Abstract
Liver transplantation (LT) represents the best treatment for end-stage chronic liver disease, acute liver failure and early stages of hepatocellular carcinoma. Radiologists should be aware of surgical techniques to distinguish a normal appearance from pathological findings. Imaging modalities, such as ultrasound, CT and MR, provide for rapid and reliable detection of vascular and biliary complications after LT. The role of imaging in the evaluation of rejection and primary graft dysfunction is less defined. This article illustrates the main surgical anastomoses during LT, the normal appearance and complications of the liver parenchyma and vascular and biliary structures.
INTRODUCTION
Liver transplantation (LT) has become an accepted therapy for acute and chronic end-stage liver diseases and the early stages of hepatocellular carcinoma (HCC).1 Although initial efforts were unsuccessful, after several years of improvements in surgical techniques and the introduction of new immunosuppressive agents, LT currently has a 5-year survival rate of approximately 75%.2–4 Deceased donor LT is the typical surgical technique adopted; however, owing to a lack of appropriately sized donors and the high mortality rate among children on the waiting list, split LT and, subsequently, living donor LT (LDLT) have been introduced since the late 1980s.
Although adult-to-adult LDLT remains the first choice among transplantation procedures in most Asian countries due to a lack of deceased donors in these areas, LDLT is less commonly undertaken in Western countries because of the greater availability of deceased donors. This fact is especially true for the UK because of a recent increase in the deceased donor pool. Despite the progressive refinements in surgical techniques and immunological therapies, complications after LT still significantly contribute to the morbidity and mortality of the patients. Post-operative imaging surveillance is important for reducing the impact of complications and increasing graft and patient survival.
IMAGING MODALITIES
The role of imaging after LT has focused on the identification and differentiation of vascular and biliary complications, and imaging shows typical findings, including rejection and graft dysfunction. The main imaging tools are ultrasound, CT and MR.
Ultrasound
Ultrasound is the primary imaging modality in the detection and follow-up of early and delayed complications of LT. First, it can be easily performed at the bedside in the intensive care unit during the early post-transplantation phase; it is accessible and non-invasive; and it avoids the use of ionizing radiation. If performed by expert operators, the results are highly reliable.5 Ultrasound examination requires greyscale and Doppler evaluation for the assessment of the liver parenchyma, biliary tree and vessels. On greyscale evaluation, the normal LT has a homogeneous echogenicity (Figure 1). Pulsed and colour Doppler examination is performed complementarily to evaluate vessel patency and flow spectra. The Doppler waveform of a normal hepatic artery shows rapid systolic upstroke with continuous diastolic flow. The acceleration time should be <80 ms and the resistive index (RI) should be between 0.5 and 0.7 (Figure 2a). Increased resistance with RI values >0.8 is a normal finding during the first 72 h post-operatively; the RI usually returns to the normally expected pattern in a few days. In the early post-transplantation period, an RI <0.6 is highly predictive of hepatic artery complication (sensitivity of 100% and specificity of 80%).6,7
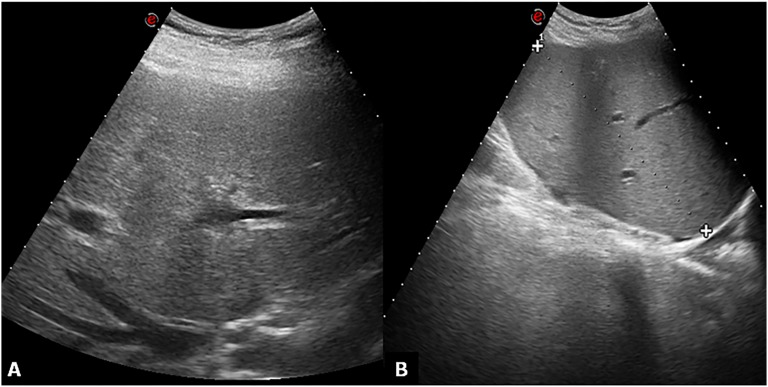
Figure 1.
Ultrasound normal appearance greyscale. (a) Subcostal oblique ultrasound image obtained through the hepatic confluence shows the right hepatic vein. The hepatic parenchyma appears homogeneous. (b) No fluid collections are found in the hepatorenal space.
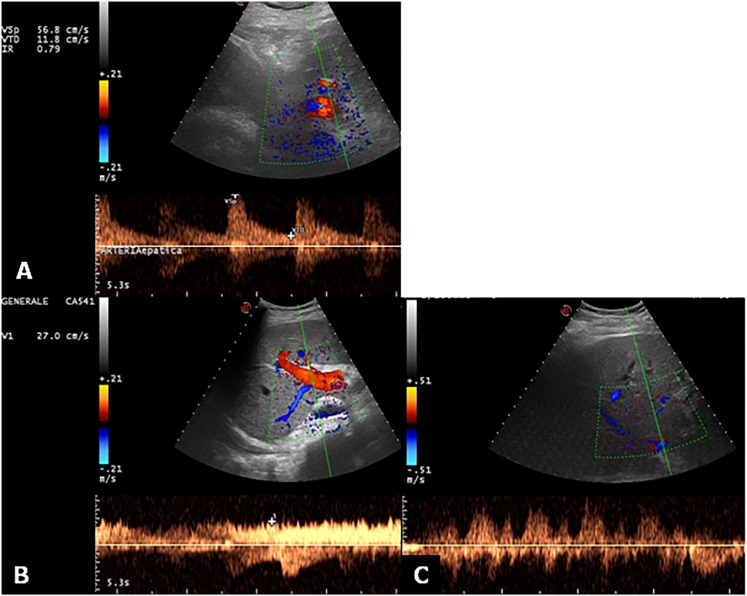
Figure 2.
Ultrasound Doppler evaluation of hepatic vessels. (a) Doppler ultrasound image and pulsed Doppler waveform of the hepatic artery in a recipient with liver transplantation. The waveform indicates a resistive index of 0.79 (normal range, 0.5–0.8). (b) The main portal vein shows a normal continuous waveform with mild velocity variations due to respiration. (c) The hepatic vein Doppler examination shows fluctuations across the baseline, which characterizes the normal triphasic pattern.
The portal vein Doppler waveform shows a continuous flow towards the liver with slight velocity variations induced by respiration. The hepatic veins and inferior vena cava (IVC) show a phasic flow pattern, reflecting the changes in the blood flow during the cardiac cycle (Figure 2b,c).8
When the interpretation of Doppler studies is inconclusive or challenging, contrast-enhanced ultrasound has been recommended for the evaluation of vascular complications after LT with sensitivity near 100% and specificity ranging from 70% to 100%.9–11
CT
CT is a second-line imaging technique that is generally used to confirm or exclude clinical suspicious and/or ultrasound findings. The introduction to clinical practice of multidetector CT (MDCT) has allowed for the acquisition of the whole volume of the abdomen, pelvis and possibly also the thorax in a few seconds with high spatial and temporal resolution, thus enabling the incorporation of both angiographic and parenchymal studies into a single acquisition. This ability is an advantage in obtaining rapid diagnosis and better image quality in critical and less co-operative patients.12 CT has shown very high sensitivity (100%), specificity (89%) and diagnostic accuracy (93%) in the evaluation of vascular complications compared with digital angiography.13,14 The typical acquisition parameters and execution protocol for liver CT are summarized in Table 1. In the first few days after LT, periportal oedema is commonly observed on CT, and it manifests as tiny narrowing fluid-attenuated signs near portal spaces due to interruption of the lymphatic system. Enlarged lymph nodes should also be noted.15
Table 1.
Multi-detector CT (64-slice) parameters and acquisition protocol for liver examination at our institution
| Parameters | Unenhanced | Late arterial phasea | Venous phase | Delayed phase |
|---|---|---|---|---|
| kVp | 120 | 120 | 120 | 120 |
| mAs | 240 | 240 | 240 | 240 |
| Scanning time (s) | 11 | 11 | 11 | 11 |
| Kernel | Soft tissue (B 20) | Soft tissue (B 20) | Soft tissue (B 20) | Soft tissue (B 20) |
| Slice thickness (mm) | 3 | 1/3 | 3 | 3 |
| Gantry revolution time (s) | 0.33 | 0.33 | 0.33 | 0.33 |
The scan delay before initiation of hepatic arterial phase imaging was determined by means of bolus tracking with automated scan triggering (CARE Bolus; Siemens Solutions, Erlangen, Germany). Arterial phase scanning begins automatically 18 s after a trigger threshold of 150 HU was reached in the supracoeliac abdominal aorta.
MR
MR, performed with high field magnets (1.5 or 3.0 T), is the preferred non-invasive modality for investigating biliary complications. The MR protocol and sequence parameters for the study of liver parenchyma after LT are detailed in Table 2. Moreover, MR cholangiography (MRC) technique is also required; it enables a detailed portrayal of the bile ducts which appear as markedly hyperintense structures. This technique is based on three-dimensional or two-dimensional heavily T2 weighted sequences using fast spin echo or single-shot fast spin echo techniques.16–18 MRC can depict the biliary system without direct contrast injection, in contrast to direct cholangiography procedures. The bile ducts are so represented in their normal state and are also visible below and above obstruction sites, regardless of the use of contrast agents.19 Alternatively, the biliary tree should be visualized by using MR hepatobiliary contrast agents (gadobenate dimegluime or gadoxetic acid).20,21 Their application is useful for demonstrating bile leakage or for evaluating bileodigestive anastomosis (AST) and bile cast syndrome.22 As previously described, periportal oedema is often observed in the immediate post-operative period. On MR images, it is seen as a periportal “collar” of low signal intensity on T1 images and high signal intensity on T2 images, respectively, and should not be interpreted as a sign of acute rejection.23
Table 2.
MR imaging and MR cholangiography parameters and acquisition protocol
| MR sequence | T2 weighted 2D TSE | T1 weighted 2D GRE | T2 weighted 3D ISO “respiratory triggered” | T2 weighted 2D SS TSE | T1 weighted 3D GREa |
|---|---|---|---|---|---|
| Fat suppression | With/without | Without | With | With | With |
| Contrast agent | Pre-contrast images assessed | Pre-contrast images assessed | Pre-contrast images assessed | Pre-contrast images assessed | Pre- and post-contrast images assessed |
| TR/TE | 4000/176 | 140/2.2–4.4 | 2500/683 | 4500/754 | 5.7/2.8 |
| Flip angle (degrees) | 150 | 90 | 140 | 180 | 10 |
| Section thickness (mm) | 5 | 5 | 1 | 50 | 2.5 |
| Matrix | 192 × 256 | 192 × 256 | 384 × 341 | 384 × 341 | 192 × 256 |
| Bandwidth (Hz/pixel) | 260 | 260 | 150 | 150 | 250 |
| Field of view (cm) | 30–50 | 30–50 | 30–50 | 30–50 | 30–50 |
2D, two-dimensional; 3D, three-dimensional; GRE, gradient recalled echo; ISO, isotropic; SS TSE, single shot turbo spin echo; TE, echo time; TR, repetition time; TSE, turbo spin echo.
T1 weighted 3D spoiled gradient recalled echo images were acquired at approximately 25, 60 and 150 s after contrast administration during the arterial, portal venous and delayed phases, respectively, and during the delayed hepatobiliary phase at approximately 20 min (gadoxetic acid ) or 75 min (gadobenate dimeglumine).
SURGICAL TECHNIQUES
Understanding surgical techniques is important for assessing recipients of LT and for finding associated complications. During LT, attention must be paid to vascular and biliary ASTs.
The most common hepatic arterial AST is end-to-end AST between the common hepatic artery of the donor and the proper hepatic artery of the recipient.15,24–26 Knowledge of this typical morphological appearance can help to prevent the erroneous diagnosis of pseudoaneurysm (Figure 3). Variants in hepatic artery ASTs encountered are the aortohepatic interposition conduit, the splenohepatic AST and the gastroduodenal–hepatic AST.15,27
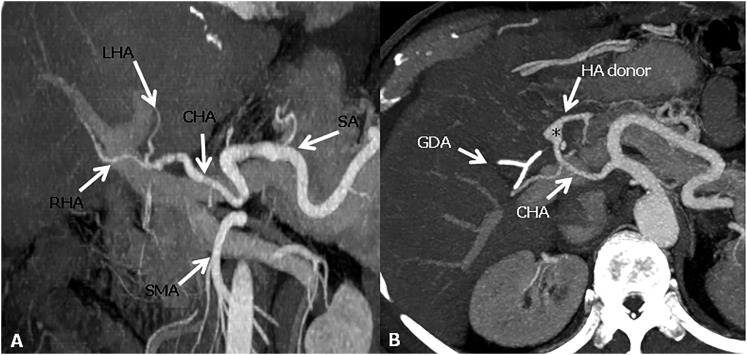
Figure 3.
Hepatic artery anastomoses. (a) The conventional “fish mouth” anastomosis between and the common hepatic artery (CHA) of the recipient and the CHA of the donor. (b) Axial maximum intensity projections image shows a pseudoaneurysm appearance (asterisk) of the anastomotic site between the gastroduodenal artery (GDA) of the recipient and the CHA of the donor. HA donor, hepatic artery donor; LHA, left hepatic artery; RHA, right hepatic artery; SA, splenic artery; SMA, superior mesenteric artery.
The portal vein AST is an end-to-end AST between the portal vein of the donor and the recipient. In cases with portal vein thrombosis (PVT), adequate portal inflow can be guaranteed using a vascular graft from the superior mesenteric vein or renal vein.15
At the beginning, for the AST of the hepatic veins, the bicaval technique was adopted, where the retrohepatic IVC of the recipient was resected, and the IVCs of the recipient and the donor were sutured with an end-to-end AST between the superior and inferior ends (Figure 4a). By now, the “piggy-back” technique is the most commonly used; it is an end-to-side AST between the donor IVC and the common stump of recipient hepatic veins. This technique has several advantages: there is no surgical dissection of the retrocaval space; normal caval flow is maintained during the anhepatic stage of surgery, thereby allowing transplantation to be performed without the need for venovenous bypass; and it reduces the overall duration of the surgery.28 The main drawback of this technique is venous outflow obstruction, which can lead to acute Budd–Chiari Syndrome: a side-to-side cavocavostomy during the piggy-back technique significantly decreased the incidence of venous outflow obstruction (Figure 4b).29
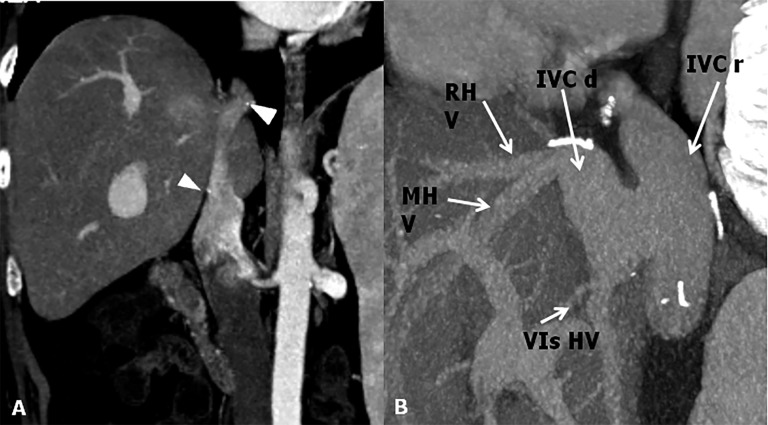
Figure 4.
Inferior vena cava (IVC) anastomoses. (a) CT post-contrast image shows end-to-end anastomosis. To note the surgical clips at the superior and inferior anastomotic sites (arrowheads). (b) Maximum intensity projections sagittal shows a cavoplasty outflow connection, where the graft of the IVC is patched directly onto an incised recipient IVC. IVC d, IVC donor; IVC r, IVC recipient; MHV, mid hepatic vein; RHV, right hepatic vein; VIs HV, hepatic vein for the sixth segment.
The most common biliary AST is an end-to-end AST between the two common bile ducts (Figure 5a). If the recipient's common bile duct is affected by primary sclerosing cholangitis, or if it is too short and too small, choledochojejunostomy is performed (Figure 5b). This technique was associated with increased complications of bacterial overgrowth, cholangitis or abscess.30
Figure 5.
Bile duct anastomoses. (a) Maximum intensity projections reconstruction of three-dimensional thin-slab fast spin-echo T2 weighted images show a regular hepaticocholedochostomy. (b) Coronal MR contrast-enhanced image obtained after intravenous administration of a hepatobiliary contrast agent (gadoxetic acid); cholangiography shows a normal hepaticojejunostomy.
COMPLICATIONS
Complications after LT should be classified into early or late (before or after 3 months) and surgical and non-surgical (Table 3).
Table 3.
Classification of complications after liver transplantation
| Complications | Surgical | Non-surgical |
|---|---|---|
| Early | Vascular complications | Acute cellular rejection |
| Biliary complications | Primary dysfunction | |
| Abscess | ||
| Haematoma | ||
| Late | Vascular complications | Chronic ductopenic rejection |
| Biliary complications | Recurrence or de novo neoplasm |
Rejection
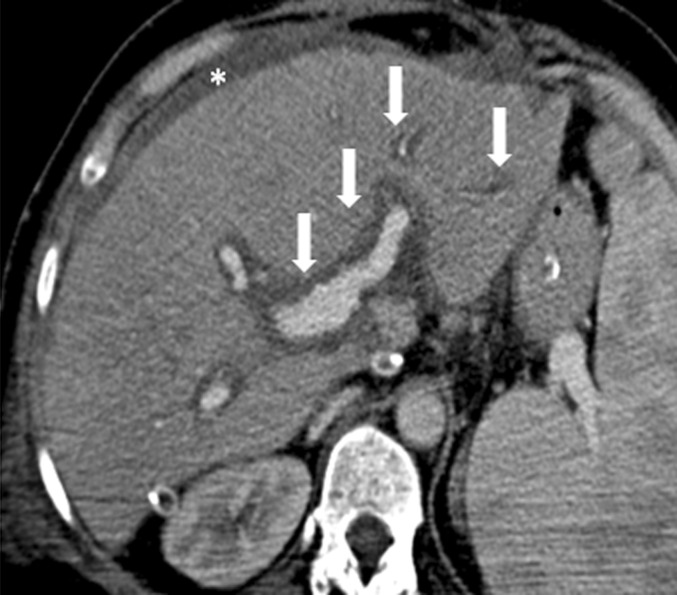
Rejection remains a common complication after LT despite improvements in immunosuppression therapy. It should be classified as acute or chronic. Acute rejection is usually of lesser significance with regard to prognosis, and it responds well to additional immunosuppression.31 Clinical and laboratory findings are non-specific and indistinguishable from those observed in other complications. The role of imaging is limited due to non-specific findings, and it consists of excluding complications with clinical signs and symptoms similar to those of rejection. On ultrasound and Doppler, acute rejection should appear as non-homogeneity of the liver parenchyma with hypoechogenicity of the periportal space due to oedema. On CT, the liver parenchyma should manifest low attenuation values in the periportal oedema space (Figure 6). On MR, the periportal oedema space appears as low signal intensity on T1 weighted images and as high signal on T2.32 Chronic rejection is caused by immunological disorders, which can lead to irreversible damage to the liver arteries, veins and bile ducts.
Figure 6.
Acute rejection. Axial contrast-enhanced CT image shows low attenuation of the liver parenchyma due to tissue patency, periportal oedema space (arrows) and ascites (asterisk).
Primary graft dysfunction
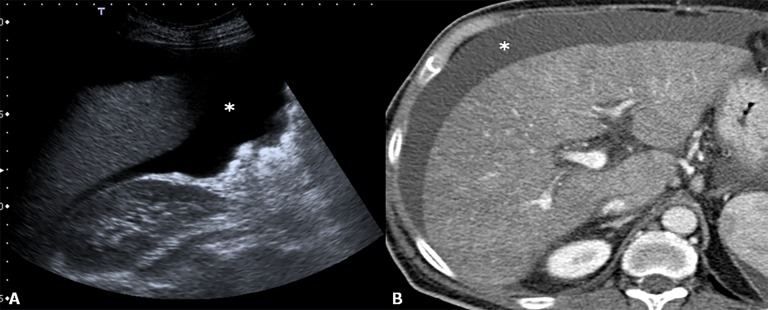
Primary graft dysfunction (PGD) is a severe complication after LT and a cause of mortality and graft loss. There is no consensus on the definition of PGD. However, it is universally accepted that PGD exhibits different degrees of severity from initial poor function (IPF) to primary non-function (PNF).33 IPF is a reversible borderline syndrome that directly influences allograft survival. PNF is immediately decreased liver function within the first 48 h leading to graft loss. The incidence of IPF ranges from 5.2% to 36.5%, whereas the incidence of PNF ranges from 0.9% to 7.2%.34 Imaging does not play a role in the diagnosis of PGD, which is achieved with laboratory chemistry (elevated serum transaminases, coagulopathy and reduced bile output, as well as metabolic acidosis, hyperkalaemia and renal failure) and liver biopsy. Non-specific findings, such as non-homogeneity of the liver parenchyma and periportal oedema space, should be noted (Figure 7). Patients with PNF need to undergo retransplantation.
Figure 7.
Primary non-function. Ultrasound (a) and CT (b); after intravenous administration of contrast agent, images revel non-homogeneity of liver parenchyma. To note the presence of ascites (asterisks) which is a sign of liver patency.
HEPATIC ARTERY COMPLICATIONS
Thrombosis
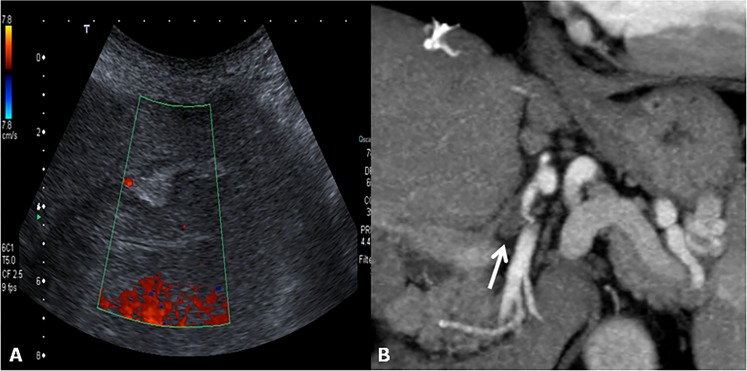
Hepatic artery thrombosis (HAT) is a common and dreaded complication after orthotopic LT (OLT), and it should be classified as early or late. The incidence of early HAT is approximately 5%, and it is a major cause of graft loss (53.1%) and mortality (33.3%) in the early post-operative period. Apart from surgical (technical) causes (kinking, stenotic AST, small donor or recipient vessels), several non-surgical causes have been described, such as acute rejection, sluggish flow through the hepatic artery, increased cold ischaemic time of the donor liver and ABO blood type incompatibility.24,35,36 Late HAT is associated with chronic rejection and sepsis. The bile ducts of a LT, unlike in a native liver, are dependent entirely on arterial blood from the hepatic artery. As a consequence, HAT has a devastating effect on the biliary epithelium, inducing ischaemia and necrosis. Initially, symptoms, signs and abnormal laboratory values are absent in early HAT; therefore, routine Doppler ultrasound screening is very important.37 On colour and pulsed Doppler, HAT manifests as the absence of flow in the proper hepatic and intrahepatic arteries (Figure 8a). Duplex Doppler imaging findings allow for a correct diagnosis in 92% of cases. Later, HAT in the arterial collateral vessels can develop, and intrahepatic flow can be identified.38 Nevertheless, the intrahepatic arterial waveform will be abnormal, displaying a tardus–parvus pattern with an acceleration time >80 ms and a RI <0.5.6 The criterion for the diagnosis of HAT is abrupt cut-off of the hepatic artery, usually at the site of the AST. CT angiography using MDCT provides for good depiction of small vessels, such as hepatic artery and its thrombosis (Figure 8b). Acute HAT can appear as high-density narrowing on unenhanced scans.39 Good correlation was found between MR angiography and conventional angiography in the detection of arterial abnormalities.40
Figure 8.
Hepatic artery thrombosis. (a) Colour Doppler image at the “porta hepatis” does not reveal blood flow within the hepatic artery. (b) The corresponding CT coronal image acquired during the arterial phase shows the thrombosis of the hepatic artery of the donor at the anastomotic site (arrow).
Stenosis
Incidence of hepatic artery stenosis is about 11% of transplantation recipients, and it occurs often at the anastomotic site.1 It usually results from clamp injury, intimal trauma caused by perfusion catheters at the time of surgery, or disrupted vasa vasorum, leading to ischaemia of the arterial ends. It can develop into biliary ischaemia, causing hepatic dysfunction.
Spectral analysis shows a velocity >3 m s−1 at the stenotic site with flow turbulence visualization distal to the stenosis.2 Intrahepatic arterial disease can display a tardus–parvus pattern with a decreased RI and prolonged acceleration time: a similar pattern should be noted in HAT with collateralization. On CT, hepatic artery stenosis is detectable as a filling defect within the hepatic artery during the arterial phase (Figure 9). Maximum intensity projection or volume rendering imaging of coronal or oblique coronal plane is essential for the depiction of focal stenosis of the hepatic artery. Treatment includes balloon angioplasty or retransplantation.
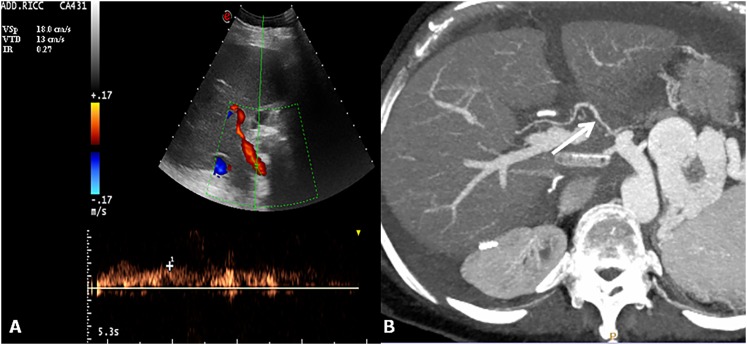
Figure 9.
Hepatic artery stenosis. Colour and spectral Doppler image of hepatic artery shows a tardus–parvus waveform with a prolonged acceleration time and decreased resistive index 0.27 (normal range, 0.5–0.8). Axial maximum intensity projection of the hepatic artery shows tiny calibre and irregular margins of the arterial vessel (arrow).
Pseudoaneurysm
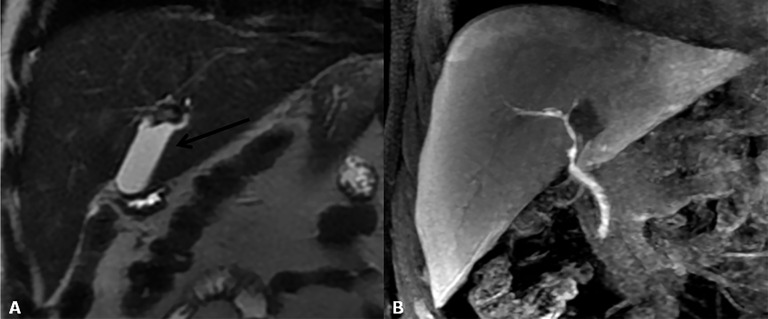
Hepatic artery pseudoaneurysm is an uncommon complication that can cause a major artery haemorrhage. It usually develops at the vascular AST as a complication of angioplasty or in an intrahepatic arterial branch as a complication of needle biopsy or local infection.25,36,41 Treatment for extrahepatic pseudoaneurysms includes surgical resection, embolization or exclusion with stent placement. Intrahepatic pseudoaneurysms can be treated with endovascular coil embolization. On ultrasound imaging, pseudoaneurysm appears as an anechoic structure near the vessel course, showing turbulent, bidirectional or even slow monophasic flow on Doppler ultrasound.25 Lesion confirmation is obtained by MDCT angiography or MR angiography, which depicts contrast distribution within the lesion similar to that of the arterial vessels (Figure 10).42
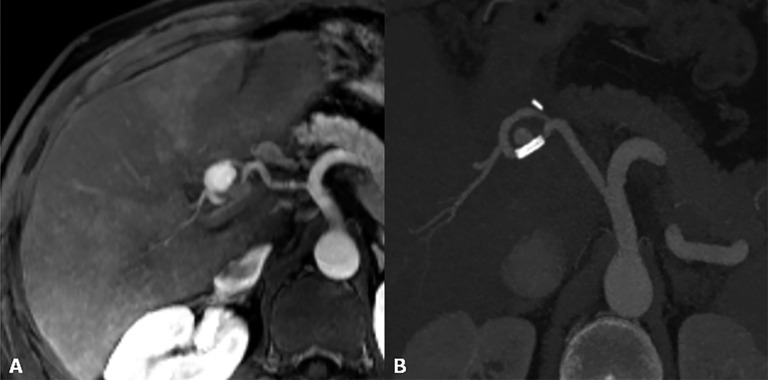
Figure 10.
Hepatic artery pseudoaneurysm. (a) MR angiography reveals a rounded lesion along the hepatic artery at the anastomotic site with homogeneous contrast enhancement. (b) After stent placement, the pseudoaneurysm is not completely excluded.
Arterial steal syndrome
Arterial steal syndrome after OLT is characterized by arterial hypoperfusion of the graft, caused by shifting of the blood flow into the splenic or gastroduodenal artery.43 It has an incidence of approximately 6% and can cause ischaemic biliary tract destruction, sepsis and graft failure. The treatment for steal syndrome is interruption of the splenic arterial flow through splenectomy, embolization or banding of the splenic artery for reduction without interrupting the blood flow.44
PORTAL VEIN COMPLICATIONS
Thrombosis and stenosis
PVT and stenosis have an incidence of approximately 1–2%.24,35 The main causes are technical problems, portal vein surgery or previous thrombosis, and hypercoagulable states.12,40,42 Clinical manifestations range from the absence of symptoms to variceal haemorrhage, ascites and graft dysfunction. The treatment for symptomatic cases is thrombolysis or surgery (thrombectomy, venous graft). When thrombosis has occurred, an echogenic filling defect can be observed in the portal vein; eventually, an acute thrombus becomes anechoic. Colour or power Doppler images and pulsed Doppler waveforms show a lack of portal venous flow.5 Partial PVT is less common and has less clinical significance than complete PVT, and it is generally incidentally found during patient follow-up. The associations among a lack of portal trunk detection, a hypertrophic hepatic artery and multiple, thin venous vessels at the porta hepatis suggest the evolution of PVT into cavernomatosis. Sometimes, the portal flow is so minimal that it is not detected on ultrasound and further evaluation is necessary. A peak anastomotic velocity >125 cm s−1 is 73% sensitive and 95% specific for the diagnosis of portal vein stenosis. An anastomotic-to-pre-anastomotic velocity ratio of 3 : 1 is 73% sensitive and 100% specific for stenosis.45 Portal vein stenosis at CT and MR manifests as an intraluminal filling defect and focal narrowing at anastomotic sites (Figure 11).
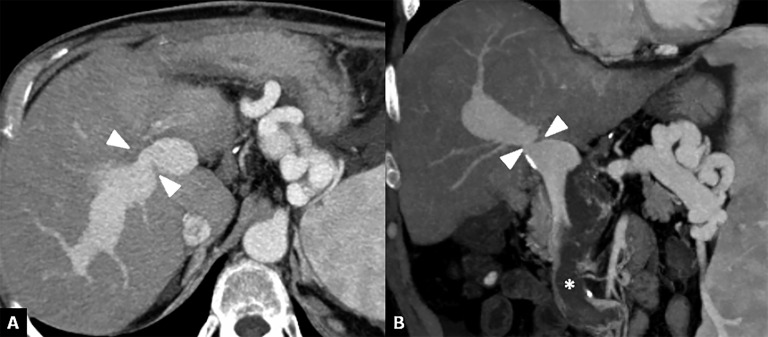
Figure 11.
Portal vein stenosis. (a, b) Axial and coronal CT images show a severe portal vein stenosis (arrowheads) at site of the anastomosis. To note the presence of thrombus within the superior mesenteric vein (asterisk).
HEPATIC VEIN COMPLICATIONS
Thrombosis and stenosis
Thrombosis and stenosis have an incidence of <1% that can reach 4% with “piggy-back” AST, and they significantly increase in frequency with LDLT.46 Generally, they occur at the anastomotic site and can be caused by surgical technique, IVC compression by the liver graft or fluid collection (Figure 12). Hepatic vein thrombosis manifests as an intraluminal filling defect and a lack of blood flow. Hepatic vein stenosis at ultrasound reveals a venous pulsatility index of <0.45 and monophasic waveforms .45,47 The treatment options for stenosis are stenting or angioplasty.
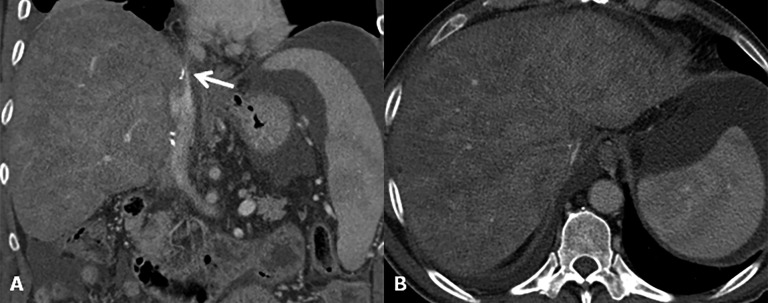
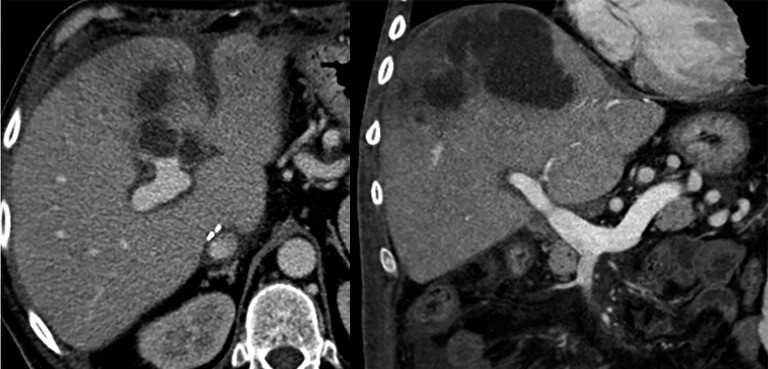
Figure 12.
Outflow obstruction. (a) Coronal CT image after contrast agent injection reveals a supraanastomotic stenosis of the inferior vena cava (arrow). To note on the axial image (b) secondary findings including hepatomegaly, ascites and signs of Budd–Chiari syndrome (liver mosaic pattern perfusion).
BILIARY COMPLICATIONS
Adverse biliary tract events are the most common complications after LT, and they represent the major source of morbidity in patients receiving LT, with an incidence of 5–32%. They include bile leaks, anastomotic and non-anastomotic strictures (NASs), biliary stones, sludge and casts, and they are more commonly discovered during the early post-operative period.48
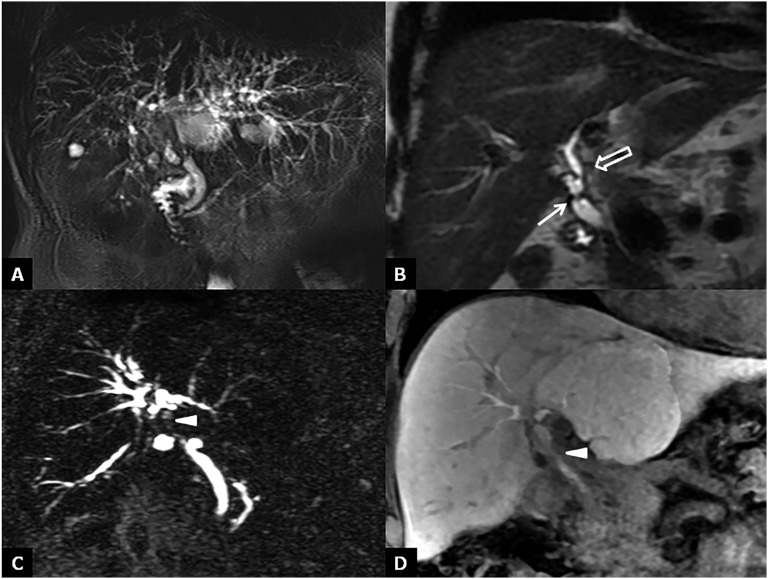
Biliary leaks
The occurrence of biliary leaks is typically in the early phase after transplantation at the anastomotic site or at the T-tube insertion site.49 The incidence of bile leaks ranges from 2% to 25% of transplanted livers, and it can be classified into two categories: early bile leaks, which present within 4 weeks of OLT, and late bile leaks, which present beyond this time.50–53 Small bile leaks tend to resolve spontaneously, and they can be monitored over time. By contrast, larger leaks translate into biloma formation, with increased risks of superinfection and sepsis. Ultrasound, CT and MRC can generally be employed to identify a biliary leak and/or bilomas.54,55 Bilomas are easily detected on ultrasound as anechoic collections in the perihepatic and subhepatic space; on CT, they appear as low-density collections, whereas on MR, they appear hypointense on T1 and hyperintense on T2 (Figure 13). However, bilomas or intraperitoneal bile is an indirect sign of leakage, and these signs are virtually indistinguishable from fluid collection and ascites. Although imaging findings provided by cross-sectional modalities might be suggestive of biliary leakage in a proper clinical setting, they are frequently non-specific, with a reported diagnostic accuracy ranging between 70% and 74%.56 Contrast-enhanced MRC with intravenous administration of hepatobiliary contrast agents can be extremely helpful in localizing bile leak, which is not generally possible on unenhanced T2 weighted MRC.57 Indeed, using contrast-enhanced MRC, we can demonstrate active biliary leakage by visualizing contrast medium extravasation into the fluid collection, so we can also localize the anatomic site of the bile leak (Figure 14). To confirm the presence of an active leak, invasive procedures, such as percutaneous transhepatic cholangiography or endoscopic retrograde cholangiopancreatography, should be finally performed to demonstrate contrast agent extravasation from the biliary system.
Figure 13.
Biloma. (a) Coronal T2 weighted image shows a fluid collection at the “porta hepatis”. (b) Contrast-enhanced MR cholangiography demonstrates the absence of connection between the bile ducts and biloma.
Figure 14.
Bile leakage. (a–c) Contrast-enhanced MR cholangiography reveals (at different levels) a biliary leakage along the surgical cut in split liver transplantation. To note the extravasation of contrast material into the fluid collection (arrows in a, b) before it has opacified the bile ducts (open arrowhead in c).
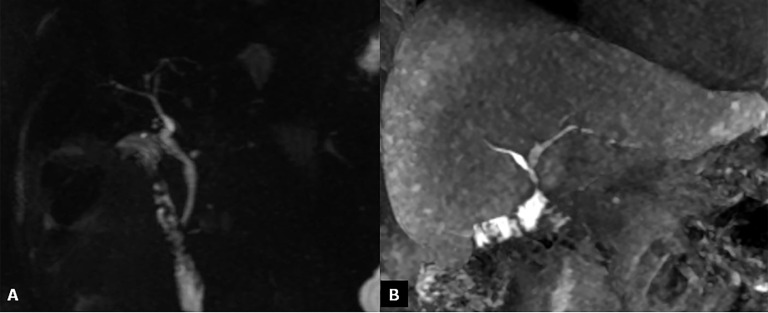
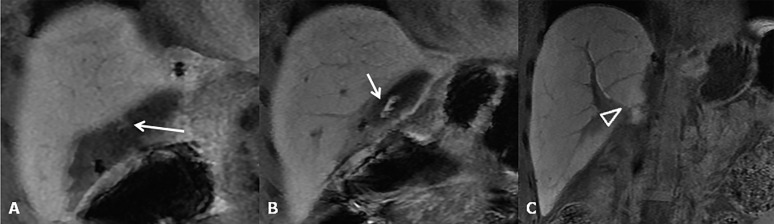
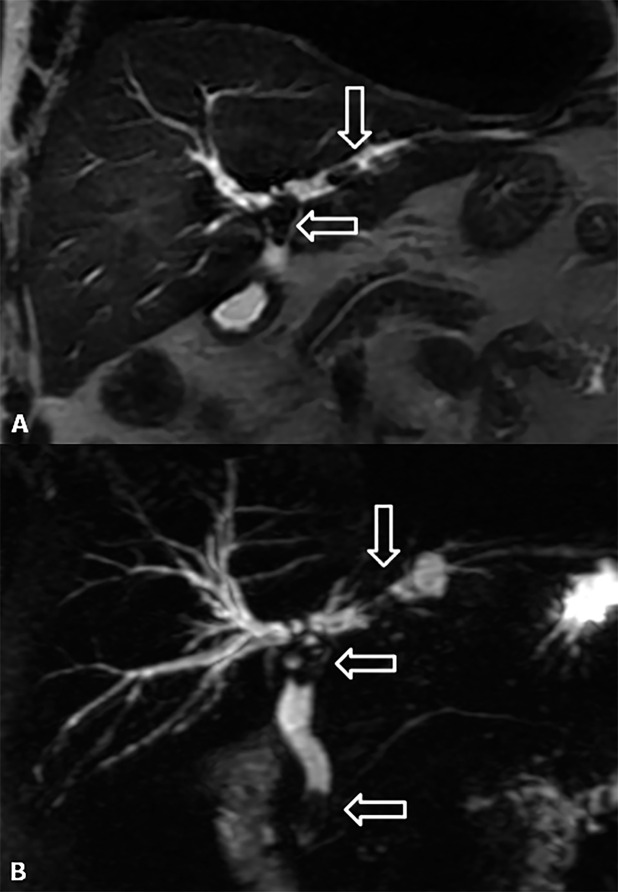
Biliary strictures
Biliary strictures constitute the most frequent type of late biliary adverse events, occurring approximately 5–8 months after OLT, and they can be classified according to their location in the strictures of the biliary AST and NASs (Figure 15a,b).58 The incidence of biliary strictures ranges from 5% to 34% of patients receiving LTs.54,59 Rapid identification of AST and NAS is important for ensuring the survival of both the organ and the patient after OLT. Ultrasound is useful for detecting biliary dilatation as an indirect sign of strictures. MRC is the best non-invasive technique in the evaluation of biliary strictures, both in the early and late post-operative periods. It shows narrowing at the level of the surgical AST associated with dilatation of the pre-anastomotic biliary tract. MR images can easily depict regular thickening of the anastomotic biliary wall, with a typical ring shape.60,61 MRC tends to overestimate biliary strictures (Figure 15c,d). Biliary stones and sludge usually complicate anastomotic or non-anastomotic strictures, occurring at both the intrahepatic or extrahepatic bile ducts as the consequence of bile stasis (Figure 16).
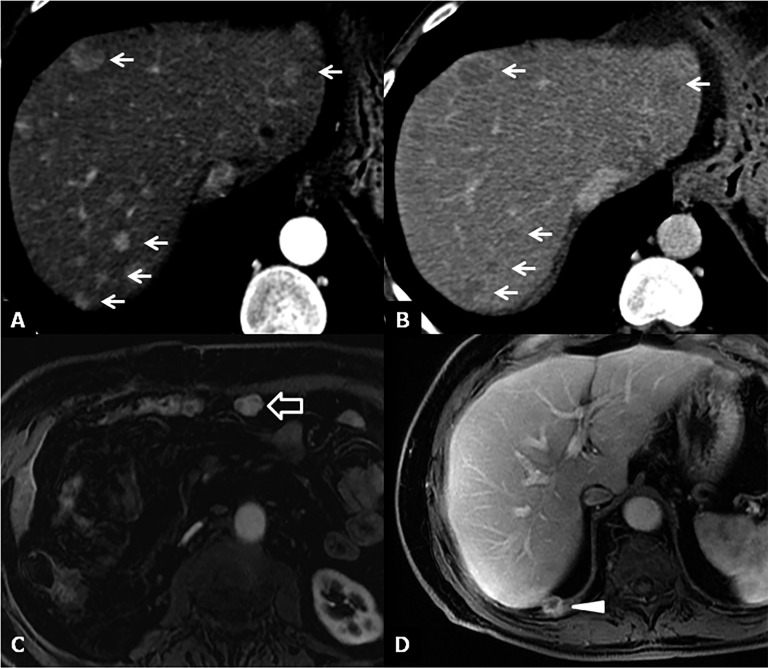
Figure 15.
Bile duct strictures. (a) Maximum intensity projections of three-dimensional (3D) thin-slab fast spin-echo T2 weighted image demonstrates the dilation and strictures of the intrahepatic biliary of both left and right lobes. (b) Coronal T2 weighted image shows intrahepatic (open arrow) and anastomotic (arrow) strictures. (c, d) Maximum intensity projections of 3D thin-slab fast spin-echo T2 weighted image and the corresponding T1 weighted MR cholangiography obtained after intravenous administration of a hepatobiliary contrast agent (gadoxetic acid) show an anastomotic stricture (arrowheads). To note that MR cholangiography tends to overestimate biliary stricture.
Figure 16.
Lithiasis of the bile ducts. (a, b) Coronal T2 weighted image and maximum intensity projections of three-dimensional thin-slab fast spin-echo T2 weighted image show the dilation of both intra- and extrahepatic biliary tracts with the presence of three stones (arrows) at the level of hepatic bifurcation, in the left hepatic duct and in juxtapapillary site.
Haematoma
Haematoma usually manifests within 2 weeks after transplantation, and it occurs near the vascular AST and/or in the perihepatic space, owing to surgical removal of the normal peritoneal reflections in the right subhepatic space.5,15 Haematoma is echogenic on ultrasound, hyperattenuating on CT and hypointense on T2 weighted MRI (Figure 17). Most haematomas will resolve spontaneously within a few weeks, but in some cases, superimposed infection can require catheter drainage or aspiration.
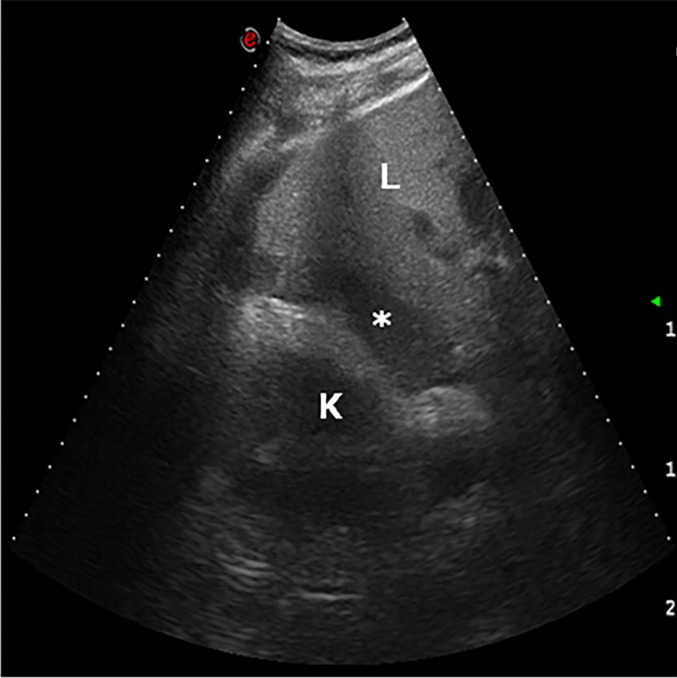
Figure 17.
Haematoma. The day after liver transplantation, ultrasound examination demonstrates a low-echogenicity fluid collection in the hepatorenal region (asterisk). K, kidney; L, liver.
ABSCESS
Intrahepatic abscess often occurs secondary to liver infarction.62 Predisposing factors include biliary stricture, arterial insufficiency and immunosuppressive medications. The presence of a complex fluid collection with a possible air–fluid level on ultrasound and CT suggests an abscess (Figure 18). The treatment consists of catheter drainage.63
Figure 18.
Abscess. Axial and coronal CT post-contrast-enhanced images show a large multiloculated lesion in the right lobe of the liver surrounded by other smaller fluid collections.
RECURRENCE OF HEPATOCELLULAR CARCINOMA AND DE NOVO NEOPLASMS
Despite the 5-year disease-free survival rate after LT of 60–80% for HCC in cases with unresectable early stages of the neoplasm, recurrence at 4 years occurs in 10% of patients with HCC according to the Milan criteria and in up to 60% of patients with HCC not using Milan criteria.64 Established tumour-related risk factors for HCC recurrence after LT include high levels of alpha-fetoprotein, tumour grade, tumour stage and vascular invasion, whereas the immunosuppression-related risk factors for HCC recurrence consist primarily of the level of immunosuppression.65–69
HCC most commonly recurs as lung metastases or as multiple lesions within the liver graft (Figure 19).70 Development of other neoplasms account for almost 30% of deaths 10 years after LT, and they represent the most common cause of mortality in patients after 1 year of LT.71 Post-transplantation lymphoproliferative disorder is the most frequent de novo malignancy after LT, accounting for approximately 20% of cases.72 Most of the commonly occurring neoplasms in patients who have undergone LT are skin cancers other than melanoma, Kaposi sarcoma and non-Hodgkin lymphoma.
Figure 19.
Recurrence of disease. (a, b) CT images 6 months after liver transplantation show multiple hypervacular nodules on the arterial phase (a) with washout during the delayed phase (b) suggestive for hepatocellular carcinoma (HCC) recurrence (arrows). (c) Axial MR image acquired during the arterial phase shows a hypervascular nodule near the anterior abdominal wall, as recurrence of HCC (open arrow). (d) Axial MR image during the venous phase demonstrates a focal lesion on the surface of the diaphragm as recurrence of hepatocholangiocarcinoma (arrowhead).
CONCLUSION
Imaging is useful for the detection of early and late complications, as well as for long-term follow-up to assess transplantation viability. Ultrasound is the primary imaging modality used in the early and late surveillance of patients after LT. CT or MRI is performed if a complication is suspected or demonstrated using ultrasound. Owing to its high temporal and spatial resolution, CT is the preferable second-level modality in the evaluation of LT complications suspected on ultrasound. MRI and mostly MRC are very useful in the evaluation of bile duct complications.
Contributor Information
Michele Di Martino, Email: micdimartino@hotmail.it.
Massimo Rossi, Email: massimo.rossi@uniroma1.it.
Gianluca Mennini, Email: gianluca.mennini@uniroma.it.
Fabio Melandro, Email: fabmelan@yahoo.it.
Michele Anzidei, Email: michele.anzidei@gmail.com.
Silvia De Vizio, Email: silvia.devizio91@gmail.com.
Kameliya Koryukova, Email: kam07@libero.it.
Carlo Catalano, Email: carlo.catalano@uniroma1.it.
REFERENCES
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–9. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 2.Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2003; 9: 612–20. doi: 10.1053/jlts.2003.50098 [DOI] [PubMed] [Google Scholar]
- 3.Hussain HK, Nghiem HV. Imaging of hepatic transplantation. Clin Liver Dis 2002; 6: 247–70. doi: 10.1016/S1089-3261(03)00075-8 [DOI] [PubMed] [Google Scholar]
- 4.Itri JN, Heller MT, Tublin ME. Hepatic transplantation: postoperative complications. Abdom Imaging 2013; 38: 1300–33. doi: 10.1007/s00261-013-0002-z [DOI] [PubMed] [Google Scholar]
- 5.Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics 2003; 23: 1093–114. doi: 10.1148/rg.235035031 [DOI] [PubMed] [Google Scholar]
- 6.Uzochukwu LN, Bluth EI, Smetherman DH, Troxclair LA, Loss GE, Jr, Cohen A, et al. Early postoperative hepatic sonography as a predictor of vascular and biliary complications in adult orthotopic liver transplant patients. AJR Am J Roentgenol 2005; 185: 1558–70. doi: 10.2214/AJR.04.1258 [DOI] [PubMed] [Google Scholar]
- 7.Dodd GD, 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 1994; 192: 657–61. doi: 10.1148/radiology.192.3.8058930 [DOI] [PubMed] [Google Scholar]
- 8.De Gaetano AM, Cotroneo AR, Maresca G, Di Stasi C, Evangelisti R, Gui B, et al. Color Doppler sonography in the diagnosis and monitoring of arterial complications after liver transplantation. J Clin Ultrasound 2000; 28: 373–80. doi: [DOI] [PubMed] [Google Scholar]
- 9.Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, et al. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology 2006; 241: 267–74. doi: 10.1148/radiol.2411050597 [DOI] [PubMed] [Google Scholar]
- 10.Berry JD, Sidhu PS. Microbubble contrast-enhanced ultrasound in liver transplantation. Eur Radiol 2004; 8(Suppl. 14): 96–103. [PubMed] [Google Scholar]
- 11.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013; 34: 11–29. doi: 10.1016/j.ultrasmedbio.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Girometti R, Como G, Bazzocchi M, Zuiani C. Post-operative imaging in liver transplantation: state-of-the-art and future perspectives. World J Gastroenterol 2014; 20: 6180–200. doi: 10.3748/wjg.v20.i20.6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katyal S, Oliver JH, 3rd, Buck DG, Federle MP. Detection of vascular complication after liver transplantation: early experience in multislice CT angiography with volume rendering. AJR Am J Roentgenol 2000; 175: 1735–9. doi: 10.2214/ajr.175.6.1751735 [DOI] [PubMed] [Google Scholar]
- 14.Boraschi P, Donati F. Complications of orthotopic liver transplantation: imaging findings. Abdom Imaging 2004; 29: 189–202. doi: 10.1007/s00261-003-0109-8 [DOI] [PubMed] [Google Scholar]
- 15.Quiroga S, Sebastià MC, Margarit C, Castells L, Boyé R, Alvarez-Castells A. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics 2001; 21: 1085–102. doi: 10.1148/radiographics.21.5.g01se061085 [DOI] [PubMed] [Google Scholar]
- 16.Hyodo T, Kumano S, Kushihata F, Okada M, Hirata M, Tsuda T, et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol 2012; 85: 887–96. doi: 10.1259/bjr/21209407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SY, Byun JH, Lee SS, Park SH, Jang YJ, Lee MG. Biliary tract depiction in living potential liver donors: intraindividual comparison of MR cholangiography at 3.0 and 1.5 T. Radiology 2010; 254: 469–78. doi: 10.1148/radiol.09090003 [DOI] [PubMed] [Google Scholar]
- 18.Laghi A, Pavone P, Catalano C, Rossi M, Panebianco V, Alfani D, et al. MR cholangiography of late biliary compilations after liver transplantation. AJR Am J Roentgenol 1999; 172: 1541–6. doi: 10.2214/ajr.172.6.10350286 [DOI] [PubMed] [Google Scholar]
- 19.Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology 1999; 211: 715–22. doi: 10.1148/radiology.211.3.r99jn17715 [DOI] [PubMed] [Google Scholar]
- 20.Salvolini L, Urbinati C, Valeri G, Ferrara C, Giovagnoni A. Contrast-enhanced MR cholangiography (MRCP) with GDEOB-DTPA in evaluating biliary complications after surgery. Radiol Med 2012; 117: 354–68. doi: 10.1007/s11547-011-0731-4 [DOI] [PubMed] [Google Scholar]
- 21.Boraschi P, Donati F. Biliary-enteric anastomoses: spectrum of findings on Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging 2013; 38: 1351–9. doi: 10.1007/s00261-013-0007-7 [DOI] [PubMed] [Google Scholar]
- 22.Cereser L, Girometti R, Como G, Molinari C, Toniutto P, Bitetto D, et al. Impact of magnetic resonance cholangiography in managing liver-transplanted patients: preliminary results of a clinical decision-making study. Radiol Med 2011; 116: 1250–66. doi: 10.1007/s11547-011-0707-4 [DOI] [PubMed] [Google Scholar]
- 23.Lang P, Schnarkowski P, Grampp S, van Dijke C, Gindele A, Steffen R, et al. Liver transplantation: significance of the periportal collar on MRI. J Comput Assist Tomogr 1995; 19: 580–5. doi: 10.1097/00004728-199507000-00014 [DOI] [PubMed] [Google Scholar]
- 24.Bhargava P, Vaidya S, Dick AA, Dighe M. Imaging of orthotopic liver transplantation: review. AJR Am J Roentgenol 2011; 196(Suppl. 3): WS15–25. [DOI] [PubMed] [Google Scholar]
- 25.Nghiem HV. Imaging of hepatic transplantation. Radiol Clin North Am 1998; 36: 429–43. doi: 10.1016/S0033-8389(05)70033-6 [DOI] [PubMed] [Google Scholar]
- 26.Saad WE, Orloff MC, Davies MG, Waldman DL, Bozorgzadeh A. Postliver transplantation vascular and biliary surgical anatomy. Tech Vasc Interv Radiol 2007; 10: 172–90. doi: 10.1053/j.tvir.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 27.Redvanly RD, Nelson RC, Steiber AC, Dodd GD, 3rd. Imaging in the preoperative evaluation of adult liver-transplant candidates: goals, merits of various procedures, and recommendations. AJR Am J Roentgenol 1995; 164: 611–17. doi: 10.2214/ajr.164.3.7863881 [DOI] [PubMed] [Google Scholar]
- 28.Jovine E, Mazziotti A, Grazi GL, Ercolani G, Masetti M, Morganti M, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int 1997; 10: 109–12. doi: 10.1111/j.1432-2277.1997.tb00550.x [DOI] [PubMed] [Google Scholar]
- 29.Pisaniello D, Marino MG, Perrella A, Russo F, Campanella L, Marcos A, et al. Side-to-side cavocavostomy in adult piggyback liver transplantation. Transplant Proc 2012; 44: 1938–41. doi: 10.1016/j.transproceed.2012.06.047 [DOI] [PubMed] [Google Scholar]
- 30.Keogan MT, McDermott VG, Price SK, Low VH, Baillie J. The role of imaging in the diagnosis and management of biliary complications after liver transplantation. AJR Am J Roentgenol 1999; 173: 215–19. doi: 10.2214/ajr.173.1.10397129 [DOI] [PubMed] [Google Scholar]
- 31.Uemura T, Ikegami T, Sanchez AZ, Jennings LW, Narasimhan G, McKenna GJ, et al. Late acute rejection after liver transplantation impacts patient survival. Clin Transplant 2008; 22: 316–23. doi: 10.1111/j.1399-0012.2007.00788.x [DOI] [PubMed] [Google Scholar]
- 32.Cappelli A, Golfieri RI. Trapianto ortotopico di fegato (OLT): ruolo dell’imaging e della radiologia interventistica nella valutazione e nella gestione delle complicanze addominale post-operatorie. Radiol Med 2014; 1: 509–52. [Google Scholar]
- 33.Chui AK, Shi LW, Rao AR. Primary graft dysfunction after liver transplantation. Transplant Proc 2000; 32: 2219–20. [DOI] [PubMed] [Google Scholar]
- 34.Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int 2014; 13: 125–37. [DOI] [PubMed] [Google Scholar]
- 35.Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW, Jr. Vascular complications after orthotopic liver transplantation. Am J Surg 1991; 161: 76–82. doi: 10.1016/0002-9610(91)90364-J [DOI] [PubMed] [Google Scholar]
- 36.Wozney P, Zajko AB, Bron KM, Point S, Starzl TE. Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol 1986; 147: 657–63. doi: 10.2214/ajr.147.4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akun E, Yaprak O, Killi R, Balci NC, Tokat Y, Yuzer Y. Vascular complications in hepatic transplantation: single-center experience in 14 years. Transplant Proc 2012; 44: 1368–72. doi: 10.1016/j.transproceed.2012.02.027 [DOI] [PubMed] [Google Scholar]
- 38.Rollins NK, Timmons CT, Superina RA, Andrews WS. Hepatic artery thrombosis in children with liver transplants: false-positive findings at Doppler sonography and arteriography in four patients. AJR Am J Roentgenol 1993; 160: 291–4. doi: 10.2214/ajr.160.2.8424338 [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Kim KW, Kim MJ, Shin YM, Lee MG, Lee SG. Multidetector row CT of various hepatic artery complications after living donor liver transplantation. Abdom Imaging 2007; 32: 635–43. doi: 10.1007/s00261-006-9145-5 [DOI] [PubMed] [Google Scholar]
- 40.Caiado AH, Blasbalg R, Marcelino AS, da Cunha Pinho M, Chammas MC, da Costa Leite C, et al. Complication liver transplantation: multimodality imaging approach. Radiographics 2007; 27: 1401–17. [DOI] [PubMed] [Google Scholar]
- 41.Sheng R, Orons PD, Ramos HC, Zajko AB. Dissecting pseudoaneurysm of the hepatic artery: a delayed complication of angioplasty in a liver transplant. Cardiovasc Intervent Radiol 1995; 18: 112–14. doi: 10.1007/BF02807234 [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Criado A, Gilabert R, Bargallo X, Bru C. Radiology in liver transplantation. Semin Ultrasound CT 2002; 23: 114–29. [DOI] [PubMed] [Google Scholar]
- 43.De Carlis L, Sansalone CV, Rondinara GF. Splenic artery steal syndrome after orthotopic liver transplantation: diagnosis and treatment. Transplant Proc 1993; 25: 2594–6. [PubMed] [Google Scholar]
- 44.Nüssler NC, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P. Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl 2003; 9: 596–602. doi: 10.1053/jlts.2003.50080 [DOI] [PubMed] [Google Scholar]
- 45.Chong WK, Beland JC, Weeks SM. Sonographic evaluation of venous obstruction in liver transplants. AJR Am J Roentgenol 2007; 188: W515–21. doi: 10.2214/AJR.06.1262 [DOI] [PubMed] [Google Scholar]
- 46.Pawlak J, Grodzicki M, Lowska E. Vascular complications after liver transplantation. Transplant Proc 2003; 35: 2313–15. doi: 10.1016/S0041-1345(03)00836-4 [DOI] [PubMed] [Google Scholar]
- 47.Ko EY, Kim TK, Kim PN, Kim AY, Ha HK, Lee MG. Hepatic vein stenosis after living donor liver transplantation: evaluation with Doppler US. Radiology 2003; 229: 806–10. doi: 10.1148/radiol.2293020700 [DOI] [PubMed] [Google Scholar]
- 48.Novellas S, Caramella T, Fournol M, Gugenheim J, Chevallier P. MR cholangiopancreatography features of the biliary tree after liver transplantation. AJR Am J Roentgenol 2008; 191: 221–7. doi: 10.2214/AJR.07.2938 [DOI] [PubMed] [Google Scholar]
- 49.Boraschi P, Donati F. Postoperative biliary adverse events following orthotopic liver transplantation: assessment with magnetic resonance cholangiography. World J Gastroenterol 2014; 20: 11080–94. doi: 10.3748/wjg.v20.i32.11080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol 2008; 14: 493–7. doi: 10.3748/wjg.14.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy 2005; 37: 857–63. doi: 10.1055/s-2005-870192 [DOI] [PubMed] [Google Scholar]
- 52.Stratta RJ, Wood RP, Langnas AN, Hollins RR, Bruder KJ, Donovan JP, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery 1989; 106: 675–83. [PubMed] [Google Scholar]
- 53.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994; 219: 40–5. doi: 10.1097/00000658-199401000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol 2004; 14: 559–78. doi: 10.1007/s00330-004-2236-1 [DOI] [PubMed] [Google Scholar]
- 55.Fayad LM, Holland GA, Bergin D, Iqbal N, Parker L, Curcillo PG, et al. Functional magnetic resonance cholangiography (fMRC) of the gallbladder and biliary tree with contrast-enhanced magnetic resonance cholangiography. J Magn Reson Imaging 2003; 18: 449–60. doi: 10.1002/jmri.10369 [DOI] [PubMed] [Google Scholar]
- 56.Sheppard D, Allan L, Martin P, McLeay T, Milne W, Houston JG. Contrast-enhanced magnetic resonance cholangiography using mangafodipir compared with standard T2W MRC sequences: a pictorial essay. J Magn Reson Imaging 2004; 20: 256–63. doi: 10.1002/jmri.20114 [DOI] [PubMed] [Google Scholar]
- 57.Aduna M, Larena JA, Martín D, Martínez-Guereñu B, Aguirre I, Astigarraga E. Bile duct leaks after laparoscopic cholecystectomy: value of contrast-enhanced MRCP. Abdom Imaging 2005; 30: 480–7. doi: 10.1007/s00261-004-0276-2 [DOI] [PubMed] [Google Scholar]
- 58.Kinner S, Dechêne A, Paul A, Umutlu L, Ladd SC, de Dechêne EM, et al. Detection of biliary stenoses in patients after liver transplantation: is there a different diagnostic accuracy of MRCP depending on the type of biliary anastomosis? Eur J Radiol 2011; 80: 20–8. doi: 10.1016/j.ejrad.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 59.Zoepf T, Maldonado-Lopez EJ, Hilgard P, Dechêne A, Malago M, Broelsch CE, et al. Diagnosis of biliary strictures after liver transplantation: which is the best tool? World J Gastroenterol 2005; 11: 2945–8. doi: 10.3748/wjg.v11.i19.2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pecchi A, De Santis M, Gibertini MC, Tarantino G, Gerunda GE, Torricelli P, et al. Role of magnetic resonance imaging in the detection of anastomotic biliary strictures after liver transplantation. Transplant Proc 2011; 43: 1132–5. doi: 10.1016/j.transproceed.2011.03.016 [DOI] [PubMed] [Google Scholar]
- 61.Girometti R, Cereser L, Como G, Zuiani C, Bazzocchi M. Biliary complications after orthotopic liver transplantation: MRCP findings. Abdom Imaging 2008; 33: 542–54. doi: 10.1007/s00261-007-9316-z [DOI] [PubMed] [Google Scholar]
- 62.Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol 2000; 175: 1145–9. doi: 10.2214/ajr.175.4.1751145 [DOI] [PubMed] [Google Scholar]
- 63.Mazariegos GV, Molmenti EP, Kramer DJ. Early complications after orthotopic liver transplantation. Surg Clin North Am 1999; 79: 109–29. doi: 10.1016/S0039-6109(05)70009-8 [DOI] [PubMed] [Google Scholar]
- 64.Roberts JP. Tumor surveillance-what can and should be done? Screening for recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2005; 11(Suppl. 2): S45–6. doi: 10.1002/lt.20605 [DOI] [PubMed] [Google Scholar]
- 65.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2009; 15: 1834–42. doi: 10.1002/lt.21953 [DOI] [PubMed] [Google Scholar]
- 66.DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011; 253: 166–72. doi: 10.1097/SLA.0b013e31820508f1 [DOI] [PubMed] [Google Scholar]
- 67.Vivarelli M, Cucchetti A, La Barba G. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg 2008; 248: 857. doi: 10.1097/SLA.0b013e3181896278 [DOI] [PubMed] [Google Scholar]
- 68.Sotiropoulos GC, Molmenti EP, Lösch C, Beckebaum S, Broelsch CE, Lang H. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res 2007; 12: 527–34. [PubMed] [Google Scholar]
- 69.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol 2010; 36: 275–88. doi: 10.1016/j.ejso.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 70.Ferris JV, Baron RL, Marsh JW, Jr. Recurrent hepatocellular carcinoma after liver transplantation: spectrum of CT findings and recurrence patterns. Radiology 1996; 198: 233–8. doi: 10.1148/radiology.198.1.8539385 [DOI] [PubMed] [Google Scholar]
- 71.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19: 3–26. doi: 10.1002/lt.23566 [DOI] [PubMed] [Google Scholar]
- 72.Baccarani U, Adani GL, Serraino D, Lorenzin D, Gambato M, Buda A, et al. De novo tumors are a major cause of late mortality after orthotopic liver transplantation. Transplant Proc 2009; 41: 1303–5. doi: 10.1016/j.transproceed.2009.03.079 [DOI] [PubMed] [Google Scholar]