Abstract
Objective:
This study investigated the value of fluorine-18 fludeoxyglucose (18F-FDG) heterogeneity as an indicator of metastatic lymph nodes (LNs) in patients with nasopharyngeal carcinoma (NPC). We further assessed whether addition of this parameter improves diagnostic performance beyond that provided by maximum standardized uptake value (SUVmax).
Methods:
We analyzed 74 LNs that were suspicious for metastasis. These LNs were measured for coefficient of variation (CV) of 18F-FDG uptake, which was used as a parameter for 18F-FDG heterogeneity.
Results:
Multivariate logistic regression analyses revealed that a high CV (hazard ratio, 20.97; 95% confidence interval, 2.26–194.62; p = 0.007) was an independent predictor of metastatic LNs. However, receiver-operating characteristic curve analysis (p = 0.278) and net reclassification (p = 0.539) were unable to show improved diagnostic performance by addition of CV to SUVmax.
Conclusion:
High CV of 18F-FDG uptake is an independent risk factor for metastatic LNs in patients with NPC displaying suspicious LNs following treatment.
Advances in knowledge:
Heterogeneity of 18F-FDG uptake has a potential as a biomarker of metastatic LNs.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a cancer of the head and neck with unique epidemiologic, biologic and clinical characteristics.1 High incidence of disease recurrence, which ranges from 15 to 58%,1–3 is an important clinical feature of this disease.1 Recent advances in treatment techniques and strategies are providing an opportunity to potentially cure some patients with recurrent NPC.4 Therefore, prompt identification of recurrence is of paramount importance during follow-up of patients with NPC.5
Fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET) has an important role in the diagnosis and management of NPC.5,6 This diagnostic modality is useful for detecting metastatic disease, defining the extent of neck nodal disease and evaluating treatment response.6 Above all, 18F-FDG PET is the modality of choice for identifying and differentiating recurrence from post-radiation change.5 Meta-analyses comparing the performance of 18F-FDG PET, CT and MRI have reported a superior diagnostic accuracy of PET for detecting recurrence.7 However, there remain limitations in identifying recurrence by 18F-FDG uptake alone.8,9 Novel parameters of FDG PET may therefore improve the diagnostic accuracy for detecting NPC recurrence.
Recently, 18F-FDG heterogeneity has been shown to help predict overall outcome in patients with cancers of the head and neck,10 oesophagus11 and lung.12 Indeed, heterogeneity of 18F-FDG uptake may have potential as a marker for distinguishing malignant from benign lymph nodes (LNs).13 However, the value of 18F-FDG heterogeneity in NPC has not been explored. Furthermore, much less is known regarding the diagnostic role of this parameter in suspected LNs.
In this study, we thus investigated the role of 18F-FDG heterogeneity as an independent marker for metastatic LNs in patients with NPC who displayed suspicious LNs on follow-up PET/CT. We further determined whether addition of this parameter improves LN characterization beyond that provided by maximum standardized uptake value (SUVmax) and short-axis diameters.
METHODS AND MATERIALS
Subjects
Study subjects were selected from 282 patients with pathology-proven NPC who had received radiotherapy or chemoradiotherapy and underwent 18F-FDG PET/CT imaging between January 2008 and November 2013 for surveillance purpose. Follow-up of patients after treatment included clinical examination with neck CT and/or PET/CT performed every 3–6 months. Other additional diagnostic work-ups were performed at the physician discretion if clinically indicated. Recurrence or distant metastasis was diagnosed based on positive biopsy results or clinical and/or radiographic evidence of progression.
Among these cases, a total of 51 PET/CT scans (of 41 patients) that showed newly appeared or growing regional LNs that were judged by two experienced nuclear medicine physicians to require tissue confirmation, further work-up or close follow-up were included in the study. None of these patients had other malignancy at the time of PET/CT. The institutional review board approved this retrospective cohort study, and the requirement to obtain informed consents was waived.
Positron emission tomography/CT imaging
All patients fasted for at least 6 h before PET/CT studies, and blood glucose levels were <200 mg dl−1 at the time of 18F-FDG injection. Imaging was performed on a GE STE scanner (GE Healthcare, Milwaukee, WI; 35 scans) or a GE Discovery LS scanner (GE Healthcare, Milwaukee, WI; 16 scans). At 45 min after injection of 370 MBq 18F-FDG, CT images were acquired first using a 16-slice helical CT (140 KeV, 30–170 mAs with an Auto A mode; section width of 3.75 mm) with an STE scanner or an 8-slice helical CT (140 KeV, 40–120 mAs adjusted to body weight; section width of 5 mm) with an LS scanner. No i.v. or oral contrast materials were used. Emission PET images were then acquired from thigh to head for 2.5 min per frame in three-dimensional mode with an STE scanner or 4 min per frame in two-dimensional mode with an LS scanner. Attenuation-corrected PET images (voxel size, 3.9 × 3.9 × 3.3 mm with STE; 4.3 × 4.3 × 3.9 mm with LS) were reconstructed using CT data by an ordered-subsets expectation maximization algorithm (20 subsets, 2 iterations with STE; 28 subsets, 2 iterations with LS).
Image analysis
All PET/CT images were analyzed by experienced nuclear medicine physicians blinded to the patient history, biopsy result and how LN appearances changed on follow-up image studies using volume viewer software on a XelerisTM workstation (GE Healthcare, Milwaukee, WI). The software provides an automatically delineated volume of interest (VOI) using an standardized uptake value (SUV)-based isocontour threshold method14 (Figure 1). LN volume contour was determined by a 41% of SUVmax adaptive threshold, which adapts the threshold relative to the local average background.15–17 From the VOI, SUVmax, mean SUV and standard deviation (SD) of SUV were automatically obtained. The coefficient of variation (CV), defined as the ratio of the SD of SUV to mean SUV, was adopted as a parameter for 18F-FDG heterogeneity. In addition, CT images of PET/CT provided short-axis diameters of LNs and information on the presence or absence of LN fatty hilum.
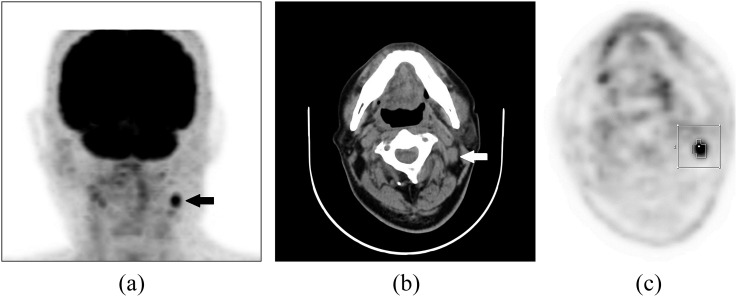
Figure 1.
Fludeoxyglucose (FDG) positron emission tomography (PET)/CT images of a 72-year-old male patient with nasopharyngeal carcinoma who underwent concurrent chemoradiotherapy: focally increased FDG uptake is well visualized in the left neck on the maximum-intensity projection image (a) (black arrow) and the enlarged cervical lymph node (LN) located at the left neck Level II is shown on the transverse CT image (b) (white arrow). Automatic volume of interest (VOI) using an isocontour threshold method was placed over the LN. Segmented VOIs are shown on the transverse PET image (c).
Lymph node status determination
Whether an LN of interest was malignant or benign was determined by pathologic confirmation or clinical or radiographic evidence of progression. LNs were classified as metastatic if confirmed by biopsy or if there was an increase in size or 18F-FDG uptake of the LN on follow-up imaging studies that led to change in management plan. All other LNs, which showed the radiographic evidence of remission on the following imaging studies, were classified as benign. LN stations were assigned according to the classification proposed by the American Head and Neck Society and the American Academy of Otolaryngology—Head and Neck Surgery.18
Statistical analysis
Difference between groups was compared with Mann–Whitney U-tests for continuous variables and χ2 tests for dichotomous variables. Specificity, sensitivity and accuracy of parameters for discriminating metastatic LNs were compared by McNemar's tests. Optimal cut-off values for discerning metastatic LNs were determined by receiver-operating characteristic (ROC) curves, and diagnostic performances of parameters were compared by areas under the curve (AUCs).
Risk factors for metastatic LNs were identified by univariate and multivariate analyses using logistic regression models. Demographics, clinical variables, SUVmax, CV and LN short axis were assessed. Since the lack of multicollinearity between variables is a basic inviolable assumption for multiple logistic regression analysis, we performed collinearity statistics to test the multicollinearity between variables, particularly between CV and SUVmax.
Continuous net reclassification indices (NRI) were estimated to examine the net effect of adding parameters to the diagnostic scheme. NRI demonstrates how many patients are moved into different clinical risk categories by using a different model. Integrated discrimination improvement (IDI) indices were estimated to evaluate the capacity of parameters to discriminate between benign and malignant LNs. IDI demonstrates the difference between competing model discrimination slopes, which tell us whether adding a new factor to a prediction model can improve the discrimination and reclassification.
Determination of optimal cut-off values was performed with MedCalc® for Windows, v. 15.5 (MedCalc Software, Ostend, Belgium). NRI, IDI estimations and AUC comparisons between diagnostic models were conducted using SAS® v. 9.4 (SAS Institute, Cary, NC). The remaining analyses were performed with SPSS® for windows v. 16.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Two-sided p-values of <0.05 were considered significant.
RESULTS
Characteristics of study subjects and lymph nodes of interest
The subjects included in the study had a mean age of 50.0 ± 13.2 years (range, 12–78 years) at the time of PET/CT, and 75.6% subjects were males. 10 patients were included twice for PET/CT studies that were performed at separate occasions following treatment. On each PET/CT scan (n = 51), there were varying numbers of suspected LNs: 34 scans showed 1 suspected LN, 14 scans showed 2 suspected LNs and 3 scans showed 4 suspected LNs. Consequently, a total of 74 suspected LNs in 51 PET/CT scans were analyzed. The characteristics of PET/CT scan-based study subjects categorized according to LN status are summarized in Table 1. In 19 of the 51 scans, all of the suspected LNs were metastatic (n = 28); in 31 scans, all of the suspected LNs were benign (n = 44); and in the remaining 1 scan, 1 suspected LN was metastatic while the other was benign. The groups with “all metastatic LNs” and “all benign LNs” differed in prior history of recurrence (Table 1).
Table 1.
Characteristics of study subjects based on fluorine-18 fludeoxyglucose positron emission tomography (PET)/CT scans (n = 51)
| Patient characteristics | Classification of scans according to LN status |
p-valuea | ||
|---|---|---|---|---|
| All metastatic (n = 19) | All benign (n = 31) | Mixed (n = 1) | ||
| Number of suspected LNs | 28 | 44 | 2 | |
| Number of subjectsb | 11c | 29c | 1 | |
| Age (years) | 50.1 ± 13.4 | 50.9 ± 14.4 | 49.0 | 0.841 |
| Male, n (%) | 12 (63.2) | 25 (80.6) | 1 (100.0) | 0.199 |
| Histology of primary tumour | ||||
| Differentiated, n (%) | 03 (15.8) | 07 (22.6) | – | 0.272 |
| Non-keratinizing, n (%) | 06 (31.6) | 04 (12.9) | – | |
| Undifferentiated, n (%) | 10 (52.6) | 20 (64.5) | 1 (100.0) | |
| Stage at initial diagnosis | ||||
| I, n (%) | 1 (05.3) | 03 (09.7) | – | 0.606 |
| II, n (%) | 2 (10.5) | 06 (19.3) | 1 (100.0) | |
| III, n (%) | 8 (42.1) | 10 (32.3) | – | |
| IV, n (%) | 8 (42.1) | 12 (38.7) | – | |
| First line of treatment | ||||
| CCRT, n (%) | 11 (57.9) | 23 (74.2) | 1 (100.0) | 0.145 |
| Chemotherapy only, n (%) | 05 (26.3) | 02 (06.5) | 0 (000.0) | |
| Radiation therapy only, n (%) | 03 (15.8) | 06 (19.3) | 0 (000.0) | |
| Prior history of recurrence, n (%) | 10 (52.6) | 7 (22.6) | 0 (000.0) | 0.037 |
| Follow-up duration, median (months) (range) | 45.1 (2.3–9.2) | 35.3 (8.4–86.4) | 0.623 | |
CCRT, concurrent chemoradiotherapy; LN, lymph node.
Comparison between “all metastatic” and “all benign” groups.
10 of the subjects had undergone 2 PET/CT studies on separate occasions included in the study.
Four subjects belong to both groups; in addition, six subjects had “all metastatic” results on two separate PET/CT studies.
The 74 suspected LNs had a mean SUVmax of 4.7 ± 3.3 (range, 1.7–22.8), CV of 0.18 ± 0.04 (range, 0.11–0.25) and short-axis diameter of 7.8 ± 2.7 mm (range, 5–17.6 mm). The characteristics of metastatic and benign LNs in our study are summarized in Table 2. Metastatic LNs had significantly greater SUVmax, CV and short-axis diameter than benign LNs. The frequent metastatic sites were Level II cervical LN and retropharyngeal LN (Table 2). The majority of LNs showed loss of fatty hilum on CT images (68/74, 91.9%). Histopathologic examination was performed in 38 (51.3%) of 74 LNs.
Table 2.
Characteristics of suspected lymph nodes (LNs) (n = 74)
| Lymph node characteristics | Metastatic LN (n = 29) | Benign LN (n = 45) | p-value |
|---|---|---|---|
| LN location, n (%) | |||
| Level I | 001 (003.5) | 07 (15.5) | 0.027 |
| Level II | 011 (037.9) | 03 (06.7) | |
| Level III | 03 (010.3) | 10 (22.2) | |
| Level IV | 05 (017.2) | 13 (28.9) | |
| Level V | 02 (006.9) | 02 (04.4) | |
| Level VI and VII | 01 (003.5) | 04 (08.9) | |
| Retropharyngeal | 04 (013.8) | 03 (06.7) | |
| Other sites | 02 (006.9) | 03 (06.7) | |
| Pathology confirmed, n (%) | 23 (079.3) | 15 (33.3) | <0.000 |
| Loss of fatty hiluma, n (%) | 29 (100.0) | 39 (86.7) | 0.075 |
| Short-axis diameter (mm) | 9.1 ± 2.5 | 6.7 ± 2.7 | <0.000 |
| SUVmax | 7.1 ± 4.3 | 3.3 ± 0.9 | <0.000 |
| CV | 0.21 ± 0.03 | 0.16 ± 0.03 | <0.000 |
CV, coefficient of variation; SUVmax, maximum standardized uptake value.
Present in 68/74 (91.9%) of the LNs assessed.
Diagnostic performance of positron emission tomography/CT parameters
On ROC analysis, the AUC of PET/CT performance for diagnosing metastasis was 0.871 for SUVmax, 0.855 for CV and 0.778 for short-axis diameter (Supplementary Figure A). The AUC for SUVmax showed a trend of being greater than that of short-axis diameter, although this did not reach statistical significance (p = 0.088). Optimal cut-off values of SUVmax, CV and short-axis diameter were 4.5, 0.194 and 6.7 mm, respectively. Using these cut-off values, LNs were categorized as having low or high parameters, with which the performance of identifying metastatic involvement was assessed (Table 3). McNemar's test results demonstrated that a high SUVmax (≥4.6) and high CV (≥0.194) had better performance in determining LN metastasis compared with a large size (≥6.8 mm). High SUVmax and high CV showed higher specificity and positive-predictive values. However, there was no significant difference in performance between high SUVmax and high CV (p = 0.508).
Table 3.
Diagnostic performance of positron emission tomography/CT parameters for detecting metastasis lymph nodes
| Parameters | Lesion-based results (n) |
Diagnostic performance (%) |
p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | Total | SN | SP | Accuracy | PPV | NPV | ||
| SUVmax (≥4.6) | 21 | 42 | 3 | 8 | 74 | 72.4 | 93.3 | 85.1 | 87.5 | 84.0 | |
| Size (≥6.8 mm) | 23 | 28 | 17 | 6 | 74 | 79.3 | 62.2 | 68.9 | 57.5 | 82.4 | 0.003a |
| CV (≥0.194) | 22 | 40 | 5 | 7 | 74 | 75.9 | 88.9 | 83.8 | 81.5 | 85.1 | 0.508a, 0.004b |
CV, coefficient of variation; FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; SN, sensitivity; SP, specificity; SUVmax, maximum standardized uptake value; TN, true negative; TP, true positive.
compared with SUVmax.
compared with size criteria.
Risk factors for metastatic lymph node
On univariate analysis, the logistic regression model demonstrated significant associations of metastatic LN with high SUVmax (≥4.6), high CV (≥0.194), large size (≥6.8 mm), female gender, first line of treatment, nodal staging (0,1 vs 2,3) and location (Table 4). Prior history of recurrence showed marginal significance, and other factors failed to show significant association with metastatic LN.
Table 4.
Logistic regression analysis for risk factors associated with metastatic lymph nodes
| Variables | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| SUVmax (≥4.6) | 36.75 | 8.83–153.04 | <0.000 | 5.45 | 0.47–062.73 | 0.174 |
| CV (≥0.194) | 25.14 | 7.13–088.64 | <0.000 | 20.97 | 2.26–194.62 | 0.007 |
| Size (≥6.8 mm) | 04.71 | 1.67–013.32 | 0.003 | 1.30 | 0.07–022.60 | 0.859 |
| Female gender | 03.97 | 1.27–012.43 | 0.018 | 21.48 | 2.38–193.93 | 0.006 |
| First line of treatment | ||||||
| Chemotherapy only | 05.33 | 1.28–022.21 | 0.021 | 2.07 | 0.08–050.39 | 0.654 |
| Location | ||||||
| Level II or retropharynx | 06.96 | 2.26–021.49 | 0.001 | 8.89 | 1.10–071.74 | 0.04 |
| Nodal staging (0, 1 vs 2, 3) | 3.01 | 1.07–8.43 | 0.037 | 1.78 | 0.25–012.65 | 0.56 |
| Prior history of recurrence | 02.47 | 0.89–006.85 | 0.082 | |||
| Stage (I vs) | ||||||
| II | 00.83 | 0.11–006.11 | 0.858 | |||
| III | 01.83 | 0.30–011.26 | 0.513 | |||
| IV | 02.31 | 0.37–014.21 | 0.367 | |||
| Histology (differentiated vs) | ||||||
| Non-keratinizing | 01.56 | 0.33–007.36 | 0.577 | |||
| Undifferentiated | 01.29 | 0.34–004.90 | 0.713 | |||
| Age (>50 years) | 00.98 | 0.38–002.48 | 0.959 | |||
| Loss of fatty hilum | 1.20 × 109 | 0.00–00NC | 0.999 | |||
CI, confidence interval; CV, coefficient of variation; HR, hazard ratio; NC, not calculable; SUVmax, maximum standardized uptake value.
Multivariate analysis performed with significant univariate factors revealed high CV [hazard ratio (HR), 20.97; 95% confidence interval (CI), 2.26–194.62; p = 0.007], female gender (HR, 21.48; 95% CI, 2.38–193.93; p = 0.006) and location (HR, 8.89; 95% CI, 1.10–71.74; p = 0.04) as significant independent risk factors for metastatic LN (Table 4). Collinearity statistics showed that variance inflation factor was <10. Tolerance, another collinearity indicator, was >0.1 between significant univariate variables. These findings indicate that collinearity is not likely to have significantly affected the results of this study (data not shown).
In addition, results of subgroup analysis performed in pathologically confirmed patient group showed that high CV and female gender have significant association with metastatic LN (Supplemental Table A).
Diagnostic value and net reclassification
We next performed ROC analysis with AUC measurements to compare the discrimination capacities of models for metastatic LN. This included SUVmax at baseline with or without addition of CV and size information. Addition of CV to the SUVmax model did not improve diagnostic performance as measured by AUC (87.1 vs 89.2, p = 0.278) (Supplemental Table B). Addition of CV and size to the SUVmax model also failed to increase the AUC of diagnostic performance (87.1 vs 89.2, p = 0.295) (Supplemental Table B).
Similarly, net reclassification of performance for discerning metastatic LNs was not significantly improved by addition of CV (NRI, 3.5%; p = 0.539) nor by addition of CV plus size to SUVmax (NRI, 3.5%; p = 0.643) (Supplemental Table B). IDI results for adding CV and size to models including SUVmax are shown in Supplemental Table B.
DISCUSSION
In our study of patients with NPC undergoing surveillance PET/CT following treatment, LNs with greater 18F-FDG heterogeneity, as represented by high CV, were a significant and independent indicator of metastatic involvement. In our study population, however, addition of 18F-FDG heterogeneity did not significantly increase diagnostic performance beyond that provided by SUVmax.
Heterogeneity of 18F-FDG uptake may have potential as a marker for distinguishing malignant from benign LNs.13 Malignant tumours are composed of substantially heterogeneous tissues19–23 and are therefore expected to have less homogeneous distribution of 18F-FDG uptake compared with benign lesions. This is partly owing to the many different tumour components, which include not only cancer cells but also infiltrating inflammatory cells, vascular cells, connective stroma, granulation tissue and necrotic tissue. Indeed, breast tumours have been shown to harbour markedly divergent proportions cancer cells, ranging from only a small number of cells up to 90% of the tumour bulk.21 Different tumour components vary in their metabolic activity19,20 and therefore contribute differently to overall tumour 18F-FDG uptake. In addition, cancer cells within the same tumour also show phenotypic and functional heterogeneity as a characteristic feature of malignant tumours.24 Furthermore, regional tumour 18F-FDG accumulation can be significantly influenced by local pathophysiological processes such as hypoxia, angiogenesis, proliferation and cell death.25–27 Hence, heterogeneity of 18F-FDG uptake could partly reflect these heterogeneous components within tumour tissue.19–22
The divergent 18F-FDG avidity of tumour components and cancer cell phenotype, as well as local pathophysiological processes, can explain a possible association between histopathologic heterogeneity and distribution of 18F-FDG uptake within tumours. Thus, the potential usefulness of tumour 18F-FDG heterogeneity as a surrogate imaging marker for tumour heterogeneity is recently gaining increasing interest. Results to date have demonstrated significant associations between baseline tumour 18F-FDG heterogeneity and overall outcome in patients with cancers of the head and neck,10 oesophagus11 and lung.12 However, while these findings support the potential prognostic role of tumour 18F-FDG heterogeneity, there are few data on tumour 18F-FDG heterogeneity in NPC as to the best of the authors' knowledge. In addition, less is known regarding the diagnostic role of this parameter. Budiawan et al13 assessed 94 LNs from 44 patients with treatment-naïve adenocarcinoma of the lung and observed more heterogeneous 18F-FDG uptake in metastatic LNs than that in inflammatory LNs.
This study tested the value of CV, defined as SD divided by the mean value of the activity concentration in the tumour volume, as a parameter of heterogeneous 18F-FDG uptake for determining metastatic involvement of LNs in patients with NPC. CV of the SUV derived from a manual VOI has previously been shown to be an independent factor for LN metastasis.13 In the patients with NPC, the diagnostic value of tumour heterogeneity as assessed by 18F-FDG PET has not been investigated. In our results, high CV was an independent risk factor associated with metastatic LNs with diagnostic performance comparable with that of SUVmax, the most popular and useful PET parameter to date. In our study subjects, however, addition of CV information to SUVmax did not significantly increase the AUC of diagnostic performance. Furthermore, NRI results did not show more appropriate reclassification of LNs by addition of CV to SUVmax. Estimated IDI, which indicates magnitudes of change in predicted risk, was also relatively small when CV was added to SUVmax. It should be noted, however, that the small number of subjects in this exploratory study may not have had sufficient power to detect an incremental value of tumour 18F-FDG heterogeneity for diagnosis of metastatic LNs.
A number of different parameters can be used as a measure of tumour 18F-FDG heterogeneity.23 Texture analysis of images, a mathematical method that describes the relationship between grey-level intensity of pixels/voxels and their position, is recently emerging as a new tool for characterizing tumour heterogeneity.23 Second-order or higher order textural features have shown to offer better tissue characterization and image segmentation and improve prediction of therapy response and survival.23 Although CV may not be the most sophisticated or highest precision parameter for tissue heterogeneity, it is widely accessible and can be easily applied in routine clinical practice without the need for specialized software. Furthermore, findings based on the results of recent studies support the possible additional role of CV.28 Calculation of baseline CV or its change between baseline and post-treatment scans may have potential utility in predicting prognosis or treatment response. Clarification of this issue could be an interesting topic for further studies regarding the prognostic value of tumour 18F-FDG heterogeneity.
Another methodological issue is lesion boundary delineation on the PET/CT images, which is important because of its significant impact on measurements of the mean value of 18F-FDG activity. Manual delineations can be an appropriate option, but are laborious and time-consuming. In this study, we applied an automatic boundary delineation method using an adaptive threshold of 41%, which adapts the threshold relative to the local average background.17 Advantages of this method over fixed threshold methods include its applicability even for small lesions with low uptake by correcting for contrast between lesion and local background,16,17 and close correlation between tumour volumes made by this method to actual values.15
Effect of pathologic tumour type could be a considerable issue in evaluating 18F-FDG heterogeneity. In this study, subjects consisting predominantly of undifferentiated carcinoma may have impact on evaluating heterogeneity. However, differentiated and undifferentiated tumours were found to have similar CVs of 0.174 ± 0.043 and 0.185 ± 0.041, respectively (p = 0.43). This finding should be confirmed in further large-scale studies with different types of tumours.
The result of our study supports the potential value of 18F-FDG heterogeneity for discriminating metastatic LNs in patients with NPC. However, it should be mentioned that there is currently insufficient evidence to recommend routine FDG PET-based surveillance in NPC. Further studies may help clarify the diagnostic value of 18F-FDG PET for NPC under different clinical settings, such as staging work-up.
This study has several limitations. First, as mentioned above, the small number of metastasized LNs provides suboptimal statistical power for robust analysis. Also, since the enrolled subjects were highly suspected for having LN metastasis, there is potential for selection-related biases that may restrict generalization of the study results. Lastly, adaptive threshold method used for lesion delineation has a limitation. This method may be challenging for LNs located close to high-uptake regions, where it is as difficult to accurately delineate lesion boundary as in the manual delineation method. Therefore, additional studies with prospective design, a larger number of lesions and a more sophisticated method for lesion delineation are warranted to confirm the findings of this study.
In our study, high CV was an independent predictor of metastatic nodes in patients with NPC but did not have an additive effect on SUVmax.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Kyunga Kim and Sun-Kyu Choi of Biostatistics and Clinical Epidemiology Center, Samsung Medical Center, for their important contributions in statistical analysis.
Contributor Information
Seung Hwan Moon, Email: seunghwan.moons.moon@samsung.com.
Young Seok Cho, Email: ysnm.cho@samsung.com.
Young-Ik Son, Email: young-ik.son@samsung.com.
Yong Chan Ahn, Email: ycahn.ahn@samsung.com.
Myung-Ju Ahn, Email: silk.ahn@samsung.com.
Joon Young Choi, Email: jynm.choi@samsung.com.
Byung-Tae Kim, Email: btnm.kim@samsung.com.
Kyung-Han Lee, Email: khnm.lee@samsung.com.
FUNDING
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (#2012R1A1A2041354).
REFERENCES
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005; 365: 2041–54. doi: 10.1016/S0140-6736(05)66698-6 [DOI] [PubMed] [Google Scholar]
- 2.Yang TS, Ng KT, Wang HM, Wang CH, Liaw CC, Lai GM. Prognostic factors of locoregionally recurrent nasopharyngeal carcinoma—a retrospective review of 182 cases. Am J Clin Oncol 1996; 19: 337–43. doi: 10.1097/00000421-199608000-00003 [DOI] [PubMed] [Google Scholar]
- 3.Chang JT, See LC, Liao CT, Ng SH, Wang CH, Chen IH, et al. Locally recurrent nasopharyngeal carcinoma. Radiother Oncol 2000; 54: 135–42. doi: 10.1016/S0167-8140(99)00177-2 [DOI] [PubMed] [Google Scholar]
- 4.Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol 2013; 20: e406–19. doi: 10.3747/co.20.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohandas A, Marcus C, Kang H, Truong MT, Subramaniam RM. FDG PET/CT in the management of nasopharyngeal carcinoma. AJR Am J Roentgenol 2014; 203: W146–57. doi: 10.2214/AJR.13.12420 [DOI] [PubMed] [Google Scholar]
- 6.Law A, Peters LJ, Dutu G, Rischin D, Lau E, Drummond E, et al. The utility of PET/CT in staging and assessment of treatment response of nasopharyngeal cancer. J Med Imaging Radiat Oncol 2011; 55: 199–205. doi: 10.1111/j.1754-9485.2011.02252.x [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Xu W, Yan WL, Ye M, Bai YR, Huang G. FDG-PET, CT, MRI for diagnosis of local residual or recurrent nasopharyngeal carcinoma, which one is the best? A systematic review. Radiother Oncol 2007; 85: 327–35. doi: 10.1016/j.radonc.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Chan SC, Ng SH, Chang JT, Lin CY, Chen YC, Chang YC, et al. Advantages and pitfalls of 18F-fluoro-2-deoxy-D-glucose positron emission tomography in detecting locally residual or recurrent nasopharyngeal carcinoma: comparison with magnetic resonance imaging. Eur J Nucl Med Mol Imaging 2006; 33: 1032–40. doi: 10.1007/s00259-005-0054-6 [DOI] [PubMed] [Google Scholar]
- 9.Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M. Clinical applications of FDG PET and PET/CT in head and neck cancer. J Oncol 2009; 2009: 208725. doi: 10.1155/2009/208725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit 2009; 42: 1162–71. doi: 10.1016/j.patcog.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 2011; 52: 369–78. doi: 10.2967/jnumed.110.082404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013; 54: 19–26. doi: 10.2967/jnumed.112.107375 [DOI] [PubMed] [Google Scholar]
- 13.Budiawan H, Cheon GJ, Im HJ, Lee SJ, Paeng JC, Kang KW, et al. Heterogeneity analysis of (18)F-FDG uptake in differentiating between metastatic and inflammatory lymph nodes in adenocarcinoma of the lung: comparison with other parameters and its application in a clinical setting. Nucl Med Mol Imaging 2013; 47: 232–41. doi: 10.1007/s13139-013-0216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck 2013; 35: 15–22. doi: 10.1002/hed.22904 [DOI] [PubMed] [Google Scholar]
- 15.Cheebsumon P, Boellaard R, de Ruysscher D, van Elmpt W, van Baardwijk A, Yaqub M, et al. Assessment of tumour size in PET/CT lung cancer studies: PET- and CT-based methods compared to pathology. EJNMMI Res 2012; 2: 56. doi: 10.1186/2191-219X-2-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004; 45: 1519–27. [PubMed] [Google Scholar]
- 17.Cheebsumon P, van Velden FH, Yaqub M, Hoekstra CJ, Velasquez LM, Hayes W, et al. Measurement of metabolic tumor volume: static versus dynamic FDG scans. EJNMMI Res 2011; 1: 35. doi: 10.1186/2191-219X-1-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 2002; 128: 751–8. doi: 10.1001/archotol.128.7.751 [DOI] [PubMed] [Google Scholar]
- 19.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33: 1972–80. [PubMed] [Google Scholar]
- 20.Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells important? J Nucl Med 1995; 36: 1854–61. [PubMed] [Google Scholar]
- 21.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med 2001; 42: 9–16. [PubMed] [Google Scholar]
- 22.Higashi K, Clavo AC, Wahl RL. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med 1993; 34: 414–19. [PubMed] [Google Scholar]
- 23.Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging 2013; 40: 133–40. doi: 10.1007/s00259-012-2247-0 [DOI] [PubMed] [Google Scholar]
- 24.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501: 328–37. doi: 10.1038/nature12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging 2011; 38: 987–91. doi: 10.1007/s00259-011-1787-z [DOI] [PubMed] [Google Scholar]
- 26.Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys 2005; 62: 545–53. doi: 10.1016/j.ijrobp.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Tixier F, Groves AM, Goh V, Hatt M, Ingrand P, Le Rest CC, et al. Correlation of intra-tumor 18F-FDG uptake heterogeneity indices with perfusion CT derived parameters in colorectal cancer. PLoS One 2014; 9: e99567. doi: 10.1371/journal.pone.0099567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, et al. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med 2014; 55: 891–7. doi: 10.2967/jnumed.113.127340 [DOI] [PubMed] [Google Scholar]