Abstract
Objective:
The aim of our preliminary study was to compare the efficacy of drug-eluting beads preloaded with irinotecan (DEBIRI) vs drug-eluting beads preloaded with doxorubicin (DEBDOX) as second-line treatment of unresectable liver metastases from cholangiocarcinoma (CCA).
Methods:
In 2013, 10 patients affected by multiple liver metastases from CCA, resistant to the first-line chemotherapy regimen, were enrolled: 5 patients were submitted to lobar/segmental transarterial chemoembolization (TACE) with DEBIRI (100-mg irinotecan/1 vial) and 5 patients with DEBDOX (50-mg doxorubicin/1 vial), performed every 3 weeks. Patients treated with DEBIRI received antipain premedication consisting of 30-mg of morphine and 3–4 ml of intra-arterial lidocaine. Complications and efficacy were assessed (response evaluation criteria in solid tumour 1.1).
Results:
A total of 32 TACE were performed (mean: 3.2 TACE/patient), all well tolerated, with only 1 case of asymptomatic cholecystitis spontaneously recovered. Response rates of patients treated with DEBDOX and DEBIRI were: 4/5 progressive disease and 1/5 partial response vs 2/5 partial response, 2/5 stable disease and 1/5 progressive disease, respectively, with the appearance of variable necrosis percentage. Progression-free survival from the first procedure and progressive disease were 12.67 weeks for DEBIRI and 15.78 weeks for DEBDOX, respectively. Overall survival from time of primary diagnosis was 176 weeks for DEBIRI and 125 weeks for DEBDOX, respectively.
Conclusion:
In our preliminary experience, DEBIRI was more effective than DEBDOX as a second-line treatment for hepatic metastases from CCA. Antipain drug administration and the use of the microcatheter led to a good treatment tolerability and a low complication rate.
Advances in knowledge:
In our preliminary experience, DEBIRI was more effective than DEBDOX as a second-line treatment of hepatic metastases from CCA; further studies involving a larger cohort of patients are needed.
Cholangiocarcinoma (CCA) is a very rare and aggressive tumour, with an average 5-year survival rate of 5–10%.1 This disease often gives metastases confined to the liver, and the surgical resectability of CCA is crucial for survival. Recently, the cisplatin and gemcitabine combination was identified as the new standard first-line systemic chemotherapy, characterized by very low response rates reported in the literature with a median progression-free survival (PFS) and median overall survival (OS) of 8.5 and 11.7 months, respectively.2 Despite the outcome improvement, disease progression is constant and approximately half of the patients failing upfront treatment have a good performance status and are willing to undergo further treatment. No standard salvage chemotherapy regimen has been identified yet.3 Hence, in these patients, locoregional therapies such as transarterial chemoembolization (TACE) aiming to control hepatic disease are vital to improve quality of life and survival. Drug-eluting beads ensure simultaneous local delivery of anticancer drugs and embolization and offer an option to control drug release over an extended period of time, which can enhance tumour response.4 The specific targeting of the drug at the site of the tumour implies enhanced efficacy by a maximized tumour dose and at the same time reduced toxicity owing to negligible systemic exposure and toxicity.5 The two drugs that are actually used to preload the beads are doxorubicin and irinotecan, based on their relative efficacy in the treatment of several advanced solid tumours (such as gastrointestinal tumours and hepatocellular carcinoma) and their high capability to be bound by the beads. Both doxorubicin [drug-eluting beads preloaded with doxorubicin (DEBDOX)]6,7 and irinotecan-eluting beads [drug-eluting beads preloaded with irinotecan (DEBIRI)]8 are beginning to be studied in the treatment of CCA, and the very limited data available suggest their efficacy in this kind of tumour, even though, to date, there is no specific indication of the use of either DEBDOX or DEBIRI.
Our purpose was to preliminarily compare the efficacy of TACE with DEBIRI vs DEBDOX in patients affected by unresectable CCA metastases confined to the liver as a second-line therapy.
METHODS AND MATERIALS
Patients
Between October 2012 and April 2014, 10 patients (4 females, 6 males) affected by unresectable metastases confined to the liver from advanced CCA were enrolled.
All the patients were previously treated with hepatic surgery and prior systemic chemotherapy based on cisplatin plus gemcitabine.
In case of disease progression at contrast-enhanced multidetector CT (MDCT) according to the response evaluation criteria in solid tumour (RECIST 1.1), patients were randomly submitted to TACE with DEBDOX or TACE with DEBIRI, as second-line treatment.
Written informed consent for all the interventional procedures was obtained from each patient before every chemoembolization with drug eluting beads (DEB-TACE).
In case of bilobar extensive liver involvement, right and left lobar DEB-TACE was alternatively performed every 3 weeks. In case of limited liver involvement, each treatment (segmental vs lobar) was planned on the basis of tumour response to the previous treatment. In case of complete response, PR or stable disease, further TACE with DEBIRI or DEBDOX was planned till tumour progression or for a maximum of 6 months.
The study was interrupted in case of liver failure or liver vascular complications, for medical decision or in case of patient will.
Procedure
For every TACE with DEBIRI, 100 mg of irinotecan preloaded in 2–4-ml beads of 70–150 μm, mixed with 4–8 cc of non-ionic contrast, was prepared 2 h before the procedure and administered. The procedure was preceded by a prophylactic treatment against pain based on a total i.v. morphine dose of 30 mg, one vial 30 min before, one vial during the slow infusion and one last vial immediately after the procedure. Intrahepatic arterial lidocaine (3–4 ml) was selectively infused into the vascular bed to be treated immediately before TACE with DEBIRI in order to reduce local pain due to irinotecan infusion.
For every TACE with DEBDOX, 50 mg of doxorubicin preloaded in 2–4-ml beads of 70–150 μm, mixed with 4–8 cc of non-ionic contrast, was prepared 20 min before the procedure and administered. Since this procedure is well tolerated, no pain medication was required.
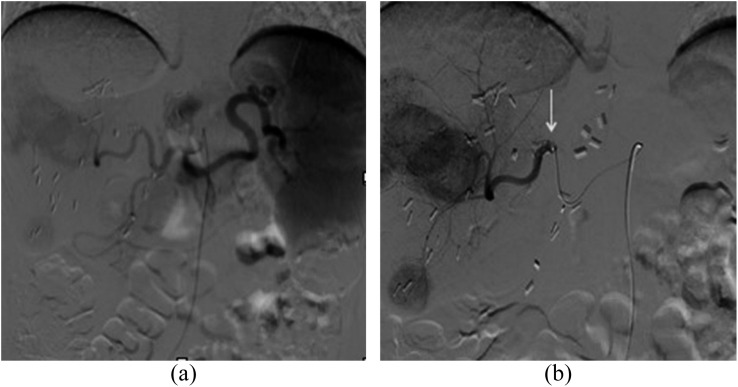
The procedures were mainly performed with a right transfemoral approach; the lobar or segmental infusion (Figure 1) of the beads was always performed using a 2.8-French microcatheter with a 0.027-inch internal lumen (Renegade™ Hi-Flo™, Boston Scientific, Marlborough, Massachusetts) advanced over a 0.014-inch guidewire through the standard angiographic catheter. The injection (one vial per patient, at every treatment) was performed very slowly, in order to avoid the backflow of the microspheres that could possibly damage extrahepatic districts; for the same reason, angiographic control was never performed immediately after the procedure.6 All the procedures were performed by the same experienced interventional radiologist (MV). Tolerability of the procedure and quality of life of the patients during the follow-up were also considered: tolerability evaluation was based on the eventual development of post-embolization syndrome, consisting of pain, fever, nausea and vomit, 48–72 h after the procedure, while quality of life assessment was based on the onset of conditions such as depression, anxiety, anorexia, insomnia and social relationship changes (pre-treatment vs 2–4 weeks after the procedure).
Figure 1.
Lobar transarterial chemoembolization with drug-eluting beads preloaded with doxorubicin (drug-eluting beads preloaded with irinotecan): (a) preliminary diagnostic angiography of the celiac trunk and (b) superselective angiography of the right hepatic artery using a microcatheter (arrow), showing some hypervascular metastases from cholangiocarcinoma.
Tumour response assessment
Tumour response imaging assessment was based on a triphasic contrast-enhanced MDCT scan (Brilliance 64; Philips Healthcare, Cleveland, OH, USA), which was performed at baseline, 72 h after each procedure to assess eventual complications (hepatic ischaemia, cholecystitis, peritoneal fluid, vascular damage), to test for lesion hypovascularization (short-term response) and, from second treatment onwards, to assess tumour response in the contralateral lobe previously treated, and 3 weeks post-treatment.
Tumour response was assessed according to RECIST 1.1 criteria. PFS and OS were evaluated, also considering the intervals between primary diagnosis of CCA and time of disease progression and death, respectively.
No complex statistical methods were necessary for this preliminary study.
RESULTS
A total of 32 TACE were performed (mean: 3.2 TACE/patient). DEB-TACE, either DEBIRI or DEBDOX, was always well tolerated and was successfully performed in all cases. In all procedures, a right transfemoral approach was used; 20 lobar TACE and 12 segmental TACE were performed.
Only one case of asymptomatic cholecystitis that spontaneously recovered without need for medication was recorded at the 72-h MDCT, after DEBDOX treatment. No cases of liver vascular complications or liver failure occurred. No cases of post-embolization syndrome or variation of quality of life were recorded during the follow-up.
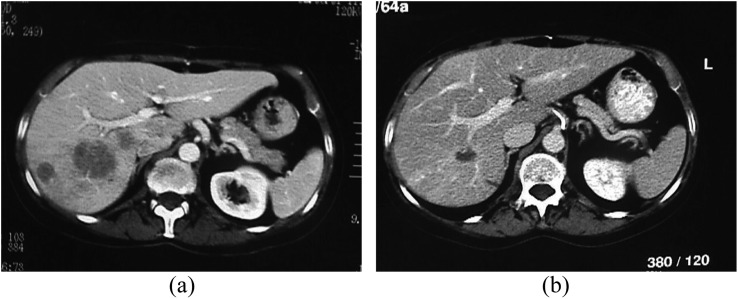
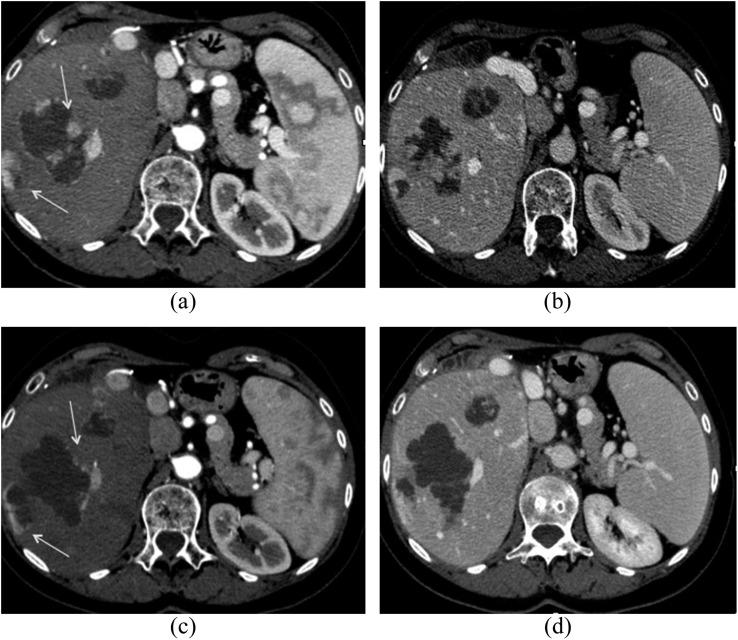
Response rates, according to the RECIST 1.1, assessed at the end of the treatment cycle of patients treated with DEBDOX were 4/5 progressive disease (PD), and 1/5 partial response (PR) while those of the patients treated with DEBIRI were 2/5 PR (Figure 2), 2/5 stable disease (SD) (Figure 3) and 1/5 PD, with the appearance of a variable necrosis percentage.
Figure 2.
Contrast-enhanced multidetector CT scan demonstrating partial response after transarterial chemoembolization with drug-eluting beads preloaded with irinotecan according to response evaluation criteria in solid tumour 1.1: (a) the pre-treatment portal-phase CT scan and (b) post-treatment portal-phase CT scan are demonstrating the shrinkage/disappearance of the metastatic lesions of the right lobe.
Figure 3.
Contrast-enhanced multidetector CT scan demonstrating stable disease (SD) after transarterial chemoembolization with drug-eluting beads preloaded with irinotecan according to response evaluation criteria in solid tumour 1.1: pre-treatment (a) arterial and (b) portal-phase CT scans and post-treatment (c) arterial and (d) portal-phase CT scans. The use of modified Response Evaluation Criteria In Solid Tumors (mRECIST) would have revealed a partial response instead of an SD in this case, owing to the high percentage of tumour necrosis (arrows).
Total PFS was 14.45 weeks: 12.67 weeks in patients submitted to TACE with DEBIRI and 15.78 weeks in patients submitted to TACE with DEBDOX.
Total OS was 47.28 weeks: 45 weeks in patients submitted to TACE with DEBIRI and 48.9 weeks in patients submitted to TACE with DEBDOX; but, considering OS from time of primary diagnosis, it resulted in 176 weeks for DEBIRI and 125 weeks for DEBDOX, respectively.
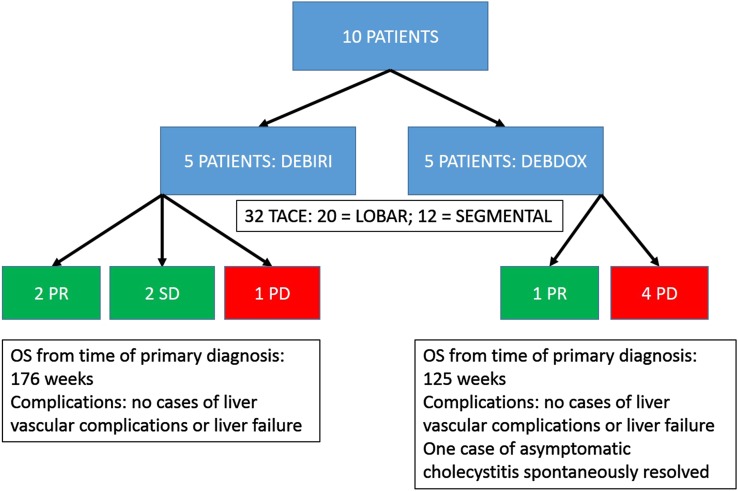
Results and complications of DEBIRI and DEBDOX are summarized separately in Figure 4.
Figure 4.
A graph summarizing separately the results and complications of drug-eluting beads preloaded with irinotecan (DEBIRI) and drug-eluting beads preloaded with doxorubicin (DEBDOX). OS, overall survival; TACE, transarterial chemoembolization.
DISCUSSION
In unresectable CCA, locoregional treatments such as chemoembolization (conventional and with drug-eluting beads) and radioembolization9 have been proven to control the hepatic disease spread, giving advantage in terms of survival, because CCA is usually characterized by metastases confined to the liver. In the literature, there are few studies on the use of drug-eluting beads in advanced CCA, but the lack of patient selection together with the fact that some authors used DEBDOX, while others used DEBIRI, does not provide a straightforward conclusion on the use of chemotherapeutic agents in case of DEB-TACE.
In our preliminary experience based on a small population, owing to the rarity of CCA, all the patients with unresectable metastases confined to the liver and unsuccessfully pre-treated with a single line of systemic chemotherapy were homogenously selected. They were then alternatively submitted to DEBIRI and DEBDOX, both used in previous reports, to preliminarily compare the two chemotherapeutic agents, although no statistical analysis is legitimate. Because of the charge interactions between the negative charges of the beads and the positive charges of both irinotecan and doxorubicin, these two drugs stably bind the beads and hence are the only ones be European Conformity (CE) marked for this use; therefore, according to the current literature,8–10 we chose to use only these two available chemotherapeutic agents, even though not specifically for CCA, but typically to be used in colorectal cancer (irinotecan) or in hepatocarcinoma (doxorubicin).
According to the RECIST 1.1, our overall response rate in patients treated with DEBIRI was higher than that of those treated with DEBDOX, revealing a higher efficacy of the former. These are encouraging results, considering the markedly aggressive behaviour of the CCA. Perhaps the use of modified RECIST along with RECIST 1.1, which also considers the percentage of tumour necrosis, could be more adequate in the assessment of tumour response to this kind of locoregional treatment, since the use of only RECIST 1.1 could lead to response underestimation (Figure 3).
Even though there was no significant difference in terms of survival between the two groups, patients in our study achieved a significant survival improvement, both patients treated with DEBIRI and those treated with DEBDOX, compared with the mean survival of 8 months of patients with advanced CCA in literature.5
The higher efficacy of DEBIRI compared with DEBDOX in our limited group of patients could be related to the pharmacology of irinotecan, as previously hypothesized by Jordan et al;11 in fact, irinotecan, owing to its weaker ionic interactions with the beads, showed a faster drug elution and a higher percentage of drug release than doxorubicin, perhaps leading to higher drug concentrations in the target site, and hence higher efficacy.
Despite the well-known collateral effects of irinotecan, TACE with DEBIRI was always well tolerated in our patients, thanks to a strict antipain premedication protocol administered at each treatment.10,12
On the other hand, TACE with DEBDOX does not require premedication against pain and, furthermore, is often better tolerated than traditional chemoembolization. Other characteristics make doxorubicin more manageable: beads have to be mixed with an irinotecan solution at least 2 h before the procedure or with a doxorubicin solution at least 20 min before the procedure. So, it is easier, during a procedure, for the interventional radiologist to prepare another vial of doxorubicin, if 50 mg is not sufficient (i.e. a right lobe bigger than normal), thus providing the delivery of a more adequate dose of anticancer drug.6,7,13
Our study limitations were first the very limited number of patients and second the low doxorubicin dose used to preload the beads (50 mg) compared with previous reports, in which doses ranged from 75 to 150 mg,8 which could also justify the lower efficacy. Further studies on a larger cohort of patients and with higher doxorubicin doses are necessary to confirm our results.
In conclusion, in our preliminary experience in the treatment of advanced CCA with unresectable metastases confined to the liver, TACE with DEBIRI and with DEBDOX was safe and well tolerated, with a low complication rate and a relative benefit in terms of survival.
Our preliminary findings could represent a new benchmark for future trials based on a larger cohort of patients and a longer follow-up, to better define the best therapeutic option in case of advanced CCA, in the effort to improve OS and disease outcome.
Contributor Information
Massimo Venturini, Email: venturini.massimo@hsr.it.
Claudio Sallemi, Email: sallemi.claudio@hsr.it.
Giulia Agostini, Email: agostini.giulia@hsr.it.
Paolo Marra, Email: marra.paolo@hsr.it.
Stefano Cereda, Email: cereda.stefano@hsr.it.
Michele Reni, Email: reni.michele@hsr.it.
Luca Aldrighetti, Email: aldrighetti.luca@hsr.it.
Francesco De Cobelli, Email: decobelli.francesco@hsr.it.
Alessandro Del Maschio, Email: delmaschio.alessandro@hsr.it.
REFERENCES
- 1.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–73; discussion 473–5. doi: 10.1097/00000658-199610000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–81. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 3.Cereda S, Belli C, Rognone A, Mazza E, Reni M. Second-line therapy in advanced biliary tract cancer: what should be the standard? Crit Rev Oncol Hematol 2013; 88: 368–74. doi: 10.1016/j.critrevonc.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 4.Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol 2006; 17(2 Pt 1): 335–42. doi: 10.1097/01.RVI.0000195323.46152.B3 [DOI] [PubMed] [Google Scholar]
- 5.Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci 2007; 30: 7–14. doi: 10.1016/j.ejps.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol 2008; 31: 883–8. doi: 10.1007/s00270-008-9336-2 [DOI] [PubMed] [Google Scholar]
- 7.Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012; 24: 437–43. doi: 10.1097/MEG.0b013e3283502241 [DOI] [PubMed] [Google Scholar]
- 8.Martin RC, Howard J, Tomalty D, Robbins K, Padr R, Bosnjakovic PM, et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol 2010; 33: 960–6. doi: 10.1007/s00270-010-9937-4 [DOI] [PubMed] [Google Scholar]
- 9.Mouli S, Memon K, Baker T, Benson AB, 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013; 24: 1227–34. doi: 10.1016/j.jvir.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res 2012; 32: 1387–95. [PubMed] [Google Scholar]
- 11.Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol 2010; 21: 1084–90. doi: 10.1016/j.jvir.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 12.Venturini M, Pilla L, Agostini G, Cappio S, Losio C, Orsi M, et al. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: tumor response and predictive value of diffusion-weighted MR imaging in five patients. J Vasc Interv Radiol 2012; 23: 937–41. doi: 10.1016/j.jvir.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 13.Kaiser J, Thiesen J, Krämer I. Stability of irinotecan-loaded drug eluting beads (DC Bead) used for transarterial chemoembolization. J Oncol Pharm Pract 2010; 16: 53–61. doi: 10.1177/1078155209337650 [DOI] [PubMed] [Google Scholar]