Abstract
Objective:
This meta-analysis aims to analyze the usefulness of contrast-enhanced ultrasonography (CEUS) for post-treatment responses evaluation of radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) management.
Methods:
Literature retrieval in three databases PubMed, Embase and Cochrane Library was conducted up to September 2015, with pre-defined criteria. The technical success rate, local tumour recurrence and local tumour progression were the measurement indexes. Cochran's Q test and I2 were used for heterogeneity detection. Subgroup analyses were performed for complete ablation rate stratified by study designs, contrast agents and post-operative testing time points. Statistical analyses were conducted using Stata® 12.0 software (Stata Corporation, College Station, TX).
Results:
12 studies consisting of 772 patients were included in this study. The CEUS-evaluated success rate of RFA for HCCs was 91%. The proportion of ablative margin <5 mm was 53%. The local tumour recurrence rate and local tumour progression rate were 4% and 8%, respectively. Subgroup analysis indicated that the CEUS-assessed technical success rate with Sonazoid™ (Daiichi-Sankyo, Tokyo, Japan) as the contrast agent was higher (95%) than those with other agents [SH U 508A (Schering AG, Berlin, Germany) 86%; SonoVue (Bracco SpA, Milan, Italy) 87%]. The success rate assessed within 24 h (94%) after treatment was higher than longer time (1–3 days 86%; 1 month 91%).
Conclusion:
The meta-analysis showed that the CEUS-evaluated success rate of RFA for HCCs was 91%. The local tumour recurrence rate and local tumour progression rate were 4% and 8%, respectively.
Advances in knowledge:
Using meta-analysis, the study provided more reliable assessment of usefulness of CEUS, which could provide guidelines for HCC treatment.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignant tumour that seriously threatens human healthy and life, causing about 690,000 deaths per year worldwide.1,2 Surgical resection of HCC is the major treatment for HCC but with a relative high post-surgical recurrent rate.3 Recently, local ablative therapy represented by radiofrequency ablation (RFA) is gradually used in clinics as the third treatment means following surgery and transhepatic arterial chemotherapy and embolization for HCC because of its effective, minimally invasive and safe properties.4–6
Post-operative tumour residue and intrahepatic reoccurrence are the main factors affecting the curative effect of RFA.7 Scholars8,9 think that because the residual tumours are not accurately found within short term following RFA treatment, the residues progressed to local recurrence focuses. Early detection and treatment of the residue lesions can substantially raise the complete coagulated rate from 77% to 99.7% by radiofrequency coagulation.10 Thus, accurate evaluation of the post-treatment curative effect of RFA is critical to improve the complete ablation rate. Imaging methods are commonly used in the evaluation of RFA curative effect, but the conventional ultrasound is limited and contrast-enhanced CT is not suitable for repeated check during short time. Contrast-enhanced ultrasonography (CEUS) due to its advantages of moderate price and ability to repeated assessment and high spatial resolution is not only used in evaluation of the local response but also in follow-up of patients.11 A number of studies have investigated the usefulness of CEUS in evaluation of the post-surgical curative effects of RFA for HCC;11–20 however, inconsistent results have been reported.
This study therefore aimed to summarize the previously published studies on the role of CEUS in evaluation of therapeutic response of RFA for HCC and systematically analyze the effect. The technical success rate, local tumour reoccurrence and local tumour progression rate were investigated by a meta-analysis.
METHODS AND MATERIALS
Search strategy
Three bibliographic databases PubMed, Embase and Cochrane library were searched up to September 2015 for studies on the post-treatment evaluation of RFA for HCCs. The keywords were radiofrequency ablation, hepatocellular carcinoma and contrast-enhanced ultrasonography. The complete search strategy was ((radiofrequency ablation) OR RFA) AND ((liver cancer) OR (hepatocellular carcinoma) OR HCC) AND ((ultrasound contrast) OR (contrast-enhanced ultrasonography) OR CEUS).
Selection of the eligible studies
Studies conforming to the following criteria were eligible for including in the meta-analysis: (1) study on post-treatment response assessment of RFA for HCCs; (2) study with at least one of the following outcome lesion detection rate, tumour resection rate and the tumour recurrence rate; (3) study in English.
Besides, duplicates, reviews, letters, meeting abstracts, and study with data could not be extracted were excluded.
Data extraction and quality assessment of the included studies
The following data were extracted by two independent investigators (authors A and B): the first author, publication year, study design, country, included patient data, tumour type, lesion size, pre-operative imaging detection method, surgical procedure, post-operative follow-up time, post-operative radiographic detection method, contrast agent, number of cases, number of lesions, ultrasonic testing time and the test results. The quality assessment of the included studies was conducted by using Agency for Healthcare Research and Quality criterion for cross-sectional study (http://www.ncbi.nlm.nih.gov/books/NBK35156/).
Statistical analyses
The technical success/complete ablation rate, local tumour recurrence and local tumour progression were pooled. Cochran's Q test and I2 test21 were used for assessment of the heterogeneity. p < 0.05 or I2 > 50% was considered as heterogeneous, and a random effects model was used for data combination; otherwise, studies were homogeneous and a fixed effect model was utilized. Subgroup analyses for the complete ablation rate based on study design (retrospective, prospective), contrast agents [SH U 508A (Schering AG, Berlin, Germany), SonoVue (Bracco SpA, Milan, Italy), Sonazoid™ (Daiichi-Sankyo, Tokyo, Japan)] and post-operative time points of CEUS detection (<1 h, 1–24 h, 1–3 days and 1 month) were performed. Stata® 12.0 (Stata Corporation, College Station, TX) was used to perform sensitivity analysis, and this analysis was conducted by eliminating one study at a time to observe the change of the pooled estimates. Reversing results indicate unstable results, whereas non-reversing indicates robust results. All analyses were conducted using Stata 12.0 software with 0.05 as the cutoff of significant difference.
RESULTS
Study selection
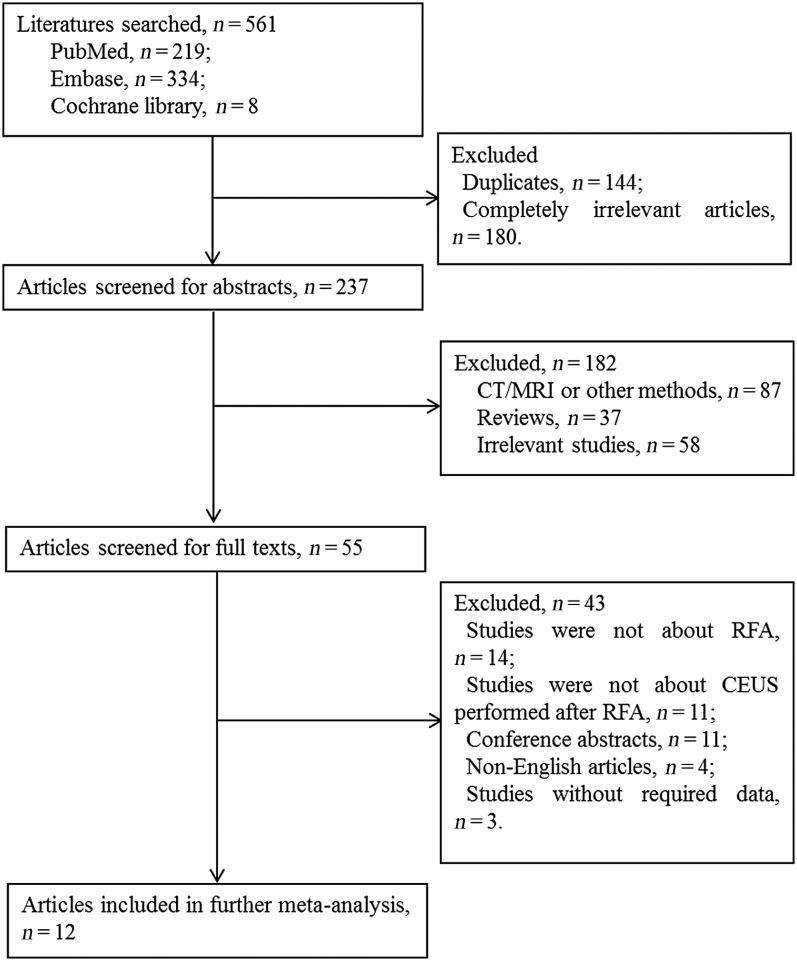
The flowchart of study selection is shown in Figure 1. The initial search obtained 561 studies (PubMed: 219; Embase: 334; Cochrane library: 8). After removing duplicates (144), 417 studies remained. By rejecting 180 obviously irrelevant studies, there were 237 studies for screening of abstracts. By eliminating 87 studies associated with CT, MRI and other methods not CEUS, 37 reviews and 58 irrelevant studies, there were 55 studies for full text reading. 11 studies of which CEUS was not the only detection method, 11 meeting abstracts, 4 non-English studies and 3 without extractable data were excluded. Finally, a total of 12 studies11–20,22,23 were enrolled in the meta-analysis.
Figure 1.
Flowchart of study selection. CEUS, contrast-enhanced ultrasonography; RFA, radiofrequency ablation.
Characteristics of the eligible studies
Table 1 shows the characteristics of the eligible studies. Among the 12 studies, there were 8 prospective studies and 4 retrospective studies. All the subjects were patients with HCC and accepted RFA for lesion resection. There were totally 772 patients with 933 lesions. All the studies reported treatment effects of RFA those were assessed by CEUS. The contrast agents included SH U 508A, SonoVue and Sonazoid. The time points for CEUS evaluation were <1 h, 1–24 h, 1–3 days and 1 month after surgery.
Table 1.
Characteristics of the included studies
| Study | Study type | Country | Patient selection period | Tumour type | Tumour size | Pre-treatment examinations | RFA technique | Evaluation of post-treatment effect of RFA |
Follow-up examination |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Contrast | Time | Number (patients/lesions) | Age (years) | Method | Time | ||||||||
| Choi et al 200312 | R | Republic of Korea | May 2000–December 2001 | Nodular HCC | 1.3–4.8 cm | Ultrasound | Real-time ultrasound-guided PRFA | CEUS | SH U 508A | <24 h (14–20 h) | 75/81 | 57 (31–72) | CT | 12–32 months |
| Dill-Macky et al 200613 | P | Canada | December 2002–February 2004 | HCC of hypervascular lesions | 1.5–3.7 cm | Multiphasic CT/dynamic MRI | Sonographic- or CT-guided RFA | CEUS | SH U 508A | <1 h (15–60 min) | 19/22 | 43–83 | Multiphasic CT, gadolinium-enhanced dynamic MRI | 3–13 months |
| Du et al 201514 | P | China | September 2011–January 2013 | HCC | 0.8–2.9 cm | Dynamic contrast-enhanced MRI | Conventional greyscale ultrasound, CEUS-guided PRFA | CEUS | SonoVue | <1 h (20–30 min) | 63/78 | 55 ± 7 (41–67) | Contrast-enhanced MRI | 11–29 months |
| Inoue et al 201315 | R | Japan | January 2007–December 2011 | HCC | 16.5 ± 7.1 mm | Dynamic CT | Ultrasound-guided PRFA | CEUS | Sonazoid™ | 1–3 days | 70/86 | 70.7 ± 7.1 | Dynamic CT | 873 ± 426 days |
| Kisaka et al 200622 | R | Japan | March 2004–August 2004 | HCC | 1.0–2.9 cm | Ultrasound | Ultrasound-guided PRFA | CEUS with VUS | SH U 508A | 3 days | 22/26 | 68.6 ± 8.08 | CECT | – |
| Kisaka et al 201016 | P | Japan | May 2005–August 2006 | HCC | 17.0 ± 6.5 mm | CT | Ultrasound-guided PRFA | CEUS with VUS | SH U 508A | 3 days | 25/25 | 67.0 ± 8.9 | Dynamic CT | 1 year |
| Luo et al 201017 | P | Japan | February 2007–November 2007 | HCC | 22 mm (10–30 mm) | 3D CECT/CEUS | Real-time ultrasound-guided PRFA | 3D CEUS | Sonazoid | 1 day | 63/63 | 70 (53–80) | 3D CECT | 3–21 months |
| Nishigaki et al 201511 | R | Japan | January 2007–June 2010 | HCC | 16.7 ± 6.1 mm | CT/MRI | CEUS-guided PRFA | CEUS | Sonazoid | 3 h | 87/87 | 71 ± 9 | 1140 days | |
| Numata et al 201223 | P | Japan | June 2009–December 2012 | HCC | 16 mm (10–30 mm) | CECT, fusion imaging combined with CEUS | Real-time ultrasound-guided RFA | Fusion imaging-CEUS | Sonazoid | 1 day | 67/80 | 73 (51–84) | CECT | 6–24 months |
| Ricci et al 200918 | P | – | January 2001–May 2004 | HCC | 3.7 ± 1.1 cm (2.6–4.8 cm) | Real time contrast-enhanced examination, helical CT | Real-time sonography-guided RFA | Low-mechanical index CEUS | SonoVue | 1 month | 100/100 | 62–76 | CT | – |
| Shimizu et al 200419 | P | Japan | October 2000–June 2001 | HCC | 10–60 mm | ADI, CECT | RFA | CEUS | SH U 508A | 1 day | 40/64 | 66.3 (50–85) | Dynamic CT | – |
| Zheng et al 201320 | P | China | May 2007–March 2011 | HCC | 2.4 cm (0.6–5.7 cm) | CECT, CEUS | Ultrasound-guided PRFA | CEUS | SonoVue | 1 month | 141/221 | 53.4 (27–81) | CECT, CEUS | 1–31 months |
3D, three-dimensional; ADI, agent detection imaging; CECT, contrast-enhanced CT; CEUS, contrast-enhanced ultrasonography; HCC, hepatocellular carcinomas; P, prospective; PRFA, percutaneous radiofrequency ablation; R, retrospective; RFA, radiofrequency ablation; VUS, virtual ultrasonography.
The quality of the included studies was relatively high because most of the studies reported the patient inclusion criteria, consecutive, the reason for exclusion and the blinding methods (Supplementary Table A).
Outcomes measures
Technical success, complete response and complete ablation
A total of 11 studies reported the complete response rate of RFA for HCCs (events: 635, total lesions: 707). Among which, two studies16,22 reported a response rate of 100%, so they were not included in this analysis. Heterogeneity was found among the remaining 9 studies (I2 = 77.3, p < 0.01), thus the random effects model was used. The pooled results indicated that CEUS evaluated success rate of RFA for HCCs was 0.91 [95% confidence interval (CI): 0.87, 0.95] (Figure 2a).
Figure 2.
Meta-analysis of the technical success rate. (a) The overall effects; (b) subgroup analysis based on the study design; (c) subgroup analysis based on the contrast agents; (d) subgroup analysis based on the time points of detection. CI, confidence interval.
Figure 2b–d shows the results of subgroup analyses. The success rate evaluated by CEUS were 0.92 (95% CI: 0.87, 0.96) and 0.90 (95% CI: 0.84, 0.96) in retrospective studies and prospective studies, respectively (Figure 2b). The success rate assessed by CEUS with Sonazoid as contrast agent (0.95, 95% CI: 0.92, 0.97) was higher than those with other contrast agents (SH U 508A 0.86, 95% CI: 0.76, 0.97; SonoVue 0.87, 95% CI: 0.68, 1.06) (Figure 2c). The success rate assessed within shorter time (<1 h 0.94, 95% CI: 0.90, 0.98; 1–24 h 0.94, 95% CI: 0.89, 0.98) after treatment showed higher success rate than longer time (1–3 days 0.86, 95% CI: 0.67, 1.05; 1 month 0.91, 95% CI: 0.87, 0.95) (Figure 2d).
Ablative margin
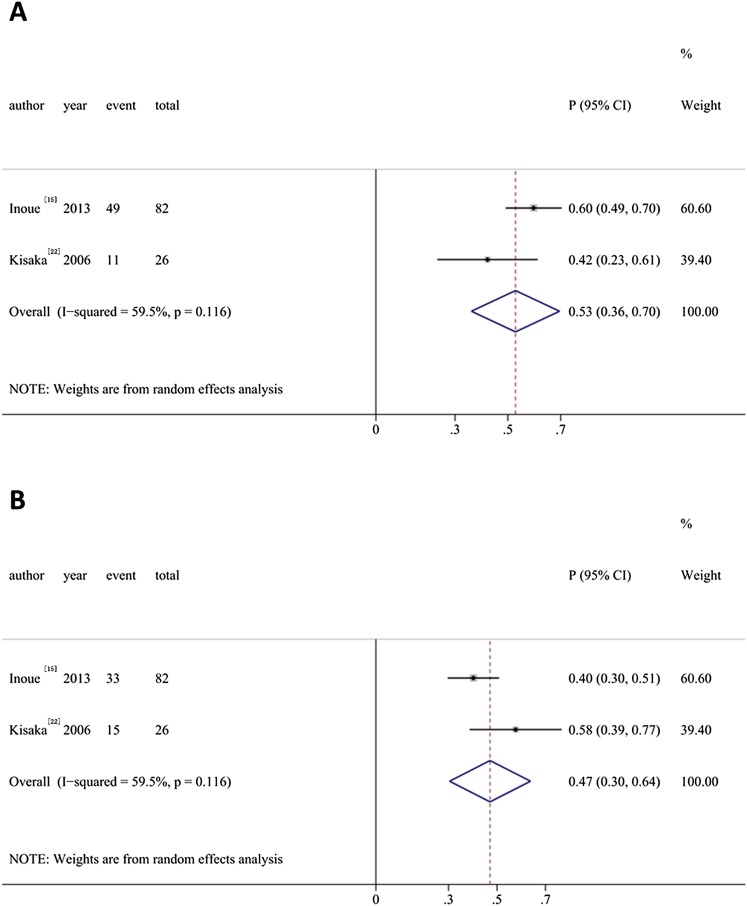
Two studies15,22 in the present study explored the success rate of CEUS for safe margin. The proportion of adequate safety margin >5 mm detected by CEUS was 0.47 (95% CI: 0.30, 0.64) while that of ablative margin <5 mm was 0.53 (95% CI: 0.36, 0.70) (Figure 3).
Figure 3.
Meta-analysis of the ablative margin. (a) Ablative margin <5 mm; (b) adequate safety margin >5 mm. CI, confidence interval.
Local tumour recurrence
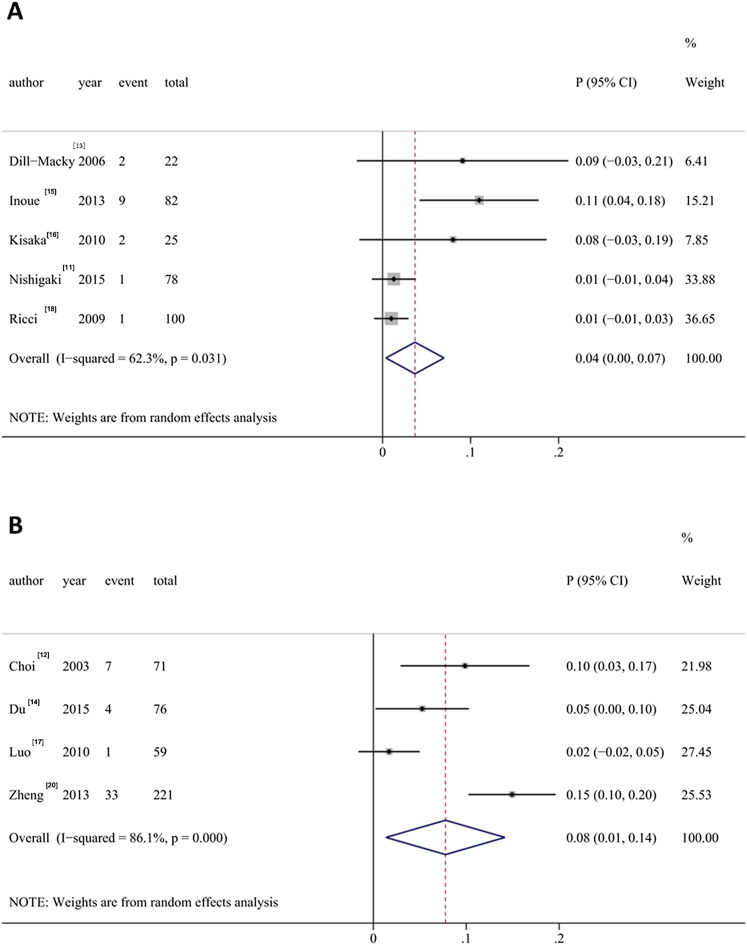
5 studies reported the local tumour recurrence of HCCs after RFA (events: 15, total lesions: 307). Significant heterogeneity was found among studies (I2 = 62.3%, p = 0.031), and the random effects model was utilized for combination of the results (Figure 4a). The pooled local tumour recurrence rate was 0.04 (95% CI: 0.00, 0.07).
Figure 4.
Meta-analysis of the local tumour recurrence (a) and local tumour progression (b). CI, confidence interval.
Local tumour progression
4 studies reported the local tumour progression of HCCs after RFA (events: 45, total lesions: 427). Significant heterogeneity was found among studies (I2 = 86.1, p < 0.01), and the random effects model was utilized for combination of the results (Figure 4b). The pooled local tumour recurrence rate was 0.08 (95% CI: 0.01, 0.14).
Sensitivity analysis
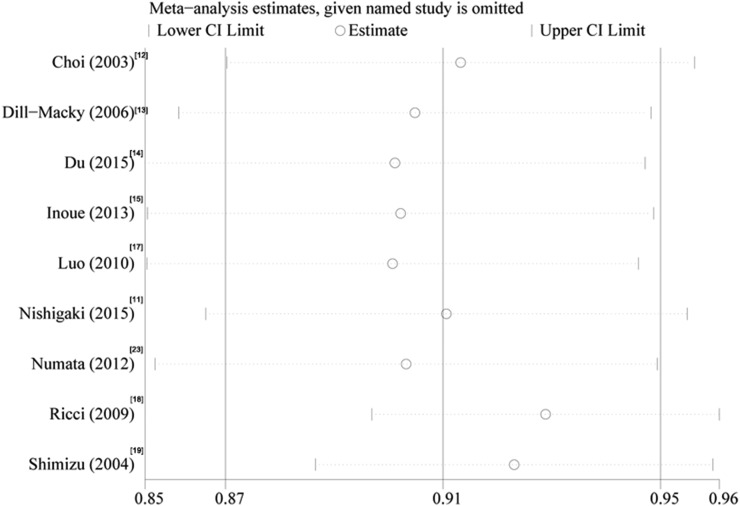
Sensitivity analysis showed that there was no reversing in result by eliminating any of the included studies, indicating the results of the present study was robust (Figure 5).
Figure 5.
Sensitivity analysis. CI, confidence interval.
DISCUSSION
CEUS has been used in assessment of post-treatment effects of RFA for HCC. Many studies have reported the results of CEUS used for the assessment of post-treatment effects of RFA for HCC, but there are inconsistencies in results and slight differences in methods. This study explored the use of CEUS for post-treatment responses evaluation of RFA for HCC by a meta-analysis of 12 studies including 772 patients with 933 lesions. Subgroup analyses based on different contrast agents and time points of test were conducted. The results indicated that the CEUS-evaluated success rate of RFA for HCCs was 91%, and it was higher (95%) with Sonazoid as the contrast agent than with the other agents. We also found that early detection within 24 h following RFA resulted in a higher success rate.
CEUS showed a comparable competence to other imaging methods in assessment of the treatment effect of RFA in HCCs. Ricci et al18 reported a CEUS accuracy of 92.3% compared with four-row spiral CT. Shimizu et al19 indicated that CEUS with agent detection imaging had similar competence to dynamic CT in assessment of the post-treatment response of RFA for HCC. The sensitivity of CEUS is >90% for detecting adequate ablation when compared with other approaches such as three-dimensional CT scans (97%)17 and contrast-enhanced CT (91.6%).22 The combined results from our study showed a 91% complete ablation rate, supporting the opinion that CEUS can be a substitute of CT scans in evaluation of the RFA effectiveness for HCCs.
This study concerned on the assessment of safe margin of RFA by CEUS. Generally, ablative margin width >5 mm after RFA was considered as safe “disease-free margin”.24 A sufficient safe margin can greatly decrease the local tumour recurrence rate, whereas with the ablative margin width of <5 mm, secondary RFA should be performed to ensure complete ablation.22 Among the included studies, only two studies15,22 have reported the complete ablated rate based on safe margin width. The results of our study indicated that the proportion of ablative margin width <5 mm was 53%, which might be useful for the judgment of the following treatment effect for patients with HCC. More attention should be drawn on the detection of safe margin in future studies.
The use of different contrast agents affects the evaluation effects of CEUS in RFA for HCC. Among the included studies in this meta-analysis, SH U 508A,12,13,18 SonoVue14,19 and Sonazoid11,15,17,23 were used in as the contrast agents. SH U 508A is the first agent approved by Europe and Canada for radiology application.25 However, Levovist (SH U 508A) has been withdrawn from the market after having been used for almost 10 years,26 and thus contrast specific modes were much less effective in Levovist times. SonoVue and Sonazoid (also NC100100) are transpulmonary vascular agents with half-life >5 min after an intravenous bolus injection.25,27 The results of the present study showed that Sonazoid had higher performance and lower heterogeneity among studies than other agents. From Figure 2c, we can also find that Sonazoid is more frequently used than other agents in recent years. The possible reasons for this situation are that Sonazoid permits real-time and precise observation of the hepatic hemodynamics during a long period,11 and Sonazoid has more stable Kupffer phase image than SH U 508A.15 However, as regards comparison between Sonazoid and SH U 508A, a bias may arise from evolution of ultrasonography equipment.
There was inconsistency among studies in the time point for detection, which also influences the evaluation results. We performed subgroup analysis to explore the suitable testing time point. The results showed that early detection by CEUS had a higher complete ablation rate (94%) in evaluation of RFA for HCC, and there was no heterogeneity among studies with time point <1 h and 1–24 h. Thus, we recommended early detection within 24 h after RFA by CEUS with Sonazoid as the contrast agent. However, the comparison of the efficiency of CEUS at different time points by well-designed study is needed to confirm the observation.
There are some concerns which should be taken into account. There was high heterogeneity among studies which might be caused by the different study design, contrast agents and time point of test. Although subgroup analyses for study design, contrast agents and time point of test were conducted, heterogeneities still existed among the analysis of perspective studies, SH U 508A, SonoVue and test point of 1–3 days. Other confounding factors such as population, age and slight modification of RFA or CEUS may also affect the results of the meta-analysis. Another concern is the small sample size. Despite the systematic analysis, there were only 772 patients with 933 lesions included. The small sample size may have influence in the statistical power. Thus, future studies with large sample size, well design by taking in account the agents, detection time and other factors, are necessary to warrant the findings in this study.
CONCLUSION
In conclusion, this meta-analysis showed that the CEUS-evaluated success rate of RFA for HCCs was 91%, and it was higher (95%) with Sonazoid as the contrast agent. The local tumour recurrence rate and local tumour progression rate were 4% and 8%, respectively. Early detection within 24 h after RFA is recommended.
FUNDING
Project supported by the social development fund of Nantong (contract grant number: HS2014063); project supported by the 12th Talent Summit of Top Six Industries in Jiangsu Province (contract grant number: WSW080).
Contributor Information
Weixiang Shi, Email: 714839331@qq.com.
Ying He, Email: Heying168@sina.com.
Wenbin Ding, Email: drdwb@126.com.
Shenchu Gong, Email: gongshenchu@msn.com.
Yilang Wang, Email: oncowang@163.com.
Jing Xiao, Email: jxiaoyz@163.com.
Bosheng He, Email: boshenghebbb@hotmail.com.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol 2009; 44(Suppl. 19): 96–101. doi: 10.1007/s00535-008-2258-6 [DOI] [PubMed] [Google Scholar]
- 3.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356: 802–7. doi: 10.1016/S0140-6736(00)02654-4 [DOI] [PubMed] [Google Scholar]
- 4.Boyvat F. Local ablation for hepatocellular carcinoma. Exp Clin Transplant 2014; 12(Suppl. 1): 55–9. doi: 10.6002/ect.25Liver.L52 [DOI] [PubMed] [Google Scholar]
- 5.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized Trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243: 321–8. doi: 10.1097/01.sla.0000201480.65519.b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laspas F, Sotiropoulou E, Mylona S, Manataki A, Tsagouli P, Tsangaridou I, et al. Computed tomography-guided radiofrequency ablation of hepatocellular carcinoma: treatment efficacy and complications. J Gastrointestin Liver Dis 2009; 18: 323–8. [PubMed] [Google Scholar]
- 7.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 2006; 59: 432–41. doi: 10.1016/j.ejrad.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007; 188: 480–8. doi: 10.2214/AJR.05.2079 [DOI] [PubMed] [Google Scholar]
- 9.Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology 2001; 221: 447–54. doi: 10.1148/radiol.2212010446 [DOI] [PubMed] [Google Scholar]
- 10.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005; 242: 158–71. doi: 10.1097/01.sla.0000171032.99149.fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishigaki Y, Hayashi H, Tomita E, Suzuki Y, Watanabe N, Watanabe S, et al. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res 2015; 45: 432–40. doi: 10.1111/hepr.12370 [DOI] [PubMed] [Google Scholar]
- 12.Choi D, Lim HK, Lee WJ, Kim SH, Kim YH, Lim JH. Early assessment of the therapeutic response to radio frequency ablation for hepatocellular carcinoma: utility of gray scale harmonic ultrasonography with a microbubble contrast agent. J Ultrasound Med 2003; 22: 1163–72. [DOI] [PubMed] [Google Scholar]
- 13.Dill-Macky MJ, Asch M, Burns P, Wilson S. Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol 2006; 186(Suppl. 5): S287–95. doi: 10.2214/AJR.04.1916 [DOI] [PubMed] [Google Scholar]
- 14.Du J, Li HL, Zhai B, Chang S, Li FH. Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol 2015; 41: 2400–11. doi: 10.1016/j.ultrasmedbio.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Kudo M, Hatanaka K, Arizumi T, Takita M, Kitai S, et al. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology 2013; 84(Suppl. 1): 51–7. doi: 10.1159/000345890 [DOI] [PubMed] [Google Scholar]
- 16.Kisaka Y, Hirooka M, Koizumi Y, Abe M, Matsuura B, Hiasa Y, et al. Contrast-enhanced sonography with abdominal virtual sonography in monitoring radiofrequency ablation of hepatocellular carcinoma. J Clin Ultrasound 2010; 38: 138–44. doi: 10.1002/jcu.20654 [DOI] [PubMed] [Google Scholar]
- 17.Luo W, Numata K, Morimoto M, Oshima T, Ueda M, Okada M, et al. Role of Sonazoid-enhanced three-dimensional ultrasonography in the evaluation of percutaneous radiofrequency ablation of hepatocellular carcinoma. Eur J Radiol 2010; 75: 91–7. doi: 10.1016/j.ejrad.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 18.Ricci P, Cantisani V, Drudi F, Pagliara E, Bezzi M, Meloni F, et al. Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall Med 2009; 30: 252–8. doi: 10.1055/s-2008-1027727 [DOI] [PubMed] [Google Scholar]
- 19.Shimizu M, Iijima H, Horibe T, Yamada M, Suzuki S, Yanagisawa K, et al. Usefulness of contrast-enhanced ultrasonography with a new contrast mode, Agent Detection Imaging, in evaluating therapeutic response in hepatocellular carcinoma treated with radio-frequency ablation therapy. Hepatol Res 2004; 29: 235–42. doi: 10.1016/j.hepres.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 20.Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, et al. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol 2013; 19: 855–65. doi: 10.3748/wjg.v19.i6.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisaka Y, Hirooka M, Kumagi T, Uehara T, Hiasa Y, Kumano S, et al. Usefulness of contrast-enhanced ultrasonography with abdominal virtual ultrasonography in assessing therapeutic response in hepatocellular carcinoma treated with radiofrequency ablation. Liver Int 2006; 26: 1241–7. doi: 10.1111/j.1478-3231.2006.01367.x [DOI] [PubMed] [Google Scholar]
- 23.Numata K, Fukuda H, Morimoto M, Kondo M, Nozaki A, Oshima T, et al. Use of fusion imaging combining contrast-enhanced ultrasonography with a perflubutane-based contrast agent and contrast-enhanced computed tomography for the evaluation of percutaneous radiofrequency ablation of hypervascular hepatocellular carcinoma. Eur J Radiol 2012; 81: 2746–53. doi: 10.1016/j.ejrad.2011.11.052 [DOI] [PubMed] [Google Scholar]
- 24.Bartolotta TV, Taibbi A, Midiri M, De Maria M. Hepatocellular cancer response to radiofrequency tumor ablation: contrast-enhanced ultrasound. Abdom Imaging 2008; 33: 501–11. doi: 10.1007/s00261-007-9294-1 [DOI] [PubMed] [Google Scholar]
- 25.Correas JM, Bridal L, Lesavre A, Méjean A, Claudon M, Hélénon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol 2001; 11: 1316–28. doi: 10.1007/s003300100940 [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol 2014; 44: 719–28. doi: 10.1007/s00247-013-2832-9 [DOI] [PubMed] [Google Scholar]
- 27.Marelli C. Preliminary experience with NC100100, a new ultrasound contrast agent for intravenous injection. Eur Radiol 1999; 9(Suppl. 3): S343–S46. doi: 10.1007/PL00014070 [DOI] [PubMed] [Google Scholar]