Abstract
Squamous cell carcinoma is the most common malignant tumour of the head and neck. The initial TNM staging, the evaluation of the tumour response during treatment, and the long-term surveillance are crucial moments in the approach to head and neck squamous cell carcinoma (HNSCC). Thus, at each of these moments, the choice of the best diagnostic tool providing the more precise and larger information is crucial. Positron emission tomography with fluorine-18 fludeoxyglucose integrated with CT (18F-FDG-PET/CT) rapidly gained clinical acceptance, and it has become an important imaging tool in routine clinical oncology. However, controversial data are currently available, for example, on the role of 18F-FDG-PET/CT imaging during radiotherapy planning, the prognostic value or its real clinical impact on treatment decisions. In this article, the role of 18F-FDG-PET/CT imaging in HNSCC during pre-treatment staging, radiotherapy planning, treatment response assessment, prognosis and follow-up is reviewed focusing on current evidence and controversial issues. A proposal on how to integrate 18F-FDG-PET/CT in daily clinical practice is also described.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the most common malignant tumour of the head and neck (HN).1 Radiotherapy has a well-established role both in the exclusive and in the adjuvant setting.2,3 Initial diagnosis and staging of HNSCC is based on physical examination, chest imaging, HN endoscopy, and HN CT or MRI. Clinical guidelines for HNSCC recommend different imaging approaches for each phase of disease.2–4 Moreover, modern imaging modalities have an essential role in the tumour response after treatment and follow-up.5–7 Each of the currently available imaging techniques present different levels of sensibility and specificity, and it is essential for the radiation oncologist to choose the better one, depending on the clinical scenario.
Positron emission tomography with fluorine-18 fludeoxyglucose integrated with CT (18F-FDG-PET/CT) rapidly gained clinical acceptance and has become an important tool in routine clinical HN oncology. According to the National Comprehensive Cancer Network guidelines, PET or PET/CT is suggested (individualized cases) for Stage III (T3, N0, M0 or T1–3N1M0) and Stage IV (T1–T4, N0–N3, M0–M1) due to the possibility of stage migration.2 The Ontario guidelines suggest that 18F-FDG-PET/CT is also indicated when the primary site is unknown or in the staging of locally advanced disease.4
It is noteworthy that not all HN guidelines agree8 upon the usefulness of PET in different potential HN clinical scenarios.
The aim of this review is to explore the most important available literature dealing with the role of 18F-FDG-PET/CT imaging in HNSCC in pre-treatment staging, radiotherapy planning, treatment response assessment, prognosis and follow-up. Evidences and controversies have been summarized, with particular attention to the point of view of the radiation oncologist.
METHODS AND MATERIALS
We performed a comprehensive literature search of the MEDLINE database without any limits to identify relevant studies (published up to the 31 October 2015) dealing with the topic of this review. We used the keywords “HNSCC” or “head and neck cancer” AND “PET-CT” or “PET” with different combinations: (a) staging, (b) clinical impact, (c) radiotherapy planning, (d) treatment response and (e) prognosis. The titles and abstracts were examined for potentially eligible studies for full-text retrieval. Results have been presented in Tables 1–5. In addition, most significant articles are detailed in the text. Additional sources were identified from references cited in the articles identified by electronic searching. Meta-analyses have been also used as source of articles and briefly described in the text when necessary.
Table 1.
Diagnostic performance of positron emission tomography (PET)/CT for unknown primary carcinoma
| Study | Year | Number of patients | Study design | Primary tumour detection rate (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Regelink et al18 | 2002 | 50 | Retrospective | 32 | 100 | 94 |
| Stockeli et al19 | 2003 | 18 | Prospective | 33 | 63 | 90 |
| Gutzeit et al20 | 2005 | 18 | Retrospective | 33 | 35 | 0 (0/1) |
| Freudenberg et al25 | 2005 | 21 | Retrospective | 57 | 86 | 100 |
| Fakrhy et al21 | 2006 | 22 | Retrospective | 32 | 70 | 75 |
| Nassenstein et al22 | 2007 | 39 | NR | 28 | 100 | 85 |
| Wartski et al23 | 2007 | 38 | Retrospective | 34 | 93 | 73 |
| Johansen et al26 | 2008 | 60 | Prospective | 37 | 87 | 68 |
| Rogh et al27 | 2009 | 44 | Retrospective | 43 | 87.5 | 82.1 |
| Zhao et al28 | 2012 | 25 | Retrospective | 84 | 73.3 | 28.6 |
| Wong et al24 | 2012 | 78 | Retrospective | 38.5 | 100 | 66.7 |
| Pereira et al29 | 2012 | 49 | Retrospective | 18.4 | 69.2 | 81.6 |
| Lee et al30 | 2015 | 56 | Prospective | 50 | 69 | 88 |
NR, not reported.
Table 5.
Studies evaluating prognostic value of pre-treatment positron emission tomography volumetric parameters
| Author | n | Year | Study design | Tumour location | Treatment | Summary results |
|---|---|---|---|---|---|---|
| La et al110 | 85 | 2009 | Retrospective | Oropharynx Hypopharynx Larynx Oral cavity CUP |
CCRT | An increase in MTV of 17.4 m was associated with an increased hazard of first event (recurrence or death) (1.9-fold, p < 0.001), and of death (2.1-fold, p < 0.001). SUVmax was not associated with DFS or OS |
| Chung et al114 | 82 | 2009 | Retrospective | Nasopharynx Oropharynx Hypopharynx |
Cisplatin-based CCRT | MTV >40 ml indicated a significantly worse DFS than MTV ≤40 ml (HR, 3.42; 95% CI, 1.04–11.26; p = 0.04). SUV did not show any prognostic impact on DFS |
| Kim et al115 | 69 | 2011 | Retrospective | Oropharynx Hypopharynx Larynx Oral cavity |
Surgery + RT (±CC) | Patients with MTV >41 ml showed short DFS and 2.4-fold higher recurrence or death than patients with MTV ≤41 (p = 0.041) |
| Park et al116 | 81 | 2013 | Retrospective | Hypopharynx Larynx |
Surgery + RT(±CC)/cisplatin-based CCRTa | MTV was an independent prognostic factor for both LRC (p = 0.018; HR = 3.141, 95% CI = 1.175–8.399) and OS (p = 0.008; HR = 3.758, 95% CI = 1.415–9.982) |
| Kao et al117 | 64 | 2012 | Retrospective | Oropharynx Hypopharynx |
CCRT | Patients with MTV2.5 > 13.6 ml had a significantly inferior 2-year PRFS compared with patients who had lower MTV2.5 tumours (39 vs 72%, respectively, p = 0.001) |
| Dibble et al118 | 45 | 2012 | Retrospective | Oropharynx Oral cavity |
Surgery + RT/CCRT | Primary tumour MTV (median cut-off point of 7.7 ml) was predictive of OS (p = 0.04). Primary tumour TGA (median cut-off point of 55 g) was predictive of OS (log rank p = 0.08) |
| Lim et al119 | 176 | 2012 | Retrospective | Oropharynx | CCRT/surgery | SUVmax was not associated with OS after adjusting for T stage (p = 0.158). In multivariate analysis, TLG and MTV remained associated with OS after correcting for T stage (p = 0.0125 and 0.0324, respectively) and HRs of 1.45 and 1.43, respectively |
| Lee et al120 | 57 | 2012 | Retrospective | Oropharynx | Surgery/surgery + adjuvant therapy | On a univariate analysis, SUVmax, SUVavg, MTV and TLG of primary tumour were significant predictors of survival. However, on multivariate analysis, only patients with high MTV (≥7.78 cm3) showed significantly worse prognoses (p = 0.037) |
| Tang et al121 | 83 | 2012 | Retrospective | Oropharynx Nasopharynx Hypopharynx Larynx Oral cavity CUP |
RT/CCRT | An increase in total MTV of 17 cm3 was associated with a 2.1-fold increase in the risk of disease progression (p = 0.0002) and a 2.0-fold increase in the risk of death (p = 0.0048). SUVmax was not associated with either outcome |
| Moon et al122 | 83 | 2013 | Retrospective | Tonsil | RT alone Surgery alone CCRT Surgery CCRT or RT |
On multivariate analyses, only TLG (HR = 1.020, 95% CI = 1.003–1.037, p = 0.023) was an independent predictive factor associated with decreased OS. MTV and SUVmax were not associated with outcomes |
| Abd El-Hafez et al123 | 126 | 2013 | Prospective | Oropharynx | Surgery/CCRT | TLG and SUVmax were independent prognostic factors for 2-year DSS. Patients with high (T)TLG (≥71.4) had a 2-year DFS of 52%, whereas 74% for those with a low (T)TLG (p = 0.007); the 2-year-DSS rates were 53% vs 84%, respectively (p < 0.001). Patients with high (N)SUVmax (≥7.5) had a 2-year DFS of 42% vs 70% for patients with a low (N)SUVmax (p = 0.001); the 2-year-DSS rates were 39% vs 78%, respectively (p < 0.001) |
| Garsa et al124 | 86 | 2013 | Retrospective | Oropharynx | CCRT | On multivariate analysis, a total MTV >20.5 ml was associated with a 13.0-fold increased risk of death (95% CI = 1.62–100; p = 0.016) for the p16-positive subgroup compared with a 4.27-fold increased risk of death (95% CI = 1.28–14.3; p = 0.018) for the p16-negative subgroup. SUVmax, SUVmean failed to predict DFS or OS |
| Romesser et al125 | 100 | 2014 | Retrospective | Oropharynx | CCRT | On multivariate analysis, a larger MTV (<9.7 cm3) retained a significant correlation with an increased risk for distant metastasis (HR = 2.47; 95% CI = 1.46–4.17; p = 0.001), disease progression or death (HR = 2.17; 95% CI = 1.40–3.38; p = 0.001), and death (HR = 2.37; 95% CI = 1.44–3.89; p = 0.001). SUVmax failed to correlate with any outcome |
| Hanamoto et al126 | 118 | 2014 | Retrospective | Nasopharynx Oropharynx Laryngohypopharyngeal |
CCRT | After multivariate analysis, high MTV (>25.0 ml) and high TLG (>144.8 g) remained as independent, significant predictors of incomplete response compared with low MTV (OR = 13.4; 95% CI = 2.5–72.9; p = 0.003) and low TLG (OR = 12.8; 95% CI = 2.4–67.9; p = 0.003), respectively |
| Alluri et al127 | 70 | 2014 | Retrospective | Oropharynx (HPV positive) | RT alone/CCRT CCRT + surgery Surgery + CCRT |
Total MTV and primary tumour MTV remained as independent prognostic markers for EFS. There was no statistically significant association of EFS with SUVmax, SUVmean and primary tumour or overall TLG |
| Picchio et al112 | 19 | 2014 | Retrospective | Oropharynx Nasopharynx Larynx |
RT/CCRT | MTV (≥32.4 cm3) and TLG (≥469.8 g) predicted patients' outcome with respect to all the considered local and distant disease control end points (LRFS, DMFS and DFS). SUVmean cut-off value predictive of LRFS and DFS were 10.8 |
| Schwartz et al128 | 74 | 2015 | Population subanalysis of Phase III trial (RTOG 0522) | Oropharynx Larynx Hypopharynx |
CCRT (cisplatin and cetuximab) | Primary tumour MTV was a strong independent prognostic factor for PFS. SUVmax was not associated with poor treatment outcomes |
| Yabuki et al129 | 118 | 2015 | Retrospective | Larynx | RT or CCRT | On multivariate analysis, the 3-year DFS for patients with a high MTV were significantly poorer than those with a low MTV (p < 0.001) |
CC, concurrent chemotherapy; CCRT, concurrent chemoradiotherapy; CI, confidence interval; CUP, carcinoma with unknown primary; DFS, disease-free survival; DMFS, distant metastasis-free survival; DSS, disease-specific survival; EFS, event free survival (either recurrence of disease at the primary site, at regional nodes, or at distant metastatic sites or overall patient mortality); HPV, human papillomavirus; HR, hazard ratio; LRC, locoregional control; LRFS, local recurrence-free survival; MTV, metabolic tumour volume; MTV2.5, PET segmentation used applying on isocontour at a SUV of 2.5; n, number of patients; (N)SUVmax, nodal SUVmax; OR, odds ratio; OS, overall survival; PFS: progression-free survival; PRFS, primary relapse-free survival; RT, radiotherapy; RTOG, Radiation Therapy Oncology Group; SUV, standardized uptake value; SUVavg, average SUV; SUVmax, maximal SUV; SUVmean, mean SUV; TGA, total glycolytic activity; TLG, total lesion glycolysis; (T)TLG, tumour total lesion glycolysis.
Several patients received induction chemotherapy.
The inclusion criteria were: articles comparing diagnostic performance (for staging and treatment response) between PET/PET-CT and conventional imaging (CT, MRI and ultrasonography); articles evaluating the role of PET vs conventional imaging for radiotherapy planning; and articles that evaluated pre-treatment PET/CT metabolic parameters to predict the outcome of patients with HNSCC undergoing radical treatment.
The exclusion criteria were: articles regarding the role of PET limited to nasopharynx carcinoma, thyroid or salivary gland tumours (considered as specific clinical entities); studies including <10 patients; and non-English written articles.
Thereafter, the articles have been classified depending on their main topic in order to be considered in each of the sections of this article (pre-therapeutic staging, impact on treatment decisions, monitoring treatment response, radiotherapy planning and prognosis value).
PRE-THERAPEUTIC STAGING
Local tumour extension (T stage)
The correct assessment of the size and extent of a primary lesion at staging is crucial to plan surgery and radiotherapy. Indeed, infiltration of adjacent structures is an important issue in clinical routine. For example, the transgression of the midline on the tongue complicates surgery9 or can modify the clinical target volume in radiotherapy treatment planning (RTP). The initial assessment of the local tumour extension is generally performed with clinical examination and endoscopy.
Even though 18F-FDG-PET/CT detects primary HNSCC with high sensitivity (>95%),10,11 contrast-enhanced (CE) CT and MRI have been considered the primary imaging modalities for evaluating T stage of HNSCC due to their superior anatomical resolution and tissue contrast.
Since it is not possible to exactly define the size and extent of a primary lesion based on 18F-FDG uptake, the PET (alone) images are not suitable to define the T stage of a patient. In addition, the main limitation of hybrid PET/CT, if performed with low-dose unenhanced CT, is its inability to accurately assess the extent of tumour spread and its relationship with adjacent structures.
Moreover, although PET/CT performed with contrast-enhanced CT provides both anatomical and metabolic details at the same time, there is no clear recommendation for routine use of PET/CT in initial T staging.
Some authors showed the high potential value of PET/CT to identify the local tumour extension.11,12 Interestingly, Baek et al11 retrospectively reviewed 69 patients with oral cavity cancer (OCC) who had non-removable dental metallic implants at the time of the pre-treatment imaging work-up and on whom CT or MRI plus PET/CT was performed for the initial staging. The aim was to analyze the clinical impact of PET/CT for primary tumour detection and volume estimation in patients presenting this particular clinical situation. A total of 64 PET/CT, 64 CT and 27 MRI were analyzed. PET/CT was more accurate in detecting primary tumours than CT in patients with OCC and dental artefacts (95.3% vs 75.0%, respectively; p = 0.0016). Among the 27 subjects who had undergone all the three diagnostic modalities, the diagnostic performance for the detection of primary tumours in the oral cavity was 96.3%, 77.8% and 85.2%, respectively (not statistically significant).
Rodrigues et al12 retrospectively evaluated 44 patients who underwent primary tumour resection and neck dissection. They compared the performance of CE-CT, a dedicated HN PET/CT (the latter being a CE-CT) and an optimized whole-body (WB) PET/CT scan. The primary tumour was correctly identified by CE-CT, WB PET/CT and HN PET/CE-CT in 71%, 92% and 95% of cases, respectively. Both (WB and HN) PET protocols demonstrated significantly better performance than did CE-CT in identifying the primary site of the tumour. However, there was no statistical difference in the detection of the primary lesion between WB PET/CT and HN PET/CE-CT protocols.12 A major limit of this study is the lack of comparison with the MRI performance. Moreover, 66% of the patients participating in the study presented an oropharyngeal carcinoma, making difficult to infer these data in HN neoplasms of different origin.
Mandibular invasion
The presence or absence of mandibular invasion is a major determinant in both therapeutic approach and prognosis of HNSCC.13 CT and MRI are commonly used to evaluate the status of the mandible. CT has been reported to be the most accurate method in evaluating discrete cortical bone involvement.14 However, MRI is superior to CT for evaluating tumour invasion into medullary cavity of the mandible.15
Gu et al16 performed a direct comparison of CT and MRI and PET/CT in the detection of mandibular invasion by OCC. The sensitivity was 47.1%, 58.3% and 58.3% for CT, MRI and PET/CT, respectively. The specificity was 100%, 97.1% and 97.1% for CT, MRI and PET/CT, respectively. No statistically significant differences in sensitivity and specificity were detected between the three imaging modalities. A recent retrospective study compared the diagnostic performance from PET/CT and MRI for the detection of bone marrow invasion of the mandible in patients with OCC (surgical specimen was used as the standard).
PET/CT was found to be more specific than MRI (83% vs 61%, respectively, p = 0.0015) but less sensitive (78% vs 97%, respectively, p = 0.0391). Given the low positive-predictive value (PPV) of MRI, a positive MRI scan should incite to confirm data with PET, which shows higher PPV, whereas a negative MRI scan can confidently exclude the presence of bone marrow invasion.15
Cancer from unknown primary
The incidence of cervical metastases from unknown primary cancer (UPC) has been estimated to be around 2–9%. The absence of information about the primary tumour strongly influences the therapeutic approach (i.e. bilateral tonsillectomies, additional pharyngeal mucosa field irradiation).17 PET/CT can identify approximately 30%18–24 of tumours in patients presenting cervical lymph node metastases from UPC, in whom the primary was not detected by the comprehensive diagnostic work-up including endoscopy and conventional imaging methods (CT or MRI). Table 1 summarizes the diagnostic performance of PET/CT in these studies.18–30 It is noteworthy that it should be performed before examination under anaesthesia for targeted panendoscopy and biopsy, avoiding potential false positives due to the inflammation that usually follows these kinds of procedures.26 Thus, a rigorous physical examination is still essential, considering that small and superficial tumours may not have enough 18F-FDG avidity to be detected by PET/CT, as showed by Thiagarajan5 and Daisne et al.31
Recently, Zhu et al32 performed a meta-analysis analyzing a total of 7 studies (246 patients). The primary tumour detection rate, sensitivity and specificity of PET/CT were 44% [95% confidence interval (CI) = 0.31–0.58], 97% (95% CI = 0.63–0.99) and 68% (95% CI = 0.49–0.83).
The largest prospective study evaluating the diagnostic performance of PET/CT in UPC has been published by Johansen et al.26 The authors report data about 60 patients presenting a primary tumour detection rate of 30%, and the sensitivity and specificity rates were 86% and 69%, respectively. However, this study had several limits, namely, it used three different PET engines, and among them one was PET/CT, whereas in two cases, it was PET alone.
Furthermore, in some cases, PET was acquired for WB, in other cases for half body. Furthermore, the authors did not perform extensive comparison with other imaging modalities.
In summary, physical examination remains essential5 for primary tumour assessment (especially regarding superficial tumour extension in the mucosa) while CE-CT and MRI continue to be the reference imaging modalities, mostly due to the lack of shown superiority of PET over morphological examination; however, PET seems to be a promising staging tool, in particular, when morphological examinations suffer from artefacts due to dental implants. Finally, when compared with conventional imaging, PET/CT is recommended to identify primary tumours in patients presenting with cervical lymph node metastases with unknown primary. Nevertheless, it needs opportune integration with other diagnostic procedure to exclude the relatively high risk of false-positive findings.
Lymph node involvement (nodal staging)
The information about nodal involvement is crucial in HNSCC, as it strongly influences the treatment and prognosis of the patients.2,3 Current non-invasive staging techniques include clinical examination, ultrasonography, CE-CT and MRI. The criteria adopted in the evaluation of the nodal status are the size, CE and radiological aspect of the nodes (presence of necrosis, analysis of the capsule to identify any sign of extracapsular extension).33 These techniques could define positive nodes with high specificity, but present limitations in the evaluation of small lymph nodes.34–38 The overall diagnostic accuracy (using pathology as the reference standard) of CT and MRI for detecting metastases in the clinical node negative neck (cN0) is relatively low. Sensitivities range from 14% to 80% for CT and from 29% to 85% for MRI, and specificities range from 80% to 100% for both CT and MRI.33,35–37
The sensitivity and specificity of the imaging techniques influence the clinical practice of the radiation oncologist; patients presenting an expected risk of nodal involvement exceeding 20%39 undergo a prophylactic treatment of the neck, including a neck dissection or unilateral and/or bilateral neck irradiation.39,40 Considering these rates of microscopic involvement, it means that there are at least two thirds of the patients who are treated on the nodal areas without presenting a nodal involvement, only because it is not possible to predict with a better accuracy the “real nodal status of the patients”.
Table 2 summarizes studies comparing the performances of different imaging approaches in the study of the nodal status of HNSCC. These studies36–38,41–43 indicate that PET ± CT is similar or slightly superior to conventional imaging for the diagnosis of neck metastasis.
Table 2.
Studies comparing positron emission tomography (PET) vs CT, MRI and ultrasonography with histopathology of cervical lymph nodes
| Author | Year | Type of study | n | CT/MRI/ultrasonographya | Sensitivity (%) |
Specificity (%) |
||
|---|---|---|---|---|---|---|---|---|
| PET/PET-CT | CT/MRI/ultrasonography | PET/PET-CT | CT/MRI/ultrasonography | |||||
| Adams et al36 | 1998 | Prospective | 60 | CT MRI Ultrasonography |
90 | 82 80 79 |
94 | 85 79 – |
| Hannah et al37 | 2002 | Prospective | 40 | CT | 82 | 81 | 94 | 81 |
| Ng et al38 | 2006 | Prospective | 134 | CT MRI |
41.2 | 20 22.2 |
96.8 | 97.3 97.4 |
| Kim et al41 | 2007 | Prospective | 32 | CT/MRIb | 96.5 | 75.9 | 90 | 90 |
| Yoon et al42 | 2009 | Retrospective | 67 | CT MRI Ultrasonography |
81.1 | 77 77 78.4 |
98.2 | 99.4 99.4 98.5 |
| Kyzas et al43 | 2008 | Meta-analysis (32 studies) | 1236 | –c CT vs PET MRI vs PET Ultrasonography vs PET |
79 (all) 82 78 45 |
– 74 78 42 |
86 (all) 86 85 88 |
76 80 96 |
Type of imaging compared to PET.
All patients underwent CT and/or MRI (reported together).
Also compared the performance of fluorine-18 fludeoxyglucose PET with that of conventional diagnostic methods (i.e. CT, MRI and ultrasonography with fine-needle aspiration).
Kyzas et al43 performed a meta-analysis on this topic in 2008. Across 32 studies (1236 patients), PET sensitivity was 79% (95% CI = 72–85%) and specificity was 86% (95% CI = 83–89%). However, for patients with cN0, sensitivity of PET was only 50% (95% CI = 37–63%), whereas specificity was 87% (95% CI = 76–93%).
Ng et al38 evaluated prospectively 134 patients with oral HNSCC with palpably negative neck with 18F-FDG-PET, CT/MRI and their visual correlation. They reported that 18F-FDG-PET was twice more sensitive than CT/MRI for detecting cervical nodal metastasis in patients with palpably negative neck (41.2% vs 21.6%, respectively; p = 0.021). Histopathological analysis was used as the gold standard to validate the results obtained with different imaging techniques. The authors concluded that 18F-FDG-PET presented a false negativity rate (of occult neck metastasis) of <15% in T1–3 tumours. However, 18F-FDG-PET, even visually correlated with CT/MRI, was unable to reduce the rate of false negative to <20% in patients with T4 tumour (neck treatment being mandatory regardless of PET results).
Kim et al41 evaluated 32 consecutive patients with oropharyngeal HNSCC undergoing 18F-FDG-PET and CT/MRI before surgery (all patients underwent curative resection of their primary tumours with also node dissection, with 7 having bilateral dissections, for a total of 39 neck sides). Each method was interpreted separately to assess primary tumour and cervical node status. Histopathology specimen (in 29 of 39 dissected neck sides and in 47 of 163 dissected cervical levels) showed that 18F-FDG-PET was more accurate than CT/MRI, both in detecting positive neck sides (22/29 vs 28/29, p < 0.05) and on a level-by-level basis (37/47 vs 45/47, p < 0.05). Interestingly, 18F-FDG-PET identified metastatic lesions in approximately two thirds of the morphologically uninvolved nodes.
Cetin et al44 studied 36 patients with HN cancers, clinically and radiographically N0, by means of PET/CT and compared data with neck dissection results. The best threshold of the maximum standardized uptake value (SUVmax) value yielded 84.2% sensitivity and 76.5% specificity for nodal-level staging.
Roh et al45 assessed prospectively 91 patients with HNSCC and negative neck palpation. PET/CT was more sensitive on a per-level basis than CT/MRI (69% vs 39%, p < 0.001), as well as on a per-patient basis 71% and 50%, respectively (p = 0.011).
Although PET/CT examination protocols without the use of contrast medium have been utilized, increasing evidence supports the use of CE-CT as a part of routine PET/CT protocols.46,47 Recently, there have been several reports of the possible superiority of PET/CE-CT over standard PET/CT in different clinical settings, including better local48 and nodal analysis.49 Other studies,50,51 confirmed the high accuracy of nodal staging by PET/CT, in particular, if CE-CT is used during PET protocol.
In summary, 18F-FDG-PET has high diagnostic performance in the overall nodal staging of patients with HNSCC. When compared with conventional imaging, PET/CT is similar or superior for detecting cervical nodal metastases (Table 2). However, this modality is not yet accurate enough to replace the accuracy of neck dissection in the identification of occult cervical metastasis in patients with cN0.36–38,41–43
Detection of distant metastasis
The presence of distant metastases is the most important predictor of patient survival in several cancers. Overall incidence of distant metastasis in HNSCC is relatively low (2–18%).52 Distant metastases frequently occur in the lungs and are routinely detected by chest CT (73% sensitivity and 80% specificity).53 It is noteworthy that early detection of metastasis has a major impact on patient management avoiding unnecessary radical treatments.54–57
Xu et al58 conducted a meta-analysis evaluating the accuracy of PET and PET-CT in the initial M staging of HNSCC. This meta-analysis suggested that 209 (14.4%) of 1445 patients had distant metastasis or a second primary tumour (SPT). PET-CT presented an overall sensibility of 87.5% (95% CI, 78.7–93.6) and an overall specificity of 95% (95% CI, 93.1–96.4).
Regarding the detection of bone metastases, Yi et al59 showed in a recent meta-analysis on >3000 patients, a sensitivity and a specificity of 81% and 99% for PET, and of 89% and 99% for PET/CT, respectively, which are better than the results obtained by bone scintigraphy. Bone scintigraphy relies on the osteoblastic response to bone destruction by cancer cells and the accompanying increase in blood flow. Therefore, 18F-FDG-PET is more efficient than bone scintigraphy for bone lesion detection considering their frequently lytic character.59
Table 3 summarizes available studies comparing the performances of different imaging approaches to detect distant metastasis of HNSCC.52,53,58–63 Globally, all these studies indicate that PET ± CT is superior to conventional imaging.
Table 3.
Results of positron emission tomography (PET) or PET/CT and other imaging tools in M staging of head and neck (HN) cancer
| Author | Year | Type of study | n | Imaging tool | DM %a | Results |
|||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity PET or PET/CT (%) | Specificity PET or PET/CT (%) | Sensitivity CT/MRI/bone scintigraphy (%) | Specificity CT/MRI/bone scintigraphy (%) | ||||||
| de Bree et al52,b | 2000 | Retrospective | 101 | HN CT/MRI Chest CT Bone scintigraphy Liver ultrasound or abdominal CT |
17 | – | – | – | – |
| Brower et al53 | 2005 | Retrospective | 109 | Chest CT | 18c | – | – | 73 | 80 |
| Ng et al60 | 2008 | Prospective | 160 | Extended CT vs PETd | 16.3 | 76.9 | 94 | 50 | 97.8 |
| Senft et al61 | 2008 | Prospective | 145 | Chest CT vs PET | 28 | 53 | 93 | 37 | 95 |
| Kim et al62 | 2007 | Prospective | 349 | HN CT/MRI + PET/CTe | 7.4 | 97.5 | 96.2 | – | – |
| Haerle et al63 | 2011 | Retrospective | 299 | PET/CTf | 10 | 96.8 | 95.4 | – | – |
| Xu et al58 | 2011 | Meta-analysis | 1445 | PET PET/CT | 14.4 | 84.8 87.5 |
95.2 95 |
– – |
– – |
| Yi et al59 | 2013 | Meta-analysisg | 2764 | PET PET/CT PET or PET/CT vs Bone scintigraphy |
– | 81 89 85 – |
99 99 98 – |
– – – 55 |
– – – 98 |
DM, distant metastasis.
DMs detected by PET.
Screening for DM without PET.
This percentage represents metastasis detected by chest CT. There are no comparisons with other imaging.
From the skull base to the lower abdomen.
All patients underwent contrast-enhanced CT or MRI of the HN. Whole-body fluorine-18 fludeoxyglucose PET/CT was also performed in all patients to identify second primary or distant metastatic cancers. There was no comparison between PET/CT and other imaging modalities.
No comparison was performed between PET/CT and other imaging modalities.
Meta-analysis to evaluate fluorine-18 fludeoxyglucose PET/PET-CT for the detection of bone metastases.
18F-FDG-PET shows higher accuracy (90–95%) than CT for the detection of distant metastasis.60,61,63 Given the very high negative-predictive value (NPV), it suggests that in case of negative PET scan, other imaging techniques are not necessary. Nevertheless, the PPV for detecting SPT or distant metastasis is around 60%, suggesting that additional diagnostic methods are still necessary to exclude false-positive results.62
Senft et al61 assessed the added value of 18F-FDG-PET (to chest CT) in the screening of distant metastases in patients with HNSCC and high-risk factors (more than or equal to three lymph node metastases, bilateral lymph node metastases, lymph node metastases of ≥6 cm, low jugular lymph node metastases, regional tumour recurrence and SPT). 145 consecutive patients with HNSCC underwent chest CT and 18F-FDG-PET. 18F-FDG-PET improved pre-treatment screening of distant metastasis compared with chest CT, showing higher sensitivity (53% vs 37%) and PPV (80% vs 75%). Moreover, the authors showed that the sensitivity of the combination of CT and 18F-FDG-PET was higher (63%) than the sensitivity of each of these techniques alone.
Ng et al60 prospectively compared 18F-FDG-PET and extended-field CE-CT (from the skull base to the lower abdomen). A total of 160 patients with HNSCC of the oropharynx or hypopharynx underwent 18F-FDG-PET and extended-field CT to detect distant metastases or SPT. In the entire study cohort, a total of 26 patients (16.3%) were found to have distant malignant lesions. Diagnostic yields of 18F-FDG-PET and extended-field CE-CT were 12.5% (20 out of 160 patients) and 8.1% (13 of 160 patients), respectively. The patient-based sensitivity of 18F-FDG-PET for detection of distant malignancies was 1.5-times higher than that of extended-field CE-CT (76.9% vs 50.0%, p = 0.039), whereas the patient-based specificity of 18F-FDG-PET was not significantly lower than that of extended-field CE-CT (94.0% vs 97.8%, p = 0.125).
In summary, when compared with conventional imaging, PET/CT is a valuable tool to rule out the presence of distant metastases in HNSCC, especially in locally advanced tumours.52,53,60–62,64
Second primaries
SPTs are detected in almost 10% of patients with HNSCC,64,65 particularly in patients who smoke and/or in patients who are negative for human papillomavirus.66
The identification of synchronic or metacronic SPT67 could occur both at the HN region (more frequently) and/or elsewhere (lungs, oesophagus, colon etc.), and it can influence the therapeutic approach61 and the prognosis of patients (especially those presenting with HNSCC).68
Strobel et al64 evaluated the role of PET/CT for the initial staging of HNSCC in 589 consecutive patients for the detection of synchronous primaries. They detected 56 secondary cancers in 44 patients. 46 (82%) were found in the aerodigestive tract as follows: lung (26%), HN (15%) and oesophagus (6%). Nine synchronous cancers were detected by endoscopy and lost at PET/CT. The prevalence of synchronous primaries according to the standard of reference (including panendoscopy or bronchoscopy or oesophageal or colon endoscopy when necessary) was 9.5%. Of these, synchronous primaries, 47 (84%) were detected in 41 patients (93%) by 18F-FDG-PET/CT. Interestingly, in 32 out of 40 patients (80%) with available follow-up, the treatment was modified because of the detection of a synchronous primary.64 They concluded that 18F-FDG-PET/CT detects a considerable number of synchronous primaries (8.0% prevalence) at the initial staging of patients with HNSCC.
According to Haerle et al65 synchronous primary tumours were detected in 4.5% of patients by panendoscopy compared with 6.1% by PET/CT. Indeed 26% of lesions detected on PET/CT were within the coverage of the panendoscopy.65 18F-FDG-PET/CT was superior to panendoscopy. The sensitivity, specificity, PPV and NPV for panendoscopy were 74%, 99.7%, 93% and 98%, respectively. The sensitivity, specificity, PPV and NPV for 18F-FDG-PET/CT were 100%, 95.7%, 59% and 100%, respectively.
According to these results64,65 with a negative 18F-FDG-PET/CT, the extent of endoscopy can be reduced to the area of the primary tumour.
In summary, PET/CT is an accurate method detecting second primaries, with a high NPV.64,65,67–69 Nonetheless, it should be stressed that due to a low PPV (approximately 60%65) in this setting, additional diagnostic methods are necessary to exclude false-positive results (inflammation and hyperplasia in the HN region or intestinal polyps can result in false positives). Moreover, whenever possible PET/CT should be performed before endoscopy and biopsy to avoid false-positive results.26
POSITRON EMISSION TOMOGRAPHY/CT AND CLINICAL IMPACT ON TREATMENT DECISIONS
Although PET/CT imaging is effective for the staging of HNSCC, its impact on patient management is somehow controversial. Indeed, to date, the overall impact of PET/CT on treatment decisions in HNSCC has been rarely explored compared with the number of studies assessing the impact of PET on staging. However, there are four prospective trials54–57 that have specifically analyzed the impact of PET/PET-CT in the treatment approach of HNSCC. These studies followed the same methodology and are detailed in Table 4. These studies addressed at the same time the issue of the impact of PET on the initial staging and management of patients with HNSCC: globally, the PET changed the original treatment plan in approximately 30% of patients.54–57
Table 4.
Clinical impact of positron emission tomography (PET) or PET/CT
| Study | Year | n | PET alone or PET/CT % (number of patients) | Change in TNM stagesa | Stageb | Moderate impactc | High impactc | PET-CT accuracy | Conventional work-up accuracy | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Connell et al56 | 2007 | 76 | PET: – PET/CT: 46% (35) |
34% (12/35) | I–IV | 29% | 11% | –d | – | – |
| Scott et al54 | 2008 | 71 | PET: 56.3% (40) PET/CT: 43.7% (31) |
31% (22) | I–IV | 15.5% | 18.3% | – | – | – |
| Lonneux et al55,e | 2010 | 233 | PET: 83% (194) PET/CT: 17% (39) |
43% (100/233) | I–IV | 5.2% | 8.6% | 78%f | 22%f | p < 0.0001 |
| Cacicedo et al57,e | 2015 | 84 | PET/CT: 100% (84) | 38% (32/84) | III–IV | 9.5%h | 16.7%h | 92.5%g 71.4%f |
73.7%g 25%f |
p < 0.001 p = 0.021 |
n, number of patients.
Discrepant TNM stages obtained between conventional work-up and the inclusion of PET or PET/CT.
Head and neck squamous cell carcinoma stages included in the study.
Medium and high impact: the impact on patient management in these studies was classified as follows: low impact (treatment modality and delivery unchanged); medium impact (change in the treatment within the same therapeutic modality: changes in the type of surgery on primary cancer and/or neck dissection, and changes in the dose or radiotherapy fields); or high impact (change in treatment intent and/or treatment modality: curative to palliation, surgery to chemoradiation or vice versa).
Not available.
In these two studies, treatment decisions were made by a tumour board (pre-PET staging and treatment management plan, and post-PET staging and treatment plan). Therefore, the pre-PET treatment decision and post-PET treatment decision were both made by the tumour board.
This accuracy was calculated for the cases presenting discordant TNMs (n = 100) between conventional work-up and from adding a PET (not for the whole population of the study).
The overall accuracy regarding the whole population of the study.
Meaning that the clinical original clinical decision (pre-PET) adopted by the tumour board was changed in approximately one out of five (9.5% + 16.7% = 26.2%) patients participating in the study, due to the PET/CT.
The largest trial, published by Lonneux et al55 included 233 patients (Stages I–IV) and reported a modification in the original treatment plan in 32 patients (13.7%). In 12 patients (5.2%), the modification was classified as medium (the therapeutic modality remained the same, but PET altered the treatment planning). In 20 patients (8.6%), the impact of PET on patient management was classified as high (change in treatment intent and/or treatment modality, e.g. curative to palliation, surgery to chemoradiation and so on). Interestingly, one of the studies57 assessed together the usefulness of PET/CT for staging and its overall impact on management plans specifically in patients with Stages III and IV HNSCC where the treatment plan was altered in 22/84 (26%) patients (Table 4). These results are in line with the current guidelines.2,3
In summary, PET/CT should be included54–57 in the routine diagnosis of patients with Stages III–IV57 HNSCC, as it significantly improves staging accuracy and also has a marked impact on management plans.
RADIOTHERAPY PLANNING
CT is the primary imaging modality in RTP. The CT images are acquired with the patient in the supine position, immobilized with an individual head support and a rigid customized mask to increase positioning accuracy and to prevent movement during image acquisition. All other imaging modalities (such as PET or MRI) are considered as secondary images.70 The secondary images in RTP will have to be registered (fused) to the primary planning CT scan. When the PET and RTP CT images are acquired on separate scanners (often in a position that does not correspond to real treatment position), a registration module in the RTP computer system can be used to fuse images. Regardless of the imaging data used (i.e. separate PET or PET/CT) correct co-registration of the PET data with the CT data used for RTP must be verified, since the difference in spatial localization of tumour may lead to false estimation of the gross tumour volume (GTV). Ideally, the fusion process can be executed automatically by a hybrid PET-CT-dedicated RTP scanner performed with the corresponding immobilization devices and reproducing radiation delivery conditions.70
Recognizing the potential of PET/CT-guided treatment planning, some authors recently investigated the role of PET in RTP, specifically for the correct delineation of lymph nodes. Schinagl et al71 compared the volume of metastatic lymph nodes between 18F-FDG-PET/CT segmentation (by ten methods) and CT with the volume as determined by pathological examination. They concluded that beyond the detection of lymph node metastasis (staging), PET has no additional value over CT for the delineation of lymph nodes.
Despite the limited role of PET-CT in improving the contouring of nodes,71 it seems to have a main role in improving the definition of the primary tumour GTV. Indeed, PET/CT information is frequently integrated in RTP. Nevertheless, the use of 18F-FDG-PET for target volume delineation in RTP for HNSCC has been mainly evaluated in single institution studies.5,31,72–75
Different segmentation methods have been proposed. Visual interpretation of the PET signal, considered the most intuitive method for segmentation, has been commonly applied in many studies. The main limit of this approach is that it is a highly operator-dependent process, and it is influenced by window-level settings.5,74–76 This is one of the major weakness in the use of PET-CT in the target volume delineation of HNSCC. This variability could be reduced by using a more objective methodology: isocontouring based on a fixed standardized uptake value (SUV) such as a SUV of 2.5–3 g l−1 or relative thresholds such as a percentage of the maximum tumour intensity (40% SUVmax, 50% SUVmax).75,77 Nevertheless, according to this method, several structures containing a high physiological 18F-FDG uptake, such as the tonsillar area or the vocal cords, can be incorrectly included in the segmented area. Therefore, models using a fixed threshold relying on SUV are somehow debatable.73
To overcome this issue, several authors successfully developed advanced adaptive relative threshold segmentation methods based on maximal tumour uptake, background uptake, tumour dimensions and tumour grade.31,75,78,79 Thereafter, other methods including gradient-based73 detections have been introduced. In brief, this method relies on the watershed transform and hierarchical cluster analysis, to allow a better estimation of the gradient intensity. Interestingly, this method allows automatic delineation and therefore is an operator-independent process. Most studies comparing GTV definitions using 18F-FDG-PET against CT or MRI reported a decrease in the GTV, especially when using more sophisticated segmentation methods.5,31,72–74,76,80
However, few groups have validated delineation process using different imaging modalities against surgical resection specimens.31,81–85 In general, all imaging modalities overestimated the tumour extension compared with surgical specimen. Nevertheless, none of the image modalities (CT, MRI or PET) completely encompassed the surgical specimen volume because of an underestimation of superficial tumour extension in the mucosa,31 as also reported by Ng et al.81
According to Daisne et al,31 the GTV delineated from 18F-FDG-PET applying an adaptive signal-to-background method was significantly smaller than GTV delineated by CT or MRI. In addition, GTV-PET was the closest volume to the pathological GTV obtained from surgical specimen. On average, the PET delineated smaller volumes than CT or MRI. Nevertheless, GTV contours at PET were not totally encompassed by those delineated with CT or MRI.
Geets et al73 validated a gradient-based method in seven patients with laryngeal carcinoma. The calculated volumes for laryngeal tumours according to this methodology73 were compared with the macroscopic specimens and, additionally, with the volumes obtained applying the source-to-background ratio developed by Daisne et al.31 Interestingly, the gradient-based method proved to be more accurate than the source-to-background ratio but neither the threshold-based nor the gradient-based volumes encompassed completely the laryngeal specimens.
Interestingly, in a recent multicentric prospective study by Leclerc et al,86 the primary tumour was automatically delineated on the 18F-FDG-PET images using a gradient-based method previously described by this group.73 They confirmed that the use of 18F-FDG-PET translated into smaller GTV, clinical target volume and planning target volume for the primary tumour volumes compared with the use of CT, lowering the dose to organs at risk.
On the other hand, there are studies with other tracers such as fluorine-18 fluorothymidine (18F-FLT) evaluating promising PET-segmentation methods for delineation of the proliferative volume (PV) of tumour. In contrast to 18F-FDG, 18F-FLT does not accumulate in inflammatory tissue,87 which is frequently found in/near primary tumours of the HN or is induced during the course of chemoradiation. Arends et al88 evaluated 46 patients who underwent 18F-FLT PET/CT prior to treatment and in the second and fourth week of therapy. The goal of the study was to compare three semiautomatic PET segmentation methods for derivation of PV in primary HNSCC on sequential 18F-FLT PET images before and during chemoradiation. The following semiautomatic segmentation methods were applied to sequential PET scans: background-subtracted relative-threshold level, a gradient-based method using the watershed transform algorithm and hierarchical clustering analysis and a fuzzy locally adaptive Bayesian algorithm. The authors88 concluded that fuzzy locally adaptive Bayesian algorithm (FLAB) was the best performing method for segmentation of the PV on repeat 18F-FLT PET/CT scans during chemoradiation. FLAB is less sensitive to image noise than the other segmentation approaches tested in the study. This finding may have other potential implications for radiotherapy indicating that FLAB is a promising candidate for radiation target volume adaptation based on sequential 18F-FLT PET scanning.
Currently, there is still no consensus (national/international) between institutions regarding the best method to use for delineation. Therefore, data from 18F-FDG-PET can complement other diagnostic imaging modalities for management decisions and guidance of RTP, but it cannot replace physical examination or MRI/CT scans to achieve significant details such as assessing invasion of tumour-surrounding tissues.85 Moreover, defining the primary tumour boundaries with PET is a difficult task.
In summary, current evidence is based on numerous heterogeneous small studies with changing methodology for different research questions. PET-based RTP is a promising modality to improve contouring accuracy. PET is the imaging modality that defines the closest volume to the pathological specimen. The main drawback is the lack of standardized method for functional volume segmentation, which highly influences the size and shape of the resulting GTV. Currently, the most accurate segmentation method seems to be the gradient-based method validated by Geets et al.73 However, it may not completely encompass the tumour specimen volume.31,73 This issue is more relevant when considering superficial mucosal spread (evaluable by physical examination).5,81 Therefore, even in some contemporary HNSCC study protocols (European Organisation for Research and Treatment of Cancer-1219; NCT01880359), PET-based target volume delineation is not allowed. Before PET can reliably be incorporated into routine high-precision RTP, operator-independent segmentation tools have to be developed and validated (international consensus), and also, clinical effect on outcomes should be reflected in clinical studies.
MONITORING RESPONSE TO CHEMORADIOTHERAPY: RESIDUAL DISEASE AND RECURRENCE (FOLLOW-UP)
Early detection of residual or recurrent disease following radiotherapy is a diagnostic challenge owing to post-treatment anatomical distortions, mostly related to oedema and fibrosis.89 The key role of a diagnostic tool evaluating treatment efficacy is to correctly identify patients requiring salvage-tailored treatments. Moreover, an early detection of the relapse could help in the selection of patients who could be successfully retreated.90 In this setting, 18F-FDG-PET/CT is an interesting modality to evaluate response to treatment, as it can assess metabolic activity-rendering malignant process.89
Isles et al91 preformed a meta-analysis reporting that 18F-FDG-PET (without CT) is a highly accurate tool for monitoring response and detecting relapse after chemoradiotherapy (for both the primary site and lymph nodes). Moreover, several studies have demonstrated that 18F-FDG-PET/CT also has a higher accuracy in the detection of recurrent lesions compared with CT/MRI.6,92–96 These results obtained with PET/CT are not significantly different from those obtained with PET alone.
The timing of PET/CT after the treatment is crucial.6,97–101 It is widely accepted that PET has a high NPV (around 90%) if it is performed at least 8 weeks after chemoradiotherapy. Therefore, a negative PET scan after treatment appears to be a consistent predictor of the absence of residual tumour.102 According to other reports, more accurate evaluation is possible when PET/CT is performed 8–12 weeks after treatment.6,97 The meta-analysis of Gupta et al showed a weighted mean (95% CI)-pooled sensitivity, specificity, PPV and NPV of post-treatment 18F-FDG-PET (CT) for the primary site of 79.9% (73.7–85.2%), 87.5% (85.2–89.5%), 58.6% (52.6–64.5%) and 95.1% (93.5–96.5%), respectively. Similar estimates for the neck were 72.7% (66.6–78.2%), 87.6% (85.7–89.3%), 52.1% (46.6–57.6%) and 94.5% (93.1–95.7%), respectively. Moreover, two recent studies showed even further increased accuracy with delayed PET/CT performed approximately 4 months after treatment with NPVs reaching 100%.100,101 Intuitively, delaying a response evaluation tool would surely increase its accuracy. However, there is no homogeneous data for optimal window for salvage treatment; probably, it would be wise not to postpone salvage surgery beyond a clinically reasonable point.
There is debate regarding the need for elective neck dissection after radical chemoradiotherapy. There are two prospective studies98,99 addressing the status of neck adenopathy of node-positive HNSCC that had 18F-FDG-PET/CT at least 12 weeks after chemoradiotherapy. Porceddu et al98 prospectively evaluated 112 patients presenting with radiological nodal complete response. Residual CT nodal abnormalities were present in 50 patients (45%): 41 were PET negative and 9 were PET positive. Patients with residual CT nodal abnormalities deemed PET negative were uniformly observed regardless of residual nodal size. Importantly, 41 of the 50 patients with a residual nodal abnormality were spared a neck dissection on the basis of negative posttherapy PET, with no subsequent nodal failures in this group. Wang et al99 prospectively evaluated 44 restaging PET/CT between 12 and 17 weeks after radiotherapy completion, and 10 PET/CT performed in the follow-up of 44 patients. Imaging data were compared with clinicopathological outcomes. For cervical lymph nodes, sensitivity was 100%, specificity was 98%, PPV was 92% and NPV was 100%. Therefore, both these prospective studies concluded that PET-guided management of the neck after chemoradiotherapy appropriately spares neck dissections in patients with complete response or presenting with PET-negative residual CT lesions.98,99
Recently, Mehanna et al103 published a prospective, randomized controlled trial assessing the non-inferiority of PET/CT-guided surveillance (evaluation was performed 12 weeks after definitive chemoradiation. Neck lymph node dissection was only indicated when PET/CT presented an incomplete or equivocal response) to planned neck dissection in a total of 564 patients with locally advanced HNSCC (Stage N2 or N3 disease), who underwent chemoradiation for primary treatment.
Patients were considered to have incomplete nodal responses when PET/CT performed 12 weeks after treatment showed high 18F-FDG uptake (with or without enlarged lymph nodes in the neck). In addition, results of PET/CT presenting mild or no 18F-FDG uptake in enlarged lymph nodes or mild 18F-FDG uptake in normal-sized nodes were classified as equivocal responses. The rest of the PET/CT scans were classified as complete responses. Patients showing an incomplete or equivocal response in the neck but presenting a complete response in the primary location underwent neck lymph node dissection within 4 weeks after PET/CT.
The survival rate was similar (2-year overall survival rate of 84.9% and 81.5% in the surveillance group and in the planned-neck dissection group, respectively) between patients who underwent PET/CT-guided surveillance policy and patients undergoing a planned surgery. Indeed, the hazard ratio for death (upper boundary of the 95% CI for the hazard ratio, <1.50; p = 0.004) favoured PET/CT-guided surveillance policy.
Moreover, surveillance resulted in considerably fewer operations (approximately 80% of patients were spared neck dissection compared with planned dissection surgery; 54 vs 221), and it was more cost effective. The per-person cost saving was £1492 (approximately $2190 in US dollars), with an additional 0.08 quality-adjusted life years per person.
However, these authors recommended that patients with an equivocal 18F-FDG uptake should continue to undergo neck dissection. In addition, when extrapolating these results to daily clinical practice, it should be noted that in this study, only a small number of patients [17/564 (3%)] presented N3 disease. Therefore, a direct extrapolation of a PET/CT-guided surveillance policy to patients presenting N3 (Stage IVb) disease should not be indicated owing to the small number of such patients recruited in the study.
18F-FDG-PET/CT could have a potential interesting role in the follow-up of patients with HNSCC. Despite that, the clinical advantages and economic costs of this issue have not yet been largely addressed. One of the largest studies has been published by a group from Pittsburg.90 They evaluated 388 patients retrospectively to assess the recurrence rate after radical chemoradiotherapy among patients who underwent PET/CT surveillance. Tumour recurrence was detected in 110 patients (73 asymptomatic and 37 symptomatic). Indeed, 95% (95% CI, 87–98%) of asymptomatic recurrences were observed within 2 years of follow-up. The authors proposed to evaluate patients for recurrence with PET/CT at 2, 5, 8 and 14 months post-treatment. The reason for this protocol is because their study demonstrated that PET/CT detected almost all HNSCC recurrences within 2 years.
IN SUMMARY
The overall diagnostic performance of 18F-FDG-PET/CT for response assessment is good, but its PPV is not optimal. By contrast, the NPV is particularly high and negative post-treatment PET/CT is very suggestive of absence of viable disease that can guide daily clinical management decisions. In this context, the timing of PET/CT after the end of the treatments is a crucial issue. Available evidences suggest waiting a minimum of 8 weeks before restaging with PET/CT, and preferably 12 weeks to increase the NPV.
Available evidences suggest that this strategy is safe to avoid neck dissection in patients presenting negative PET/CT after CRT.98,99,102 The safety of this attitude is also confirmed by the results of the PET-NECK study,103 where PET/CT-guided active surveillance showed similar survival outcomes compared with planned neck dissection, and considerably fewer neck dissections, and it was more cost effective. However, extrapolation of a PET-CT-guided surveillance policy to patients with N3 (Stage IVb) disease cannot currently be justified.
Evidence-based recommendations to guide the utilization of PET/CT in the follow-up of patients with HNSCC do not exist.2,90
PROGNOSTIC VALUE
Treatment outcome of HNSCC cancer remains heterogeneous. Identification of novel pre-treatment factors (other than tumour stage, lymph node involvement, anatomical subsite or human papillomavirus status) that potentially predict long-term outcome is of great interest.
Quantifying the prognostic value of PET is challenging. In general, the results of prognostic value of SUV remain undetermined because of the small sample of most of these studies. Moreover, it should be considered that HNSCC prognosis also depends on the initial tumour site; data regarding the prognostic value of PET/CT depending on different tumour locations is scarce in the literature.
Two meta-analyses have been conducted to estimate the effect of SUV on the prognosis of HNSCC. First, Zhang et al104 analyzed the potential of SUV [SUVmax and mean SUV (SUVmean)] as a prognostic marker. These authors concluded that increased SUVmax/mean of the primary tumour is a poor prognosis factor and has a potential value in predicting local control, disease-free survival and overall survival. Thereafter, Xie et al105 performed another meta-analysis to evaluate the prognostic value of SUV, confirming that low primary tumour SUV was associated with better survival prognosis. It should be stressed that SUV estimates suffer from poor reproducibility between centres because of the lack of standardization of the acquisition and processing protocols.
Globally, studies evaluating the prognostic utility of PET/CT in HN cancer are quite heterogeneous. Most of them have focused mainly on the SUVmax. Some studies have demonstrated worse clinical outcomes with higher pre-treatment SUVmax.106–108 Other studies observed the correlation of survival with several PET data such as SUVmean or metabolic tumour volume (MTV).109,110 Kitajima et al reported111 that the pre-treatment SUVmax of nodal disease (rather than the primary tumour) in patients with laryngeal cancer was prognostic of recurrence. However, a prospective trial7 conducted at the MD Anderson Cancer Center, Houston, TX, evaluated this particular question and failed to demonstrate any significant clinical correlation between pre-radiotherapy PET-CT SUV parameters and treatment outcomes.
According to other authors, SUVmean109,112 may be a better prognosis marker than SUVmax, with inferior disease-free survival in patients presenting higher pre-treatment SUVmean.109 These results could be explained considering that SUVmax reflects the highest intensity of 18F-FDG uptake as measured in the highest pixels within a concrete region of interest, whereas the SUVmean represents the average of the intensity of the uptake providing a more global picture of tumour metabolism than SUVmax.109 Nevertheless, a potential pitfall of SUVmean is the lesser degree of reproducibility relative to SUVmax.109
More recently, there has been an increasing interest in the use of volumetric parameters of metabolism such as the MTV and total lesion glycolysis (TLG), which weights the volumetric burden and volumetric activity of tumours. Pak et al113 conducted a meta-analysis (13 studies including 1180 patients) on volumetric parameters addressing the prognostic value of MTV and TLG in patients with HN cancer. Despite the various methods adopted between studies, these authors concluded that MTV and TLG are accurate prognostic indicators of outcome in patients with HN cancer. Indeed, high MTV and TLG increased the risk of disease progression and death. The studies evaluated in the meta-analysis and more recent contemporary studies110,112,114–129 are described in Table 5.
In summary, meta-analysis and several studies showed that PET-quantified data such as SUVmax, SUVmean, MTV, TLG are strongly and negatively correlated with survival. However, given that some uncertainty still exists on which of these parameters are the best predictors, prospective trials are needed to definitively settle this issue.
POSITRON EMISSION TOMOGRAPHY/MRI: ADDING NEW POSSIBILITIES
Since information provided by PET/CT and MRI is complementary in many clinical situations, it seems to make sense to combine the two modalities. The high soft-tissue contrast and the different functional imaging techniques of MRI might help to ameliorate the informative value of a hybrid imaging study. Consequently, the discussion about potential applications of this new hybrid technology in oncological imaging and especially in the HN has been generated.130
The feasibility131 and diagnostic performance (sensitivity, specificity and accuracy) of clinical PET/MRI have been demonstrated in a significant number of studies,51,85 but technological and logistic challenges, such as errors in attenuation correction and long scan duration continue to be a major focus of the PET/MRI literature.132
PET/MRI has many potential advantages over PET/CT (including perineural spread of tumours and the infiltration of important anatomical landmarks, such as the pre-vertebral fascia and great vessel walls; lower radiation exposure, higher soft-tissue contrast and several functional techniques).133 Realizing this potential in clinics will likely require new radiopharmaceuticals and applications other than WB cancer staging.132
Although PET/MRI is still in the early stages of clinical development, it is clear that the clinical adoption of PET/MRI is slower than that of PET/CT. This slower evolution is not only due partly to ongoing technological challenges (e.g. accurate attenuation correction of PET images) but also to the complex logistics of combining a WB PET scan with WB or organ-specific MRI. Key applications of PET/MRI that provide information that is clinically relevant and different from that provided by PET/CT still need to be defined.134
Further studies that exploit the functional MRI and molecular PET capabilities may report substantial contributions of PET/MRI for treatment response predictions, radiotherapy planning, tumour phenotyping and treatment monitoring.131,135,136
Future research involving larger patient series is needed to assess the true impact of this technique in HNSCC and will show whether PET/MRI outperforms PET/CT, MRI, diffusion-weighted MRI or their combination.
FUTURE APPLICATIONS OF POSITRON EMISSION TOMOGRAPHY/CT IN RADIATION ONCOLOGY
Table 6 summarizes some of the ongoing clinical trials (www.ClinicalTrials.gov) dealing with the issue of PET/CT in HNSCC treated with radiotherapy.
Table 6.
Currently ongoing trials evaluating the role of positron emission tomography (PET)/CT in clinical practice
| ClinicalTrials.gov identifier | Study type | Study design | Clinical scenario | Purpose |
|---|---|---|---|---|
| NCT01179360 | Observational | Prospective | Treatment response assessment | To determine the performance of 18F-FDG-PET/CT with respect to detecting residual lymph node involvement after chemoradiation in order to omit planned neck dissections in patients with locally advanced potentially operable, N2 and N3 HNSCC |
| NCT02372890 | Observational | Prospective | Staging | To determine the sensitivity and specificity of lymph node staging with high-resolution 18F-FDG-PET/CT in HNSCC by correlating PET/CT with histopathology after neck dissection |
| NCT02047201 | Interventional | Prospective (safety/efficacy study) | Prognosis | To evaluate the safety and efficacy of cisplatin plus IMRT based on 18F-FDG-PET/CT after induction chemotherapy for locally advanced HNSCC. To evaluate correlation of OS, PFS and LRC with metabolic tumour response, anatomical tumour response, baseline SUV and HPV |
| NCT00606294 | Interventional | Prospective (non-randomized) | New tracers | To evaluate low oxygen areas called hypoxia within tumours in order to improve the accuracy of hypoxia imaging for head and neck cancers through pixel-by-pixel kinetic analysis of 18F-FMISO tracer of dynamic PET images |
| NCT01341535 | Interventional | Phase II randomized | Radiotherapy planning | To compare standard IMRT, using only pre-treatment planning 18F-FDG-PET/CT scans to adaptive (dose painting by numbers) 18F-FDG-PET-voxel intensity-based IMRT using repetitive per-treatment planning 18F-FDG-PET/CT to obtain increase in local control |
| NCT02273778 | Interventional | Pilot study | Radiotherapy planning | To investigate the use of co-registered 18F-FDG-PET-CT and MRI for radiotherapy planning in locally advanced HNSCC |
| NCT00147472 | Interventional | Prospective (non-randomized) | Residual disease | To determine the ability of PET to detect residual cancer in neck lymph nodes of patients following curative treatment with radiation therapy. Then, patients undergo neck dissection surgery (the PET and CT results are compared with the presence or absence of tumours in the neck nodes) |
| NCT01235052 | Interventional | Prospective | Treatment response assessment | Non-invasive assessment of hypoxia in cancer. Correlation between a hypoxic volume determined by 18F-FMISO PET-CT and a treatment response 2 years after radical treatment |
| NCT02262221 | Interventional | Randomized (Phase II) | Follow-up | To evaluate the cost effectiveness of two different follow-up programs in head and neck cancer survivors. ARM A (non-intensive follow-up) and ARM B (intervention foreseen is scheduled radiologic evaluations: CT or MRI scan and PET scan if patients ≥50 years old and with a smoking history of ≥20 packs per year) |
| NCT00954148 | Interventional | Randomized | Follow-up | To show that PET/CT will be superior (15% improvement) to conventional methods of follow-up in terms of 5-year survival, cost and time to identification of new disease |
| NCT00159978 | Observational | Prospective (Phase I) | New tracers | To validate 18F-FMISO-PET for detection of tumour hypoxia and 18F-FLT-PET for detection of tumour cell proliferation by immunohistochemical assessment of hypoxia and proliferation in head and neck cancer resection specimen |
18F-FDG-PET/CT, PET with fluorine-18 fludeoxyglucose integrated with CT; 18F-FLT, fluorine-18 fluorothymidine; 18F-FMISO, fluorine-18 fluoromisonidazole; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; IMRT, intensity-modulated radiotherapy; LRC, locoregional control; OS, overall survival; PFS, progression-free survival; SUV, standardized uptake value.
Dose escalation to 18F-FDG-avid subvolumes of the tumour as well as adapting the RTP during treatment regarding the functional tumour changes induced during radiation are two important issues that are currently under investigation.137–139 Madani et al140 performed a Phase I trial to establish the maximum tolerated dose, when the dose was escalated in 18F-FDG-PET GTV within the anatomically (CT/MRI) based GTV. They demonstrated the feasibility of heterogeneous dose delivery with dose escalation up to 77.5 Gy for 18F-FDG-avid tumour areas (the so called “dose painting” approach). Another approach would be adaptation of the biological target volume during the course of radiotherapy in order to reduce the treated volume as radiotherapy progresses. Duprez et al138 used adaptive intensity-modulated radiotherapy planning based on dose painting by numbers according to 18F-FDG-PET voxel intensities concluding that replanning was possible reaching a total dose of 80.9 Gy. It is noteworthy that these kinds of approaches require extreme attention, as a slight shift of the anatomy will not only cause a mismatch of dose and intratumour anatomy but also a higher dose into nearby healthy tissue. Moreover, another unsolved issue is the monitoring of the shift of high SUV regions during the course of treatment.
Last but not least, the possibility of targeting radiation resistance within the tumour on the basis of biological information (intratumoural hypoxic and proliferation states) obtained from functional imaging with other tracers than 18F-FDG is an emergent strategy.141–143 However, this issue is still under investigation and should still be considered as experimental.144,145
CONCLUSION
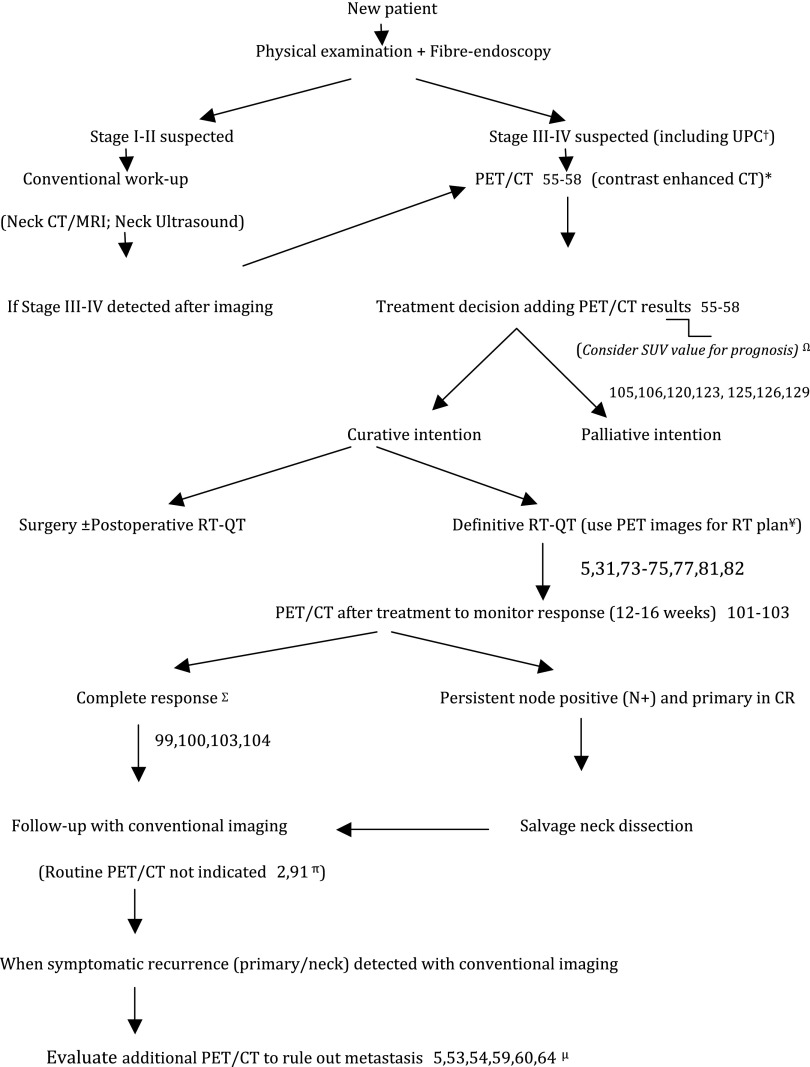
PET/CT is an important diagnostic tool in HN oncology, especially in the initial staging and in monitoring response to definitive chemoradiotherapy. Moreover, approaches of neck dissection sparing based on the results of restaging PET/CT are safe and should be implemented in daily clinical practice. The summary of indications and controversies are detailed in Table 7. Based on the results of our review, we summarized in Figure 1 a proposal for integrating PET/CT in daily clinical practice.
Table 7.
Summary: recommendations and key issues
| 1. | Unknown primary: 18F-FDG-PET/CT is recommended to identify primary tumours in patients presenting with cervical lymph node metastases with unknown primary |
| 2. | Pre-treatment staging: Multiple studies have demonstrated that adding PET (or PET/CT) to a conventional work-up resulted in a higher staging accuracy; nodal classification is improved, especially in terms of specificity; and 18F-FDG-PET or PET/CT detects more distant metastases and second malignancy than conventional staging |
| 3. | Clinical impact: A more refined staging has demonstrated to have clinical impact on treatment decisions. PET/CT should be included in the routine diagnosis of patients with Stages III–IV HNSCC, as it significantly improves staging accuracy and also has a marked impact on management plans. However, there is no evidence with regard to a possible benefit in outcomes due to PET/CT |
| 4. | Radiotherapy: The use of 18F-FDG-PET translates into smaller GTV for the primary tumour volumes than with the use of CT or MRI. PET can complement other diagnostic imaging modalities for management decisions and guidance of radiotherapy planning, but it cannot replace physical examination or MRI/CT. There is no current standardized method for functional volume segmentation recommended for daily practice |
| 5. | Monitoring response to treatment: PET/CT is recommended to check for residual disease at least 8 and, preferably, 12–16 weeks after definitive chemoradiotherapy in node-positive HNSCC (NPV >90%), avoiding unnecessary neck dissections in patients presenting complete response. Mehanna et al103 indicated that patients with incomplete (presenting high 18F-FDG uptake at 12 weeks after chemoradiotherapy, with or without enlarged lymph nodes in the neck), or equivocal response (mild or no 18F-FDG uptake in enlarged nodes or mild 18F-FDG uptake in normal-sized nodes) should undergo neck dissection. Few patients in this trial had N3 (Stage IVb) disease [17/564 (3%)]. Therefore, a PET-CT-guided surveillance policy to patients presenting N3 disease is not currently justified due to the small number of patients presenting N3 disease in this study |
| 6. | Surveillance: PET/CT is not indicated in routine follow-up |
| 7. | Prognosis: Patients presenting increased SUV (SUVmax, SUVmean) or higher MTV or TLG seem to have worse prognosis (higher risk of treatment failure). There is no currently evidence to support that this type of patients should receive different treatment approach. Nevertheless, these parameters could be used to stratify patients in future clinical trials |
| 8. | To further validate incorporating PET/CT into daily practice also, clinical effect on outcomes and cost effectiveness should be reflected in clinical studies |
18F-FDG, fluorine-18 fludeoxyglucose; GTV, gross tumour volume; HNSCC, head and neck squamous cell carcinoma; MTV, metabolic tumour volume; NPV, negative-predictive value; PET, positron emission tomography; SUV, standardized uptake value; SUVmax, maximal SUV; SUVmean, mean SUV; TLG, total lesion glycolysis.
Figure 1.
Proposal to incorporate positron emission tomography (PET)/CT (contrast-enhanced CT) into routine clinical practice for head and neck squamous cell carcinoma. †Cervical metastases from unknown primary cancer (UPC). *Evaluate to include MRI to assess soft-tissue invasion or perineural spread if needed depending on primary tumour location.16 ΩPrognosis: uptake parameters and volumetric parameters can potentially help in identifying patients with worse outcomes (still an area of active investigation). ¥Radiotherapy (RT) plan: PET/CT may be helpful, improving contouring accuracy. However, standardized method is lacking. πFollow-up with conventional imaging (CT/MRI) is recommended. When equivocal findings additional PET/CT can be performed. μOnce recurrence is detected, additional PET/CT may be of interest to improve patient counselling; restaging the tumour and planning additional therapy, especially when “aggressive” interventions (extended surgery or reirradiation) may be needed. ΣHowever, Mehanna et al103 indicated that patients with incomplete [patients presenting high fluorine-18 fludeoxyglucose (18F-FDG) uptake at 12 weeks after chemoradiotherapy, with or without enlarged lymph nodes in the neck] or equivocal response (mild or no 18F-FDG uptake in enlarged nodes or mild 18F-FDG uptake in normal-sized nodes) should undergo neck dissection. In addition, it should be noted that few patients in this trial had N3 (Stage IVb) disease [17/564 (3%)]. Therefore, a PET-CT-guided surveillance policy to patients presenting N3 disease is not justified due to the small number of patients presenting N3 disease in this study. CR, complete response; QT, chemotherapy.
Contributor Information
Jon Cacicedo, Email: jon.cacicedofernandezbobadilla@osakidetza.net.
Arturo Navarro, Email: anavarro@iconcologia.net.
Olga del Hoyo, Email: olgamanuela.hoyodelalvarez@osakidetza.net.
Alfonso Gomez-Iturriaga, Email: Alfonso.gomezdeiturriagapina@osakidetza.net.
Filippo Alongi, Email: filippo.alongi@sacrocuore.it.
Jose A Medina, Email: jmedinacarmona@gmail.com.
Olgun Elicin, Email: olgunelicin@gmail.com.
Andrea Skanjeti, Email: francesco.giammarile@chu-lyon.fr.
Francesco Giammarile, Email: francesco.giammarile@chu-lyon.fr.
Pedro Bilbao, Email: Pedro.bilbaozulaica@osakidetza.eus.
Francisco Casquero, Email: francisco.casqueroocio@osakidetza.net.
Berardino de Bari, Email: berardino.de-bari@chuv.ch.
Alan Dal Pra, Email: alan.dalpra@insel.ch.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2. NCCN Clinical Practice Guidelines in Oncology. [Internet.] [Cited 9 March 2016]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 3. National Cancer Institute guidelines. [Internet.] [Cited 9 March 2016]. Available from: http://www.cancer.gov/types/head-and-neck/hp.
- 4.Yoo J, Henderson S, Walker-Dilks C. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer. Clin Oncol (R Coll Radiol) 2013; 25: e33–66. doi: 10.1016/j.clon.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Thiagarajan A, Caria N, Schöder H, Iyer NG, Wolden S, Wong RJ, et al. Target volume delineation in oropharyngeal cancer: impact of PET, MRI, and physical examination. Int J Radiat Oncol Biol Phys 2012; 83: 220–7. doi: 10.1016/j.ijrobp.2011.05.060 [DOI] [PubMed] [Google Scholar]
- 6.Andrade RS, Heron DE, Degirmenci B, Filho PA, Branstetter BF, Seethala RR, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys 2006; 65: 1315–22. doi: 10.1016/j.ijrobp.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 7.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective risk-adjusted [18F] fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol 2009; 27: 2509–15. doi: 10.1200/JCO.2008.19.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong WL, Ross P, Corcoran M. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer from Ontario and guidelines in general—some observations. Clin Oncol (R Coll Radiol) 2013; 25: 242–5. [DOI] [PubMed] [Google Scholar]
- 9.Goerres GW, Schmid DT, Grätz KW, von Schulthess GK, Eyrich GK. Impact of whole body positron emission tomography on initial staging and therapy in patients with squamous cell carcinoma of the oral cavity. Oral Oncol 2003; 39: 547–51. doi: 10.1016/S1368-8375(03)00016-2 [DOI] [PubMed] [Google Scholar]
- 10.Roh JL, Yeo NK, Kim JS, Lee JH, Cho KJ, Choi SH, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol 2007; 43: 887–93. doi: 10.1016/j.oraloncology.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Baek CH, Chung MK, Son YI, Choi JY, Kim HJ, Yim YJ, et al. Tumor volume assessment by 18F-FDG PET/CT in patients with oral cavity cancer with dental artifacts on CT or MR images. J Nucl Med 2008; 49: 1422–8. doi: 10.2967/jnumed.108.051649 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues RS, Bozza FA, Christian PE, Hoffman JM, Butterfield RI, Christensen CR, et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J Nucl Med 2009; 50: 1205–13. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Brown JS, Woolgar JA, Lowe D, Rogers SN, Vaughan ED. The influence of the pattern of mandibular invasion on recurrence and survival in oral squamous cell carcinoma. Head Neck 2004; 26: 861–9. doi: 10.1002/hed.20036 [DOI] [PubMed] [Google Scholar]
- 14.Handschel J, Naujoks C, Depprich RA, Kübler NR, Kröpil P, Kuhlemann J, et al. CT-scan is a valuable tool to detect mandibular involvement in oral cancer patients. Oral Oncol 2012; 48: 361–6. doi: 10.1016/j.oraloncology.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 15.Abd El-Hafez YG, Chen CC, Ng SH, Lin CY, Wang HM, Chan SC, et al. Comparison of PET/CT and MRI for the detection of bone marrow invasion in patients with squamous cell carcinoma of the oral cavity. Oral Oncol 2011; 47: 288–95. doi: 10.1016/j.oraloncology.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 16.Gu DH, Yoon DY, Park CH, Chang SK, Lim KJ, Seo YL, et al. CT, MR, (18)F-FDG PET/CT, and their combined use for the assessment of mandibular invasion by squamous cell carcinomas of the oral cavity. Acta Radiol 1987; 51: 1111–19. doi: 10.3109/02841851.2010.520027 [DOI] [PubMed] [Google Scholar]
- 17.Villeneuve H, Després P, Fortin B, Filion E, Donath D, Soulières D, et al. Cervical lymph node metastases from unknown primary cancer: a single-institution experience with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 1866–71. doi: 10.1016/j.ijrobp.2011.02.031 [DOI] [PubMed] [Google Scholar]
- 18.Regelink G, Brouwer J, de Bree R, Pruim J, van der Laan BF, Vaalburg W, et al. Detection of unknown primary tumours and distant metastases in patients with cervical metastases: value of FDG-PET versus conventional modalities. Eur J Nucl Med Mol Imaging 2002; 29: 1024–30. doi: 10.1007/s00259-002-0819-0 [DOI] [PubMed] [Google Scholar]
- 19.Stoeckli SJ, Mosna-Firlejczyk K, Goerres GW. Lymph node metastasis of squamous cell carcinoma from an unknown primary: impact of positron emission tomography. Eur J Nucl Med Mol Imaging 2003; 30: 411–16. doi: 10.1007/s00259-002-1078-9 [DOI] [PubMed] [Google Scholar]
- 20.Gutzeit A, Antoch G, Kühl H, Egelhof T, Fischer M, Hauth E, et al. Unknown primary tumors: detection with dual-modality PET/CT—initial experience. Radiology 2005; 234: 227–34. doi: 10.1148/radiol.2341031554 [DOI] [PubMed] [Google Scholar]
- 21.Fakhry N, Jacob T, Paris J, Barberet M, Mundler O, Giovanni A, et al. Contribution of 18-F-FDG PET for detection of head and neck carcinomas with an unknown primary tumor. [In French.] Ann Otolaryngol Chir Cervicofac 2006; 123: 17–25. [DOI] [PubMed] [Google Scholar]
- 22.Nassenstein K, Veit-Haibach P, Stergar H, Gutzeit A, Freudenberg L, Kuehl H, et al. Cervical lymph node metastases of unknown origin: primary tumor detection with whole-body positron emission tomography/computed tomography. Acta Radiol 1987; 48: 1101–8. [DOI] [PubMed] [Google Scholar]
- 23.Wartski M, Le Stanc E, Gontier E, Vilain D, Banal A, Tainturier C, et al. In search of an unknown primary tumour presenting with cervical metastases: performance of hybrid FDG-PET-CT. Nucl Med Commun 2007; 28: 365–71. doi: 10.1097/MNM.0b013e3280708edf [DOI] [PubMed] [Google Scholar]
- 24.Wong WL, Sonoda LI, Gharpurhy A, Gollub F, Wellsted D, Goodchild K, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers—an audit and review of published studies. Clin Oncol (R Coll Radiol) 2012; 24: 190–5. [DOI] [PubMed] [Google Scholar]
- 25.Freudenberg LS, Fischer M, Antoch G, Jentzen W, Gutzeit A, Rosenbaum SJ, et al. Dual modality of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in patients with cervical carcinoma of unknown primary. Med Princ Pract 2005; 14: 155–60. doi: 10.1159/000084632 [DOI] [PubMed] [Google Scholar]
- 26.Johansen J, Buus S, Loft A, Keiding S, Overgaard M, Hansen HS, et al. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck 2008; 30: 471–8. doi: 10.1002/hed.20734 [DOI] [PubMed] [Google Scholar]
- 27.Roh JL, Kim JS, Lee JH, Cho KJ, Choi SH, Nam SY, et al. Utility of combined (18)F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol 2009; 45: 218–24. doi: 10.1016/j.oraloncology.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 28.Zhao K, Luo XM, Zhou SH, Liu JH, Yan SX, Lu ZJ, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography as an effective diagnostic workup in cervical metastasis of carcinoma from an unknown primary tumor. Cancer Biother Radiopharm 2012; 27: 685–93. [DOI] [PubMed] [Google Scholar]
- 29.Pereira G, Silva JC, Monteiro E. Positron emission tomography in the detection of occult primary head and neck carcinoma: a retrospective study. Head Neck Oncol 2012; 4: 34. doi: 10.1186/1758-3284-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JR, Kim JS, Roh JL, Lee JH, Baek JH, Cho KJ, et al. Detection of occult primary tumors in patients with cervical metastases of unknown primary tumors: comparison of (18)F FDG PET/CT with contrast-enhanced CT or CT/MR imaging-prospective study. Radiology 2015; 274: 764–71. doi: 10.1148/radiol.14141073 [DOI] [PubMed] [Google Scholar]
- 31.Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004; 233: 93–100. doi: 10.1148/radiol.2331030660 [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Wang N. 18F-fluorodeoxyglucose positron emission tomography-computed tomography as a diagnostic tool in patients with cervical nodal metastases of unknown primary site: a meta-analysis. Surg Oncol 2013; 22: 190–4. doi: 10.1016/j.suronc.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 33.van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177: 379–84. doi: 10.1148/radiology.177.2.2217772 [DOI] [PubMed] [Google Scholar]
- 34.van den Brekel MW, Castelijns JA, Stel HV, Golding RP, Meyer CJ, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol 1993; 250: 11–17. [DOI] [PubMed] [Google Scholar]
- 35.Curtin HD, Ishwaran H, Mancuso AA, Dalley RW, Caudry DJ, McNeil BJ. Comparison of CT and MR imaging in staging of neck metastases. Radiology 1998; 207: 123–30. doi: 10.1148/radiology.207.1.9530307 [DOI] [PubMed] [Google Scholar]
- 36.Adams S, Baum RP, Stuckensen T, Bitter K, Hör G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med 1998; 25: 1255–60. doi: 10.1007/s002590050293 [DOI] [PubMed] [Google Scholar]
- 37.Hannah A, Scott AM, Tochon-Danguy H, Chan JG, Akhurst T, Berlangieri S, et al. Evaluation of 18F-fluorodeoxyglucose positron emission tomography and computed tomography with histopathologic correlation in the initial staging of head and neck cancer. Ann Surg 2002; 236: 208–17. doi: 10.1097/00000658-200208000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng SH, Yen TC, Chang JT, Chan SC, Ko SF, Wang HM, et al. Prospective study of [18F] fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol 2006; 24: 4371–6. [DOI] [PubMed] [Google Scholar]
- 39.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994; 120: 699–702. doi: 10.1001/archotol.1994.01880310005001 [DOI] [PubMed] [Google Scholar]
- 40.Grégoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000; 56: 135–50. [DOI] [PubMed] [Google Scholar]
- 41.Kim MR, Roh JL, Kim JS, Lee JH, Cho KJ, Choi SH, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography in the preoperative staging of squamous cell carcinoma of the oropharynx. Eur J Surg Oncol 2007; 33: 633–8. doi: 10.1016/j.ejso.2007.02.016 [DOI] [PubMed] [Google Scholar]
- 42.Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol 2009; 19: 634–42. [DOI] [PubMed] [Google Scholar]
- 43.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst 2008; 100: 712–20. doi: 10.1093/jnci/djn125 [DOI] [PubMed] [Google Scholar]
- 44.Cetin B, Atasever T, Akdemir UO, Senturk S, Tufan G, Turan N, et al. The role of positron emission tomography with 18F-fluorodeoxyglucose in nodal staging of clinical and radiological N0 head and neck cancers. Eur Arch Otorhinolaryngol 2013; 270: 2307–13. [DOI] [PubMed] [Google Scholar]
- 45.Roh JL, Park JP, Kim JS, Lee JH, Cho KJ, Choi SH, et al. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology 2014; 271: 153–61. doi: 10.1148/radiol.13131470 [DOI] [PubMed] [Google Scholar]
- 46.Pfannenberg AC, Aschoff P, Brechtel K, Müller M, Klein M, Bares R, et al. Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol 2007; 80: 437–45. doi: 10.1259/bjr/34082277 [DOI] [PubMed] [Google Scholar]
- 47.Aschoff P, Plathow C, Beyer T, Lichy MP, Erb G, Öksüz MÖ, et al. Multiphase contrast-enhanced CT with highly concentrated contrast agent can be used for PET attenuation correction in integrated PET/CT imaging. Eur J Nucl Med Mol Imaging 2012; 39: 316–25. doi: 10.1007/s00259-011-1919-5 [DOI] [PubMed] [Google Scholar]
- 48.Morbelli S, Conzi R, Campus C, Cittadini G, Bossert I, Massollo M, et al. Contrast-enhanced [18 F] fluorodeoxyglucose-positron emission tomography/computed tomography in clinical oncology: tumor-, site-, and question-based comparison with standard positron emission tomography/computed tomography. Cancer Imaging 2014; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haerle SK, Strobel K, Ahmad N, Soltermann A, Schmid DT, Stoeckli SJ. Contrast-enhanced 18F-FDG-PET/CT for the assessment of necrotic lymph node metastases. Head Neck 2011; 33: 324–9. [DOI] [PubMed] [Google Scholar]
- 50.Matsubara R, Kawano S, Chikui T, Kiyosue T, Goto Y, Hirano M, et al. Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad Radiol 2012; 19: 708–17. doi: 10.1016/j.acra.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Kanda T, Kitajima K, Suenaga Y, Konishi J, Sasaki R, Morimoto K, et al. Value of retrospective image fusion of 18F-FDG PET and MRI for preoperative staging of head and neck cancer: comparison with PET/CT and contrast-enhanced neck MRI. Eur J Radiol 2013; 82: 2005–10. doi: 10.1016/j.ejrad.2013.06.025 [DOI] [PubMed] [Google Scholar]
- 52.de Bree R, Deurloo EE, Snow GB, Leemans CR. Screening for distant metastases in patients with head and neck cancer. Laryngoscope 2000; 110: 397–401. doi: 10.1097/00005537-200003000-00012 [DOI] [PubMed] [Google Scholar]
- 53.Brouwer J, de Bree R, Hoekstra OS, Golding RP, Langendijk JA, Castelijns JA, et al. Screening for distant metastases in patients with head and neck cancer: is chest computed tomography sufficient? Laryngoscope 2005; 115: 1813–17. doi: 10.1097/01.mlg.0000174954.51514.b7 [DOI] [PubMed] [Google Scholar]
- 54.Scott AM, Gunawardana DH, Bartholomeusz D, Ramshaw JE, Lin P. PET changes management and improves prognostic stratification in patients with head and neck cancer: results of a multicenter prospective study. J Nucl Med 2008; 49: 1593–600. [DOI] [PubMed] [Google Scholar]
- 55.Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, et al. Positron emission tomography with [18F] fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol 2010; 28: 1190–5. [DOI] [PubMed] [Google Scholar]
- 56.Connell CA, Corry J, Milner AD, Hogg A, Hicks RJ, Rischin D, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck 2007; 29: 986–95. doi: 10.1002/hed.20629 [DOI] [PubMed] [Google Scholar]
- 57.Cacicedo J, Fernandez I, Del Hoyo O, Dolado A, Gómez-Suarez J, Hortelano E, et al. Should PET/CT be implemented in the routine imaging work-up of locally advanced head and neck squamous cell carcinoma? A prospective analysis. Eur J Nucl Med Mol Imaging 2015; 42: 1378–89. doi: 10.1007/s00259-015-3071-0 [DOI] [PubMed] [Google Scholar]
- 58.Xu GZ, Zhu XD, Li MY. Accuracy of whole-body PET and PET-CT in initial M staging of head and neck cancer: a meta-analysis. Head Neck 2011; 33: 87–94. doi: 10.1002/hed.21400 [DOI] [PubMed] [Google Scholar]
- 59.Yi X, Fan M, Liu Y, Zhang H, Liu S. 18 FDG PET and PET-CT for the detection of bone metastases in patients with head and neck cancer. A meta-analysis. J Med Imaging Radiat Oncol 2013; 57: 674–9. doi: 10.1111/1754-9485.12077 [DOI] [PubMed] [Google Scholar]
- 60.Ng SH, Chan SC, Liao CT, Chang JT, Ko SF, Wang HM, et al. Distant metastases and synchronous second primary tumors in patients with newly diagnosed oropharyngeal and hypopharyngeal carcinomas: evaluation of (18)F-FDG PET and extended-field multi-detector row CT. Neuroradiology 2008; 50: 969–79. doi: 10.1007/s00234-008-0426-2 [DOI] [PubMed] [Google Scholar]
- 61.Senft A, de Bree R, Hoekstra OS, Kuik DJ, Golding RP, Oyen WJ, et al. Screening for distant metastases in head and neck cancer patients by chest CT or whole body FDG-PET: a prospective multicenter trial. Radiother Oncol 2008; 87: 221–9. doi: 10.1016/j.radonc.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 62.Kim SY, Roh JL, Yeo NK, Kim JS, Lee JH, Choi SH, et al. Combined 18F-fluorodeoxyglucose-positron emission tomography and computed tomography as a primary screening method for detecting second primary cancers and distant metastases in patients with head and neck cancer. Ann Oncol 2007; 18: 1698–703. [DOI] [PubMed] [Google Scholar]
- 63.Haerle SK, Schmid DT, Ahmad N, Hany TF, Stoeckli SJ. The value of (18)F-FDG PET/CT for the detection of distant metastases in high-risk patients with head and neck squamous cell carcinoma. Oral Oncol 2011; 47: 653–9. doi: 10.1016/j.oraloncology.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 64.Strobel K, Haerle SK, Stoeckli SJ, Schrank M, Soyka JD, Veit-Haibach P, et al. Head and neck squamous cell carcinoma (HNSCC)—detection of synchronous primaries with (18)F-FDG-PET/CT. Eur J Nucl Med Mol Imaging 2009; 36: 919–27. doi: 10.1007/s00259-009-1064-6 [DOI] [PubMed] [Google Scholar]
- 65.Haerle SK, Strobel K, Hany TF, Sidler D, Stoeckli SJ. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck 2010; 32: 319–25. doi: 10.1002/hed.21184 [DOI] [PubMed] [Google Scholar]
- 66.Peck BW, Dahlstrom KR, Gan SJ, Caywood W, Li G, Wei Q, et al. Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck 2013; 35: 794–9. doi: 10.1002/hed.23033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 2011; 29: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narayana A, Vaughan AT, Fisher SG, Reddy SP. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol Biol Phys 1998; 42: 557–62. doi: 10.1016/S0360-3016(98)00250-8 [DOI] [PubMed] [Google Scholar]
- 69.Schwartz DL, Rajendran J, Yueh B, Coltrera M, Anzai Y, Krohn K, et al. Staging of head and neck squamous cell cancer with extended-field FDG-PET. Arch Otolaryngol Head Neck Surg 2003; 129: 1173–8. doi: 10.1001/archotol.129.11.1173 [DOI] [PubMed] [Google Scholar]
- 70.Scripes PG, Yaparpalvi R. Technical aspects of positron emission tomography/computed tomography in radiotherapy treatment planning. Semin Nucl Med 2012; 42: 283–8. doi: 10.1053/j.semnuclmed.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 71.Schinagl DA, Span PN, van den Hoogen FJ, Merkx MA, Slootweg PJ, Oyen WJ, et al. Pathology-based validation of FDG PET segmentation tools for volume assessment of lymph node metastases from head and neck cancer. Eur J Nucl Med Mol Imaging 2013; 40: 1828–35. doi: 10.1007/s00259-013-2513-9 [DOI] [PubMed] [Google Scholar]
- 72.Geets X, Daisne J-F, Tomsej M, Duprez T, Lonneux M, Grégoire V. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol 2006; 78: 291–7. [DOI] [PubMed] [Google Scholar]
- 73.Geets X, Lee JA, Bol A, Lonneux M, Grégoire V. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging 2007; 34: 1427–38. doi: 10.1007/s00259-006-0363-4 [DOI] [PubMed] [Google Scholar]
- 74.Riegel AC, Berson AM, Destian S, Ng T, Tena LB, Mitnick RJ, et al. Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys 2006; 65: 726–32. doi: 10.1016/j.ijrobp.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 75.Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69: 1282–9. doi: 10.1016/j.ijrobp.2007.07.2333 [DOI] [PubMed] [Google Scholar]
- 76.Delouya G, Igidbashian L, Houle A, Bélair M, Boucher L, Cohade C, et al. 18F-FDG-PET imaging in radiotherapy tumor volume delineation in treatment of head and neck cancer. Radiother Oncol 2011; 101: 362–8. [DOI] [PubMed] [Google Scholar]
- 77.Moule RN, Kayani I, Moinuddin SA, Meer K, Lemon C, Goodchild K, et al. The potential advantages of (18) FDG PET/CT-based target volume delineation in radiotherapy planning of head and neck cancer. Radiother Oncol 2010; 97: 189–93. [DOI] [PubMed] [Google Scholar]
- 78.Henriques de Figueiredo B, Barret O, Demeaux H, Lagarde P, De-Mones-Del-Pujol E, Kantor G, et al. Comparison between CT- and FDG-PET-defined target volumes for radiotherapy planning in head-and-neck cancers. Radiother Oncol 2009; 93: 479–82. [DOI] [PubMed] [Google Scholar]
- 79.Perez-Romasanta LA, Bellon-Guardia M, Torres-Donaire J, Lozano-Martin E, Sanz-Martin M, Velasco-Jimenez J. Tumor volume delineation in head and neck cancer with 18-fluor-fluorodeoxiglucose positron emission tomography: adaptive thresholding method applied to primary tumors and metastatic lymph nodes. Clin Transl Oncol 2013; 15: 283–93. [DOI] [PubMed] [Google Scholar]
- 80.Deantonio L, Beldì D, Gambaro G, Loi G, Brambilla M, Inglese E, et al. FDG-PET/CT imaging for staging and radiotherapy treatment planning of head and neck carcinoma. Radiat Oncol 2008; 3: 29. doi: 10.1186/1748-717X-3-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med 2005; 46: 1136–43. [PubMed] [Google Scholar]
- 82.Caldas-Magalhaes J, Kasperts N, Kooij N, van den Berg CA, Terhaard CH, Raaijmakers CP, et al. Validation of imaging with pathology in laryngeal cancer: accuracy of the registration methodology. Int J Radiat Oncol Biol Phys 2012; 82: e289–98. doi: 10.1016/j.ijrobp.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 83.Burri RJ, Rangaswamy B, Kostakoglu L, Hoch B, Genden EM, Som PM, et al. Correlation of positron emission tomography standard uptake value and pathologic specimen size in cancer of the head and neck. Int J Radiat Oncol Biol Phys 2008; 71: 682–8. doi: 10.1016/j.ijrobp.2007.10.055 [DOI] [PubMed] [Google Scholar]
- 84.Zaidi H, Abdoli M, Fuentes CL, El Naqa IM. Comparative methods for PET image segmentation in pharyngolaryngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2012; 39: 881–91. doi: 10.1007/s00259-011-2053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang SH, Chien CY, Lin WC, Fang FM, Wang PW, Lui CC, et al. A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin Nucl Med 2011; 36: 518–25. doi: 10.1097/RLU.0b013e318217566f [DOI] [PubMed] [Google Scholar]
- 86.Leclerc M, Lartigau E, Lacornerie T, Daisne JF, Kramar A, Grégoire V. Primary tumor delineation based on (18) FDG PET for locally advanced head and neck cancer treated by chemo-radiotherapy. Radiother Oncol 2015; 116: 87–93. [DOI] [PubMed] [Google Scholar]
- 87.van Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med 2004; 45: 695–700. [PubMed] [Google Scholar]
- 88.Arens AI, Troost EG, Hoeben BA, Grootjans W, Lee JA, Grégoire V, et al. Semiautomatic methods for segmentation of the proliferative tumour volume on sequential FLT PET/CT images in head and neck carcinomas and their relation to clinical outcome. Eur J Nucl Med Mol Imaging 2014; 41: 915–24. doi: 10.1007/s00259-013-2651-0 [DOI] [PubMed] [Google Scholar]
- 89.Bronstein AD, Nyberg DA, Schwartz AN, Shuman WP, Griffin BR. Soft-tissue changes after head and neck radiation: CT findings. AJNR Am J Neuroradiol 1989; 10: 171–5. [PMC free article] [PubMed] [Google Scholar]
- 90.Beswick DM, Gooding WE, Johnson JT, Branstetter BF. Temporal patterns of head and neck squamous cell carcinoma recurrence with positron-emission tomography/computed tomography monitoring. Laryngoscope 2012; 122: 1512–17. doi: 10.1002/lary.23341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 2008; 33: 210–22. doi: 10.1111/j.1749-4486.2008.01688.x [DOI] [PubMed] [Google Scholar]
- 92.Branstetter BF, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology 2005; 235: 580–6. doi: 10.1148/radiol.2352040134 [DOI] [PubMed] [Google Scholar]
- 93.Chen AY, Vilaseca I, Hudgins PA, Schuster D, Halkar R. PET-CT vs contrast-enhanced CT: what is the role for each after chemoradiation for advanced oropharyngeal cancer? Head Neck 2006; 28: 487–95. doi: 10.1002/hed.20362 [DOI] [PubMed] [Google Scholar]
- 94.Ong SC, Schöder H, Lee NY, Patel SG, Carlson D, Fury M, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med 2008; 49: 532–40. doi: 10.2967/jnumed.107.044792 [DOI] [PubMed] [Google Scholar]
- 95.Nayak JV, Walvekar RR, Andrade RS, Daamen N, Lai SY, Argiris A, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope 2007; 117: 2129–34. doi: 10.1097/MLG.0b013e318149e6bc [DOI] [PubMed] [Google Scholar]
- 96.Gupta T, Jain S, Agarwal JP, Rangarajan V, Purandare N, Ghosh-Laskar S, et al. Diagnostic performance of response assessment FDG-PET/CT in patients with head and neck squamous cell carcinoma treated with high-precision definitive chemoradiation. Radiother Oncol 2010; 97: 194–9. [DOI] [PubMed] [Google Scholar]
- 97.Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope 2000; 110: 1493–7. doi: 10.1097/00005537-200009000-00016 [DOI] [PubMed] [Google Scholar]
- 98.Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck 2011; 33: 1675–82. doi: 10.1002/hed.21655 [DOI] [PubMed] [Google Scholar]
- 99.Wang YF, Liu RS, Chu PY, Chang FC, Tai SK, Tsai TL, et al. Positron emission tomography in surveillance of head and neck squamous cell carcinoma after definitive chemoradiotherapy. Head Neck 2009; 31: 442–51. doi: 10.1002/hed.20978 [DOI] [PubMed] [Google Scholar]
- 100.Zundel MT, Michel MA, Schultz CJ, Maheshwari M, Wong SJ, Campbell BH, et al. Comparison of physical examination and fluorodeoxyglucose positron emission tomography/computed tomography 4–6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 81: e825–32. doi: 10.1016/j.ijrobp.2010.11.072 [DOI] [PubMed] [Google Scholar]
- 101.Slevin F, Subesinghe M, Ramasamy S, Sen M, Scarsbrook AF, Prestwich RJ. Assessment of outcomes with delayed (18)F-FDG PET-CT response assessment in head and neck squamous cell carcinoma. Br J Radiol 2015; 88: 20140592. doi: 10.1259/bjr.20140592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brkovich VS, Miller FR, Karnad AB, Hussey DH, McGuff HS, Otto RA. The role of positron emission tomography scans in the management of the N-positive neck in head and neck squamous cell carcinoma after chemoradiotherapy. Laryngoscope 2006; 116: 855–8. doi: 10.1097/01.mlg.0000214668.98592.d6 [DOI] [PubMed] [Google Scholar]
- 103.Mehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016; 374: 1444–54. doi: 10.1056/NEJMoa1514493 [DOI] [PubMed] [Google Scholar]
- 104.Zhang B, Li X, Lu X. Standardized uptake value is of prognostic value for outcome in head and neck squamous cell carcinoma. Acta Otolaryngol 2010; 130: 756–62. doi: 10.3109/00016480903402981 [DOI] [PubMed] [Google Scholar]
- 105.Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 2011; 137: 1085–93. doi: 10.1007/s00432-010-0972-y [DOI] [PubMed] [Google Scholar]
- 106.Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol 2009; 192: W156–60. doi: 10.2214/AJR.08.1429 [DOI] [PubMed] [Google Scholar]
- 107.Halfpenny W, Hain SF, Biassoni L, Maisey MN, Sherman JA, McGurk M. FDG-PET. A possible prognostic factor in head and neck cancer. Br J Cancer 2002; 86: 512–16. doi: 10.1038/sj.bjc.6600114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys 2004; 59: 1295–300. doi: 10.1016/j.ijrobp.2003.12.039 [DOI] [PubMed] [Google Scholar]
- 109.Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys 2012; 82: 548–53. doi: 10.1016/j.ijrobp.2010.11.050 [DOI] [PubMed] [Google Scholar]
- 110.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009; 74: 1335–41. doi: 10.1016/j.ijrobp.2008.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitajima K, Suenaga Y, Kanda T, Miyawaki D, Yoshida K, Ejima Y, et al. Prognostic value of FDG PET imaging in patients with laryngeal cancer. PLoS One 2014; 9: e96999. doi: 10.1371/journal.pone.0096999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Picchio M, Kirienko M, Mapelli P, Dell'Oca I, Villa E, Gallivanone F, et al. Predictive value of pre-therapy (18)F-FDG PET/CT for the outcome of (18)F-FDG PET-guided radiotherapy in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 2014; 41: 21–31. doi: 10.1007/s00259-013-2528-2 [DOI] [PubMed] [Google Scholar]
- 113.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014; 55: 884–90. doi: 10.2967/jnumed.113.133801 [DOI] [PubMed] [Google Scholar]
- 114.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009; 15: 5861–8. [DOI] [PubMed] [Google Scholar]
- 115.Kim G, Kim YS, Han EJ, Yoo IR, Song JH, Lee SN, et al. FDG-PET/CT as prognostic factor and surveillance tool for postoperative radiation recurrence in locally advanced head and neck cancer. Radiat Oncol J 2011; 29: 243–51. doi: 10.3857/roj.2011.29.4.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park GC, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol 2013; 24: 208–14. doi: 10.1093/annonc/mds247 [DOI] [PubMed] [Google Scholar]
- 117.Kao CH, Lin SC, Hsieh TC, Yen KY, Yang SN, Wang YC, et al. Use of pretreatment metabolic tumour volumes to predict the outcome of pharyngeal cancer treated by definitive radiotherapy. Eur J Nucl Med Mol Imaging 2012; 39: 1297–305. doi: 10.1007/s00259-012-2127-7 [DOI] [PubMed] [Google Scholar]
- 118.Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med 2012; 53: 709–15. [DOI] [PubMed] [Google Scholar]
- 119.Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med 2012; 53: 1506–13. doi: 10.2967/jnumed.111.101402 [DOI] [PubMed] [Google Scholar]
- 120.Lee SJ, Choi JY, Lee HJ, Baek CH, Son YI, Hyun SH, et al. Prognostic value of volume-based 18F-fluorodeoxyglucose PET/CT parameters in patients with clinically node-negative oral tongue squamous cell carcinoma. Korean J Radiol 2012; 13: 752. doi: 10.3348/kjr.2012.13.6.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012; 83: 1514–20. doi: 10.1016/j.ijrobp.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck 2013; 35: 15–22. doi: 10.1002/hed.22904 [DOI] [PubMed] [Google Scholar]
- 123.Abd El-Hafez YG, Moustafa HM, Khalil HF, Liao CT, Yen TC. Total lesion glycolysis: a possible new prognostic parameter in oral cavity squamous cell carcinoma. Oral Oncol 2013; 49: 261–8. doi: 10.1016/j.oraloncology.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 124.Garsa AA, Chang AJ, Dewees T, Spencer CR, Adkins DR, Dehdashti F, et al. Prognostic value of (18)F-FDG PET metabolic parameters in oropharyngeal squamous cell carcinoma. J Radiat Oncol 2013; 2: 27–34. doi: 10.1007/s13566-012-0065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romesser PB, Lim R, Spratt DE, Setton J, Riaz N, Lok B, et al. The relative prognostic utility of standardized uptake value, gross tumor volume, and metabolic tumor volume in oropharyngeal cancer patients treated with platinum based concurrent chemoradiation with a pre-treatment (18)F-fluorodeoxyglucose positron emission tomography scan. Oral Oncol 2014; 50: 802–8. doi: 10.1016/j.oraloncology.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanamoto A, Tatsumi M, Takenaka Y, Hamasaki T, Yasui T, Nakahara S, et al. Volumetric PET/CT parameters predict local response of head and neck squamous cell carcinoma to chemoradiotherapy. Cancer Med 2014; 3: 1368–76. doi: 10.1002/cam4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alluri KC, Tahari AK, Wahl RL, Koch W, Chung CH, Subramaniam RM. Prognostic value of FDG PET metabolic tumor volume in human papillomavirus-positive stage III and IV oropharyngeal squamous cell carcinoma. AJR Am J Roentgenol 2014; 203: 897–903. doi: 10.2214/AJR.14.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwartz DL, Harris J, Yao M, Rosenthal DI, Opanowski A, Levering A, et al. Metabolic tumor volume as a prognostic imaging-based biomarker for head-and-neck cancer: pilot results from Radiation Therapy Oncology Group protocol 0522. Int J Radiat Oncol Biol Phys 2015; 91: 721–9. doi: 10.1016/j.ijrobp.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yabuki K, Shiono O, Komatsu M, Sano D, Nishimura G, Takahashi M, et al. Predictive and prognostic value of metabolic tumor volume (MTV) in patients with laryngeal carcinoma treated by radiotherapy (RT)/concurrent chemoradiotherapy (CCRT). PLoS One 2015; 10: e0117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Becker M, Zaidi H. Imaging in head and neck squamous cell carcinoma: the potential role of PET/MRI. Br J Radiol 2014; 87: 20130677. doi: 10.1259/bjr.20130677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Czernin J, Ta L, Herrmann K. Does PET/MR imaging improve cancer assessments? Literature evidence from more than 900 patients. J Nucl Med 2014; 55: 59S–62. doi: 10.2967/jnumed.114.141838 [DOI] [PubMed] [Google Scholar]
- 132.Weber WA. PET/MR imaging: a critical appraisal. J Nucl Med 2014; 55: 56S–58. doi: 10.2967/jnumed.113.129270 [DOI] [PubMed] [Google Scholar]
- 133.Queiroz MA, Huellner MW. PET/MR in cancers of the head and neck. Semin Nucl Med 2015; 45: 248–65. doi: 10.1053/j.semnuclmed.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 134.Bailey DL, Barthel H, Beuthin-Baumann B, Beyer T, Bisdas S, Boellaard R, et al. Combined PET/MR: where are we now? Summary report of the second international workshop on PET/MR imaging April 8–12, 2013, Tubingen, Germany. Mol Imaging Biol 2014; 16: 295–310. [DOI] [PubMed] [Google Scholar]
- 135.Covello M, Cavaliere C, Aiello M, Cianelli MS, Mesolella M, Iorio B, et al. Simultaneous PET/MR head-neck cancer imaging: preliminary clinical experience and multiparametric evaluation. Eur J Radiol 2015; 84: 1269–76. doi: 10.1016/j.ejrad.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 136.Leibfarth S, Mönnich D, Welz S, Siegel C, Schwenzer N, Schmidt H, et al. A strategy for multimodal deformable image registration to integrate PET/MR into radiotherapy treatment planning. Acta Oncol 2013; 52: 1353–9. doi: 10.3109/0284186X.2013.813964 [DOI] [PubMed] [Google Scholar]
- 137.Geets X, Tomsej M, Lee JA, Duprez T, Coche E, Cosnard G, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol 2007; 85: 105–15. [DOI] [PubMed] [Google Scholar]
- 138.Duprez F, De Neve W, De Gersem W, Coghe M, Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 80: 1045–55. doi: 10.1016/j.ijrobp.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 139.Grégoire V, Jeraj R, Lee JA, O'Sullivan B. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol 2012; 13: e292–300. doi: 10.1016/S1470-2045(12)70237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madani I, Duthoy W, Derie C, De Gersem W, Boterberg T, Saerens M, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 68: 126–35. doi: 10.1016/j.ijrobp.2006.12.070 [DOI] [PubMed] [Google Scholar]
- 141.Chang JH, Wada M, Anderson NJ, Lim Joon D, Lee ST, Gong SJ, et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using (18)F-FMISO PET: a biological modeling study. Acta Oncol 2013; 52: 1723–9. doi: 10.3109/0284186X.2012.759273 [DOI] [PubMed] [Google Scholar]
- 142.Servagi-Vernat S, Differding S, Sterpin E, Hanin FX, Labar D, Bol A, et al. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol 2015; 54: 1008–16. doi: 10.3109/0284186X.2014.990109 [DOI] [PubMed] [Google Scholar]
- 143.Bollineni VR, Koole MJ, Pruim J, Brouwer CL, Wiegman EM, Groen HJ, et al. Dynamics of tumor hypoxia assessed by 18F-FAZA PET/CT in head and neck and lung cancer patients during chemoradiation: possible implications for radiotherapy treatment planning strategies. Radiother Oncol 2014; 113: 198–203. [DOI] [PubMed] [Google Scholar]
- 144.Carlin S, Zhang H, Reese M, Ramos NN, Chen Q, Ricketts SA. A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J Nucl Med 2014; 55: 515–21. doi: 10.2967/jnumed.113.126615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoeben BA, Bussink J, Troost EG, Oyen WJ, Kaanders JH. Molecular PET imaging for biology-guided adaptive radiotherapy of head and neck cancer. Acta Oncol 2013; 52: 1257–71. doi: 10.3109/0284186X.2013.812799 [DOI] [PubMed] [Google Scholar]