for Health and Care Excellence guidelines in conjunction with systemic chemotherapy.91 Overall response rate was reported as 82–86% (Figure 10).9,11,17
Brentuximab vedotin
Brentuximab vedotin is a CD30-directed antibody–drug conjugate that is used for treatment of CD30-positive lymphomas. Brentuximab vedotin was approved by the FDA in 2011 for treating patients with systemic anaplastic large cell lymphoma after failure of at least one prior chemotherapy regimen. In a study of 49 patients with DLBCL, Jacobsen et al15 demonstrated that brentuximab vedotin was active (44% objective response rate) across a range of CD30 expression and responses occurred in 44% of refractory patients.
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody targeting CD52 antigen which is widely expressed on B or T cells as well as malignant lymphoid cells.92 Alemtuzumab was initially registered for the treatment of fludarabine-refractory chronic lymphatic leukaemia and has shown clinical activity as a first-line treatment for patients with chronic lymphatic leukaemia. However, alemtuzumab has limited evidence for DLBCL. In a study of 11 patients with refractory DLBCL, Cetin et al8 showed limited effectiveness of alemtuzumab as salvage chemotherapy. Alemtuzumab is no longer recommended as a first-line treatment option for chronic lymphatic leukaemia except in the setting of del(17p).75
ROLE OF IMAGING: HAEMATOLOGICAL MALIGNANCIES
Treatment response assessment
The molecular targeted drugs in the lymphoma treatment are different from drugs used in epithelial or mesenchymal malignancies. Therefore, treatment response assessment of lymphoma is also different from those of epithelial or mesenchymal malignancies. Recent advances in imaging modalities, molecular profiling techniques and the use of prognostic indices have the potential to improve disease characterization and outcomes in lymphoma. Positron emission tomography (PET)/CT plays a major role in objectively assessing response to therapy during lymphoma treatment. The Malignant Lymphomas Imaging Working Group response criteria, introduced in 1999 and revised in 2007 and 2014, have been widely adopted.93–95 These include guidance on reporting of PET-CT for staging and response assessment of DLBCL. The use of Deauville five-point scale proposed in 2009 for assessment of interim PET/CT in patients with lymphoma has been shown to have significant prognostic value and permit interim PET/CT-adapted strategies (Figure 10). The five-point scale scores the most intense uptake in a site of initial disease, if present, as follows: 1, no uptake; 2, uptake ≤ mediastinal blood pool; 3, uptake > mediastinal blood pool but ≤ liver; 4, uptake moderately higher than liver; 5, uptake markedly higher than liver and/or new lesions; and X, new areas of uptake unlikely to be related to lymphoma. Quantitative imaging parameters (e.g. δSUVmax) for assessing disease burden and response have to be explored as potential prognosticators.95
Toxicities
Rituximab can cause serious adverse events including tumour lysis syndrome, infections and pulmonary toxicity (interstitial pneumonitis).96 Pulmonary toxicity associated with rituximab manifests clinically with cough, shortness of breath and fever and radiologically as diffuse ground-glass opacities.97 Rituximab and brentuximab vedotin are reportedly associated with progressive multifocal leukoencephalopathy,98,99 a rare but fatal central nervous system infection caused by reactivation of the latent John Cunningham (JC) virus.100
CONCLUSION
We have attempted to provide a comprehensive review of the targeted therapies used in gynaecological malignancies and the radiological findings associated with these agents. Several gynaecological malignancies have characteristic genetic alterations that can be targeted at a molecular level, and an increasing number of gynaecological malignancies are being treated with molecular targeted therapy. The use of these drugs will likely increase in the future and make the role of the radiologist critical in treatment response assessment and detection of toxicities of these agents. Accurate response assessment and recognizing the class-specific drug toxicities are important for optimal patient management.
Contributor Information
Chong Hyun Suh, Email: jhsuh04@gmail.com.
Sree H Tirumani, Email: sreeharsha_tirumani@dfci.harvard.edu.
Abhishek Keraliya, Email: Abhishek_Keraliya@dfci.harvard.edu.
Kyung Won Kim, Email: medimash@gmail.com.
Nikhil H Ramaiya, Email: nramaiya@partners.org.
Atul B Shinagare, Email: ashinagare@partners.org.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. doi: 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2. Referenced from the National Institute for Health and Care Excellence (NICE) technology appraisal guidance. Olaparib for maintenance treatment of relapsed, platinum-sensitive, BRCA mutation-positive ovarian, fallopian tube and peritoneal cancer after response to second-line or subsequent platinum-based chemotherapy. Accessed June 7, 2016. Available from: http://nice.org.uk/guidance/ta381.
- 3.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(Suppl. 7): vii92–9. doi: 10.1093/annonc/mds253 [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012; 30: 2039–45. doi: 10.1200/jco.2012.42.0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol 2009; 10: 1188–98. doi: 10.1016/s1470-2045(09)70226-8 [DOI] [PubMed] [Google Scholar]
- 6.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010; 376: 245–51. doi: 10.1016/s0140-6736(10)60893-8 [DOI] [PubMed] [Google Scholar]
- 7.Cassier PA, Dufresne A, Blay JY, Fayette J. Trabectedin and its potential in the treatment of soft tissue sarcoma. Ther Clin Risk Manag 2008; 4: 109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cetin B, Coskun U, Yildiz R, Buyukberber S, Baykara M, Benekli M. Acute cholecystitis in a patient with metastatic renal cell carcinoma treated with everolimus. J Oncol Pharm Pract 2011; 17: 274–8. doi: 10.1177/1078155210363317 [DOI] [PubMed] [Google Scholar]
- 9.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 235–42. doi: 10.1056/NEJMoa011795 [DOI] [PubMed] [Google Scholar]
- 10.Elit L, Hirte H. Palliative systemic therapy for women with recurrent epithelial ovarian cancer: current options. Onco Targets Ther 2013; 6: 107–18. doi: 10.2147/ott.s30238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005; 23: 4117–26. doi: 10.1200/jco.2005.09.131 [DOI] [PubMed] [Google Scholar]
- 12.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010; 28: 2512–19. doi: 10.1200/jco.2009.26.9589 [DOI] [PubMed] [Google Scholar]
- 13.Gajdos C, Elias A. Trabectedin: safety and efficacy in the treatment of advanced sarcoma. Clin Med Insights Oncol 2011; 5: 35–43. doi: 10.4137/cmo.s4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011; 12: 852–61. doi: 10.1016/s1470-2045(11)70214-5 [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015; 125: 1394–402. doi: 10.1182/blood-2014-09-598763 [DOI] [PubMed] [Google Scholar]
- 16.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366: 1382–92. doi: 10.1056/NEJMoa1105535 [DOI] [PubMed] [Google Scholar]
- 17.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379–91. doi: 10.1016/s1470-2045(06)70664-7 [DOI] [PubMed] [Google Scholar]
- 18.Pink D, Lindner T, Mrozek A, Kretzschmar A, Thuss-Patience PC, Dorken B, et al. Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol 2006; 101: 464–9. doi: 10.1016/j.ygyno.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 19.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol 2014; 32: 1302–8. doi: 10.1200/jco.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- 20.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010; 28: 835–40. doi: 10.1200/jco.2009.25.2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams CJ. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev 2001; 1: CD001034. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol 2013; 121: 1226–34. doi: 10.1097/AOG.0b013e3182922a17 [DOI] [PubMed] [Google Scholar]
- 23.Burger RA. Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol Oncol 2011; 121: 230–8. doi: 10.1016/j.ygyno.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan S, Subramanian IV, Yokoyama Y, Geller M. Angiogenesis in normal and neoplastic ovaries. Angiogenesis 2005; 8: 169–82. doi: 10.1007/s10456-005-9001-1 [DOI] [PubMed] [Google Scholar]
- 25.Brown MR, Blanchette JO, Kohn EC. Angiogenesis in ovarian cancer. Baillieres Best Pract Res Clin Obstet Gynaecol 2000; 14: 901–18. doi: 10.1053/beog.2000.0134 [DOI] [PubMed] [Google Scholar]
- 26.Iglehart JD, Silver DP. Synthetic lethality—a new direction in cancer-drug development. N Engl J Med 2009; 361: 189–91. doi: 10.1056/NEJMe0903044 [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 2010; 28: 3570–6. doi: 10.1200/jco.2009.27.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groen RS, Gershenson DM, Fader AN. Updates and emerging therapies for rare epithelial ovarian cancers: one size no longer fits all. Gynecol Oncol 2015; 136: 373–83. doi: 10.1016/j.ygyno.2014.11.078 [DOI] [PubMed] [Google Scholar]
- 29. Referenced from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Uterine Neoplasms V.2.2015. © National Comprehensive Cancer Network, Inc. 2015. All Rights Reserved. Accessed January 23, 2015. To view the most recent and complete version of the guideline, National Comprehensive Cancer Network®, NCCN®, NCCN Guidelines®, and all other NCCN content are trademarks owned by the National Comprehensive Cancer Network, Inc. Available from: www.nccn.org.
- 30.Altman AD, Thompson J, Nelson G, Chu P, Nation J, Ghatage P. Use of aromatase inhibitors as first- and second-line medical therapy in patients with endometrial adenocarcinoma: a retrospective study. J Obstet Gynaecol Can 2012; 34: 664–72. doi: 10.1016/S1701-2163(16)35320-8 [DOI] [PubMed] [Google Scholar]
- 31.Barker LC, Brand IR, Crawford SM. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin 2009; 25: 1105–9. doi: 10.1185/03007990902860549 [DOI] [PubMed] [Google Scholar]
- 32.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 2007; 17: 964–78. doi: 10.1111/j.1525-1438.2007.00897.x [DOI] [PubMed] [Google Scholar]
- 33.Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: a review of North American trials. Expert Rev Anticancer Ther 2009; 9: 905–16. doi: 10.1586/era.09.54 [DOI] [PubMed] [Google Scholar]
- 34.Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004; 92: 10–14. doi: 10.1016/j.ygyno.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 35.Herzog TJ. What is the clinical value of adding tamoxifen to progestins in the treatment [correction for treament] of advanced or recurrent endometrial cancer? Gynecol Oncol 2004; 92: 1–3. doi: 10.1016/j.ygyno.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 36.Kauppila A. Oestrogen and progestin receptors as prognostic indicators in endometrial cancer. A review of the literature. Acta Oncol 1989; 28: 561–6. doi: 10.3109/02841868909092271 [DOI] [PubMed] [Google Scholar]
- 37.Quinn MA, Campbell JJ. Tamoxifen therapy in advanced/recurrent endometrial carcinoma. Gynecol Oncol 1989; 32: 1–3. doi: 10.1016/0090-8258(89)90839-1 [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 2007; 106: 325–33. doi: 10.1016/j.ygyno.2007.03.042 [DOI] [PubMed] [Google Scholar]
- 39.Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol 1999; 17: 1736–44. [DOI] [PubMed] [Google Scholar]
- 40.Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2001; 19: 364–7. [DOI] [PubMed] [Google Scholar]
- 41.Whitney CW, Brunetto VL, Zaino RJ, Lentz SS, Sorosky J, Armstrong DK, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004; 92: 4–9. doi: 10.1016/j.ygyno.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 42.Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 2008; 108: 577–83. doi: 10.1016/j.ygyno.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 1998; 42: 79–85. doi: 10.1016/S0360-3016(98)00193-X [DOI] [PubMed] [Google Scholar]
- 44.Maggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer 2000; 89: 116–22. doi: [DOI] [PubMed] [Google Scholar]
- 45.Berchuck A, Rodriguez G, Kamel A, Soper JT, Clarke-Pearson DL, Bast RC, Jr. Expression of epidermal growth factor receptor and HER-2/neu in normal and neoplastic cervix, vulva, and vagina. Obstet Gynecol 1990; 76: 381–7. [PubMed] [Google Scholar]
- 46.Oonk MH, de Bock GH, van der Veen DJ, Ten Hoor KA, de Hullu JA, Hollema H, et al. EGFR expression is associated with groin node metastases in vulvar cancer, but does not improve their prediction. Gynecol Oncol 2007; 104: 109–13. doi: 10.1016/j.ygyno.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 47.Johnson GA, Mannel R, Khalifa M, Walker JL, Wren M, Min KW, et al. Epidermal growth factor receptor in vulvar malignancies and its relationship to metastasis and patient survival. Gynecol Oncol 1997; 65: 425–9. doi: 10.1006/gyno.1997.4660 [DOI] [PubMed] [Google Scholar]
- 48.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 2005; 23: 2445–59. doi: 10.1200/jco.2005.11.890 [DOI] [PubMed] [Google Scholar]
- 49.Horowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, Matulonis UA, et al. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol Oncol 2012; 127: 141–6. doi: 10.1016/j.ygyno.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 50.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the US. Cancer 2005; 103: 1000–7. doi: 10.1002/cncr.20866 [DOI] [PubMed] [Google Scholar]
- 51.Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol 2008; 9: 973–81. doi: 10.1016/s1470-2045(08)70254-7 [DOI] [PubMed] [Google Scholar]
- 52.Creasman WT, Phillips JL, Menck HR. The National Cancer Data Base report on cancer of the vagina. Cancer 1998; 83: 1033–40. doi: [DOI] [PubMed] [Google Scholar]
- 53.Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011; 29: 2904–9. doi: 10.1200/jco.2010.33.9275 [DOI] [PubMed] [Google Scholar]
- 54.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–16. doi: 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–19. doi: 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006; 24: 4340–6. doi: 10.1200/jco.2006.06.2984 [DOI] [PubMed] [Google Scholar]
- 58.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 59.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 60.Baccala A, Jr, Hedgepeth R, Kaouk J, Magi-Galluzzi C, Gilligan T, Fergany A. Pathological evidence of necrosis in recurrent renal mass following treatment with sunitinib. Int J Urol 2007; 14: 1095–7; discussion 7. doi: 10.1111/j.1442-2042.2007.01902.x [DOI] [PubMed] [Google Scholar]
- 61.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 2010; 195: 281–9. doi: 10.2214/ajr.09.4110 [DOI] [PubMed] [Google Scholar]
- 62.Burger RA, Brady MF, Bookman MA, Monk BJ, Walker JL, Homesley HD, et al. Risk factors for GI adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2014; 32: 1210–17. doi: 10.1200/jco.2013.53.6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 2008; 19: 577–82. doi: 10.1093/annonc/mdm508 [DOI] [PubMed] [Google Scholar]
- 64.Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol 2007; 25: 2902–8. doi: 10.1200/jco.2007.12.1509 [DOI] [PubMed] [Google Scholar]
- 65.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol 2007; 105: 3–6. doi: 10.1016/j.ygyno.2007.01.038 [DOI] [PubMed] [Google Scholar]
- 66.Shinagare AB, Howard SA, Krajewski KM, Zukotynski KA, Jagannathan JP, Ramaiya NH. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol 2012; 199: 1259–65. doi: 10.2214/ajr.12.8782 [DOI] [PubMed] [Google Scholar]
- 67.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007; 14: 1860–9. doi: 10.1245/s10434-006-9337-9 [DOI] [PubMed] [Google Scholar]
- 68.Chow H, Jung A, Talbott J, Lin AM, Daud AI, Coakley FV. Tumor fistulization associated with targeted therapy: computed tomographic findings and clinical consequences. J Comput Assist Tomogr 2011; 35: 86–90. doi: 10.1097/RCT.0b013e3181fce2cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009; 361: 123–34. doi: 10.1056/NEJMoa0900212 [DOI] [PubMed] [Google Scholar]
- 70.Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, et al. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol 2013; 200: W468–74. doi: 10.2214/ajr.12.9751 [DOI] [PubMed] [Google Scholar]
- 71.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 2010; 116: 131–9. doi: 10.1016/j.ygyno.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 72.Vang R, Kempson RL. Perivascular epithelioid cell tumor ('PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol 2002; 26: 1–13. doi: 10.1097/00000478-200201000-00001 [DOI] [PubMed] [Google Scholar]
- 73.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, Cha SS, Sloan JA, Keeney GL, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol 2003; 89: 460–9. doi: 10.1016/S0090-8258(03)00137-9 [DOI] [PubMed] [Google Scholar]
- 74.Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, Le Cesne A, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007; 8: 595–602. doi: 10.1016/s1470-2045(07)70175-4 [DOI] [PubMed] [Google Scholar]
- 75.Leitao MM, Jr, Hensley ML, Barakat RR, Aghajanian C, Gardner GJ, Jewell EL, et al. Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol 2012; 124: 558–62. doi: 10.1016/j.ygyno.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 76.George S, Feng Y, Manola J, Nucci MR, Butrynski JE, Morgan JA, et al. Phase 2 trial of aromatase inhibition with letrozole in patients with uterine leiomyosarcomas expressing estrogen and/or progesterone receptors. Cancer 2014; 120: 738–43. doi: 10.1002/cncr.28476 [DOI] [PubMed] [Google Scholar]
- 77.Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2009; 2: 45. doi: 10.1186/1756-8722-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin Liberal J, Lagares-Tena L, Sainz-Jaspeado M, Mateo-Lozano S, Garcia Del Muro X, Tirado OM. Targeted therapies in sarcomas: challenging the challenge. Sarcoma 2012; 2012: 626094. doi: 10.1155/2012/626094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007; 25: 1753–9. doi: 10.1200/jco.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- 80.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol 2010; 194: 157–65. doi: 10.2214/ajr.09.2941 [DOI] [PubMed] [Google Scholar]
- 81.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, attenuation, size, and structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on anti-angiogenic targeted therapy. AJR Am J Roentgenol 2010; 194: 1470–8. doi: 10.2214/ajr.09.3456 [DOI] [PubMed] [Google Scholar]
- 82.Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, et al. High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology 2009; 251: 447–56. doi: 10.1148/radiol.2512081403 [DOI] [PubMed] [Google Scholar]
- 83.Albiges L, Chamming's F, Duclos B, Stern M, Motzer RJ, Ravaud A, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol 2012; 23: 1943–53. doi: 10.1093/annonc/mds115 [DOI] [PubMed] [Google Scholar]
- 84.Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics 2006; 26: 1637–53. doi: 10.1148/rg.266065004 [DOI] [PubMed] [Google Scholar]
- 85.Tirumani SH, Jagannathan JP, Shinagare AB, Kim K, Krajewski KM, Ramaiya NH. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology 2013; 13: 461–7. doi: 10.1016/j.pan.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 86.Rohatgi S, Jagannathan JP, Rosenthal MH, Kim KW, Ramaiya NH, Krajewski KM. Vascular toxicity associated with chemotherapy and molecular targeted therapy: what should a radiologist know? AJR Am J Roentgenol 2014; 203: 1353–62. doi: 10.2214/ajr.13.11967 [DOI] [PubMed] [Google Scholar]
- 87.Pham PT, Pham PC, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, et al. Sirolimus-associated pulmonary toxicity. Transplantation 2004; 77: 1215–20. doi: 10.1097/01.TP.0000118413.92211.B6 [DOI] [PubMed] [Google Scholar]
- 88.Dabydeen DA, Jagannathan JP, Ramaiya N, Krajewski K, Schutz FA, Cho DC, et al. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer 2012; 48: 1519–24. doi: 10.1016/j.ejca.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 89.Trenhaile TR, Killackey MA. Primary pelvic non-Hodgkin's lymphoma. Obstet Gynecol 2001; 97: 717–20. [DOI] [PubMed] [Google Scholar]
- 90.Lagoo AS, Robboy SJ. Lymphoma of the female genital tract: current status. Int J Gynecol Pathol 2006; 25: 1–21. doi: 10.1097/01.pgp.0000183049.30212.f9 [DOI] [PubMed] [Google Scholar]
- 91. Referenced from the National Institute for Health and Care Excellence (NICE) technology appraisal guidance 65. Rituximab for aggressive non-Hodgkin's lymphoma. Accessed June 7, 2016. Available from: http://guidance.nice.org.uk/ta65.
- 92.Rodig SJ, Abramson JS, Pinkus GS, Treon SP, Dorfman DM, Dong HY, et al. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H). Clin Cancer Res 2006; 12: 7174–9. doi: 10.1158/1078-0432.ccr-06-1275 [DOI] [PubMed] [Google Scholar]
- 93.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244. [DOI] [PubMed] [Google Scholar]
- 94.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. doi: 10.1200/jco.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 95.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32: 3048–58. doi: 10.1200/jco.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burton C, Kaczmarski R, Jan-Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med 2003; 348: 2690–1. doi: 10.1056/nejm200306263482619 [DOI] [PubMed] [Google Scholar]
- 97.Liote H, Liote F, Seroussi B, Mayaud C, Cadranel J. Rituximab-induced lung disease: a systematic literature review. Eur Respir J 2010; 35: 681–7. doi: 10.1183/09031936.00080209 [DOI] [PubMed] [Google Scholar]
- 98.Wagner-Johnston ND, Bartlett NL, Cashen A, Berger JR. Progressive multifocal leukoencephalopathy in a patient with Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma 2012; 53: 2283–6. doi: 10.3109/10428194.2012.676170 [DOI] [PubMed] [Google Scholar]
- 99.von Geldern G, Pardo CA, Calabresi PA, Newsome SD. PML-IRIS in a patient treated with brentuximab. Neurology 2012; 79: 2075–7. doi: 10.1212/WNL.0b013e3182749f17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113: 4834–40. doi: 10.1182/blood-2008-10-186999 [DOI] [PMC free article] [PubMed] [Google Scholar]