Abstract
Objective:
Dynamic jaws (DJ) are expected to be useful in stereotactic radiotherapy (SRT) for brain metastases (BM). The efficacy and optimal dose fractionation were investigated.
Methods:
In a planning study, 63 treatment plans were generated for the following 3 conditions: 1.0-cm fixed jaws (FJ), 2.5-cm FJ and 2.5-cm DJ. In a clinical study, 30 Gy/3 fr, 35 Gy/5 fr or 37.5 Gy/5 fr were prescribed depending on tumour size. Clinical results of groups treated with 2.5-cm DJ plans and 1.0-cm FJ were compared.
Results:
In the planning study, the treatment times in 2.5-cm DJ and FJ plans were less than that in 1.0-cm FJ plans (p < 0.001). The brain doses in 1.0-cm FJ plans and 2.5-cm DJ plans were smaller than those in 2.5-cm FJ plans (p < 0.05). In the clinical study, 34 patients with 68 BM were treated with SRT. Of those, 15 patients with 34 BM were treated with 2.5-cm DJ plans and 19 patients with 34 BM were treated with 1.0-cm FJ plans. The overall survival and local tumour control (LC) rates were 52 and 93% at 12 months, respectively. The DJ system achieved favourable LC and 29% shorter treatment time compared with the FJ system (p < 0.001). Grade 2 or 3 necrosis occurred more frequently in patients with 15 cc or larger tumour volumes (p = 0.05).
Conclusion:
DJ technology enables treatment time to be reduced without worsening the dose distribution and clinical efficacy. The prescribed doses in this study may be acceptable for patients with small tumour volumes.
Advances in knowledge:
DJ technology enables treatment time to be reduced without worsening the dose.
INTRODUCTION
Management of newly diagnosed brain metastases (BM) includes various treatment options: surgery, whole-brain radiotherapy and stereotactic radiotherapy (SRT).1,2 Tomotherapy is a radiation delivery system that combines dynamic intensity-modulated radiation therapy and an on-board imaging system, providing high-precision radiotherapy.3 The role of helical tomotherapy has now been established for the treatment of various targets.3–7 SRT for BM can be delivered with helical tomotherapy in the clinical setting.8,9 However, the optimal dose and fractionation schedules have not been established yet. Our previous dose escalation study showed that fractionated SRT (30 Gy/3 fr or 35 Gy/5 fr) was acceptable against large BM (≥10 cc) in the cyberknife radiosurgery system.10 According to that study, similar dose fractionation regimens have been used in tomotherapy treatment in our institution. Thus, the first hypothesis of this study is that those suggested regimens for SRT can be used with tomotherapy.
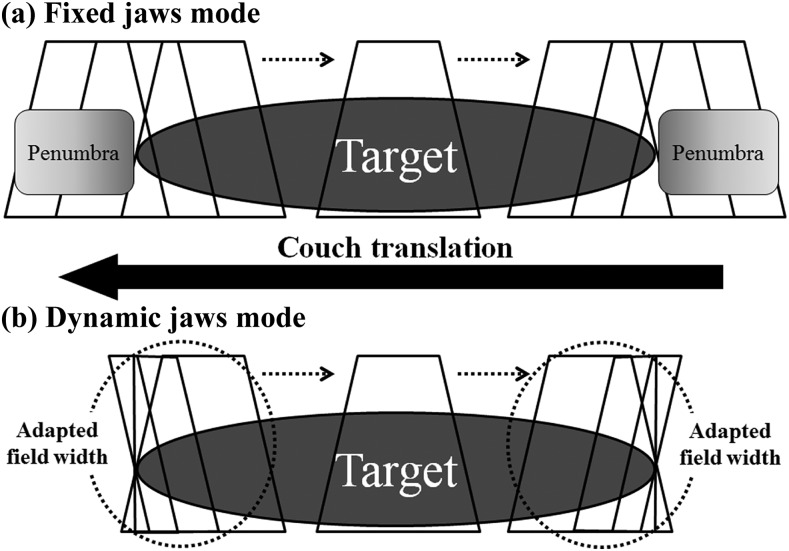
Second, with the conventional tomotherapy delivery mode, the craniocaudal “penumbra”, i.e. dose scattering at the craniocaudal edges of a target, has been an issue in need of improvement. In 2014, our institution was the first in Japan to implement the use of a newly developed technology, dynamic jaws (DJ). Using DJ, radiation doses to the craniocaudal edges of the target can be lowered by narrowing the jaws around the edges (Figure 1).5,6 Thus, this system can be expected to provide favourable dose distribution compared with the same jaw-size fixed jaws (FJ) mode. Treatment time can also be reduced compared with using smaller jaws modes. To evaluate the feasibility of these hypotheses, we first conducted a planning study to compare two treatment plans with or without the use of the DJ system. In addition, the efficacy was evaluated in clinical treatments.
Figure 1.
Schema for the conventional fixed jaws system (a) and the new dynamic jaw system (b). Reproduced from SAGE, Sugie et al.5
METHODS AND MATERIALS
Planning study
In the planning study, axial non-contrast-enhanced CT with a slice thickness of 2 mm was performed for treatment planning. Imaginary spherical planning target volumes (PTVs) with diameters of 10, 12, 14, 16, 18, 20 or 22 mm were contoured on head CT images of three patients. All the contouring of target volumes and normal structures was performed on the Pinnacle v. 9 treatment planning system (Philips Medical System, Eindhoven, Netherlands).3 The contours created in the treatment planning system were exported to the Tomotherapy Hi-Art treatment planning system v. 4.0 (Accuray Inc., Sunnyvale, CA). Treatment plans were calculated for the following three conditions: 1.0-cm field width with FJ, 2.5-cm field width with FJ and 2.5-cm field width with DJ. Thus, in total, 63 plans were analyzed. The prescribed dose was 30 Gy/3 fr in this planning study. Evaluated parameters were treatment time, monitor unit, uniformity index (UI) and conformity index (CI) for the PTV and V20, V10 and V5 Gy (cc) for the brain. The PTV doses satisfied the criteria; (1) D95% > 90% of the prescribed dose and (2) V90% ≥ 95%. D95% was defined as the minimum dose delivered to 95% of the PTV. V90% was defined as the percentage of the PTV receiving at least 90% of the prescribed dose. V20, V10 and V5 Gy (cc) were the volumes of the brain receiving at least 20, 10 and 5 Gy, respectively. In the tomotherapy planning system, the parameters set before optimization were field width, modulation factor and pitch. The same pitch and modulation factor were used for the same lesions in this planning study. The CI and UI were calculated according to the following formulae:4
| (1) |
| (2) |
In these formulae, the abbreviations indicate the following: VPTV = PTV (cc), TVPV = lesion volume (cc) covered by the prescribed isodose, VTV = prescribed isodose volume (cc) and D5% = minimum dose delivered to 5% of the PTV. Lower CI indicates higher conformity and lower UI indicates better homogeneity. Ideal CI and UI are both 1.
Clinical study
SRT for BM was carried out in a single institution from September 2012 according to a prospective protocol. Patients who fulfilled the following inclusion criteria were treated in the protocol: (1) World Health Organization performance status of 0–2, (2) patient conditions allowing the same body position in an immobilizing device for more than 20 min and (3) five or less brain metastases. The exclusion criteria were: (1) previous surgery or radiotherapy history for BM, (2) meningitis carcinomatosa, (3) pregnancy or potential pregnancy, (4) psychiatric disorder and (5) contraindication to iodine or gadolinium. Informed consent had to be obtained from all patients or their guardians. The DJ system was introduced in January 2014. After the introduction of the DJ system, SRT was generally delivered using this system. The DJ plans were generated with 2.5-cm-width DJ. The FJ plans were made with 1.0-cm-width FJ. Multiple BM were simultaneously treated in one plan. Even large tumours with a maximum diameter of 3 cm or greater were treated with SRT, assuming that the fractionated treatment schedule could avoid severe neurotoxicities.
The patients were placed in a supine position and a thermoplastic mask was moulded to the head and attached to the head support. The clinical target volume was defined as an abnormal contrast-enhanced lesion on CT and MRI. The PTV margin was 2 mm. The basic prescribed dose was 35 Gy/5 fr. For small lesions (maximum diameter <1.5 cm), 30 Gy/3 fr was permitted to shorten the total treatment period. For large lesions (≥3 cm), 37.5 Gy/5 fr could be used to improve local tumour control (LC). Treatment was performed three times a week to efficiently utilize reoxygenation phenomena. Dose constraints were applied to nearby critical structures based on the total dose and fractionation schedules. The maximum doses to the brain stem, optic nerve and optic chiasm were limited to <25 Gy/5 fr or 18 Gy/3 fr, as described previously.10 The maximum dose to the lens was lowered to <7 Gy/3 fr or 10 Gy/5 fr.10 To satisfy these limitations, dose-painting technique was applied.
We evaluated the overall survival (OS), LC and toxicities of all patients treated with SRT from September 2012 to July 2015. Local recurrence was defined as a >20% increase in the maximum diameter of the contrast-enhanced tumour on MRI or CT. Toxicities were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v. 4. Brain necrosis was pathologically verified or diagnosed by MRI or C-11 methionine positron emission tomography.11 To evaluate the clinical efficacy and toxicity of the DJ system, groups treated with the 1.0-cm-width FJ and 2.5-cm-width DJ were compared. This study was approved by the institutional review board (no. 1313).
Statistical analyses
DJ and FJ plan dose–volume parameters and treatment times were compared using the Kruskal–Wallis test and Mann–Whitney U-test. The OS and LC were calculated using the Kaplan–Meier method from the start of initial SRT and from the start of each SRT, respectively. The cumulative incidence of local recurrence was calculated by accounting for death as a competing risk. All statistical analyses were performed in R v. 2.13.0 R Development Core Team, Vienna, Austria for Windows.
RESULTS
Planning study
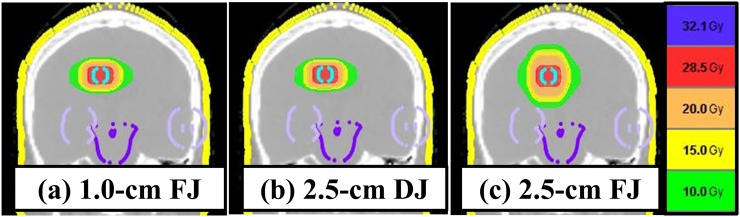
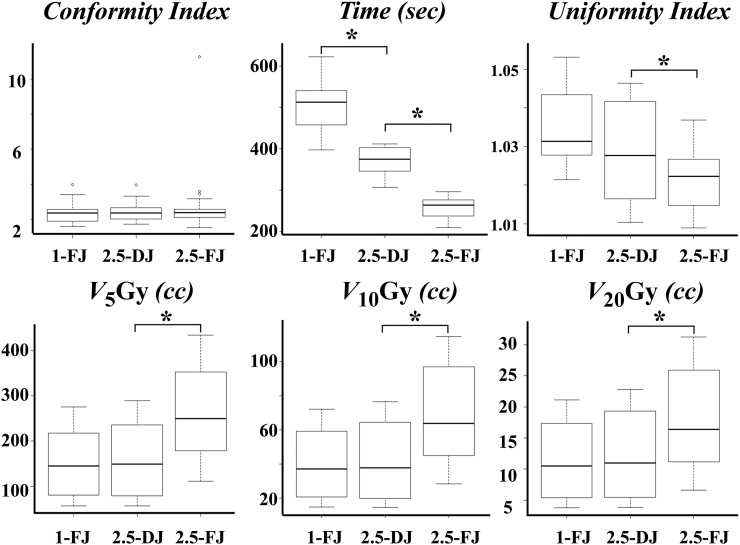
Typical dose distributions are shown in Figure 2. Table 1 and Figure 3 summarize the treatment and dose–volume parameters and treatment times of the 63 plans. The V20, V10 and V5 Gy (cc) in 2.5-cm DJ plans were comparable with those in 1.0-cm FJ plans. The V20, V10 and V5 Gy in 1.0-cm FJ plans and 2.5-cm DJ plans were significantly smaller than those in 2.5-cm FJ plans (p < 0.05). The CI did not differ significantly among 2.5-cm FJ, 2.5-cm DJ and 1.0-cm FJ plans. The planning study showed that treatment time significantly decreased to 73% in 2.5-cm DJ plans and 50% in 2.5-cm FJ plans compared with 1.0-cm FJ plans (p < 0.001). Irrespective of the target diameter, treatment plans with a 2.5-cm field width with DJ could shorten treatment time, and the DJ plans showed comparable dose distribution with those in 1.0-cm field width FJ plans in SRT for BM.
Figure 2.
Dose distributions of 1.0-cm fixed jaws (FJ) (a), 2.5-cm dynamic jaws (DJ) (b) and 2.5-cm FJ plans (c).
Table 1.
Planning study results
| Treatment system |
1.0-cm FJ | 2.5-cm DJ | 2.5-cm FJ | p-value | |
|---|---|---|---|---|---|
| Plan number | 21 | 21 | 21 | ||
| (Mean ± SD) | |||||
| CI |
2.4 ± 0.6 | 2.5 ± 0.6 | 2.8 ± 2.0 | 0.78 | |
| UI |
1.03 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | <0.001 | |
| Maximum dose (Gy) |
31.2 ± 0.4 | 31.1 ± 0.4 | 30.8 ± 0.2 | 0.07 | |
| Minimum dose (Gy) |
29.9 ± 0.2 | 30 ± 0.2 | 29.9 ± 0.1 | 0.28 | |
| Time (s)a |
509 ± 61 | 372 ± 34 | 257 ± 26 | <0.001 | |
| Monitor unita |
7123 ± 911 | 5155 ± 480 | 3515 ± 373 | <0.001 | |
| Brain | V5 Gy (cc) | 153 ± 74 | 159 ± 81 | 260 ± 107 | 0.002 |
| V10 Gy (cc) | 40 ± 20 | 41 ± 22 | 68 ± 29 | 0.003 | |
| V20 Gy (cc) | 11 ± 6 | 12 ± 7 | 18 ± 8 | 0.02 | |
CI, conformity index; DJ, dynamic jaws; FJ, fixed jaws; SD, standard deviation; UI, uniformity index; V5, V10 and V20, volumes of the brain receiving at least 5, 10 and 20 Gy.
Per fraction.
Figure 3.
Comparisons of 1.0-cm fixed jaws (FJ), 2.5-cm dynamic jaws (DJ) and 2.5-cm FJ plans. V5, V10 and V20, volumes of the brain receiving at least 5, 10 and 20 Gy. * Significant difference (p-value ≤0.05).
Clinical study
Patient characteristics and treatment details
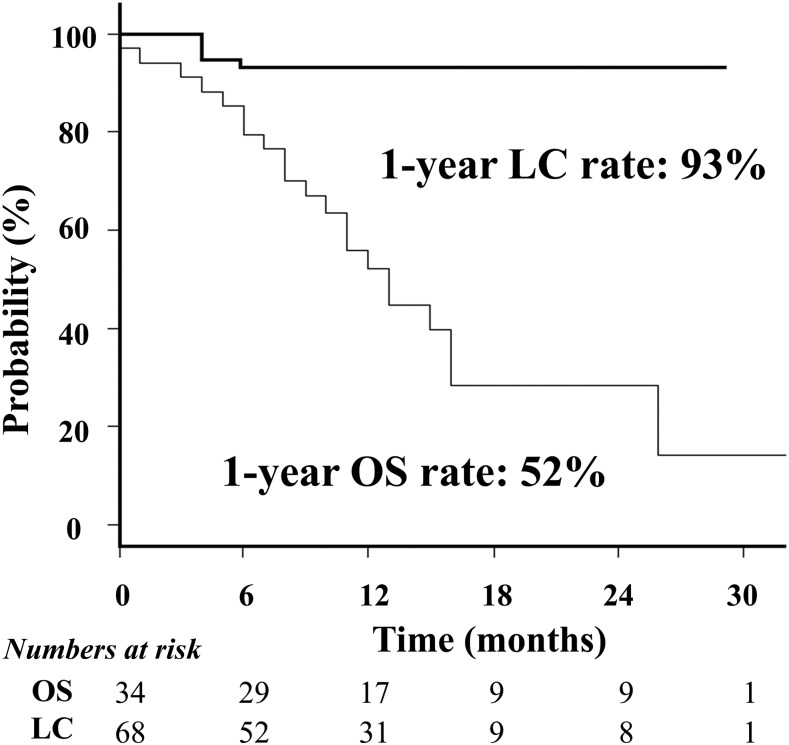
Details of the patients and treatment are summarized in Tables 2 and 3. In total, 34 patients with 68 BM were treated with SRT. All patients had extracranial tumours. They were classified as recursive partitioning analysis Class II.12 The most frequent primary tumour site was the lung. Four patients were retreated once or twice with SRT against new BM. Thus, a total of 40 SRT sessions were carried out. The DJ system was applied to 23 treatments. 5 fractions were applied to 57 of 68 BM. The mean PTV (cc) of each BM was 7.7 ± 10.1 cc (range: 0.2–42.1 cc). The total irradiated tumour volume of each patient was 15.3 ± 11.7 cc (< vs ≥15 cc, 21 vs 13). Based on the planning study described above, 15 patients with 34 BM were treated with 2.5-cm DJ plans since 2014. The CI and UI were similar between the two groups. The OS and LC curves are shown in Figure 4. The OS rate was 52% at 12 months (median, 13 months). The LC rate was 93% at 12 months for all patients; it was 100% for patients treated with DJ and 84% for those treated with FJ (p = 0.02). Four of the five local recurrences occurred in the same patient. Treatment with the DJ system achieved 29% shorter treatment times compared with the FJ system.
Table 2.
Patient characteristics at first stereotactic radiotherapy
| Treatment system |
1.0-cm FJ |
2.5-cm DJ |
|---|---|---|
| Patient number | 19 | 15 |
| Age (mean ± SD) | 63 ± 10 | 64 ± 11 |
| Sex (male/female) | 9/10 | 9/6 |
| Performance status (0/1/2) | 4/14/1 | 0/15/0 |
| BM number (1/2/3/4/5) | 14/3/0/2/0 | 7/6/0/2/0 |
| Primary cancer | ||
| Lung | 11 | 9 |
| Breast | 2 | 0 |
| Colon/rectum | 1 | 1 |
| Oesophagus | 0 | 1 |
| Bladder | 2 | 0 |
| Sarcoma | 0 | 1 |
| Thyroid | 0 | 2 |
| Kidney | 0 | 1 |
| Unknown | 1 | 0 |
| Ovary | 2 | 0 |
BM, brain metastases; DJ, dynamic jaws; FJ, fixed jaws; SD, standard deviation.
Table 3.
Characteristics of the 68 treated lesions
| Treatment system |
1.0-cm FJ | 2.5-cm DJ | p-value | |
|---|---|---|---|---|
| BM number | 34 | 34 | ||
| PTV (cc) | (mean ± SD) | 7.2 ± 9.3 | 8.6 ± 11.3 | 0.89 |
| <1 | 9 | 8 | ||
| ≥1, <4 | 8 | 9 | ||
| ≥4, <15 | 11 | 11 | ||
| ≥15 | 6 | 6 | ||
| Fraction number | 30 Gy/3 fr | 5 | 6 | |
| 35 Gy/5 fr | 12 | 14 | ||
| 37.5 Gy/5 fr | 17 | 14 | ||
| CI (mean ± SD) |
4.9 ± 12.7 | 2.2 ± 1.7 | 0.53 | |
| UI (mean ± SD) |
1.1 ± 0.07 | 1.1 ± 0.06 | 0.41 | |
| Monitor unita (mean ± SD) |
7910 ± 2434 | 5484 ± 1186 | <0.001 | |
| Time (s)a (mean ± SD) |
559 ± 164 | 395 ± 83 | <0.001 | |
| Tumour location | ||||
| Cerebellum |
10 | 4 | ||
| Frontal lobe |
7 | 7 | ||
| Occipital lobe |
2 | 6 | ||
| Parietal lobe |
5 | 3 | ||
| Temporal lobe |
8 | 14 | ||
| Basal Ganglia |
2 | 0 | ||
| Primary cancer | ||||
| Lung |
22 | 15 | ||
| Breast |
2 | 0 | ||
| Colon |
1 | 9 | ||
| Oesophagus |
1 | 4 | ||
| Bladder |
3 | 0 | ||
| Sarcoma |
0 | 2 | ||
| Thyroid |
0 | 3 | ||
| Kidney |
0 | 1 | ||
| Unknown |
2 | 0 | ||
| Ovary | 3 | 0 | ||
BM, brain metastases; CI, conformity index; DJ, dynamic jaws; FJ, fixed jaws; PTV, planning target volume; SD, standard deviation; UI, uniformity index.
Per fraction.
Figure 4.
Overall survival (OS) and local tumour control (LC) curves.
Toxicities
Regarding toxicities, grade 2 seizure was observed in two patients. Grade 2 intratumour bleeding occurred in one patient. Grade 1 brain necrosis occurred in four patients. They all were asymptomatic. One patient was diagnosed with biopsy, another with positron emission tomography and the other two patients with functional and perfusion MRI. Grade 2 brain necrosis was observed in two lesions in two patients treated with the DJ system, and grade 3 necrosis was seen in two lesions in one patient treated with the FJ system. One grade 2 necrosis was pathologically confirmed following craniotomy for treatment of local recurrence. The other grade 2 or 3 necroses were diagnosed with functional and perfusion MRI. No local recurrence was suspected in these cases after careful follow-up. The incidence of grade 2 or 3 brain necrosis did not differ between the DJ (2 of 34 tumours) and FJ groups (2 of 34 tumours) (Fisher test; p = 1). Surgery was performed on lesions in two of these patients, but no recurrence was observed; thus, the patients were considered to be in complete remission. Grade 2 or 3 necrosis occurred more frequently in patients with larger total irradiated tumour volume (Fisher test; p < 0.05). In 21 patients with small total irradiated tumour volume (< 15 cc), these necrosis was not seen at all. In contrast, the necrosis was observed in 3 of 13 patients with 15 cc or more tumour volume.
DISCUSSION
The DJ mode offers DJ alignment throughout the treatment, but the application to patients has not been reported yet. To the best of our knowledge, the present study is the first to report on the planning evaluation and clinical application of the DJ mode in patients with BM. These planning and clinical analyses demonstrate that the DJ system can reduce treatment time. In general, treatment time is influenced by jaw size, pitch and modulation factors in tomotherapy treatment delivery.5,6 A larger jaw size generally leads to reduction in the monitor unit and treatment time and makes target coverage more uniform. The plan conformity decreases, resulting in structures around the target receiving higher doses. Owing to this trade-off, treatment time has been controversial in tomotherapy planning. DJ technology can provide solutions to this dilemma. The present planning study demonstrated that the 2.5-cm DJ mode could reduce total treatment time and achieve almost equal CI, as compared with the 1.0-cm FJ mode. In contrast, the UI was higher and treatment time was longer in the 2.5-cm DJ plans than in the 2.5-cm FJ plans. These results are reasonable because the 2.5-cm DJ mode is a combination of 1.0-cm and 2.5-cm jaws and a part of the targets is treated with 1.0-cm jaws. In the clinical study, treatment time reduction was also observed in the group treated with 2.5-cm DJ plans, compared with the group treated with 1.0-cm FJ plans. Thus, this new technology is useful in SRT for BM.
The study results suggest that 30 Gy/3 fr, 35 Gy/5 fr or 37.5 Gy/5 fr are feasible for patients with small tumour volumes (<15 cc) in SRT using tomotherapy. Peñagarícano et al13 conducted a planning study of radiosurgery to compare tomotherapy and gamma knife. In their report, although the PTV coverage and CIs were comparable between gamma knife and tomotherapy, the volume of low-dose spillage was larger in tomotherapy than in gamma knife. In stereotactic radiosurgery, it is well known that a low-dose irradiated volume of the brain is a useful predictor of brain necrosis. Flickinger et al14 analyzed 85 patients treated with radiosurgery for arteriovenous malformation and suggested that a brain volume receiving 12 Gy could be a predictor of brain necrosis after radiosurgery. In fractionated SRT, biologically equivalent doses to the brain can be lowered by fractionation. Therefore, toxicity can be reduced in fractionated SRT. In addition, anticancer effects can be increased by reoxygenation phenomena during fractionation.15 These advantages of fractionation can be fully utilized in the treatment of large BM. Nagai et al9 reported fractionated SRT (28 Gy/4 fr) outcomes of 122 BM. The LC rate at 12 months was 91%, while no severe radiation necrosis was observed. The authors suggested that the LC improvement was the result of using fractionated SRT. However, the number of large BM (≥10 cc) in their study was only 6 of 122. It has not been shown that the same dose fractionation can be used on large BM as well. On the other hand, we previously conducted a dose escalation study using a cyberknife.10 In that study, 27–30 Gy/3 fr and 30–35 Gy/5 fr were acceptable and feasible against large BM (≥10 cc). Based on those findings, the current dose fractionation schedules, 30 Gy/3 fr, 35 Gy/5 fr or 37.5 Gy/5 fr, were adopted in the present study. In the present study, 15 of 68 lesions were ≥10 cc and the 1-year LC rate was 93%. Hence, these dose fractionation regimens appear sufficient to control large BM. With respect to adverse events, the toxicities were acceptable (grade 1 or 0) for patients with small tumour volumes (<15 cc). Grade 2 necrosis was observed only in patients with larger lesions (≥15 cc). These results suggest the following. First, dose fractionation of 30 Gy/3 fr, 35 Gy/5 fr or 37.5 Gy/5 fr are acceptable for patients with small tumour volumes (<15 cc). Second, a larger number of fractions are needed in SRT for patients with a BM of 15 cc or larger. At present, we have revised our SRT regimen, applying 40 Gy/10 fr to these patients.
Regarding the efficacy of DJ technology, the present planning study showed that low-dose irradiation to the brain could be reduced by the 2.5-cm DJ mode as compared with the same-size FJ mode. The low-dose irradiation to the brain in 2.5-cm DJ plans was similar to that in 1.0-cm FJ plans. Thus, it may be reasonable that toxicity after SRT with the DJ system is almost equal to that with the FJ system. The present clinical results support this hypothesis. The incidence of brain necrosis did not differ between the two groups. The LC rate in the FJ group was lower than that in the DJ group. However, this result needs to be interpreted with caution. Among the five local recurrences treated with the DJ system, four recurrences were observed in the same patient. All BM in this patient could not be controlled. Thus, these recurrences may be explained by tumour-specific radioresistance. Excluding the patient data, the LC rate at 12 months was 95% for patients treated with FJ (p = 0.27). Thus, the result can be considered strongly influenced by this patient data. Even in cases with large BM, the use of a 2.5-cm field width with DJ could achieve an LC rate comparable with that with FJ. Thus, the DJ system can be clinically effective in delivering SRT for BM.
In conclusion, DJ technology can reduce treatment time compared with the conventional 1.0-cm field width FJ mode in SRT for BM. No significant differences in dose distribution, clinical efficacy and toxicity were observed in this study. Thus, this new technology is useful in SRT for BM. And, the current dose fractionation of 30 Gy/3 fr, 35 Gy/5 fr or 37.5 Gy/5 fr may be acceptable for patients with small tumour volumes (<15 cc) in SRT for BM.
Contributor Information
Taro Murai, Email: taro8864@yahoo.co.jp.
Akihiro Hayashi, Email: taro8864@yahoo.co.jp.
Yoshihiko Manabe, Email: manabe.ncu@gmail.com.
Chikao Sugie, Email: chikao@bg8.so-net.ne.jp.
Taiki Takaoka, Email: gkwtt762@yahoo.co.jp.
Takeshi Yanagi, Email: tyana116@gmail.com.
Tetsuya Oguri, Email: t-oguri@med.nagoya-cu.ac.jp.
Masayuki Matsuo, Email: masa-uw@hotmail.co.jp.
Yoshimasa Mori, Email: yoshim@aichi-med-u.ac.jp.
Yuta Shibamoto, Email: yshiba@med.nagoya-cu.ac.jp.
REFERENCES
- 1.Dahele M, Senan S. The role of stereotactic ablative radiotherapy for early-stage and oligometastatic non-small cell lung cancer: evidence for changing paradigms. Cancer Res Treat 2011; 43: 75–82. doi: 10.4143/crt.2011.43.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibamoto Y, Sugie C, Iwata H. Radiotherapy for metastatic brain tumors. Int J Clin Oncol 2009; 14: 281–8. doi: 10.1007/s10147-009-0915-2 [DOI] [PubMed] [Google Scholar]
- 3.Murai T, Murata R, Manabe Y. Intensity modulated stereotactic body radiation therapy for single or multiple vertebral metastases with spinal cord compression. Pract Radiat Oncol 2014; 4: e231–7. doi: 10.1016/j.prro.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Murai T, Shibamoto Y, Manabe Y, Murata R, Sugie C, Hayashi A, et al. Intensity-modulated radiation therapy using static ports of tomotherapy (TomoDirect): comparison with the TomoHelical mode. Radiat Oncol 2013; 8: 68. doi: 10.1186/1748-717X-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugie C, Manabe Y, Hayashi A, Murai T, Takaoka T, Hattori Y, et al. Efficacy of the dynamic jaw mode in helical tomotherapy with static ports for breast cancer. Technol Cancer Res Treat 2015; 14: 459–65. doi: 10.1177/1533034614558746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manabe Y, Shibamoto Y, Sugie C. Helical and static-port tomotherapy using the newly-developed dynamic jaws technology for lung cancer. Technol Cancer Res Treat 2015; 14: 583–91. doi: 10.7785/tcrtexpress.2013.600280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manabe Y, Shibamoto Y, Sugie C, Baba F, Ayakawa S, Nagai A, et al. Toxicity and efficacy of three dose-fractionation regimens of intensity-modulated radiation therapy for localized prostate cancer. J Radiat Res 2014; 55: 494–501. doi: 10.1093/jrr/rrt124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammirati M, Kshettry VR, Lamki T, Wei L, Grecula JC. A prospective phase II trial of fractionated stereotactic intensity modulated radiotherapy with or without surgery in the treatment of patients with 1 to 3 newly diagnosed symptomatic brain metastases. Neurosurgery 2014; 74: 586–94; discussion 594. doi: 10.1227/NEU.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 9.Nagai A, Shibamoto Y, Yoshida M, Wakamatsu K, Kikuchi Y. Treatment of single or multiple brain metastases by hypofractionated stereotactic radiotherapy using helical tomotherapy. Int J Mol Sci 2014; 15: 6910–24. doi: 10.3390/ijms15046910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, et al. Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 2014; 26: 151–8. doi: 10.1016/j.clon.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 11.Shah R, Vattoth S, Jacob R. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics 2012; 32: 1343–59. doi: 10.1148/rg.325125002 [DOI] [PubMed] [Google Scholar]
- 12.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997; 37: 745–51. doi: 10.1016/S0360-3016(96)00619-0 [DOI] [PubMed] [Google Scholar]
- 13.Peñagarícano JA, Yan Y, Shi C, Linskey ME, Ratanatharathorn V. Dosimetric comparison of helical tomotherapy and Gamma Knife stereotactic radiosurgery for single brain metastasis. Radiat Oncol 2006; 1: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flickinger JC, Kondziolka D, Lunsford LD, Kassam A, Phuong LK, Liscak R, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys 2000; 46: 1143–8. doi: 10.1016/S0360-3016(99)00513-1 [DOI] [PubMed] [Google Scholar]
- 15.Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Miyakawa A, Murai T, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 2015; 10: 960–4. doi: 10.1097/JTO.0000000000000525 [DOI] [PubMed] [Google Scholar]