Abstract
Objective:
To study the impact of brachial plexus MR neurography (MRN) in the diagnostic thinking and therapeutic management of patients with suspected plexopathy.
Methods:
MRN examinations of adult brachial plexuses over a period of 18 months were reviewed. Relevant data collection included—patient demographics, clinical history, pre-imaging diagnostic impression, pre-imaging treatment plan, post-imaging diagnosis, post-imaging treatment plan, surgical notes and electrodiagnostic (ED) results. Impact of imaging on the pre-imaging clinical diagnosis and therapeutic management were classified as no change, mild change or substantial change.
Results:
Final sample included 121 studies. The common aetiologies included inflammatory in 31 (25.6%) of 121 patients, trauma in 29 (23.9%) of 121 patients and neoplastic in 26 (21.5%) of 121 patients. ED tests were performed in 47 (38.8%) of 121 patients and these showed concordance with MRN findings in 31 (66.0%) of 47 patients. Following MRN, there was change in the pre-imaging clinical impression for 91 (75.2%) of 121 subjects, with a mild change in diagnosis in 57 (47.1%) of 121 patients and a substantial change in 34 (28.0%) of 121 patients. 19 (15.7%) of 121 patients proceeded to therapies that would not have been performed in the same manner without the information obtained from MRN.
Conclusion:
MRN of the brachial plexus significantly impacts clinical decision-making and should be routinely performed in suspected brachial plexopathy.
Advances in knowledge:
MRN significantly impacts the diagnostic thinking and therapeutic management of patients with suspected brachial plexopathy. MRN not only provides concordant information to ED tests in majority of cases, but also supplements with additional diagnostic data in patients who are ED negative.
INTRODUCTION
The brachial plexus is a complex network of nerves, which gives rise to large mixed peripheral nerves to the upper extremities.1 Brachial plexopathy can be caused by trauma, neoplasia, inflammation or autoimmune aetiologies.2 Clinically, brachial plexopathy commonly mimics the symptoms and signs of cervical spondylosis-related radiculopathy, and it is important to differentiate these pathologies as the management strategies can differ. In addition, owing to deep location of the brachial plexus and its complex architecture, the plexus lesions are difficult to diagnose, characterize and treat, often leading to inconclusive electrodiagnostic (ED) testing.3–5 Clinicians frequently face management challenges in terms of whether these patients should undergo surgery, pursue further work-up, be treated conservatively or receive treatment for diagnosis unrelated to neuropathy.5,6 Conventional MRI has been used for a long time for the evaluation of brachial plexopathy and is useful for the diagnosis of mass lesions, gross nerve root avulsions or post-radiation changes.3,6,7 However, MRI in the neck is frequently limited owing to vascular signal contamination, fat suppression inhomogeneity and resolution. MR neurography (MRN), using a combination of two-dimensional and three-dimensional imaging, is increasingly being used in the diagnosis of peripheral neuropathy for a variety of peripheral nerve disorders.8–12 It has been shown to change the decision-making and therapeutic plans of peripheral nerve surgeons, thereby leading to better patient care.13,14 Exquisite evaluation of brachial plexus and its various segments is also possible on 3.0-T scanners.10,15 A large-scale assessment of the impact of MRN in suspected but not established brachial plexopathy is lacking in literature. 1.5-T magnets are more widely available and the role of 1.5-T MRN in this domain is also not known. In this study, we retrospectively analyzed the impact of brachial plexus MRN performed on 1.5-T scanners in the clinical diagnosis and therapeutic management of a large series of consecutive patients who presented for suspected but not known brachial plexopathy.
METHODS AND MATERIALS
Institutional review board approval was obtained for this Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective study and informed consent was waived. All consecutive brachial plexus MRN examinations performed on subjects at our institution between April 2013 and October 2014 were reviewed and patient demographics were extracted (Table 1). All examinations were performed on 1.5-T MR scanners (Avanto; Siemens, Erlangen, Germany) employing a uniform imaging protocol (Table 2) incorporating a torso array coil with a neck coil for anterior coverage linked to a spine coil for posterior coverage. The picture archiving and communication system was queried for “Brachial Plexus MR Neurography” to retrieve the reports of examinations performed during the above-mentioned time period. The MRN interpretation reports were evaluated by two observers (CM and SF). All reports had been previously generated as part of clinical care by expert musculoskeletal radiologists in our institution. Respective clinical charts were reviewed to retrieve pertinent data, when available, including: clinical presentation, pre-imaging diagnostic impression, pre-imaging therapeutic treatment plan, relevant comorbidities, referrals related to post-imaging diagnosis, surgical notes and ED test results. Following the MRN interpretation, effects on the pre-imaging clinical diagnosis were classified as concordant, mild change or substantial change. Mild change was defined as a difference in disease severity unlikely to affect treatment planning. Substantial change was defined as a separate disease aetiology, actionable and previously unknown incidental findings, or a large deviation from expected degree of disease severity. Changes in management from conservative to surgical and vice versa were also classified as substantial changes. Data were recorded and analyzed in Excel® 2013 (Microsoft Inc., Seattle, WA).
Table 1.
Patient demographics
| Demographic | Number |
|---|---|
| Number of male patients | 79 |
| Number of female patients | 69 |
| Mean male age (SD) (years) | 46 (16) |
| Mean female age (SD) (years) | 48 (14) |
| Combined mean age (SD) (years) | 47 (15) |
| Age range (years) | 14–79 |
SD, standard deviation.
Table 2.
MR neurography imaging protocol
| Sequence | Slice thickness (mm) | Gap | TR(ms)/TE(ms) | Additional instructions |
|---|---|---|---|---|
| Coronal 3D STIR SPACE (TI 160 ms) | 1.2–1.5 isotropic (ISO) | 0 | 1500–2000/75–80 | Cover both sides to shoulder; make 7-mm coronal MIPs |
| Sagittal 3D T2 SPACE | 0.9 ISO | 0 | 1500–2000/110–120 | Recon 0.9 mm in axial and coronal planes |
| Coronal Fast Imaging Employing Steady-state Acquisition (FIESTA) | 0.6 ISO | 0 | Focus on spinal column | Recon in 0.6-mm sagittal and axial planes |
| Axial 2D T1 weighted | 3–4 × 0.5 × 0.6 | 10% | 700/6–9 | Cover both sides to shoulder |
| Sagittal STIR—right | 3 × 0.5 × 0.6 | 10% | 1500–2000/25–35 | Cover left paramidline to right shoulder |
| Sagittal STIR—left | 3 × 0.5 × 0.6 | 10% | 1500–2000/25–35 | Cover right paramidline to left shoulder |
| Axial DTI | 4 × 1.5 × 1.5 | 0 | 7000/90 | B-value 50 and 800; 12 directions |
2D, two dimensional; 3D, three dimensional; DTI, diffusion tensor imaging; MIP, maximum intensity projection; STIR, short tau inversion recovery; TE, echo time; TI, inversion time; TR, repetition time.
RESULTS
The picture archiving and communication system query returned a total of 148 studies, of which 121 studies were included in the study. 27/148 studies were excluded owing to reasons including: study never performed,9 research protocols,8 clinical information unavailable7 and body part labelled incorrectly.3
The mean age ± standard deviation of subjects included 46 ± 16 (14–73) years and 48 ± 14 (19–79) years for males and females, respectively. The common aetiologies included inflammatory [in 31/121 (25.6%) patients], trauma [in 29/121 (23.9%) patients] and neoplasia [in 26/121 (21.5%) patients]. The other disease processes identified included cervical spine spondylosis [in 19/121 (15.7%) cases], post-radiation changes or surgery [in 11/121 (9.1%) cases], thoracic outlet syndrome [in 6/121 (5.0%) cases] and 1 (0.8%) case each of Chiari 1 malformation, spinal cord infarction and carpal tunnel syndrome (in a subject who had a forearm MRN performed concurrently).
ED tests were performed in 47 (38.8%) of 121 cases and these were concordant with MRN findings in 31 (66.0%) of 47 cases. MRN imaging preceded the ED tests in 21 (44.7%) of 47 cases. Among the 16 (34.0%) of 47 subjects in whom there was discordance in the diagnosis between electromyography (EMG) and MRN, 12 (75%) of 16 subjects demonstrated findings not detected by EMG and 4 (25%) of 16 subjects showed no MRN correlates for the described EMG findings. The subjects with discordant and negative MRN and positive ED tests included suspected lower cervical radiculopathy in 2 (4.3%) of 47 subjects and non-specific brachial plexopathy in 2 (4.3%) of 47 subjects.
A few specific examples of discordant and positive MRN include: Patient 1: ED reported suspected neuropathy of lateral cord or median nerve and MRN reported avulsed C8 and T1 nerve roots and neuropathy of C7–T1 nerve roots, middle and lower trunks, multiple divisions, posterior and middle cords and median and ulnar nerves (Figure 1); Patient 2: ED showed only carpal tunnel syndrome, while MRN showed diffuse myelopathy from C4 down with cord oedema and diffuse bilateral brachial plexopathy (Figure 2); Patient 3: ED showed ulnar motor neuropathy, while MRN showed bilateral thoracic outlet syndrome findings with superimposed inflammatory diffuse right plexopathy (Figure 3); and finally, Patient 4: ED showed subacute right upper trunk plexopathy and no radiculopathy or focal mononeuropathy, while MRN showed severe right C5–8 plexopathy, neuroma in continuity of C7 and multiple rib metastases (Figure 4). Following MRN, there was change in the pre-imaging clinical impression for 91 (75.2%) of 121 subjects, with a mild change in diagnosis in 57 (47.1%) of 121 subjects and a substantial change in 34 (28.0%) of 121 subjects (Table A1). 19 (15.7%) of 121 subjects proceeded to therapies that would not have been performed in the same manner without the information obtained from MRN, including neurolysis and entrapment releases in 6 of 19 subjects, nerve grafting in 5 of 19 subjects, decompressive spinal surgery in 3 of 19 subjects, mass resection in 3 of 19 subjects, botox injection in 1 of 19 subjects and epidural steroid injection in 1 of 19 subjects.
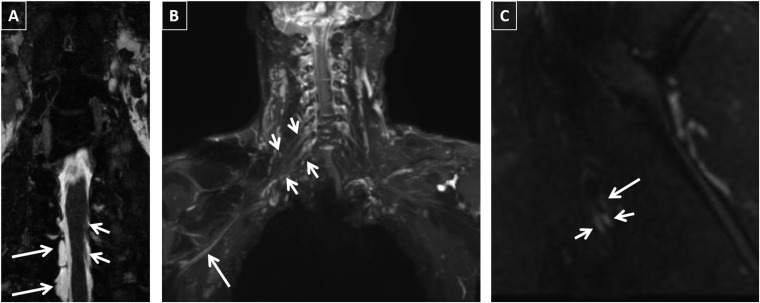
Figure 1.
A 34-year-old male with Motor Vehicle Accident (MVA): electrodiagnostic study revealed neuropathy of the lateral cord or median nerve. MR neurography images are showing multiple abnormalities. (a) Pseudomeningoceles on three-dimensional (3D) constructive interference in steady state (CISS) are seen at C8 and T1 levels on the right at the site of nerve root avulsions (long arrows). Normal nerve rootlets can be noticed on the left (small arrows). (b) Abnormal nerve root thickening and hyperintensity are demonstrated on coronal 3D short tau inversion recovery (STIR) Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) maximum intensity projections from C7 through T1 on the right (small arrows). In addition, there is abnormal signal in the median nerve more peripherally (long arrow). (c) Sagittal two-dimensional STIR image is showing bright and thickened median and ulnar nerves (small arrows), while the radial nerve is normal (long arrow).
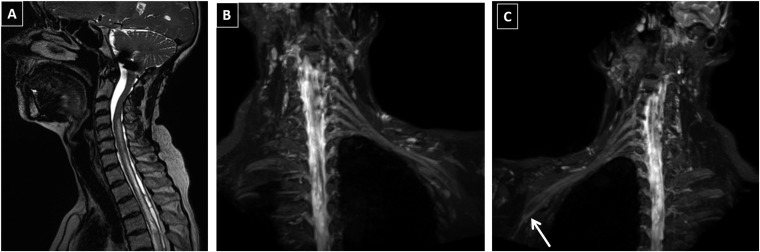
Figure 2.
A 59-year-old male with bilateral suspected brachial plexopathy: electrodiagnostic study only showed carpal tunnel syndrome and no other nerve involvement. MR neurography of the brachial plexus showed multiple findings. (a) Diffuse myelopathy and cavitation from the C4 level extending beyond the visualized upper thoracic cord. Diffuse cord oedema is present. (b) Diffusely bright and thickened left plexus with loss of normal distal fading of the nerve signal and loss of differentiation of dorsal nerve root ganglion from the distal nerves due to neuropathy. (c) Diffusely bright and thickened right plexus with distal extension of signal into the ulnar nerve (arrow).
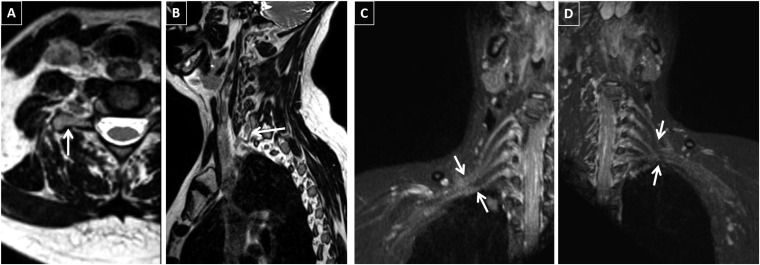
Figure 3.
A 44-year-old female with right arm weakness and pain: electrodiagnostic study suggested right ulnar motor neuropathy vs C8 neuropathy. MR neurography showed bilateral findings. (a) Elongated morphology of the right C7 transverse process (arrow). (b) Fibrous band is noted to be extending from the C7 transverse process on the right continuing on to the vertebral body (arrow), consistent with anatomic morphology of thoracic outlet syndrome. Identical findings on the left are not shown. (c) Increased thickness and signal intensity of the right C6–T1 nerve roots and more distal plexus components (arrows). Crowding of the right distal plexus can be noticed at the thoracic outlet. (d) Normal left plexus signal and only crowding of the nerve roots is seen (arrows).
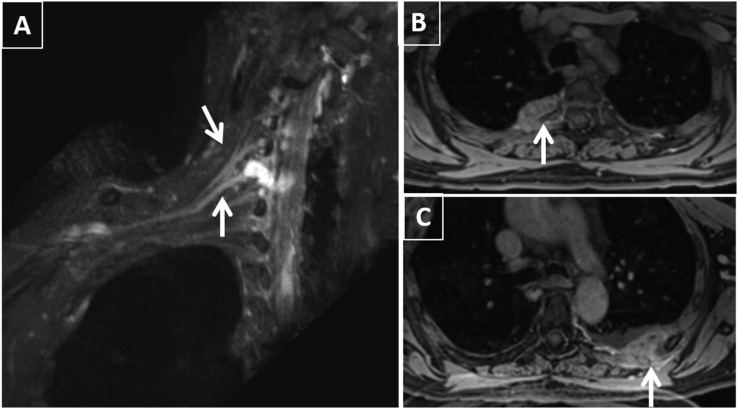
Figure 4.
A 72-year-old female with suspected right brachial plexopathy: electromyography showed subacute right upper trunk plexopathy and no radiculopathy or focal mononeuropathy. MR neurography showed the following findings: (a) right C5–C8 plexopathy with high-grade injury of the C7 nerve root (arrows) consistent with neuroma in continuity (Sunderland Class 4) and pseudomeningocele formation. (b, c) Contrast-enhanced fat-suppressed T1 weighted image is showing bilateral enhancing rib metastatic lesions (arrows).
DISCUSSION
This retrospective analysis of a large series of clinically suspected brachial plexopathy subjects in a tertiary care setting confirms the ability of brachial plexus MRN to significantly impact their diagnosis and therapeutic management. Our analysis suggests that this examination can add considerable value to the evaluation of these patients, since the clinical findings and examination are often fraught with false negatives and positives. Inclusion of cervical spine evaluation in brachial plexus protocol provides important value, as the spine, spinal cord, nerves (pre- and post-ganglionic) and adjacent muscles can be assessed in the same setting.16,17 This was seen in many cases where additional findings of radiculopathy, cord cavitation and Wallerian degeneration were observed along with peripheral neural abnormalities. Also, these results show that MRN performed on 1.5-T scanners can significantly impact patient management similar to what has been shown in the literature using 3.0-T imaging. It should be noted that it takes longer (about 50 min to 1 h) to accomplish similar imaging quality on a 1.5-T scanner as compared with 40–45 min on a 3.0-T scanner.18
Diagnostic accuracy of MRN has been widely reported in literature.8,19–21 However, studies demonstrating the impact on clinical management and patient outcomes are limited. Andreisek et al22 found moderate to major impact in the evaluation of the majority of upper extremity neuropathies (84%) with MRI. In addition, in studies where no change in diagnosis was suggested, the results were also found to be valuable. Negative examination results allow the patients to forgo further expensive work-ups, and surgery can be avoided in such cases. Recently, a prospective study was published describing the impact of MRN in a heterogeneous group of subjects suspected to have peripheral neuropathies.14 However, this brachial plexus study is the largest to date demonstrating the impact of MRN in a uniform population with suspected plexopathy. A change in preclinical impression after MRN evaluation was found in the majority of the patients, with substantial impact in 28% of patients. MRN therefore allows clinicians to make the right management decision so that the patients can receive optimal care.
The results from this study show that MRN significantly supplements and complements the results from ED tests. The overall sensitivity and specificity of ED testing (electromyography and Nerve Conduction Velocity (NCV) studies) have been debated and can be variable depending on the condition being tested. The test is particularly limited in the evaluation of deeper (plexus) nerves.23,24 In addition, lack of anatomic capability lowers its value, since it is often not possible to distinguish spine pathology from plexus pathology, as observed in many of our cases (Figures 2 and 4). MRN provides direct objective evidence and lesion localization information that is essential for pre-surgical planning. In addition, MRN altered the location and extent of nerve abnormalities in many of our cases, demonstrating its additive value to these patients compared with ED tests. Owing to the retrospective nature of this study, clinicians were not able to survey as to which study was relied upon to plan treatment when results were discordant, although this would be an interesting future study.
Our study has several limitations. First, the analysis includes the retrospective methodology and a degree of subjectivity in the categorization of mild or substantial change in diagnosis, although the definitions are provided. Second, we did not obtain future outcomes data for many of these patients, since many patients were lost to follow-up. Third, when ED testing was performed for included patients, the results were available prior to imaging almost 55% of the time, which may have influenced interpretation of the MRN examinations; however, this study was performed retrospectively with data that represent our clinical practice. Finally, owing to the retrospective nature of the examination, no interobserver performance in the reading of the MRN examinations was obtained. Our attempt was to evaluate the clinical impact of routine reads of these examinations in a tertiary care setting.
To conclude, MRN significantly impacts the diagnostic and therapeutic management of patients with brachial plexopathy.
CONFLICTS OF INTEREST
AC is a MSK CAD consultant with Siemens, Grants (Siemens, GEAUR, Integra Life Sciences), Royalties (Jaypee, Elsevier, Wolters).
Appendix A
Table A1.
Summary of the 34 patients with substantial change to clinical diagnosis based on MR neurography
| Patient number | Age (years) | Gender | Pre-imaging clinical diagnosis | Imaging findings | Treatment |
|---|---|---|---|---|---|
| 1 | 26 | M | Right upper extremity weakness after long surgery, suspect stretch injury | Stretch injury involving right upper and middle trunks with underlying neuroma suspected; multiple thoracic disc herniations; heterogeneous solid and cystic neck mass | Neck mass was biopsied and found to be papillary thyroid cancer, which was ablated and resected |
| 2 | 64 | M | Right hand weakness with rapid rise in International Normalized Ratio (INR); suspected spinal subdural bleed or thoracic outlet region haematoma | Extensive right brachial plexopathy possibly due to diabetes or Parsonage–Turner | Patient was readmitted with diabetic ketoacidosis and hepatic encephalopathy but was lost to follow-up |
| 3 | 61 | M | Right upper extremity pain status post (s/p) C3–5 Anterior cervical discectomy and fusion (ACDF); concern for surgical complication | Stretch injury of right C5–C7 nerve roots; no focal neuroma or discontinuity; incidental right labral tear and paralabral cyst noted | Pain resolved prior to discharge with conservative management |
| 4 | 44 | F | Right upper extremity pain with ulnar distribution; question prior traumatic injury to brachial plexus | Right thoracic outlet syndrome with superimposed right inflammatory plexopathy | Referred to thoracic surgery, who recommended trial of conservative management; symptoms did not improve with 2 months prothrombin time (PT) and the patient stopped going and was lost to follow-up |
| 5 | 30 | F | Shoulder pain with chronic left hand deficits; do not suspect brachial plexus inflammation | Left Parsonage–Turner | Admitted for i.v. steroids |
| 6 | 35 | F | History of neurofibromas with chronic pain in neck and back, now subjective left upper extremity weakness; “benign” physical examination | Large suprascapular nerve sheath tumour centred in scalene triangle involving the C7–T1 nerves with epidural extension and severe cord compression | Admitted for resection of the mass and hemilaminectomy from C5–T1 |
| 7 | 56 | F | Right arm sensory symptoms with right upper lobe lung cancer; concerned for brachial plexus involvement | Normal plexus | Proceeded with Right upper limb (RUL) lobectomy and chemotherapy |
| 8 | 26 | F | Suspect stretch injury with right upper extremity sensorimotor deficits | Right C5–T1 avulsions with multiple pseudomeningoceles; Wallerian degeneration at C5 level in poster column | Brachial plexus exploration with multiple neuroplasty/neurolysis; cross C7 nerve transfer to right median nerve; right spinal accessory nerve to right suprascapular nerve transfer |
| 9 | 27 | F | Torticollis with left upper extremity weakness and numbness; possible lateral and posterior cord injury | Normal plexus bilaterally; cervical spondylosis with left-sided foraminal narrowings | Referred to Physical Medicine & Rehabilitation (PM&R) |
| 10 | 43 | M | Diabetic vs metabolic or infectious neuropathy/plexopathy | Healed clavicular fracture with underlying Sunderland Grade 3 injury to posterior and medial cords; additional neuropathy of left suprascapular nerve with denervation signal in supraspinatus and infraspinatus | Referred to orthopaedics and neurology; was lost to follow-up |
| 11 | 56 | F | Motor pedestrian collision; right shoulder pain with reduced range of motion at shoulder and hand tingling; suspect brachial plexus injury | Focal full thickness supraspinatus tears; intratrabecular fracture of the right humeral head; Sunderland Grade 1–2 injury of the right plexus cords extending into peripheral branches | Referred to orthopaedics, who performed subacromial steroid and analgesic injection with continued improvement; pain and other symptoms resolved with 9 months of physical therapy |
| 12 | 31 | F | Left arm weakness, concern for metastases causing impingement; history of triple-negative Invasive ductal carcinoma (IDC) | Multilobulated nodal mass encasing entire plexus with denervation change of the left shoulder girdle; mass extends into cervical and thoracic neural foramina; vasculature encasement with occlusion of the subclavian vein; mass also involves left common carotid, left jugular vein and upper chest wall | i.v. steroids and radiation were given emergently |
| 13 | 45 | F | Cervical cancer with rib lesions and pain and sensory loss in right upper extremity; concern for nerve impingement or perineural metastases | Diffuse right inflammatory plexopathy; no evidence of mass lesions or perineural involvement; mild scapulothoracic bursitis; right rib metastasis noted without involvement of the plexus or peripheral nerves | Was treated with radiation to spine for other osseous metastases |
| 14 | 43 | F | Concern for thoracic outlet syndrome; bilateral radicular arm pain | No anatomy of thoracic outlet syndrome; bilateral brachial plexopathy, either autoimmune or inflammatory | Referred to neurology but never came to clinic appointments |
| 15 | 36 | M | Right upper extremity weakness; suspect drug-related peripheral neuropathy | Sunderland Grade 2–3 stretch injury of the right brachial plexus trunks | Referred to neurology and EMG was consistent with acute injury |
| 16 | 51 | M | Parsonage–Turner vs electrical induced neuropathy | Spinal stenosis at C5–6 with proximal nerve signal at left C5 and bilateral C6 nerves associated with severe foraminal stenosis; right rotator cuff tear with haemorrhage and reparative inflammatory tissue | Received biceps botox injection by PM&R and was lost to follow-up after discharge |
| 17 | 61 | M | Right upper extremity weakness; suspect nerve impingement or cord compression | Sequelae of prior episodes of brachial neuritis, autoimmune or inflammatory; bilateral rotator cuff tears | Referred to neurology and lost to follow-up |
| 18 | 62 | M | Left brachial plexitis with wrist drop and pain in first and second fingers | Diffuse left brachial plexus and radial nerve inflammatory neuropathy; pattern consistent with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) | Treated with Intravenous Immunoglobulin (IVIG) Every 2 weeks for 8 months without significant improvement |
| 19 | 56 | M | Lymphoedema and pain; history of lung adenocarcinoma and bone metastases | Diffuse left brachial plexitis appears inflammatory but is possibly perineural spread; enhancing retropectoral lymph nodes could represent new nodal disease | Offered palliative radiation but patient moved out of the country |
| 20 | 54 | F | Right upper lobe tumour; unclear clinically whether there is brachial plexus involvement | Right T1 and T2 nerve encasement by extrapleural spread; abuts but does not clearly involve C8 nerve, although there is evidence of C8 neuropathy | Went to surgery and had chest wall resection with multilevel intercostal nerve block and a vascularized intercostal muscle pedicle |
| 21 | 34 | M | Neck pain and right hand atrophy; history of left C7 foraminal stenosis | Symmetrical brachial plexopathy bilaterally suggesting Parsonage–Turner syndrome; evaluation for ulnar entrapment recommended | Had C7/T1 interlaminar epidural steroid injection by PM&R and then the patient moved out of state |
| 22 | 64 | M | Right upper extremity weakness proximal to ulnar distribution; suspect cervical spine or brachial plexus pathology | Reintrapment of the ulnar nerve with extensive perineural scar formation and denervation along Flexor Carpi Ulnaris (FCU) and Flexor Digitorum Profundus (FDP) muscles; multilevel spondylosis with myelomalacia at C3–C4 | Conservatively managing |
| 23 | 49 | F | Methicillin Resistant Staphylococcus Aureus (MRSA) bacteraemia with left hand weakness, right shoulder pain and right hand weakness and numbness; concern for epidural abscess | Multilevel lower cervical infectious spondylitis with surrounding myositis but no abscess; right worse than left inflammatory brachial plexitis | i.v. course of vancomycin was prolonged to 6 weeks |
| 24 | 68 | M | Lymphoedema; left arm pain and history of metastatic melanoma | Normal plexus with cervical spondylosis. Axillary masses noted suspicious for nodal metastases with chronic venous thrombosis of the subclavian and axillary veins on the left | Went for repeat lymph node dissection avoiding brachial plexus exploration |
| 25 | 66 | M | Myofascial pain along the left anterior deltoid muscle | Severe C6–7 bilateral neuroforaminal stenosis with bilateral C7 nerve radicular signal abnormalities | Lost to follow-up |
| 26 | 33 | F | Supraclavicular shoulder pain; aetiology unclear | Suggestive of right thoracic outlet syndrome along the lower aspect of the scalene triangle with prominence of the lower trunk; long neck morphology | Referred to PM&R and no follow-up has occurred |
| 27 | 73 | M | Right brachial plexopathy with progressive right upper extremity weakness; history of thyroid cancer | Severe plexopathy from C5–C8 with high-grade injury of C7 with focal truncation of the nerve root and a neuroma in continuity (Sunderland Grade 4); multiple erosive masses noted in the upper ribs suspect metastases | Conservative management |
| 28 | 45 | F | Cervical spine pathology involving suprascapular nerve and axillary nerve | Normal plexus; bubbly enhancing mass below left lobe of thyroid, likely a venous malformation. | Moved her care elsewhere |
| 29 | 60 | F | Plexus involvement of possible post-viral myelitis due to numbness spells | Cavitary myelopathic changes from C3 through visualized upper thoracic regions; bilateral diffuse brachial plexopathy | Referred to neurosurgery and was decompressed for suspected intradural adhesions |
| 30 | 35 | M | Carpal tunnel syndrome symptoms after MVA with clavicle fracture | Sunderland Grade 5 injury of the lower right brachial plexus with more mild stretch injury of the upper plexus; mild ascending Wallerian degeneration at T1 level | Referred to surgery and had distal clavicular and neuroma excisions |
| 31 | 76 | F | Cervical spondylosis with right shoulder bursitis/impingement | Right brachial plexopathy consistent with Parsonage–Turner syndrome | Continued receiving subacromial injections |
| 32 | 55 | F | Brachial plexus neuropathy or cervical spine pathology | Normal plexus; minimal scarring in right thoracic outlet, likely due to radiation | Treated for carpal tunnel syndrome |
| 33 | 53 | F | Right C5–6 weakness and numbness; brachial plexitis not favoured | Findings consistent with Parsonage–Turner | Admitted for treatment and discharged to outpatient rehab |
| 34 | 46 | M | C5 avulsion and axillary nerve injury suspected; loss of deltoid function after motorcycle collision | Syringohydromyelia at T1–2 level; upper right brachial plexopathy with denervation changes; neuroma in continuity of axillary nerve near deltoid insertion; suspect transection of suprascapular nerve | Nerve graft planned in home city |
F, female; M, male.
Contributor Information
Stephen Fisher, Email: stephen.fisher@phhs.org.
Vibhor Wadhwa, Email: vibhorwadhwa90@gmail.com.
Christine Manthuruthil, Email: Christine.manthuruthil@utsouthwestern.edu.
Jonathan Cheng, Email: Jonathan.Cheng@utsouthwestern.edu.
Avneesh Chhabra, Email: avneesh.chhabra@utsouthwestern.edu.
REFERENCES
- 1.Leffert RD. Brachial plexus injuries. London, UK: Churchill Livingstone; 1985. [Google Scholar]
- 2.Tung TH, Mackinnon SE. Brachial plexus injuries. Clin Plast Surg 2003; 30: 269–87. doi: 10.1016/S0094-1298(02)00094-9 [DOI] [PubMed] [Google Scholar]
- 3.Blum A, Lecocq S, Louis M, Wassel J, Moisei A, Teixeira P. The nerves around the shoulder. Eur J Radiol 2013; 82: 2–16. doi: 10.1016/j.ejrad.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 4.Nardin RA, Rutkove SB, Raynor EM. Diagnostic accuracy of electrodiagnostic testing in the evaluation of weakness. Muscle Nerve 2002; 26: 201–5. doi: 10.1002/mus.10192 [DOI] [PubMed] [Google Scholar]
- 5.O'Shea K, Feinberg JH, Wolfe SW. Imaging and electrodiagnostic work-up of acute adult brachial plexus injuries. J Hand Surg Eur Vol 2011; 36: 747–59. doi: 10.1177/1753193411422313 [DOI] [PubMed] [Google Scholar]
- 6.Sakellariou VI, Badilas NK, Mazis GA, Stavropoulos NA, Kotoulas HK, Kyriakopoulos S, et al. Brachial plexus injuries in adults: evaluation and diagnostic approach. ISRN Orthop 2014; 2014: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasparotti R, Ferraresi S, Pinelli L, Crispino M, Pavia M, Bonetti M, et al. Three-dimensional MR myelography of traumatic injuries of the brachial plexus. AJNR Am J Neuroradiol 1997; 18: 1733–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Chhabra A, Chalian M, Soldatos T, Andreisek G, Faridian-Aragh N, Williams E, et al. 3-T high-resolution MR neurography of sciatic neuropathy. AJR Am J Roentgenol 2012; 198: W357–64. doi: 10.2214/AJR.11.6981 [DOI] [PubMed] [Google Scholar]
- 9.Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin 2004; 22: 643–82. doi: 10.1016/j.ncl.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol 2013; 34: 486–97. doi: 10.3174/ajnr.A3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz D, Weiler M, Pham M, Heiland S, Bendszus M, Baumer P. Diagnostic signs of motor neuropathy in MR neurography: nerve lesions and muscle denervation. Eur Radiol 2015; 25: 1497–503. doi: 10.1007/s00330-014-3498-x [DOI] [PubMed] [Google Scholar]
- 12.Behrens L, Baumer P, Veltkamp R, Meinck HM, Bendszus M, Pham M. MR neurography of acute and regenerated brachial plexus pressure palsy. J Neurol 2013; 260: 3176–7. doi: 10.1007/s00415-013-7173-y [DOI] [PubMed] [Google Scholar]
- 13.Upadhyaya V, Upadhyaya DN, Kumar A, Gujral RB. MR neurography in traumatic brachial plexopathy. Eur J Radiol 2015; 84: 927–32. doi: 10.1016/j.ejrad.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Chhabra A, Belzberg AJ, Rosson GD, Thawait GK, Chalian M, Farahani SJ, et al. Impact of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol 2016; 26: 1235–44. doi: 10.1007/s00330-015-3958-y [DOI] [PubMed] [Google Scholar]
- 15.Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M. New approaches in imaging of the brachial plexus. Eur J Radiol 2010; 74: 403–10. doi: 10.1016/j.ejrad.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 16.Chhabra A. Peripheral MR neurography: approach to interpretation. Neuroimaging Clin N Am 2014; 24: 79–89. doi: 10.1016/j.nic.2013.03.033 [DOI] [PubMed] [Google Scholar]
- 17.Filler A. Magnetic resonance neurography and diffusion tensor imaging: origins, history, and clinical impact of the first 50,000 cases with an assessment of efficacy and utility in a prospective 5000-patient study group. Neurosurgery 2009; 65(Suppl. 4): A29–43. doi: 10.1227/01.NEU.0000351279.78110.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliafico A, Succio G, Emanuele Neumaier C, Serafini G, Ghidara M, Calabrese M, et al. MR imaging of the brachial plexus: comparison between 1.5-T and 3-T MR imaging: preliminary experience. Skeletal Radiol 2011; 40: 717–24. doi: 10.1007/s00256-010-1050-x [DOI] [PubMed] [Google Scholar]
- 19.Baumer P, Kele H, Kretschmer T, Koenig R, Pedro M, Bendszus M, et al. Thoracic outlet syndrome in 3T MR neurography-fibrous bands causing discernible lesions of the lower brachial plexus. Eur Radiol 2014; 24: 756–61. doi: 10.1007/s00330-013-3060-2 [DOI] [PubMed] [Google Scholar]
- 20.Baumer P, Dombert T, Staub F, Kaestel T, Bartsch AJ, Heiland S, et al. Ulnar neuropathy at the elbow: MR neurography—nerve T2 signal increase and caliber. Radiology 2011; 260: 199–206. [DOI] [PubMed] [Google Scholar]
- 21.Baumer P, Weiler M, Ruetters M, Staub F, Dombert T, Heiland S, et al. MR neurography in ulnar neuropathy as surrogate parameter for the presence of disseminated neuropathy. PLoS One 2012; 7: e49742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreisek G, Burg D, Studer A, Weishaupt D. Upper extremity peripheral neuropathies: role and impact of MR imaging on patient management. Eur Radiol 2008; 18: 1953–61. doi: 10.1007/s00330-008-0940-y [DOI] [PubMed] [Google Scholar]
- 23.Wilbourn AJ. Thoracic outlet syndrome is overdiagnosed. Muscle Nerve 1999; 22: 130–6; discussion 6–7. doi: [DOI] [PubMed] [Google Scholar]
- 24.Roos DB. Thoracic outlet syndrome is underdiagnosed. Muscle Nerve 1999; 22: 126–9; discussion 137–8. doi: [DOI] [PubMed] [Google Scholar]