Abstract
We reviewed the literature on the use of margins in radiotherapy of patients with prostate cancer, focusing on different options for image guidance (IG) and technical issues. The search in PubMed database was limited to include studies that involved external beam radiotherapy of the intact prostate. Post-prostatectomy studies, brachytherapy and particle therapy were excluded. Each article was characterized according to the IG strategy used: positioning on external marks using room lasers, bone anatomy and soft tissue match, usage of fiducial markers, electromagnetic tracking and adapted delivery. A lack of uniformity in margin selection among institutions was evident from the review. In general, introduction of pre- and in-treatment IG was associated with smaller planning target volume (PTV) margins, but there was a lack of definitive experimental/clinical studies providing robust information on selection of exact PTV values. In addition, there is a lack of comparative research regarding the cost–benefit ratio of the different strategies: insertion of fiducial markers or electromagnetic transponders facilitates prostate gland localization but at a price of invasive procedure; frequent pre-treatment imaging increases patient in-room time, dose and labour; online plan adaptation should improve radiation delivery accuracy but requires fast and precise computation. Finally, optimal protocols for quality assurance procedures need to be established.
INTRODUCTION
Recent advances in radiation therapy (RT) allowed for more conformal dose delivery to the target and improved sparing of the healthy organs. Higher gradients of dose distribution require precise determination of the target position or we can miss the target “precisely”.1,2 This is particularly relevant for prostate cancer RT since the prostate gland position within the pelvis is likely to change between treatment fractions.3–5 With increasing evidence for the benefit of dose escalation and, in parallel, an increasing use of sophisticated image guidance (IG) techniques in RT of prostate cancer, there is a growing interest to the questions of appropriate margins for the planning target volume (PTV) and optimization of IG. The selection of the PTV margin is critical for safe dose escalation using intensity-modulated RT (IMRT) with smaller margins permitting higher doses in modelling studies. For example, Goulet et al6 have estimated the maximum achievable dose to the prostate gland at 83.0, 113.1 and 135.9 Gy for the PTV margins of 10, 5 and 3 mm, respectively. In this review, we examine the range of PTV margins in relation to IG strategies utilized and discuss various practical approaches to optimization of image-guided RT (IGRT) for patients with prostate cancer.
METHODS AND MATERIALS
A PubMed search was performed for articles published in the past 22 years (from December 1992 to March 2016) using the terms “margin” AND “prostate” AND “radiotherapy” (686 items) and “margin” AND “prostate” AND “radiation” (616 items) to identify relevant studies. The search was limited to studies that involved external beam treatment of the intact prostate and were published in the English language. Post-prostatectomy studies, brachytherapy and particle beam therapy were excluded. We identified 155 publications.
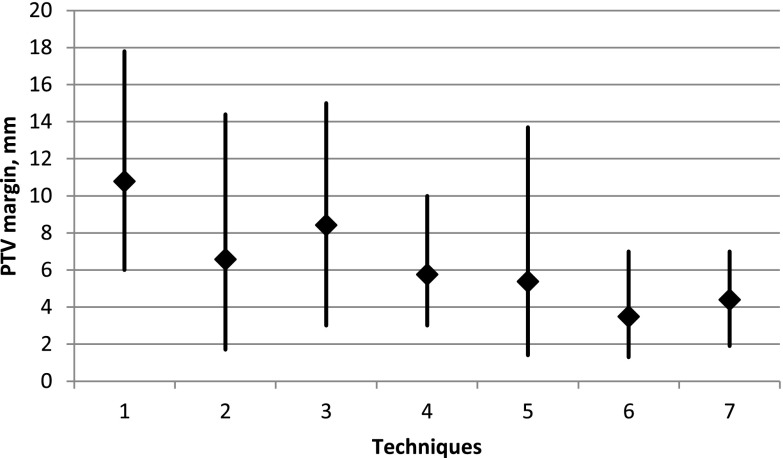
We categorized each article according to the IG option that was investigated. Seven key areas of applications were identified: (1) positioning on external marks using room lasers (9 references), (2) non-specified IGRT (40 references), (3) bone anatomy match (6 references), (4) soft tissue match (3 references), (5) fiducial markers (28 references), (6) electromagnetic tracking (5 references) and (7) adapted delivery (16 references). Articles that belonged to one of these seven categories were included for review. Average values with maximum/minimum range for the PTV margins proposed in the literature for different techniques are shown in Figure 1. The values of recommended PTV margins, employed IG options and 155 references are shown in Table S1 of the Supplementary material.
Figure 1.
Average values with maximum/minimum range for the planning target volume (PTV) margins proposed in the literature for different techniques: (1) positioning on external marks using room lasers (9 references), (2) image-guided radiation therapy (40 references), (3) bone anatomy match (6 references), (4) soft tissue match (3 references), (5) fiducial markers (28 references), (6) electromagnetic tracking (5 references) and (7) adapted delivery (16 references).
RESULTS AND DISCUSSION
Image guidance strategies
With the introduction of pre-treatment IG, one would expect that PTV margins could be decreased allowing for better sparing of the bladder and, especially, of the rectum, organs in very close proximity to the prostate gland.7 However, the literature data do not provide a single consensus recipe, and a wide range of PTV margins from 0 to 20 mm were described in the articles reviewed. Several factors in delivery strategies influence the proposed margins: (1) imaging before or during radiation delivery; (2) the type of imaging device [two-dimensional or three-dimensional (3D) ultrasound, electronic portal imaging (EPID), kilovoltage (kV) and megavoltage (MV) cone beam CT (CBCT), megavoltage fan-beam CT (MVCT), CT on rail]; (3) frequency of IGRT sessions; (4) a choice of immobilization device, if any; (5) patient preparation protocol; (6) method of radiation delivery [conformal four-field box technique, static IMRT, helical tomotherapy or volumetric modulated are therapy (VMAT)]; (7) considerations of interobserver target volume delineation variation; (8) target definition of the prostate only vs prostate plus seminal vesicles vs inclusion of lymph nodes as well. For example, Mzenda et al8 considered a model where delineation, set-up and organ motion-induced errors were included; the derived margin was on average 0.5 mm bigger than currently used margins in the region of small treatment uncertainties.
How many patients and how often the set-up errors should be measured to obtain a reliable PTV margin evaluation? Matsumoto et al9 summarized the data for 35 patients and used different amounts of position verification and found that a minimum of 15 patients and >15 verifications were required.
In another study, PTV margins 8 mm (6 mm posterior) used in IMRT plans were considered adequate to deliver a dose to the prostate with conventional patient positioning using skin tattoos or bony anatomy while the use of IG allowed for significant PTV margin reduction to 4 mm (3 mm posterior) with improved organ-at-risk (OAR) sparing.10 Similarly, Tsai et al11 recommended a 4.5-mm margin when IGRT-IMRT techniques were employed.
Seminal vesicles are more prone for inter- and intrafraction motion and require larger PTV margins (e.g. 4.5 mm, whereas 3 mm for the prostate with online IG12). Inclusion of elective pelvic lymph nodes in the target volume due to the increased spatial uncertainly required additional 9-mm in the anterioposterior (AP) and 7-mm in the left–right (LR) directions to account for their daily displacement relative to the prostate.13 Most of the investigations considered translational corrections only; the impact on target coverage due to prostate rotation was shown to be almost equal for the PTV margins of 2, 4, 6 and 8 mm.14
Nairz et al15 looked for the optimal balance between advantages of a frequent IG and additional cost by a restriction of daily IGRT. They found that three imaging sessions at the beginning of the treatment (when uncertainties in the prostate motion and the doctor's delineation are included in calculation) required the total PTV margins amounted to 8.6 mm in the LR, 10.4 mm in the superior–inferior (SI) and 14.4 mm the AP directions.
Pelvis bone matching is the most common implementation of IG. In Japan, in a survey of 70 facilities that could perform IGRT, 33 (47.1%) facilities conducted bone matching, 28 (40.0%) conducted prostate matching and 9 (12.9%) used metal markers. Prostate or metal marker matching tended to produce a smaller margin than bone matching.16
Multiple strategies for IG in prostate cancer radiotherapy were evaluated by Mayyas et al.17 They compared ultrasound, CBCT, planar kV images and electromagnetic transponders for 27 patients. The use of IG was shown to reduce these margins to <5 mm compared with 10–11 mm, when skin tattoos only were used for guidance with all IGRT strategies providing similar benefit. Margins to compensate for both residual set-up (interfraction) errors as well as intrafraction motion were 6.6, 6.8 and 3.9 mm in the AP, SI and LR directions, respectively.
Alignment of images performed by an operator depends on his/her experience, training and matching protocol. Deegan et al18 concluded that in a study of interobserver variability for alignment of CBCT and planning CT between three radiation therapists, 95% limits of agreement with the mean were 2 mm if fiducial markers were used for alignment and were 3 mm in the case of soft tissue matching. Zahra et al19 reported a good interobserver reproducibility for CBCT registration. The question of education for optimal alignment has not been addressed in the literature.
Thus, while there is variability in the literature with regard to strategy and recommended PTV margins, the literature would support the use of online IG using either implanted fiducial markers or soft tissue matching with in-room CT or ultrasound. With such IG, PTV margins in the range of 6–8 mm (3–5 mm posteriorly) were the most commonly reported.
Immobilization options
Immobilization devices may be external or internal and are employed with the intent of reducing positioning error (external devices) or prostate motion (internal devices). In one study, external patient immobilization reduced the calculated clinical target volume (CTV) to PTV margin to 1.9, 8.3 and 2.3 mm for the Hipfix system (CIVCO Medical Solutions, Kalona, IA) and 3.4, 2.1 and 1.8 mm for the whole-body alpha cradle in the LR, CC and AP axes, respectively.20
The most popular internal immobilization device to reduce prostate motion is endorectal balloon, and this can serve both as a localization tool as well as an immobilization tool. The use of an endorectal balloon was associated with PTV margins of 3–5 mm to correct for intrafraction prostate movements.21,22 Other studies23,24 suggested that margins to account for intrafraction motion for patients with endorectal balloon were dependent on treatment time, with margins in the 3–5 mm range for treatments under 6 min and 4–8 mm for treatments beyond 6 min in duration.
Others noted that the choice of treatment technique (four-field box vs four-field box plus IMRT boost vs IMRT only) was associated with variations in calculated PTV margins with the use of rectal balloon, with smaller margins (2–4 mm) calculated for the four-field box technique than for IMRT techniques (4–6 mm).25 Thus, compared with other forms of IG, the endorectal balloon does not seem to carry a dramatic benefit in terms of PTV selection when IMRT delivery is selected, and if such a device is used, efficient positioning and treatment delivery would seem necessary to realize maximum benefit.
One study examined the use of prostatic calcifications as natural, internal fiducials for tracking with a kilovoltage X-ray IG system (ExacTrac® X-ray system; BrainLab Inc., Feldkirchen, Germany).26 In this study, the required PTV margins were found to be 1.4, 9.7 and 6.1 mm in the LR, SI and AP directions, respectively. Although the ability to use intrinsic fiducials is attractive, not all patients have such calcifications, and the PTV margins seem larger (6–10 mm) than in other IG strategies.
Daily alignment to skin marks
The PTV margins 7.5, 11.4 and 16.3 mm were required with 3-point skin mark alignment in the LR, SI and AP directions, respectively, in a study of 14 patients.27 For the same directions, Pérez-Romasanta et al28 suggested patient-specific ranges of the PTV margin values: 9–10.5, 10.6–12.4 and 15.2–17.8 mm, respectively. In other studies, 5- to 10-mm margins (depending on the direction) using skin marks as reference were proposed with IG, allowing a margin reduction to 5 mm.29,30 Overall, if skin tattoos only are used, the literature would suggest that generous margins (at least 10 mm) are required.
Use of daily ultrasound
The first reported use of ultrasound for IG for prostate treatment employed a portable ultrasound-based system [B-mode acquisition and targeting (BAT)] that was shown to provide prostate localization with the mean absolute magnitude compared with daily CT localizations of 3, 2.4 and 4.6 mm in the AP, LR and SI directions, respectively.31,32 The localization process where transverse and sagittal suprapubic ultrasound images were captured, and the system overlaid the corresponding CT contours relative to the machine isocentre, could be completed in <5 min. Daily BAT system localization was compared with weekly orthogonal portal imaging.33 Prostate internal organ motion appeared to predominate over set-up error as the major component of variation in target localization. One concern was that the BAT procedure itself was found to produce an average motion of 1 mm in the AP and SI directions.34 This effect of prostate displacement by the ultrasound probe was further investigated by Artignan et al35 who found that good-quality ultrasound images could be achieved by the probe displacement of about 1.2 cm, and this pressure on the abdomen resulted in an average prostate displacement of 3.1 mm. Large retrospective analysis of the results of a two-dimensional ultrasound daily pre-treatment localization revealed a systematic 6.1-mm shift posterior due to differences in planning conditions between the CT simulation and the treatment room.36 In general, the literature suggests that 7.5- to 10-mm margins are recommended when daily ultrasound localization is utilized. Comparison of the online MV-CBCT and fiducial IG data showed that for treatments that include imaging of fiducial markers or MV-CBCT, a CTV-to-PTV margin could be 4 mm smaller than the one suggested by ultrasound data.37
Use of daily cross-sectional imaging (fan beam or cone beam)
Tomotherapy
Zhou et al38 used pre-treatment MVCT-based corrections to evaluate the required set-up margins for patients with prostate cancer and reported that accounting for interfraction target motion required margins of 7.4, 6.6 and 5.4 mm in the LR, SI and AP directions, respectively.
Another study analysed the difference between tomotherapy MVCT pre- and post-treatment correction shifts to evaluate the margins required to account for interfraction motion. Margins of 2.2, 2.1 and 2.1 in the LR, SI and AP directions were required for translational motion only, and inclusion of the effect of rotations and matching errors increased these margins to approximately 4 mm in the LR and 5 mm in the SI and AP directions.39 In a similar analysis, larger margins of 11.30, 9.95 and 13.49 mm in the same directions were proposed by Murthy et al.40
Kilovoltage cone beam CT
Hirose et al41 evaluated dosimetric consequences using kV CBCT guidance with either 8-mm margins (5-mm margin posteriorly and superiorly) or 5-mm margins isotropically. They showed that even with bony set-up correction, the target coverage was sufficient with both margins. They also noted better sparing of OARs, if the Hipfix immobilization system was used.
Lerma et al42 demonstrated that with CT IG and online planning, a 3-mm PTV margin was sufficient. Kim et al43 argued that the PTV margin should be no <4 mm if daily CBCT imaging is used for target matching. For the CBCT pre-treatment imaging used in the first 5 days, the 8-mm PTV margin may not be enough.44 Similar margins of 7.3, 7.0 and 9.0 mm in the LR, SI and AP directions were proposed for the prostate gland by quantitative assessment based on CBCT data.45 Target dose coverage was evaluated by the proportion of the CTV encompassed by the 95% isodose for two options of 10- and 7-mm safety margins by Paluska et al.46 Kliton et al47 suggested 9.3-, 6.5- and 8.9-mm PTV margins in the LR, SI and AP directions if in-room kV CT is used for IG. The pre-treatment CBCT images of radio-opaque markers provided information about the motion of the target immediately preceding the treatment useful for PTV margin evaluation,48 but no correlation of these data with pre- and post-CBCT data comparison was found. Normal tissue complication probability (NTCP) prediction values for late rectal bleeding of 3.5%, 2.8% and 2.4% were obtained by simulating three sets of PTV margins: (1) 10 and 6 mm at the prostate/anterior rectal wall interface (10/6 mm), (2) 5/3 mm and (3) uniformly 3 mm, respectively.49 More elaborate strategies using online CTV translation correction by repeated CT imaging for confirmation suggested that a 7-mm margin was sufficient. When the correction of rotation errors was included, a smaller 4-mm margin was possible.50 Similarly, repeat CT scans of 19 patients were rigidly aligned with the planning CT scan using intraprostatic implanted markers, followed by deformable registrations; statistical motion models determined PTV expansions of 5 mm.51 Finally, differences in PTV margins with the use of soft-tissue-based kV CT vs megavoltage guidance were found with 5-mm margins possible with kV CT.52
On balance, the literature would suggest, with in-room CT guidance (either fan-beam CT or CBCT), the use of 5- to 8-mm PTV margins on the gland with 5-mm posterior margins. More elaborate strategies such as the use of kV CT localization, deformable registration and online planning may allow smaller margins (3–4 mm) but at the cost of added time and complexity for the localization process.
Use of internal fiducials with or without cross-sectional imaging
Insertion of gold fiducial markers (usually three) in the prostate gland facilitates visualization of the target position and allows for smaller PTV margins due to the ability to localize the prostate on conventional kV portal imaging.18,53,54 In one study, implantation of fiducial markers allowed PTV margin reduction from 10 to 7 mm as verified by reconstruction of dose distributions after correction using verification CBCT studies.55,56 In another study, set-up margins calculated to encompass 98% of prostate set-up shifts were 11–14 mm with offline corrections based on alignment to the bone, and the PTV margins needed were reduced to 4–7 mm with the use of online correction using fiducial markers.57 Similarly, Skarsgard et al58 using set-up to skin markings without IG noted that margins of 5.7 cm, and 7.9 and 7.7 mm, along the LP, and SI and AP axes, respectively, were required to give 95% probability of complete CTV coverage each day. Using daily portal images with implanted fiducial markers, the PTV margins could be reduced to 3.6, 3.7 and 3.7 mm, respectively. Similar margins of 2.69, 3.22 and 3.37 mm for the LR, SI and AP axes using this technique to account for intrafraction motion was proposed by Siow et al.59 Graf et al60 investigated EPID with implanted fiducials using weekly and daily position correction options: the 7.0-, 9.5- and 9.5-mm PTV margins in the LR, SI and AP directions (using bone alignment only) could be lowered, correspondingly, to 6.7, 8.2 and 8.7 mm with weekly correction and down to 4.9, 5.1 and 4.8 mm with daily correction. The same group reported that usage of kV X-rays for more precise position correction lowered the margins calculated in the LR, SI and AP directions from 2.3, 3.7 and 3.0 mm to 1.8, 1.8 and 2.1 mm if a second repositioning was applied.61
Seo et al62 noted that a margin of 4.97 mm around the CTV provided adequate coverage when two oblique kV images were used for bone alignment corrected by the position of fiducial markers. The results of the NTCP modelling by Gauthier et al63 suggest that the reduction in the PTV margin from 15 mm (10 mm posteriorly) to 10 mm (6 mm posteriorly) afforded by kV fiducial marker localization should decrease the rate of late rectal toxicities and allow moderate dose escalation. Clinically, Engels et al64 reported on conformal arc therapy in treatment of 213 patients with prostate cancer without implanted markers, with 6-mm LR and 10-mm SI and AP margins and compared with clinical results for 25 patients treated with fiducial markers with 3-mm LR and 5-mm SI and AP margins. They noted similar rectal toxicity in-between two cohorts. Shimizu et al65 reported on 110 patients treated with real-time tracking of fiducial markers and a PTV margin of 3 mm. They noted low adverse events with favourable relapse-free survival suggesting that the small margins did not compromise prostate coverage with their localization protocol. Other reports of localization with fiducials by continuous tracking allowed a 3-mm margin instead of 5-mm margin, without a significant compromise in dose.66 In addition to real-time tracking correction for rotations as well as translation can improve accuracy.67 Fiducial markers tracking, combined with a possibility to adjust patient position in six dimensions using ExacTrac/Novalis Body™ (ET/NB) System and a robotic couch, allowed for smaller PTV margins of 2.9 and 2.8 mm in the SI and AR directions.68
Unlike localization of the prostate, inclusion of the seminal vesicles can still be problematic. For example, a margin of 8 mm was found insufficient for seminal vesicles owing to deformations even in the case of continuous fiducial markers tracking, whereas 5-mm PTV margin for the prostate provided sufficient dosimetric coverage.69 Likewise, others noted that a margin of 8.2 mm for the seminal vesicles was necessary, even if daily marker-based rotation correction of the seminal vesicles is performed.70
The use of fiducial markers is attractive given the relative ease of localization on a variety of imaging platforms (EPID, kV imaging, CBCT). The literature would suggest that margin reductions to 5 mm are possible with the use of implanted fiducials with possible further reductions to 3 mm if sophisticated fiducial marker strategies are used such as incorporation of rotation correction or real-time tracking.
Optimizing image guidance strategies
Frequency of imaging/repeat cross-sectional imaging
Given the time required for daily localization, exploration of alternative imaging schedules has been investigated. For example, Yan et al71 investigated an offline adaptive planning process and noted no advantage to 2 weeks of daily CT in constructing a custom PTV margin compared with imaging during the first week for the conventional beam delivery. Nairz et al15 looked for the optimal balance between advantages of a frequent IG and additional cost. They found that three imaging sessions at the beginning of the treatment resulted in total PTV margins of 8.6 mm in the LR, 10.4 mm in the SI and 14.4 mm in the AP directions, suggesting that more frequent imaging may be necessary to reduce margins.
Bortfeld et al72 found a simple formula for the optimum number n of measurements of patient positions during the first n treatment fractions (n = 4 for typical cases) followed by patient position of new external marks without imaging and integrated this principle into a “no action level” protocol. This idea was used in the analysis of daily position corrections by pre-treatment MVCT imaging for patients with prostate cancer and simulation of the residual errors if the no-action-level protocol was applied.73,74 The adaptive procedure based on the online set-up correction enabled Piziorska et al75 to reduce the PTV margin to 7, 7 and 4 mm for the vertical, longitudinal and lateral directions, respectively.
While tracking the intrafraction motion with the Calypso system (Varian, Palo Alto, CA), smaller margins require more frequent imaging to achieve adequate geometric target coverage.76
Accounting for interobserver and intermachine variability
Image-guided therapy QA center recalculated the registration shifts using three independent software systems [MIMvista, (MIM Software Inc. Cleveland, OH), FocalSim (CMS Inc., St Louis, MO) and VelocityAI (Velocity Medical Solutions, Atlanta, GA)] and compared these with the data sent from three IG systems: Tomotherapy Hi-ART (Accuray Inc., Sunnyvale, CA), Synergy (Elekta, Crawley, UK) and Trilogy (Varian, Palo Alto, CA).77 For the 66 comparisons in prostate cases, the differences of 1.1 ± 1.0, 2.1 ± 1.7 and 2.0 ± 1.8 mm in the LR, SI and AP directions, respectively, were found. Quality assurance of the image registration process with a phantom is needed before considering margin reduction using IGRT.
Patient-specific margins and adapted radiotherapy
Non-representative patient anatomy (distended rectum or bladder) at the time of planning may require larger PTV margins to account for systematic error while on treatment. For example, more generous margins were needed for patients with the combination of rectal volume >60 cm3 and bladder volumes >40 cm3 from the study of prostate displacement observed by CTs before, during and after radiotherapy.78 Likewise, patients with large rectum and bladder volumes required larger margins in a cohort of 213 patients without implanted markers (6-mm LR and 10-mm SI and AP margins) and in a cohort of 25 patients in whom implanted markers were used for positioning (3-mm LR and 5-mm SI and AP margins).64 Similarly, larger body habitus, as measured body mass index (BMI) of the patient may lead to more set-up uncertainty, requiring either larger PTV margins or more frequent imaging. For example, one study demonstrated benefits to daily imaging for obese and overweight patients (BMI >25), whereas the patients with normal BMI could be treated with imaging guidance restricted to the first few initial treatment fractions with calculated patient-specific set-up corrections applied thereafter.79
Schulze et al80 evaluated the possible effect of online daily replanning and estimated better organ-at-risk sparing: the rectum and bladder doses were lower by 11% and 14%, respectively. With CBCT pre-treatment imaging, matching based on bony anatomy, soft tissue and online reoptimization was evaluated using the 10- and 5-mm PTV margins.81 Dose calculation cumulated >40 fractions revealed a drop in minimum dose to the prostate of approximately 8% between a 5-mm and 3-mm PTV margin plan.66
A review of image-guided adaptive techniques included discussion of online replanning through direct beam aperture modification for conformal radiotherapy, multileaf collimator segment adjustment for IMRT, online inverse planning, hybrid online correction or offline replanning and full online adaptive inverse planning.82 Replanning of the dose delivery based on the data from CT-on-rail technology allowed for PTV margin reduction from 5 mm (3 mm towards rectum) to 2 mm.83
The adaptive strategy based on the five first fractions resulted in margins ranging from 1 to 3.2 mm in the RL, 2–6.6 mm in the SI and 2–7 mm in the AP directions depending on the patient, whereas population margins to include the same percentage of motion were 1.7, 4.1 and 4.0 mm.84 Therefore, population-based approach results in overdosing of some of the patients and in underdosing of the others. Offline adaptive dose compensation technique could effectively reduce the required margin by 1–2 mm compared with online IG-only strategy.85,86 The online aperture adaptation allowed safe reduction of PTV margins down to 5 mm when only interfractional corrections were applied.87
Given the time required for adaptive replanning using current platforms, the generation of patient-specific margins and/or imaging schedules based on analysis of early treatment imaging histories and/or measurements of patient-specific factors such as BMI and rectal and bladder volumes may be more practical strategies. Such strategies may allow a reduction in imaging frequency and/or PTV margins. In an exploration of the dosimetric consequences of various adaptive and imaging strategies, Battista et al88 noted that one offline replanning using personalized margins calculated from images acquired during the first 6 days of a 7-week radiation schedule yielded the best OAR sparing without compromising target volume coverage.
Accounting for intrafraction motion
Prostate gland motion during radiation treatment itself adds an uncertainty for the target location that requires addition of “intrafraction motion” margin. Taking into account prostate gland motion during treatment presents a significant challenge. Several techniques were reported: continuous EPID,89 CBCT before and after treatment,90 and electromagnetic tracking system.27,91–95 Kupelian et al91 using electromagnetic tracking (single responder) with Calypso system showed that intrafraction motion was not only strongly patient dependent but also changed between treatment fractions. The same approach made it possible to determine anisotropic character of intrafraction motion leading to margins for the LR, SI and AP axes of 8.4, 10.8 and 14.7 mm for skin mark-only set-up and 1.3, 2.3 and 2.8 mm using the online set-up correction.93
Margins of 6 mm were needed to compensate for intrafraction motion when this was estimated from pre- and post-radiation CBCT.90 Rassiah-Szegedi et al94 showed that individualized margin design based on the data obtained from electromagnetic transponders can provide better OAR sparing than conventional 7-mm or even 5-mm uniform margins. A 3-mm margin at the prostate–rectal interface and 5 mm elsewhere was recommended if electromagnetic tracking is used for VMAT treatment.95 A larger 8-mm uniform PTV margin was proposed by Budiharto et al96 for online IMRT adjustment with IG between gantry angle changes. Kurosawa et al97 evaluated that 4.73-mm PTV margin is sufficient to account for intrafractional set-up and organ motion.
With real-time couch adjustment and real-time tracking to account for intrafraction motion, Li et al92 estimated the necessary posterior margin of 6.4 and 4.6 mm (the latter with prostate rotation correction) compared with 7.5 and 7.2 mm for 5- and 3-mm threshold gated treatments. These margins were relatively large because other uncertainties (target delineation, immobilization device and beam delivery errors) were included in these calculations. The longer the treatment, the more pronounced the effect of intrafraction motion, and they noted a 3-mm internal margin may sufficiently account for 95% of intrafraction prostate movement for up to 6 min of treatment time if endorectal balloon and fiducial markers are used for prostate immobilization and localization.27
The intrafraction motion pattern has been shown to differ between patients and even in different fractions for the same treatment course.91 Intratreatment motion has been evaluated by imaging before and after radiation delivery39,48,90,98–100 or directly during treatment using implanted electromagnetic transponders (beacons).20,101,102 Fluoroscopy can also be used for prostate tracking at the expense of increased imaging dose, but Crocker et al103 argued that this dose could be significantly reduced if the imaging field size is tailored to specific patients. Margin requirements accounting for intrafraction motion were evaluated by Tanyi et al27 for different localization techniques: 1.4, 2.6 and 2.3 mm for implanted electromagnetic transponder detection; 2.1, 9.4 and 10.5 mm for volumetric imaging with bony landmark registration; and 2.8, 3.7 and 3.2 mm for volumetric imaging with implanted fiducial marker registration in the LR, SI and AP directions, respectively. Introduction of hypofractionated stereotactic body radiotherapy to treat prostate cancer and rotational techniques for delivery presented additional challenges for OARs sparing, and Tree et al104 evaluated the need of a 5-mm isotropic margin, except 3 mm posteriorly, aiming to deliver 47.5 Gy in five fractions to the boost whilst treating the whole prostate to 36.25 Gy in five fractions using RapidArc (Varian). This margin is possible if intrafraction tracking is implemented, otherwise an 8-mm isotropic margin, except 5 mm posteriorly, is required with more OAR toxicity.
Thus, the literature would suggest that real-time tracking to correct for intrafraction motion may yield benefits in terms of reduction of PTV margins to the range of 3–4 mm compared with 5–8 mm for image localization without tracking. The benefits of this margin reduction of 1–2 mm need to be considered against the need for invasive markers required for such real-time tracking.
Margin calculations
Most publications use “van Herk” equation for the PTV margin that provides a minimum 95% of the prescription dose to the CTV for 90% of patients calculated as 2.5 ∑ + 0.7σ.105,106 The 2.5 ∑ component accounts for the systematic errors. A further margin of 0.7σ is added to account for random set-up and organ-motion uncertainties during treatment. Owing mainly to its simplicity, this equation has been universally used in spite of various proposed modifications: McKenzie et al107 presented a table of the required random error component of the margin depending on the number of beams; Witte et al108 used an analytic model to show that the random error margin increased with tissue density and decreased with target size, leading to significant changes in the minimum margin required for random errors. There were attempts to include radiobiology considerations in margin calculations by Witte et al109 and Mzenda et al.110 Other models for random errors have been considered by Herschtal et al111 who looked for the optimal statistical model to account for random errors and by Suzuki et al112 who assumed a non-Gaussian distribution. Also, the original caution113 that 2.5 ∑ + 0.7σ margin excludes rotational errors and shape deviations and must be considered as a lower limit for safe radiotherapy has been generally ignored. In principle, ∑ and σ should include other uncertainties and be calculated as quadratic sums of errors in target delineation, inter- and intrafraction translation and rotation motion; errors in localization device; and errors in beam delivery system.114,115
Instead of specifying a “hard margin”, Li and Xing116 described an inverse planning algorithm which takes into consideration positional uncertainty in terms of spatial probability distribution. Witte et al109 proposed a probabilistic planning method with biological cost functions that does not require the definition of margins. A target probabilistic planning approach that defines safety margins by directly including the effect of geometric uncertainty into the evaluation of objective functions during optimization was further developed by Bohoslavsky et al.117 This approach resulted in more regular isodoses and in reduced dose, on average, to OARs, up to >6 Gy, while maintaining target coverage and keeping the maximum dose to limiting structures within requirements. Using custom software plugins in a commercial treatment planning system, Fontanarosa et al118 evaluated this method by replanning three prostate cases' probabilistic objective functions and found that the new plans achieved similar or better dose distributions than the original clinical plans in terms of expected target coverage and rectum wall sparing. A probabilistic treatment planning was shown to improve conformality and decrease “dosimetric” margin defined as the specific isodose line compared with standard plan.119–122 Barriers to implementing such probabilistic planning techniques include incorporating such algorithms into commercial treatment planning platforms, determination of accurate input parameters into such algorithms (also necessary for traditional margin calculation “recipes”) and validation of the safety and efficacy of such plans in clinical cohorts.
Impact of image guidance in prostate cancer
Clinical outcomes
The ultimate reason for using more sophisticated (and more expensive) technology is improvement of patients' health. Prior to the introduction of relatively cheap ultrasound devices for localization of the prostate and seminal vesicles, relatively large (1.5-cm) anterior and lateral safety margins along with a posterior margin encompassing the anterior one third of the rectal circumference contributed to rectal toxicity that decreased with the use of tighter margins afforded by ultrasound localization.123 3D ultrasound was tested for two groups of patients treated with 5- or 10-mm margin with conclusion that margin size had no impact either on biochemical control or on late toxicity.124 Good outcomes were achieved by Patel et al125 by using a uniform 7-mm margin and daily ultrasound IGRT. Electromagnetic real-time tracking combined with 3-mm PTV margins led to less radiotherapy-related morbidity.126
Toxicity in patients with prostate cancer treated to 78 Gy, based on daily IG of fiducial markers and 5- to 7-mm PTV margins, was less than in those treated to 76 Gy with 3D conformal radiation therapy and 1- to 2-cm PTV margins.127 Biochemical failure occurred in 13.6% of 140 patients treated with 1-cm PTV margin with late grade 3 genitourinary and gastrointestinal toxicity rates in 1.6% and 3% of patients, respectively.128 No obvious detriment using the smaller radiotherapy margin of 1 cm instead of 1.5 cm was observed in a clinical trial including 126 patients treated with 3D CRT.129 At median follow-up of 23 months, biochemical relapse-free survival rate was 95.2%, and only one patient required premature conclusion at 45 Gy due to grade 3 genitourinary toxicity in hypofractionated treatment on tomotherapy with the PTV margins of 11.30, 9.95 and 13.49 mm in the LR, SI and AP directions, respectively.40 Likewise, Kupelian et al130 reported on a large cohort (n = 488) of males with prostate cancer treated with moderately hypofractionated radiotherapy (70 Gy/28 fractions) using daily ultrasound guidance. They noted low rates of toxicity and high rates of biochemical control. They also noted an independence of their results from the rectal volume at the time of CT planning, suggesting that daily IG offset the systematic errors introduced by extremes of anatomy at the time of simulation.
Cost/benefit ratio of new technology in prostate cancer
Many factors should be taken in assessment of potential benefits in introduction of sophisticated new technology. Improved patients' quality of life will be reflected in better patient performance in his/her workplace. Reduced acute and late toxicity will result in less expense for follow-up medical care and drugs. 19 years ago, Perez et al131 predicted an overall benefit of 3D conformal radiotherapy in treatment of localized prostate cancer due to better local tumour control and decreased treatment morbidity in spite of increased initial reimbursement for introduction of conformal radiotherapy (28% higher than standard radiotherapy and 12% higher than radical prostatectomy). Introduction of IG is still under investigation from the economy point of view. Ploquin and Dunscombe132 argued that IG used solely for translational patient repositioning for prostate cancer adds costs with relatively little improvement in dosimetric quality. However, full exploitation of the potential of IGRT, particularly through margin reduction, can be expected to result in a reduction in the cost–outcome ratios. Recent clinical series suggest benefits of the addition of IG to either 3D conformal radiotherapy or IMRT.133
CONCLUSION
Our approach to the choice of appropriate PTV margin in RT of patients with prostate cancer is still far from being uniform in different institutions. Although everyone feels that an introduction of pre- and in-treatment IG should allow for tighter margins with improved OARs sparing, the exact values are on a research/discussion stage, and the experimental/clinical studies providing robust information on this subject are scarce. And always there is a question of the optimal balance: insertion of fiducial markers or electromagnetic transponders facilitate prostate gland localization but at a price of invasive procedure; frequent pre-treatment imaging increases patient in-room time, dose and labour; online plan adaptation should improve radiation delivery accuracy but requires fast and precise computation. The role and protocols for quality assurance procedures should be established. Careful analysis of clinical outcomes with respect to employed PTV margins and including patient-specific data could lead to personalized choice for optimal treatment of prostate cancer. In general, the literature would support PTV margins of 10 mm or more when using set-up based on alignment to skin marks or bony anatomy; PTV margins of 5–8 mm when using daily cross-sectional imaging based on soft-tissue registration or when using implanted fiducial markers; and PTV margins of 3-mm when using highly sophisticated techniques such as rapid delivery coupled with real-time tracking, adaptive replanning or protocols that incorporate corrections for rotations as well as translations.
Contributor Information
Slav Yartsev, Email: slav.yartsev@lhsc.on.ca.
Glenn Bauman, Email: glenn.bauman@lhsc.on.ca.
REFERENCES
- 1.Wu VW, Law MY, Star-Lack J, Cheung FW, Ling CC. Technologies of image guidance and the development of advanced linear accelerator systems for radiotherapy. Front Radiat Ther Oncol 2011; 43: 132–64. doi: 10.1159/000322414 [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Meyer JL, Dawson LA. Image guidance and the new practice of radiotherapy: what to know and use from a decade of investigation. Front Radiat Ther Oncol 2011; 43: 196–216. doi: 10.1159/000322429 [DOI] [PubMed] [Google Scholar]
- 3.Kupelian P, Meyer JL. Prostate cancer: image guidance and adaptive therapy. Front Radiat Ther Oncol 2007; 40: 289–314. doi: 10.1159/0000106043 [DOI] [PubMed] [Google Scholar]
- 4.Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol (R Coll Radiol) 2010; 22: 698–706. doi: 10.1016/j.clon.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 5.Heemsbergen WD, Al-Mamgani A, Witte MG, van Herk M, Lebesque JV. Radiotherapy with rectangular fields is associated with fewer clinical failures than conformal fields in the high-risk prostate cancer subgroup: results from a randomized trial. Radiother Oncol 2013; 107: 134–9. doi: 10.1016/j.radonc.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 6.Goulet CC, Herman MG, Hillman DW, Davis BJ. Estimated limits of IMRT dose escalation using varied planning target volume margins. Phys Med Biol 2008; 53: 3777–88. doi: 10.1088/0031-9155/53/14/005 [DOI] [PubMed] [Google Scholar]
- 7.Tudor GS, Rimmer YL, Nguyen TB, Cowen MA, Thomas SJ. Consideration of the likely benefit from implementation of prostate image-guided radiotherapy using current margin sizes: a radiobiological analysis. Br J Radiol 2012; 85: 1263–71. doi: 10.1259/bjr/27924223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mzenda B, Hosseini-Ashrafi M, Palmer A, Liu H, Brown DJ. A simulation technique for computation of the dosimetric effects of setup, organ motion and delineation uncertainties in radiotherapy. Med Biol Eng Comput 2010; 48: 661–9. doi: 10.1007/s11517-010-0616-z [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Ohta S, Ohno Y, Ogata Y. [Study of setup margins in radiation therapy: how many verification times and patients are required]. Nihon Hoshasen Gijutsu Gakkai Zasshi 2010; 66: 1186–96. doi: 10.6009/jjrt.66.1186 [DOI] [PubMed] [Google Scholar]
- 10.Pawlowski JM, Yang ES, Malcolm AW, Coffey CW, Ding GX. Reduction of dose delivered to organs at risk in prostate cancer patients via image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: 924–34. doi: 10.1016/j.ijrobp.2009.06.068 [DOI] [PubMed] [Google Scholar]
- 11.Tsai JS, Micaily B, Miyamoto C. Optimization and quality assurance of an image-guided radiation therapy system for intensity-modulated radiation therapy radiotherapy. Med Dosim 2012; 37: 321–33. doi: 10.1016/j.meddos.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Wu Q, Yan D. The role of seminal vesicle motion in target margin assessment for online image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2009; 73: 935–43. doi: 10.1016/j.ijrobp.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton BK, Fiveash JB, Wu X, Dobelbower MC, Kim RY, Jacob R. Optimal planning target volume margins for elective pelvic lymphatic radiotherapy in high-risk prostate cancer patients. ISRN Oncol 2013; 2013: 941269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lips IM, van der Heide UA, Kotte AN, van Vulpen M, Bel A. Effect of translational and rotational errors on complex dose distributions with off-line and on-line position verification. Int J Radiat Oncol Biol Phys 2009; 74: 1600–8. doi: 10.1016/j.ijrobp.2009.02.056 [DOI] [PubMed] [Google Scholar]
- 15.Nairz O, Merz F, Deutschmann H, Kopp P, Schöller H, Zehentmayr F, et al. A strategy for the use of cone-beam CT IGRT with alignment on pelvic bones and its impact on treatment margins for prostate cancer patients. Strahlenther Onkol 2008; 184: 663–7. doi: 10.1007/s00066-008-1874-7 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Akimoto T, Mizowaki T, Hatano K, Kodaira T, Nakamura N, et al. Patterns of practice in intensity-modulated radiation therapy and image-guided radiation therapy for prostate cancer in Japan. Jpn J Clin Oncol 2012; 42: 53–7. doi: 10.1093/jjco/hyr175 [DOI] [PubMed] [Google Scholar]
- 17.Mayyas E, Chetty IJ, Chetvertkov M, Wen N, Neicu T, Nurushev T, et al. Evaluation of multiple image-based modalities for image-guided radiation therapy (IGRT) of prostate carcinoma: a prospective study. Med Phys 2013; 40: 041707. [DOI] [PubMed] [Google Scholar]
- 18.Deegan T, Owen R, Holt T, Fielding A, Biggs J, Parfitt M, et al. Assessment of cone beam CT registration for prostate radiation therapy: fiducial marker and soft tissue methods. J Med Imaging Radiat Oncol 2015; 59: 91–8. doi: 10.1111/1754-9485.12197 [DOI] [PubMed] [Google Scholar]
- 19.Zahra N, Monnet C, Bartha E, Bouilhol G, Boydev C, Courbis M, et al. Interobserver variability study for daily cone beam computed tomography registration of prostate volumetric modulated arc therapy. Cancer Radiother 2015; 19: 303–7. doi: 10.1016/j.canrad.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 20.White P, Yee CK, Shan LC, Chung LW, Man NH, Cheung YS. A comparison of two systems of patient immobilization for prostate radiotherapy. Radiat Oncol 2014; 9: 29. doi: 10.1186/1748-717X-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeenk RJ, Louwe RJ, Langen KM, Shah AP, Kupelian PA, van Lin EN, et al. An endorectal balloon reduces intrafraction prostate motion during radiotherapy. Int J Radiat Oncol Biol Phys 2012; 83: 661–9. doi: 10.1016/j.ijrobp.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 22.Jones BL, Gan G, Diot Q, Kavanagh B, Timmerman RD, Miften M. Dosimetric and deformation effects of image-guided interventions during stereotactic body radiation therapy of the prostate using an endorectal balloon. Med Phys 2012; 39: 308013–18. [DOI] [PubMed] [Google Scholar]
- 23.Both S, Wang KK, Plastaras JP, Deville C, Bar Ad V, Tochner Z, et al. Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int J Radiat Oncol Biol Phys 2011; 81: 1302–9. doi: 10.1016/j.ijrobp.2010.08.052 [DOI] [PubMed] [Google Scholar]
- 24.Wang KK, Vapiwala N, Deville C, Plastaras JP, Scheuermann R, Lin H, et al. A study to quantify the effectiveness of daily endorectal balloon for prostate intrafraction motion management. Int J Radiat Oncol Biol Phys 2012; 83: 1055–63. doi: 10.1016/j.ijrobp.2011.07.038 [DOI] [PubMed] [Google Scholar]
- 25.Steiner E, Georg D, Goldner G, Stock M. Prostate and patient intrafraction motion: impact on treatment time-dependent planning margins for patients with endorectal balloon. Int J Radiat Oncol Biol Phys 2013; 86: 755–61. doi: 10.1016/j.ijrobp.2013.02.035 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda I, Mizowaki T, Sawada Y, Nakata M, Norihisa Y, Ogura M, et al. Assessment of interfractional prostate motion in patients immobilized in the prone position using a thermoplastic shell. J Radiat Res 2014; 55: 168–74. doi: 10.1093/jrr/rrt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanyi JA, He T, Summers PA, Mburu RG, Kato CM, Rhodes SM, et al. Assessment of planning target volume margins for intensity-modulated radiotherapy of the prostate gland: role of daily inter- and intrafraction motion. Int J Radiat Oncol Biol Phys 2010; 78: 1579–85. doi: 10.1016/j.ijrobp.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Romasanta LA, Lozano-Martín E, Velasco-Jiménez J, Mendicote-León F, Sanz-Martín M, Torres-Donaire J, et al. CTV to PTV margins for prostate irradiation. Three-dimensional quantitative assessment of interfraction uncertainties using portal imaging and serial CT scans. Clin Transl Oncol 2009; 11: 615–21. doi: 10.1007/s12094-009-0413-z [DOI] [PubMed] [Google Scholar]
- 29.Røthe Arnesen M, Eilertsen K, Malinen E. Optimal treatment margins for radiotherapy of prostate cancer based on interfraction imaging. Acta Oncol 2008; 47: 1373–81. [DOI] [PubMed] [Google Scholar]
- 30.Khalifa J, Commandeur F, Bachaud JM, de Crevoisier R. Choice of optimal margins in prostate conformal radiotherapy. Cancer Radiother 2013; 17: 461–9. doi: 10.1016/j.canrad.2013.06.031 [DOI] [PubMed] [Google Scholar]
- 31.Lattanzi J, McNeeley S, Pinover W, Horwitz E, Das I, Schultheiss TE, et al. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int J Radiat Oncol Biol Phys 1999; 43: 719–25. doi: 10.1016/S0360-3016(98)00496-9 [DOI] [PubMed] [Google Scholar]
- 32.Lattanzi J, McNeeley S, Donnelly S, Palacio E, Hanlon A, Schultheiss TE, et al. Ultrasound-based stereotactic guidance in prostate cancer—quantification of organ motion and set-up errors in external beam radiation therapy. Comput Aided Surg 2000; 5: 289–95. [DOI] [PubMed] [Google Scholar]
- 33.Little DJ, Dong L, Levy LB, Chandra A, Kuban DA. Use of portal images and BAT ultrasonography to measure setup error and organ motion for prostate IMRT: implications for treatment margins. Int J Radiat Oncol Biol Phys 2003; 56: 1218–24. doi: 10.1016/S0360-3016(03)00290-6 [DOI] [PubMed] [Google Scholar]
- 34.Trichter F, EnnisInt RD. Prostate localization using transabdominal ultrasound imaging. J Radiat Oncol Biol Phys 2003; 56: 1225–33. doi: 10.1016/S0360-3016(03)00269-4 [DOI] [PubMed] [Google Scholar]
- 35.Artignan X, Smitsmans MH, Lebesque JV, Jaffray DA, van Herk M, Bartelink H. Online ultrasound image guidance for radiotherapy of prostate cancer: impact of image acquisition on prostate displacement. Int J Radiat Oncol Biol Phys 2004; 59: 595–601. doi: 10.1016/j.ijrobp.2004.01.043 [DOI] [PubMed] [Google Scholar]
- 36.Poli ME, Parker W, Patrocinio H, Souhami L, Shenouda G, Campos LL, et al. An assessment of PTV margin definitions for patients undergoing conformal 3D external beam radiation therapy for prostate cancer based on an analysis of 10,327 pretreatment daily ultrasound localizations. Int J Radiat Oncol Biol Phys 2007; 67: 1430–7. doi: 10.1016/j.ijrobp.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 37.Gayou O, Miften M. Comparison of mega-voltage cone-beam computed tomography prostate localization with online ultrasound and fiducial markers methods. Med Phys 2008; 35: 531–8. doi: 10.1118/1.2830381 [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Uhl B, Dewit K, Young M, Taylor B, Fei DY, et al. Analysis of daily setup variation with tomotherapy megavoltage computed tomography. Med Dosim 2010; 35: 31–7. doi: 10.1016/j.meddos.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 39.Thomas SJ, Ashburner M, Tudor GS, Treeby J, Dean J, Routsis D, et al. Intra-fraction motion of the prostate during treatment with helical tomotherapy. Radiother Oncol 2013; 109: 482–6. doi: 10.1016/j.radonc.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 40.Murthy V, Krishnatry R, Mallik S, Master Z, Mahantshetty U, Shrivastava S. Helical tomotherapy-based hypofractionated radiotherapy for prostate cancer: a report on the procedure, dosimetry and preliminary clinical outcome. J Cancer Res Ther 2013; 9: 253–60. doi: 10.4103/0973-1482.113378 [DOI] [PubMed] [Google Scholar]
- 41.Hirose Y, Nakamura M, Tomita T, Kitsuda K, Notogawa T, Miki K, et al. Evaluation of different set-up error corrections on dose-volume metrics in prostate IMRT using CBCT images. J Radiat Res 2014; 55: 966–75. doi: 10.1093/jrr/rru033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerma FA, Liu B, Wang Z, Yi B, Amin P, Liu S, et al. Role of image-guided patient repositioning and online planning in localized prostate cancer IMRT. Radiother Oncol 2009; 93: 18–24. doi: 10.1016/j.radonc.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Hammoud R, Pradhan D, Zhong H, Jin RY, Movsas B, et al. Prostate localization on daily cone-beam computed tomography images: accuracy assessment of similarity metrics. Int J Radiat Oncol Biol Phys 2010; 77: 1257–65. doi: 10.1016/j.ijrobp.2009.09.068 [DOI] [PubMed] [Google Scholar]
- 44.Perks J, Turnbull H, Liu T, Purdy J, Valicenti R. Vector analysis of prostate patient setup with image-guided radiation therapy via kV cone beam computed tomography. Int J Radiat Oncol Biol Phys 2011; 79: 915–19. doi: 10.1016/j.ijrobp.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 45.Juan-Senabre XJ, López-Tarjuelo J, Conde-Moreno A, Santos-Serra A, Sánchez-Iglesias AL, Quirós-Higueras JD, et al. Uncertainties and CTV to PTV margins quantitative assessment using cone-beam CT technique in clinical application for prostate, and head and neck irradiation tumours. Clin Transl Oncol 2011; 13: 819–25. doi: 10.1007/s12094-011-0740-8 [DOI] [PubMed] [Google Scholar]
- 46.Paluska P, Hanus J, Sefrova J, Rouskova L, Grepl J, Jansa J, et al. Utilization of cone-beam CT for offline evaluation of target volume coverage during prostate image-guided radiotherapy based on bony anatomy alignment. Rep Pract Oncol Radiother 2012; 17: 134–40. doi: 10.1016/j.rpor.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kliton J, Agoston P, Major T, Polgár C. Patient positioning using in-room kV CT for image-guided radiotherapy (IGRT) of prostate cancer. Magy Onkol 2012; 56: 193–8. [PubMed] [Google Scholar]
- 48.Bernchou U, Agergaard SN, Brink C. Radiopaque marker motion during pre-treatment CBCT as a predictor of intra-fractional prostate movement. Acta Oncol 2013; 52: 1168–74. doi: 10.3109/0284186X.2012.747698 [DOI] [PubMed] [Google Scholar]
- 49.Wen N, Kumarasiri A, Nurushev T, Burmeister J, Xing L, Liu D, et al. An assessment of PTV margin based on actual accumulated dose for prostate cancer radiotherapy. Phys Med Biol 2013; 58: 7733–44. doi: 10.1088/0031-9155/58/21/7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rijkhorst EJ, Lakeman A, Nijkamp J, de Bois J, van Herk M, Lebesque JV, et al. Strategies for online organ motion correction for intensity-modulated radiotherapy of prostate cancer: prostate, rectum, and bladder dose effects. Int J Radiat Oncol Biol Phys 2009; 75: 1254–60. doi: 10.1016/j.ijrobp.2009.04.034 [DOI] [PubMed] [Google Scholar]
- 51.Thörnqvist S, Hysing LB, Zolnay AG, Söhn M, Hoogeman MS, Muren LP, et al. Treatment simulations with a statistical deformable motion model to evaluate margins for multiple targets in radiotherapy for high-risk prostate cancer. Radiother Oncol 2013; 109: 344–9. doi: 10.1016/j.radonc.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 52.Godley A, Ahunbay E, Peng C, Li XA. Accumulating daily-varied dose distributions of prostate radiation therapy with soft-tissue-based kV CT guidance. J Appl Clin Med Phys 2012; 13: 3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duffton A, McNee S, Muirhead R, Alhasso A. Clinical commissioning of online seed matching protocol for prostate radiotherapy. Br J Radiol 2012; 85: e1273–81. doi: 10.1259/bjr/72368557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deegan T, Owen R, Holt T, Roberts L, Biggs J, McCarthy A, et al. Interobserver variability of radiation therapists aligning to fiducial markers for prostate radiation therapy. J Med Imaging Radiat Oncol 2013; 57: 519–23. doi: 10.1111/1754-9485.12055 [DOI] [PubMed] [Google Scholar]
- 55.Paluska P, Hanus J, Sefrova J, Rouskova L, Grepl J, Jansa J, et al. Utilization of cone beam CT for reconstruction of dose distribution delivered in image-guided radiotherapy of prostate carcinoma—bony landmark setup compared to fiducial markers setup. J Appl Clin Med Phys 2013; 14: 4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasaova L, Sirak I, Jansa J, Paluska P, Petera J. Quantitative evaluation of the benefit of fiducial image-guidance for prostate cancer intensity modulated radiation therapy using daily dose volume histogram analysis. Technol Cancer Res Treat 2014; 13: 47–55. [DOI] [PubMed] [Google Scholar]
- 57.Greer PB, Dahl K, Ebert MA, Wratten C, White M, Denham JW. Comparison of prostate set-up accuracy and margins with off-line bony anatomy corrections and online implanted fiducial-based corrections. J Med Imaging Radiat Oncol 2008; 52: 511–16. doi: 10.1111/j.1440-1673.2008.02005.x [DOI] [PubMed] [Google Scholar]
- 58.Skarsgard D, Cadman P, El-Gayed A, Pearcey R, Tai P, Pervez N, et al. Planning target volume margins for prostate radiotherapy using daily electronic portal imaging and implanted fiducial markers. Radiat Oncol 2010; 5: 52. doi: 10.1186/1748-717X-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siow TR, Ngoi CL, Tan WK. Inter-fraction prostate motion during intensity-modulated radiotherapy for prostate cancer. Singapore Med J 2011; 52: 405–9. [PubMed] [Google Scholar]
- 60.Graf R, Wust P, Budach V, Boehmer D. Potentials of on-line repositioning based on implanted fiducial markers and electronic portal imaging in prostate cancer radiotherapy. Radiat Oncol 2009; 4: 13. doi: 10.1186/1748-717X-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graf R, Boehmer D, Budach V, Wust P. Residual translational and rotational errors after kV X-ray image-guided correction of prostate location using implanted fiducials. Strahlenther Onkol 2010; 186: 544–50. doi: 10.1007/s00066-010-2030-8 [DOI] [PubMed] [Google Scholar]
- 62.Seo YE, Kim TH, Lee KS, Cho WY, Lee HS, Hur WJ, et al. Interfraction prostate movement in bone alignment after rectal enema for radiotherapy. Korean J Urol 2014; 55: 23–8. doi: 10.4111/kju.2014.55.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gauthier I, Carrier JF, Béliveau-Nadeau D, Fortin B, Taussky D. Dosimetric impact and theoretical clinical benefits of fiducial markers for dose escalated prostate cancer radiation treatment. Int J Radiat Oncol Biol Phys 2009; 74: 1128–33. doi: 10.1016/j.ijrobp.2008.09.043 [DOI] [PubMed] [Google Scholar]
- 64.Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys 2009; 74: 388–91. doi: 10.1016/j.ijrobp.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 65.Shimizu S, Nishioka K, Suzuki R, Shinohara N, Maruyama S, Abe T, et al. Early results of urethral dose reduction and small safety margin in intensity-modulated radiation therapy (IMRT) for localized prostate cancer using a real-time tumor-tracking radiotherapy (RTRT) system. Radiat Oncol 2014; 9: 118. doi: 10.1186/1748-717X-9-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noel CE, Santanam L, Olsen JR, Baker KW, Parikh PJ. An automated method for adaptive radiation therapy for prostate cancer patients using continuous fiducial-based tracking. Phys Med Biol 2010; 55: 65–82. doi: 10.1088/0031-9155/55/1/005 [DOI] [PubMed] [Google Scholar]
- 67.Shang Q, Sheplan Olsen LJ, Stephans K, Tendulkar R, Xia P. Prostate rotation detected from implanted markers can affect dose coverage and cannot be simply dismissed. J Appl Clin Med Phys 2013; 14: 4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badakhshi H, Wust P, Budach V, Graf R. Image-guided radiotherapy with implanted markers and kilovoltage imaging and 6-dimensional position corrections for intrafractional motion of the prostate. Anticancer Res 2013; 33: 4117–21. [PubMed] [Google Scholar]
- 69.Mutanga TF, de Boer HC, van der Wielen GJ, Hoogeman MS, Incrocci L, Heijmen BJ. Margin evaluation in the presence of deformation, rotation, and translation in prostate and entire seminal vesicle irradiation with daily marker-based setup corrections. Int J Radiat Oncol Biol Phys 2011; 81: 1160–7. doi: 10.1016/j.ijrobp.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 70.de Boer J, van Herk M, Pos FJ, Sonke JJ. Hybrid registration of prostate and seminal vesicles for image guided radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 177–82. doi: 10.1016/j.ijrobp.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 71.Yan D, Lockman D, Brabbins D, Tyburski L, Martinez A. An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer. Int J Radiat Oncol Biol Phys 2000; 48: 289–302. doi: 10.1016/S0360-3016(00)00608-8 [DOI] [PubMed] [Google Scholar]
- 72.Bortfeld T, van Herk M, Jiang SB. When should systematic patient positioning errors in radiotherapy be corrected? Phys Med Biol 2002; 47: N297–302. doi: 10.1088/0031-9155/47/23/401 [DOI] [PubMed] [Google Scholar]
- 73.Beldjoudi G, Yartsev S, Bauman G, Battista J, Van Dyk J. Schedule for CT image guidance in treating prostate cancer with helical tomotherapy. Br J Radiol 2010; 83: 241–51. doi: 10.1259/bjr/28706108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beavis AW. Image-guided radiation therapy: what is our utopia? Br J Radiol 2010; 83: 191–3. doi: 10.1259/bjr/26132255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piziorska M, Kukołowicz P, Zawadzka A, Pilichowska M, Pęczkowski P. Adaptive off-line protocol for prostate external radiotherapy with cone beam computer tomography. Strahlenther Onkol 2012; 188: 1003–9. doi: 10.1007/s00066-012-0226-9 [DOI] [PubMed] [Google Scholar]
- 76.Curtis W, Khan M, Magnelli A, Stephans K, Tendulkar R, Xia P. Relationship of imaging frequency and planning margin to account for intrafraction prostate motion: analysis based on real-time monitoring data. Int J Radiat Oncol Biol Phys 2013; 85: 700–6. doi: 10.1016/j.ijrobp.2012.05.044 [DOI] [PubMed] [Google Scholar]
- 77.Cui Y, Galvin JM, Straube WL, Bosch WR, Purdy JA, Li XA, et al. Multi-system verification of registrations for image-guided radiotherapy in clinical trials. Int J Radiat Oncol Biol Phys 2011; 81: 305–12. doi: 10.1016/j.ijrobp.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zelefsky MJ, Crean D, Mageras GS, Lyass O, Happersett L, Ling CC, et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol 1999; 50: 225–34. doi: 10.1016/S0167-8140(99)00011-0 [DOI] [PubMed] [Google Scholar]
- 79.Piotrowski T, Kaczmarek Jodda A, Ryczkowski A, Bajon T, Rodrigues G, Yartsev S. Image guidance procedures in radiotherapy for prostate cancer and the influence of BMI. J Radiother Pract 2014; 13: 410–17. doi: 10.1017/S1460396914000193 [DOI] [Google Scholar]
- 80.Schulze D, Liang J, Yan D, Zhang T. Comparison of various online IGRT strategies: The benefits of online treatment plan re-optimization. Radiother Oncol 2009; 90: 367–76. doi: 10.1016/j.radonc.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thongphiew D, Wu QJ, Lee WR, Chankong V, Yoo S, McMahon R, et al. Comparison of online IGRT techniques for prostate IMRT treatment: adaptive vs repositioning correction. Med Phys 2009; 36: 1651–62. doi: 10.1118/1.3095767 [DOI] [PubMed] [Google Scholar]
- 82.Ghilezan M, Yan D, Martinez A. Adaptive radiation therapy for prostate cancer. Semin Radiat Oncol 2010; 20: 130–7. doi: 10.1016/j.semradonc.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahunbay EE, Peng C, Holmes S, Godley A, Lawton C, Li XA. Online adaptive replanning method for prostate radiotherapy. Int J Radiat Oncol Biol Phys 2010; 77: 1561–72. doi: 10.1016/j.ijrobp.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 84.Adamson J, Wu Q. Prostate intrafraction motion assessed by simultaneous kV fluoroscopy at MV delivery II: adaptive strategies. Int J Radiat Oncol Biol Phys 2010; 78: 1323–30. doi: 10.1016/j.ijrobp.2009.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu H, Wu Q. Dosimetric and geometric evaluation of a hybrid strategy of offline adaptive planning and online image guidance for prostate cancer radiotherapy. Phys Med Biol 2011; 56: 5045–62. doi: 10.1088/0031-9155/56/15/024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H, Wu Q. Evaluations of an adaptive planning technique incorporating dose feedback in image-guided radiotherapy of prostate cancer. Med Phys 2011; 38: 6362–70. doi: 10.1118/1.3658567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deutschmann H, Kametriser G, Steininger P, Scherer P, Schöller H, Gaisberger C, et al. First clinical release of an online, adaptive, aperture-based image-guided radiotherapy strategy in intensity-modulated radiotherapy to correct for inter- and intrafractional rotations of the prostate. Int J Radiat Oncol Biol Phys 2012; 83: 1624–32. doi: 10.1016/j.ijrobp.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 88.Battista JJ, Johnson C, Turnbull D, Kempe J, Bzdusek K, Van Dyk J, et al. Dosimetric and radiobiological consequences of computed tomography-guided adaptive strategies for intensity modulated radiation therapy of the prostate. Int J Radiat Oncol Biol Phys 2013; 87: 874–80. doi: 10.1016/j.ijrobp.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 89.Månsson Haskå T, Honore H, Muren LP, Høyer M, Poulsen PR. Intrafraction changes of prostate position and geometrical errors studied by continuous electronic portal imaging. Acta Oncol 2008; 47: 1351–7. doi: 10.1080/02841860802256509 [DOI] [PubMed] [Google Scholar]
- 90.Polat B, Guenther I, Wilbert J, Goebel J, Sweeney RA, Flentje M, et al. Intra-fractional uncertainties in image-guided intensity-modulated radiotherapy (IMRT) of prostate cancer. Strahlenther Onkol 2008; 184: 668–73. doi: 10.1007/s00066-008-1875-6 [DOI] [PubMed] [Google Scholar]
- 91.Kupelian P, Willoughby T, Mahadevan A, Djemil T, Weinstein G, Jani S, et al. Multi-institutional clinical experience with the calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys 2007; 67: 1088–98. doi: 10.1016/j.ijrobp.2006.10.026 [DOI] [PubMed] [Google Scholar]
- 92.Li JS, Jin L, Pollack A, Horwitz EM, Buyyounouski MK, Price RA, Jr, et al. Gains from real-time tracking of prostate motion during external beam radiation therapy. Int J Radiat Oncol Biol Phys 2009; 75: 1613–20. doi: 10.1016/j.ijrobp.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 93.Su Z, Zhang L, Murphy M, Williamson J. Analysis of prostate patient setup and tracking data: potential intervention strategies. Int J Radiat Oncol Biol Phys 2011; 81: 880–7. doi: 10.1016/j.ijrobp.2010.07.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rassiah-Szegedi P, Wang B, Szegedi M, Tward J, Zhao H, Huang YJ, et al. Individualized margins for prostate patients using a wireless localization and tracking system. J Appl Clin Med Phys 2011; 12: 3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang P, Mah D, Happersett L, Cox B, Hunt M, Mageras G. Determination of action thresholds for electromagnetic tracking system-guided hypofractionated prostate radiotherapy using volumetric modulated arc therapy. Med Phys 2011; 38: 4001–8. doi: 10.1118/1.3596776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Budiharto T, Slagmolen P, Haustermans K, Maes F, Junius S, Verstraete J, et al. Intrafractional prostate motion during online image guided intensity-modulated radiotherapy for prostate cancer. Radiother Oncol 2011; 98: 181–6. doi: 10.1016/j.radonc.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 97.Kurosawa Y, Ishikawa H, Hoshino Y, Higuchi H, Ogano T, Kawamura H, et al. Intra-fractional set-up and organ motion errors in intensity-modulated radiation therapy for prostate cancer. Nihon Hoshasen Gijutsu Gakkai Zasshi 2012; 68: 290–8. doi: 10.6009/jjrt.2012_JSRT_68.3.290 [DOI] [PubMed] [Google Scholar]
- 98.Drabik DM, MacKenzie MA, Fallone GB. Quantifying appropriate PTV setup margins: analysis of patient setup fidelity and intrafraction motion using post-treatment megavoltage computed tomography scans. Int J Radiat Oncol Biol Phys 2007; 68: 1222–8. doi: 10.1016/j.ijrobp.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 99.Kron T, Thomas J, Fox C, Thompson A, Owen R, Herschtal A, et al. Intra-fraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother Oncol 2010; 95: 191–7. doi: 10.1016/j.radonc.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 100.Iwama K, Yamazaki H, Nishimura T, Oota Y, Aibe H, Nakamura S, et al. Analysis of intrafractional organ motion for patients with prostate cancer using soft tissue matching image-guided intensity-modulated radiation therapy by helical tomotherapy. Anticancer Res 2013; 33: 5675–9. [PubMed] [Google Scholar]
- 101.Willoughby TR, Kupelian PA, Pouliot J, Shinohara K, Aubin M, Roach M, et al. Target localization and real-time tracking using the calypso 4D localization system in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2006; 65: 528–34. doi: 10.1016/j.ijrobp.2006.01.050 [DOI] [PubMed] [Google Scholar]
- 102.Li HS, Chetty IJ, Enke CA, Foster RD, Willoughby TR, Kupellian PA, et al. Dosimetric consequences of intrafraction prostate motion. Int J Radiat Oncol Biol Phys 2008; 71: 801–12. doi: 10.1016/j.ijrobp.2007.10.049 [DOI] [PubMed] [Google Scholar]
- 103.Crocker JK, Ng JA, Keall PJ, Booth JT. Measurement of patient imaging dose for real-time kilovoltage X-ray intrafraction tumour position monitoring in prostate patients. Phys Med Biol 2012; 57: 2969–80. doi: 10.1088/0031-9155/57/10/2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tree A, Jones C, Sohaib A, Khoo V, van As N. Prostate stereotactic body radiotherapy with simultaneous integrated boost: which is the best planning method? Radiat Oncol 2013; 8: 228. doi: 10.1186/1748-717X-8-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Herk M, Remeijer P, Rasch C. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 1121–35. doi: 10.1016/S0360-3016(00)00518-6 [DOI] [PubMed] [Google Scholar]
- 106.Stroom JC, Koper PC, Korevaar GA. Internal organ motion in prostate cancer patients treated in prone and supine treatment position. Radiother Oncol 1999; 51: 237–48. doi: 10.1016/S0167-8140(99)00061-4 [DOI] [PubMed] [Google Scholar]
- 107.McKenzie AL, van Herk M, Mijnheer B. The width of margins in radiotherapy treatment plans. Phys Med Biol 2000; 45: 3331–42. [DOI] [PubMed] [Google Scholar]
- 108.Witte MG, van der Geer J, Schneider C, Lebesque JV, van Herk M. The effects of target size and tissue density on the minimum margin required for random errors. Med Phys 2004; 31: 3068–7. doi: 10.1118/1.1809991 [DOI] [PubMed] [Google Scholar]
- 109.Witte MG, van der Geer J, Schneider C, Lebesque JV, Alber M, van Herk M. IMRT optimization including random and systematic geometric errors based on the expectation of TCP and NTCP. Med Phys 2007; 34: 3544–559. doi: 10.1118/1.2760027 [DOI] [PubMed] [Google Scholar]
- 110.Mzenda B, Hosseini-Ashrafi M, Gegov A, Brown DJ. A fuzzy convolution model for radiobiologically optimized radiotherapy margins. Phys Med Biol 2010; 55: 3219–35. doi: 10.1088/0031-9155/55/11/015 [DOI] [PubMed] [Google Scholar]
- 111.Herschtal A, Foroudi F, Greer PB, Eade TN, Hindson BR, Kron T. Finding the optimal statistical model to describe target motion during radiotherapy delivery—a Bayesian approach. Phys Med Biol 2012; 57: 2743–55. doi: 10.1088/0031-9155/57/9/2743 [DOI] [PubMed] [Google Scholar]
- 112.Suzuki J, Tateoka K, Shima K, Yaegashi Y, Fujimoto K, Saitoh Y, et al. Uncertainty in patient set-up margin analysis in radiation therapy. J Radiat Res 2012; 53: 615–19. doi: 10.1093/jrr/rrs003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 1121–35. doi: 10.1016/S0360-3016(00)00518-6 [DOI] [PubMed] [Google Scholar]
- 114.Bidmead M, Coffey M, Crellin A, Dobbs J, Driver D, Greener T, et al. , eds. Geometric uncertainties in radiotherapy: defining the planning target volume. London, UK: British Institute of Radiology; 2003. [Google Scholar]
- 115.Wilkinson JM. Geometric uncertainties in radiotherapy. Br J Radiol 2004; 77: 86–7. doi: 10.1259/bjr/25924254 [DOI] [PubMed] [Google Scholar]
- 116.Li JG, Xing L. Inverse planning incorporating organ motion. Med Phys 2000; 27: 1573–8. doi: 10.1118/1.599023 [DOI] [PubMed] [Google Scholar]
- 117.Bohoslavsky R, Witte MG, Janssen TM, van Herk M. Probabilistic objective functions for margin-less IMRT planning. Phys Med Biol 2013; 58: 3563–80. doi: 10.1088/0031-9155/58/11/3563 [DOI] [PubMed] [Google Scholar]
- 118.Fontanarosa D, van der Laan HP, Witte M, Shakirin G, Roelofs E, Langendijk JA, et al. An in silico comparison between margin-based and probabilistic target-planning approaches in head and neck cancer patients. Radiother Oncol 2013; 109: 404–8. doi: 10.1016/j.radonc.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 119.Gordon JJ, Siebers JV. Coverage-based treatment planning: optimizing the IMRT PTV to meet a CTV coverage criterion. Med Phys 2009; 36: 961–73. doi: 10.1118/1.3075772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moore JA, Gordon JJ, Anscher MS, Siebers JV. Comparisons of treatment optimization directly incorporating random patient setup uncertainty with a margin-based approach. Med Phys 2009; 36: 3880–90. doi: 10.1118/1.3176940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore JA, Gordon JJ, Anscher M, Silva J, Siebers JV. Comparisons of treatment optimization directly incorporating systematic patient setup uncertainty with a margin-based approach. Med Phys 2012; 39: 1102–11. doi: 10.1118/1.3679856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu H, Vile DJ, Sharma M, Gordon JJ, Siebers JV. Coverage-based treatment planning to accommodate deformable organ variations in prostate cancer treatment. Med Phys 2014; 41: 101705. doi: 10.1118/1.4894701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bohrer M, Schröder P, Welzel G, Wertz H, Lohr F, Wenz F, et al. Reduced rectal toxicity with ultrasound-based image guided radiotherapy using BAT (B-mode acquisition and targeting system) for prostate cancer. Strahlenther Onkol 2008; 184: 674–8. doi: 10.1007/s00066-008-1837-z [DOI] [PubMed] [Google Scholar]
- 124.Crehange G, Mirjolet C, Gauthier M, Martin E, Truc G, Peignaux-Casasnovas K, et al. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol 2012; 103: 244–6. doi: 10.1016/j.radonc.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 125.Patel N, Faria S, Cury F, David M, Duclos M, Shenouda G, et al. Hypofractionated radiation therapy (66 Gy 22 fractions 3 Gy per fraction) favorable-risk prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys 2013; 86: 534–9. doi: 10.1016/j.ijrobp.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 126.Sandler HM, Liu PY, Dunn RL, Khan DC, Tropper SE, Sanda MG, et al. Reduction in patient-reported acute morbidity in prostate cancer patients treated with 81-Gy Intensity-modulated radiotherapy using reduced planning target volume margins and electromagnetic tracking: assessing the impact of margin reduction study. Urology 2010; 75: 1004–8. doi: 10.1016/j.urology.2009.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sveistrup J, Rosenschöld PM, Deasy JO, Oh JH, Pommer T, Petersen PM, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol 2014; 9: 44. doi: 10.1186/1748-717X-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gadia R, Leite ET, Gabrielli FG, Marta GN, Arruda FF, Abreu CV, et al. Outcomes of high-dose intensity-modulated radiotherapy alone with 1 cm planning target volume posterior margin for localized prostate cancer. Radiat Oncol 2013; 8: 285. doi: 10.1186/1748-717X-8-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Creak A, Hall E, Horwich A, Eeles R, Khoo V, Huddart R, et al. Randomised pilot study of dose escalation using conformal radiotherapy in prostate cancer: long-term follow-up. Br J Cancer 2013; 109: 651–7. doi: 10.1038/bjc.2013.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Impact of image guidance on outcomes after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 70: 1146–50. doi: 10.1016/j.ijrobp.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 131.Perez CA, Michalski J, Ballard S, Drzymala R, Kobeissi BJ, Lockett MA, et al. Cost benefit of emerging technology in localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1997; 39: 875–83. doi: 10.1016/S0360-3016(97)00453-7 [DOI] [PubMed] [Google Scholar]
- 132.Ploquin N, Dunscombe P. A cost-outcome analysis of image-guided patient repositioning in the radiation treatment of cancer of the prostate. Radiother Oncol 2009; 93: 25–31. doi: 10.1016/j.radonc.2009.03.023 [DOI] [PubMed] [Google Scholar]
- 133.Wortel RC, Incrocci L, Pos FJ, Lebesque JV, Witte MG, van der Heide UA, et al. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys 2015; 91: 737–44. doi: 10.1016/j.ijrobp.2014.12.017 [DOI] [PubMed] [Google Scholar]