Abstract
Objective:
Single-energy metal artefact reduction (SEMAR), a new technique that can now be used in routine CT examinations, has recently become applicable to volume data acquired with electrocardiography gating. We evaluated the effect of this technique on the visualization of the coronary arteries in patients harboring cardiac devices.
Methods:
We subjected 8 patients (7 males, 1 female; mean age 65.5 ± 11.3 years) with implanted cardiac devices to coronary CT angiography on a 320-slice CT scanner (Aquilion ONE Vision™; Toshiba Medical Systems Corp., Tokyo, Japan). Image data sets were reconstructed with and without SEMAR. Two radiologists visually evaluated the image quality based on metal artefacts from the electronic device leads using a four-point scale (1 = vessel not visible to 4 = minimal or no metal artefacts). Images with a score of 3 or 4 were considered diagnostic.
Results:
In both SEMAR and non-SEMAR data sets, 94 coronary artery segments were available for evaluation. Without SEMAR, 11 segments (11.7%) were rated as non-diagnostic; SEMAR improved the image quality of 9 of the 11 segments (81.8%), and the images became diagnostic.
Conclusion:
SEMAR reduced metal artefacts from the electronic device leads and improved the image quality of the coronary arteries in patients with cardiac devices.
Advances in knowledge:
SEMAR has recently become applicable to volume data acquired with electrocardiography gating. SEMAR reduces metal artefacts elicited by electronic device leads and improves the image quality of the coronary arteries in patients with cardiac devices.
INTRODUCTION
Coronary CT angiography (CTA), a robust non-invasive imaging modality, can yield an accurate diagnosis and exclude coronary artery disease (CAD) with high diagnostic accuracy.1–3 However, in patients with pacemakers or implanted cardioverter defibrillators (ICDs), the pacemaker or ICD leads produce metal artefacts. This limits the precise assessment of the coronary arteries.4,5 The artefacts are due to scattering, X-ray beam hardening and photon starvation in the shadow of the metal object.6,7
Single-energy metal artefact reduction (SEMAR) is a new technique developed by Toshiba Medical Systems Corporation on 320-detector CT scanners (Aquilion ONE Vision™; Toshiba Medical Systems Corp., Tokyo, Japan). The algorithm consists of a raw data- and image-based technique; metal artefacts are eliminated and metal-artefact-free CT images are theoretically obtained for non-electrocardiography (ECG)-gated volume data.8 Earlier studies suggested the usefulness of this technique in patients with total hip prostheses, dental fillings or metal coils in the abdomen.9–11
SEMAR is now applicable to volume data acquired with ECG gating. To the best of our knowledge, there are no clinical studies that evaluated the effect of SEMAR on coronary CTA images. We hypothesized that SEMAR reduces metal artefacts from cardiac devices on these images and improves the visual assessment of the coronary arteries. The purpose of this study was to evaluate the effect of SEMAR on the visualization of the coronary arteries in patients with implanted cardiac devices.
METHODS AND MATERIALS
Patients
Institutional review board approval was obtained for this retrospective study; patient informed consent was waived. Using entries into our radiologic database made between November 2015 and March 2016, we retrospectively reviewed the coronary CTA images of eight consecutive patients (seven males, one female; mean age 65.5 ± 11.3 years) with implanted pacemakers (n = 5) or ICDs (n = 3) (Table 1). In six patients, CAD was suspected due to dyspnoea (n = 1), atypical chest pain (n = 3) or high cardiovascular risk (n = 2). The other two patients were scheduled for catheter ablation. Patients with a resting heart rate exceeding 65 beats per minute (bpm) received 20–40 mg of oral metoprolol (Selokeen; AstraZeneca, Zoetermeer, Netherlands) 60 min before the CT studies.
Table 1.
Patient characteristics, radiation dose and number of diagnostic segments in the single-energy metal artefact reduction (SEMAR) and non-SEMAR images
| Case | Age (years) | Sex | BMI | Cardiac device | Number of leads | DLP (mGy cm) | Effective dose (mSv) | Number of diagnostic segments |
|
|---|---|---|---|---|---|---|---|---|---|
| Non-SEMAR | SEMAR | ||||||||
| 1 | 74 | M | 22.8 | Pacemaker | 1 | 672.1 | 11.4 | 11 | 12 |
| 2 | 58 | M | 26.4 | ICD | 2 | 533.9 | 9.1 | 11 | 12 |
| 3 | 83 | F | 29.2 | Pacemaker | 2 | 226.6 | 3.9 | 11 | 12 |
| 4 | 48 | M | 28.1 | Pacemaker | 2 | 307.7 | 5.2 | 13 | 14 |
| 5 | 61 | M | 27.8 | ICD | 2 | 295.9 | 5.0 | 10 | 11 |
| 6 | 66 | M | 26.0 | ICD | 3 | 705.9 | 12.0 | 6 | 8 |
| 7 | 59 | M | 22.1 | Pacemaker | 3 | 548.0 | 9.3 | 11 | 12 |
| 8 | 75 | M | 27.9 | Pacemaker | 3 | 717.6 | 12.2 | 10 | 11 |
BMI, body mass index; DLP, dose–length product; F, female; ICD, implantable cardioverter defibrillator; M, male.
CT scanning
All CT scans were performed on a 320-detector CT scanner (Aquilion ONE Vision™) with prospective ECG triggering. Using a dual-shot injector (Nemoto Kyorindo, Tokyo, Japan), we delivered 0.6 ml kg−1 of non-ionic contrast material (Iomeprol, Iomeron 350 mgI ml−1; Eisai, Tokyo, Japan) at a fixed duration of 10 s. This was followed by 20 ml of saline solution injected at the same flow rate. The scan delay was determined with an automatic bolus-tracking system (Real Prep Technique; Toshiba). A region of interest was placed in the ascending aorta; triggering was at a threshold of 150 Hounsfield units.
The scan parameters were collimation, 320 × 0.5 mm; rotation time, 0.275 s; tube voltage, 100 kV or 120 kV; and tube current, 700–750 mA. In patients with a body mass index (BMI) <25 kg m−2, the tube voltage was 100 kVp (n = 2); in patients with a BMI >25 kg m−2, it was 120 kVp (n = 6). The phase window during which the patient was exposed was limited to 70–80% of the cardiac cycle for patients with a heart rate <65 bpm and to 40–80% of the cardiac cycle for patients with a heart rate 65–69 bpm. In patients with a heart rate >69 bpm, 2 heartbeats were scanned for CT data acquisition.
The reconstruction phase with minimum artefacts was identified on the CT console by cardiac-phase search software (Phase Navi; Toshiba). Axial images were reconstructed with a slice thickness of 0.5 mm; the reconstruction interval was 0.25 mm. Non-SEMAR images were reconstructed with the “mild” setting of hybrid iterative reconstruction (adaptive iterative dose reduction 3D; Toshiba Medical Systems) alone, that is the routine setting in our institution. SEMAR images were reconstructed with adaptive iterative dose reduction 3D plus the SEMAR technique. All images were transferred to a computer workstation (Virtual Place v. 3.3; Aze, Tokyo, Japan) for post-processing.
The dose–length product provided by the CT scanner was recorded for each patient. The effective radiation dose was calculated as the product of the dose–length product and a conversion coefficient for the chest (k = 0.017 mSv mGy−1 cm−1).12
Methods of evaluation
Coronary arteries were classified into 15 segments based on the guidelines of the American Heart Association.13 Overall image quality was assessed by two board-certified radiologists with 10 and 13 years of experience in cardiac radiology. They were blinded to the reconstruction method. If their data analysis disagreed, the final decision was reached by consensus. Using a four-point scale where 1 = poor (vessel not visible due to severe metal artefacts), 2 = fair (marked metal artefacts limiting diagnostic information), 3 = acceptable (some metal artefacts, sufficient diagnostic information), and 4 = good (minimal or no metal artefacts), the overall image quality of each coronary artery segment was rated. Images with a score of 1 or 2 were considered non-diagnostic. We compared the image quality score of the coronary arteries between SEMAR and non-SEMAR images. Interobserver agreement was assessed with the Cohen kappa κ coefficient.
RESULTS
CT scans were acquired without complications in all eight patients; their mean BMI was 26.3 ± 2.6 (range 22.1–29.2). The mean heart rate during the acquisition of CT images was 64.4 ± 5.6 bpm (range 57–70 bpm); one (n = 5) or two (n = 3) heartbeats were scanned for CT data acquisition. The mean effective radiation dose was 8.5 ± 3.4 mSv (range 3.9–12.2 mSv) (Table 1). The mean time required for reconstruction without and with SEMAR was 27.4 s (range 24–31 s) and 94.1 s (range 77–110 s), respectively.
In the SEMAR and non-SEMAR data sets a total of 94 coronary artery segments were available for evaluation. Without SEMAR, 11 (11.7%) were rated as non-diagnostic (Score 1 or 2) due to metal artefacts from the cardiac devices, 6 of these were non-diagnostic due to electrodes at the tip, and the other 5 were affected by shock coils. The affected segments were in the right coronary artery (n = 7), the distal segments of the left anterior descending artery (n = 3) or the left circumflex coronary arteries (n = 1); visualization of 9 of these 11 segments (81.8%) was improved by SEMAR and the image quality score became diagnostic (Table 1). Two of the 94 segments (2.1%) remained non-diagnostic despite the application of SEMAR. There was substantial interobserver agreement with respect to the overall image quality (κ = 0.76). A representative case is shown in Figure 1.
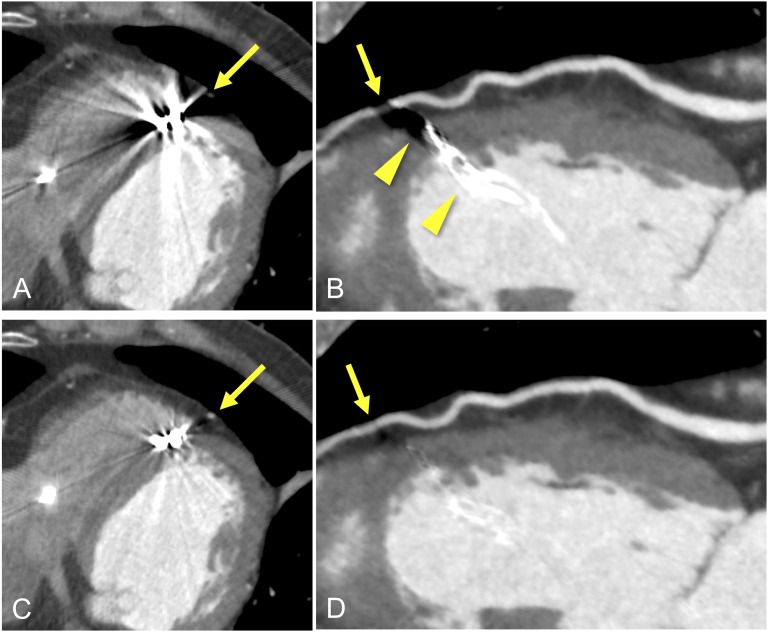
Figure 1.
Axial- and curved-planar reconstruction images of the left anterior descending coronary artery (LAD) in a 74-year-old male who underwent pacemaker implantation due to atrial fibrillation. The images in (a) and (b) were reconstructed with the conventional method. Single-energy metal artefact reduction (SEMAR) was applied in images (c) and (d). On non-SEMAR images (a, b), severe metal artefacts (b, arrowhead) from the tip of the leads affected assessment of the LAD (arrows). On SEMAR images (c, d), the metal artefacts are reduced and the visibility of the LAD is considerably improved (arrows).
DISCUSSION
This is the first study to evaluate the effect of SEMAR on the image quality of coronary CTA scans. SEMAR drastically reduced metal artefacts elicited by electronic device leads and improved the image quality of the coronary arteries in patients harbouring cardiac devices.
Pacing leads typically feature an electrode pair at the tip for sensing and pacing. ICD leads have similar pacing electrodes at the tip and shock coils that are most commonly located in the superior vena cava and the right ventricle. Despite the small size of the electrodes and shock coils, they create substantial streak artefacts on CT images.14 Metal artefacts arise from metal elements of the pacemaker or ICD leads and are a result of two processes: a beam hardening phenomenon due to the dense metallic component and the exponential edge-gradient effect due to disparity between the high-density metal and the low-density surrounding tissue.7 In addition, the extent of artefacts may also be affected by the lead position, the CT acquisition parameters and respiratory and cardiac motion. Therefore, the evaluation of coronary CTA scans of patients with cardiac devices remains challenging.
There are only a few reports that focus on CT in patients with implanted devices.4,5 Among the constituent materials, electrodes at the tip exert a destructive influence on the image quality of the right coronary artery.5,14 Also, in patients with ICDs, the shock coils produce significantly large artefacts most likely at the inferoseptal region of the left ventricle.4 Thus, in some patients harbouring cardiac devices, invasive coronary angiography should be considered after CT examination.
SEMAR is advantageous for the accurate segmentation of the metal part and for the classification of tissues in the metal-free part.8 It uses various steps for data segmentation and a feedback mode with forward and backward projections on the basis of projection and image data.11 Reconstruction applies the following steps: (1) segment the metal parts in the original filtered back projection image, and metal-only data are forward-projected to generate a sinogram of metal-only data; (2) replaced the metal data points in the sinogram with interpolated values using the neighbouring non-metal data points; (3) reconstructed the interpolated sinogram; (4) classify tissues in the metal-free component by repeated correction to remove the artefact; and (5) blend the metal-free images with the metal images to obtain the final image.
In this study, as in earlier studies, the right or distal segments of the left coronary arteries were affected by metal artefacts. ECG-gated SEMAR eliminated metal artefacts from both the tip of the electrodes and from shock coils and improved the image quality of the coronary arteries. We did not assess the diagnostic accuracy of the coronary CTA images by comparing our findings with the reference standard for invasive coronary angiography. However, we expect that the number of patients who require conventional coronary angiography after coronary CTA would be decreased by using SEMAR.
Another technique to reduce metal artefacts is monoenergetic imaging of dual-energy CT. Secchi et al15 reported the clinical usefulness of monoenergetic imaging for reducing artefacts from metal and high iodine contrast concentration. However, the optimal keV level for dual-energy metal artefact reduction protocols remains controversial. SEMAR is a single-energy-based technique not requiring an extra acquisition or radiation dose; therefore, it can be applied retrospectively to routine volume data. Also, it is reported that the tube current did not affect the effect of this technique on the visual evaluation nor the signal-to-artefact ratios.8 Thus, SEMAR would be more suitable and feasible for clinical use.
Our study has some limitations. First, the small number of patients limits the informational value of our findings. Studies on larger patient populations are under way to confirm our preliminary results. Second, the two readers were blinded to the reconstruction methods (SEMAR or non-SEMAR), but the reconstruction method used might be obvious to the readers because of the major differences of their image characteristics. Third, we did not assess the diagnostic accuracy of the coronary CTA images by comparing our findings with the reference standard for invasive coronary angiography. Further studies must evaluate whether the diagnostic accuracy and the exclusion of CAD are superior on coronary CTA images reconstructed with SEMAR.
In conclusion, SEMAR reduces metal artefacts elicited by electronic device leads and improves the image quality of the coronary arteries in patients with cardiac devices.
FUNDING
Dr Kazuo Awai is the recipient of a research grant from Toshiba Medical Systems Ltd.
Contributor Information
Fuminari Tatsugami, Email: sa104@rg8.so-net.ne.jp.
Toru Higaki, Email: higaki@hiroshima-u.ac.jp.
Hiroaki Sakane, Email: sakaneh@hiroshima-u.ac.jp.
Wataru Fukumoto, Email: wfukumoto@hiroshima-u.ac.jp.
Makoto Iida, Email: edamako@hiroshima-u.ac.jp.
Yasutaka Baba, Email: ybaba@hiroshima-u.ac.jp.
Chikako Fujioka, Email: fujioka@hiroshima-u.ac.jp.
Yasuki Kihara, Email: ykihara@hiroshima-u.ac.jp.
So Tsushima, Email: so.tsushima@glb.toshiba.co.jp.
Kazuo Awai, Email: awai@hiroshima-u.ac.jp.
REFERENCES
- 1.Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J 2005; 26: 1482–7. doi: 10.1093/eurheartj/ehi261 [DOI] [PubMed] [Google Scholar]
- 2.Nikolaou K, Knez A, Rist C, Wintersperger BJ, Leber A, Johnson T, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol 2006; 187: 111–7. doi: 10.2214/AJR.05.1697 [DOI] [PubMed] [Google Scholar]
- 3.Herzog C, Zwerner PL, Doll JR, Nielsen CD, Nguyen SA, Savino G, et al. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology 2007; 244: 112–20. doi: 10.1148/radiol.2441060332 [DOI] [PubMed] [Google Scholar]
- 4.DiFilippo FP, Brunken RC. Do implanted pacemaker leads and ICD leads cause metal-related artifact in cardiac PET/CT? J Nucl Med 2005; 46: 436–43. [PubMed] [Google Scholar]
- 5.Sosnowski M, Mlynarski R, Wlodyka A, Brzoska J, Kargul W, Tendera M. The presence of endocardial leads may limit applicability of coronary CT angiography. Scand Cardiovasc J 2010; 44: 31–6. doi: 10.3109/14017430903114453 [DOI] [PubMed] [Google Scholar]
- 6.Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics 2004; 24: 1679–91. doi: 10.1148/rg.246045065 [DOI] [PubMed] [Google Scholar]
- 7.Meyer LT, Boll DT. Novel technique for addressing streak artifact in gated dual-source MDCT angiography utilizing ECG-editing. Eur Radiol 2008; 18: 2446–8. doi: 10.1007/s00330-008-1013-y [DOI] [PubMed] [Google Scholar]
- 8.Funama Y, Taguchi K, Utsunomiya D, Oda S, Hirata K, Yuki H, et al. A newly-developed metal artifact reduction algorithm improves the visibility of oral cavity lesions on 320-MDCT volume scans. Phys Med 2015; 31: 66–71. doi: 10.1016/j.ejmp.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Gondim Teixeira PA, Meyer JB, Baumann C, Raymond A, Sirveaux F, Coudane H, et al. Total hip prosthesis CT with single-energy projection-based metallic artifact reduction: impact on the visualization of specific periprosthetic soft tissue structures. Skeletal Radiol 2014; 43: 1237–46. doi: 10.1007/s00256-014-1923-5 [DOI] [PubMed] [Google Scholar]
- 10.Hirata K, Utsunomiya D, Oda S, Kidoh M, Funama Y, Yuki H, et al. Added value of a single-energy projection-based metal-artifact reduction algorithm for the computed tomography evaluation of oral cavity cancers. Jpn J Radiol 2015; 33: 650–6. doi: 10.1007/s11604-015-0471-9 [DOI] [PubMed] [Google Scholar]
- 11.Kidoh M, Utsunomiya D, Ikeda O, Tamura Y, Oda S, Funama Y, et al. Reduction of metallic coil artefacts in computed tomography body imaging: effects of a new single-energy metal artefact reduction algorithm. Eur Radiol 2016; 26: 1378–86. doi: 10.1007/s00330-015-3950-6 [DOI] [PubMed] [Google Scholar]
- 12.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007; 116: 1290–305. doi: 10.1161/CIRCULATIONAHA.107.688101 [DOI] [PubMed] [Google Scholar]
- 13.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation 1975; 51(Suppl. 4): 5–40. doi: 10.1161/01.CIR.51.4.5 [DOI] [PubMed] [Google Scholar]
- 14.Mak GS, Truong QA. Cardiac CT imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5: 328–36. doi: 10.1007/s12410-012-9150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Secchi F, De Cecco CN, Spearman JV, Silverman JR, Ebersberger U, Sardanelli F, et al. Monoenergetic extrapolation of cardiac dual energy CT for artifact reduction. Acta Radiol 2015; 56: 413–18. doi: 10.1177/0284185114527867 [DOI] [PubMed] [Google Scholar]