Abstract
The adequate treatment of non-resectable liver metastases from colorectal cancer which are resistant to systemic chemotherapy currently provides a great challenge. The aim is to identify and review key strategies in the treatment of colorectal liver metastases. A search for current literature on the topic of interventional strategies for colorectal metastases was performed in Medline in order to achieve this goal. Studies before 2005 and with <20 patients treated for colorectal metastases were excluded. Transarterial chemoembolization (TACE), transarterial embolization and selective internal radiation therapy (SIRT) were identified as examples of regional strategies for colorectal liver metastases, utilizing the unique blood supply of the liver. Radiofrequency ablation (RFA), microwave ablation (MWA) and cryoablation were selected as examples for currently available ablative techniques. Median survival in the key studies reviewed ranged from 7.7 to 28.6 for TACE, 8.3–12.6 for SIRT, 8.2–53.2 for RFA and 29–43 months for MWA. After review of the literature, it can be concluded that interventional oncologic therapies are a safe and effective method for treating colorectal liver metastases. The use of new chemotherapeutic agents for local therapy and new ablation technologies and techniques may increase patient survival and allows a neoadjuvant therapy setting. In addition, a combination of local therapies may be used to increase effectiveness in the future, which is subject to further research.
INTRODUCTION
Colorectal cancer is one of the most common cancers that shows fatal progression.1 Colorectal carcinoma most commonly metastasises to the liver due to haematogenous spread.2 At the time of diagnosis, 20–30% of patients have liver metastases, and during the course of disease, up to 60% of patients develop hepatic metastases.3 Although surgical excision is the first-line treatment for colorectal metastases, only 10–25% of patients are candidates for surgical resection.4 Neoadjuvant systemic chemotherapy only allows 10–30% of patients with unresectable colorectal liver metastases sufficient downsizing for resection.5
The high rate of inoperable patients and intrahepatic recurrence rates are another relevant problem. In case of inoperability of liver metastases and in the absence of response to systemic chemotherapy with FOLFIRI or FOLFOX (with or without addition of biological agents), various interventional radiology procedures are considered as an alternative treatment method in the interdisciplinary therapy management. Such treatment methods include transarterial chemoembolization (TACE), transarterial embolization (TAE), chemoembolization with chemotherapeutically loaded beads, radiofrequency ablation (RFA), cryotherapy, microwave ablation (MWA) and selective internal radiation therapy (SIRT). For the remaining patients, locoregional therapies can be used for neoadjuvant, symptomatic and palliative treatments.6,7
CURRENT TRENDS IN INTERVENTIONAL TREATMENT OF COLORECTAL CANCER LIVER METASTASES
The main focus of this article is to review current options in local treatment of colorectal liver metastases. In order to achieve this goal, literature was searched in Medline and then reviewed. Studies before 2005 and with <20 patients with colorectal metastases were excluded; of the remaining studies, key literature was reviewed. In this review, current strategies are presented along with the authors' opinion on them.
Transarterial chemoembolization
TACE is a catheter-based locoregional therapy for treating inoperable liver metastases of colorectal cancer. In TACE, one or more chemotherapeutic drugs and embolizing material are administered into the hepatic artery.8 TACE was introduced by Yamada et al9 in the late 1970s and was introduced in other clinics in the 1980s.
The primary indication for TACE is as a second-line treatment after failure of systemic chemotherapy.10 It generally includes multiple procedures in a 4- to 6-week interval. CT/MRT is used to evaluate the results of the procedures (Figure 1).8
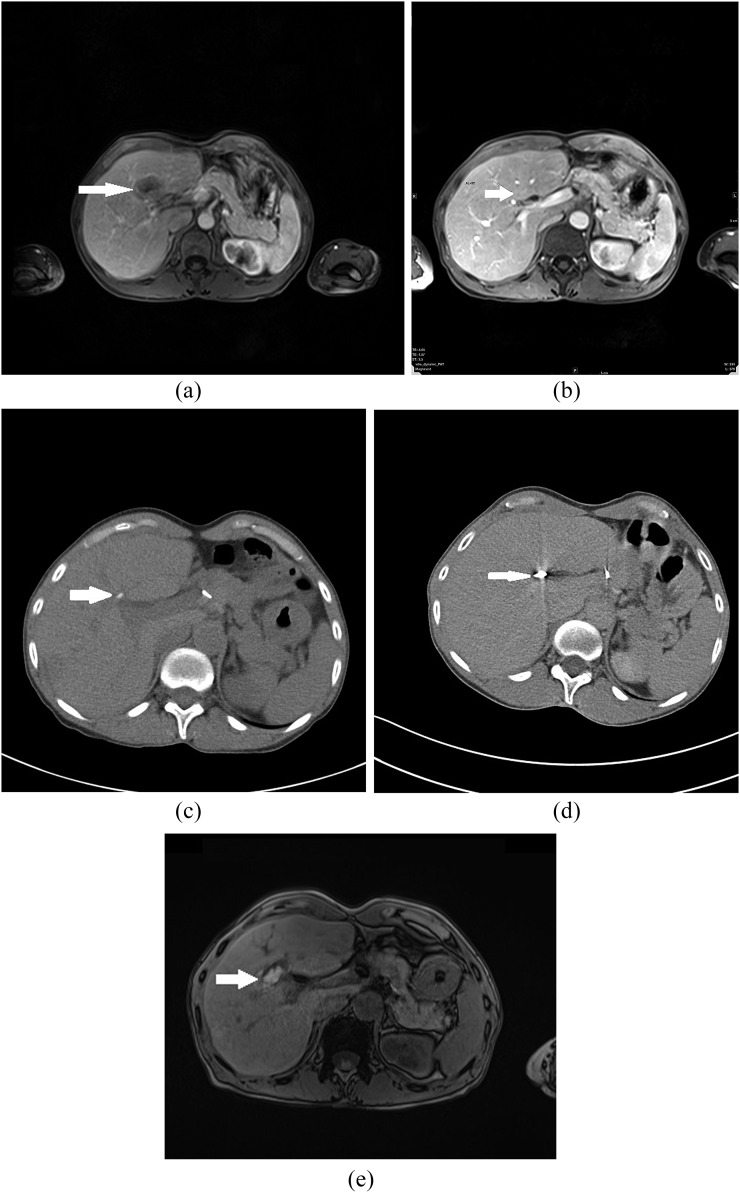
Figure 1.
A 46-year-old female with liver metastases of colorectal cancer (T3N1M1) and morphological features of complete response after treatment with transarterial embolization and microwave ablation (MWA). (a) Pre-treatment contrast-enhanced axial MRI scan shows metastatic liver lesion (arrow) in segment IV. (b) Post-treatment contrast-enhanced axial T1 weighted MR image after three sessions of chemoembolization shows partial response of liver metastases (arrow). (c) CT scan after selective transarterial embolization shows lower degree of Lipiodol retention in metastatic lesion in segment IV (arrow). (d) CT image obtained during MWA shows a microwave antenna positioned in the tumour (arrow). (e) Transverse contrast-enhanced T1 weighted MR image obtained 24 h after MWA demonstrates ablated volume (arrow). Area of necrosis is larger than original lesion.
During TACE, a catheter is introduced into the femoral artery using the Seldinger technique.11 Using the femoral approach, a catheter is passed through the coeliacal trunk into the main hepatic artery and then into the relevant branch that is providing the tumours with blood flow. Angiography is used to confirm the correct position of the tip of the catheter and to detect any anatomical abnormalities which may require repositioning of the catheter to avoid inadvertant complications resulting from the pharmaceutical agents being delivered to incorrect anatomical sites and structures.
Embolizing agents can either be temporary, such as degradable starch microspheres, collagen and gelatine sponge (Gelofam), or permanent, such as polyvinyl alcohol and Lipiodol® (Guerbet, Sulzbach, Germany). The lipophilic nature of Lipiodol has the advantage of delivering the drugs directly to the targeted liver cells.12 The embolizing agents administered significantly reduce flow through the relevant hepatic artery branch, although total occlusion is not achieved. Therefore, this significantly increases the time that the tumour cells are exposed to the chemotherapeutic agents and also increases the effective local concentration of these agents, thereby enhancing the activity of these agents.8 Most commonly, the mixture of chemotherapeutics and Lipiodol is injected first, followed by the injection of embolizing particles until stagnation or reflux in the hepatic artery is documented.13 Mitomycin, cisplatin and doxorubicin are frequently used chemotherapeutic drugs for intra-arterial infusion (Table 1).5
Table 1.
Key studies employing transarterial chemoembolization
| Study (years) | Number of patients | Used chemotherapeutic | Used embolization | Median follow-up (months) | Median survival (months) |
|---|---|---|---|---|---|
| Hong et al14 (2009) | 21 | Cisplatin, doxyrubicin, mitomycin | PVA | NR | 7.7 |
| Vogl et al7 (2009) | 463 | (1) Mitomycin and iriinotecan (2) Gemcitabine and mitomycin (3) Mitomycin |
Lipiodol and starch microspheres | NR | (1) 14 (2) 13.9 (3) 14 |
| Martin et al15 (2010) | 55 | DEBIRI | DC beads | NR | 28.6 |
| Albert et al10 (2011) | 121 | Cisplatin, doxyrubicin, mitomycin | Lipidol, PVA | NR | 11 |
| Aliberti et al16 (2011) | 82 | DEBIRI | DC beads | NR | 25 |
| Fiorentini et al17 (2012) | 35 | DEBIRI | DC Beads | 50 | 22 |
DC, drug-eluting; DEBIRI, irinotecan-loaded drug-eluting beads; NR, not reported; PVA, polyvinyl alcohol.
TACE usually causes no serious adverse effects. Most common effects of post-embolization syndrome are pain, nausea, vomiting and fatigue.7,10
Post-embolization pain represents one of the most common side effects of chemoembolization. Intra-arterial injection of lidocaine has been reported to reduce the severity and duration of post-embolization pain.18,19 The usage of corticosteroids to reduce post-embolization pain has been shown to be none beneficial.19 In our experience, the usage of intravenous analgesics during the procedure and oral analgesics such as Metamizole (Ratiopharm, Ulm, Germany) provide sufficient pain control after chemoembolization. Before discharge, patients are informed about the possible post-embolization side effects including slight elevation of temperature, fatigue over the next few days, nausea and vomiting, in addition to pain. Patients are requested to use oral analgesics and antiemetics. In addition, patients are instructed to immediately seek medical service in case of high fever, severe uncontrollable pain, bleeding from the puncture site or any other major symptoms.
Patients are either treated as outpatients or spend approximately 1 day in the hospital.7,10 Several previous studies addressed the safety of performing TACE on an outpatient basis. Mitchell et al20 reported the safety of the outpatient concept in carefully selected patients, where 131 from 133 sessions were performed on outpatient cases and only 2 required readmission. Nasser et al21 also reported the safety of treating the patients on outpatient basis. In our experience, most patients can be treated on outpatient basis; hospital stay is required in case of complications.
In recent times, new chemotherapeutic and embolizing agents have been introduced in clinical practice, including irinotecan-loaded drug-eluting beads (DEBIRIs). The DEBIRI is composed of microspheres that bind irinotecan and release the drug for a period of time after injection at the tumour side.18 The use of drug-eluting beads results in a lower peak plasma concentration of the chemotherapeutic drugs compared with traditional TACE and longer exposure of the tumour to the therapeutic agents. The lower peak concentration results in lower exposure of healthy liver tissue.13
Transarterial embolization
The aim of TAE is to create total arterial occlusion, resulting in tumour necrosis of the tumour.14 Embolizing agents are classified as temporary agents such as gelatine sponge, autologous blood clot and degradable starch microspheres or permanent/semi-permanent agents such as polyvinyl alcohol and steel coils.14 In comparison to TACE, TAE uses more frequent embolization procedures in order to minimize the creation of a collateral circulation. Therapeutic agents that lead to capillary occlusion, such as Lipiodol, are advantageous.8
Selective internal radiation therapy
SIRT is minimal invasive locoregional treatment that uses the principles similar to brachytherapy. SIRT was introduced in Australia in the 1990s.15 Radioembolization uses the same physiological principle as TACE. By administering the radioactive material in the direct proximity of the tumour, higher radioactive doses can be achieved and normal liver tissue absorbs less radiation.16
The most common isotope for SIRT is yttrium-90. It undergoes beta minus decay, has a penetration of approximately 2.4 mm and a half-life of 64.2 h. The most commonly used microspheres are composed of resin or glass. The microspheres are smaller than those used in TACE.17 Selective infusion of yttrium-90 microspheres is achieved through closed circuit delivery using proprietary delivery system specific to that particular device.16 Close monitoring using angiography whilst delivering the microspheres is necessary to ensure that there is no significant inadvertant reflux into normal hepatic tissue or elsewhere, which would lead to inadvertant tissue damage. Following ablation, the lesions are monitored using either contrast-enhanced CT or MRI to ensure the lesions have undergone total necrosis and to ensure there is no residual tumour, which would otherwise continue to grow (Figure 2).22 SIRT generally has few risks of complications. It can cause unspecific symptoms such as fever, nausea, pain, fatigue and anorexia. If the microspheres accidentally enter the gastrointestinal system, they can cause local inflammation and ulceration. A very rare but potentially life-threatening complication is the radiation-induced liver disease (Table 2).26
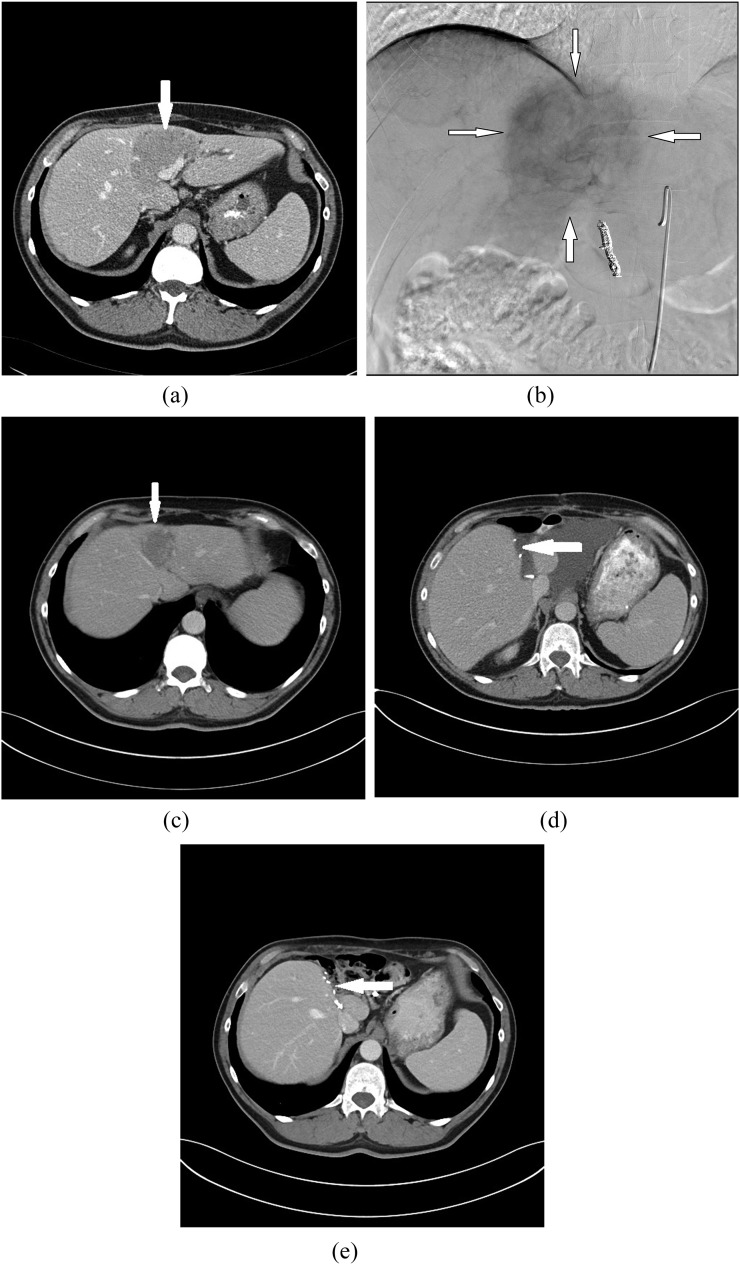
Figure 2.
Images in a 51-year-old male with bilobar liver metastases of colorectal cancer (T2N1M1) show partial response after selective internal radiation therapy (SIRT). The patient then became eligible for a hepatic resection, which was subsequently successfully undertaken. (a) Pre-treatment contrast-enhanced CT image shows 42 × 36-mm target lesion in Segments II, III and IV with irregular borders (arrow). (b) Selective digital subtraction angiogram obtained during SIRT reveals the hypervascularity of the lesion (arrows). (c) Contrast-enhanced CT image after SIRT shows partial response of liver metastases (arrow). (d) CT image after liver resection shows fluid area at the resection margin (arrow). (e) Contrast-enhanced transverse CT image 3 years after liver resection. There is no evidence of recurrent disease (arrow).
Table 2.
Key studies employing selective internal radiation therapy
| Study (years) | Number of patients | Local reoccurrences (%) | Median follow-up (months) | Median survival (months) | Major complications |
|---|---|---|---|---|---|
| Kennedy et al23 (2006) | 209 | NR | 13 | Responders: 10.5 Non-responders: 4.5 |
NR |
| Cosimelli et al24 (2010) | 50 | NR | 11 | 12.6 | NR |
| Seidensticker et al25 (2012) | 29 | NR | 12 | 8.3 | 3 grade 2 GIT ulcers, 3 RELID |
GIT, gastrointestinal; NR, not reported; RELID, radiation-induced liver disease.
Radiofrequency ablation
RFA is another minimal invasive procedure that can be used in the treatment of liver tumours. RFA was first introduced in 1990. It gained the Food and Drug Administration approval in 2001.27 RFA is used for treating unresectable tumours. It also can be used as a first-line therapy in colorectal liver metastases <3 cm in size.23 RFA uses electromagnetic energy with a frequency of <900 kHz. Coagulation necrosis is achieved by inserting an electrode in the target lesion and administering high-frequency-altering current. This induces ionization and friction. Temperatures of >100 °C increase the electrical impedance and limit the amount of energy that can be administered. This can be avoided by using cooled-wet electrodes, which are cooled by interstitial saline infusion and internal cooling. The reduction in impedance is used to increase the size of the ablative zone.24 No standardized protocol exists for the use of RFA.25 The RFA probe is usually inserted under image guidance, such as ultrasound or CT.27 After the lesion has been ablated, the needle is withdrawn whilst still emitting a low energy frequency in order to prevent spreading of the tumour by needle track seeding.27 Thereafter lesions are generally monitored using contrast CT and MRI.22
The most common complications during RFA are bile duct injury, haemorrhage, liver infarction and refractory pleural effusions. Intraperitoneal haemorrhage can be a fatal complication of RFA (Table 3).42
Table 3.
Key studies employing radiofrequency ablation
| Study (years) | Number of patients | Local reoccurrences (%) | Median follow-up (months) | Median survival (months) | Major complications (%) |
|---|---|---|---|---|---|
| Elias et al28 (2005) | 63 | 7.1 | 27.6 | 36 | 27 |
| Gillams and Lees29 (2005) | 73 | NR | NR | 38 | 4 |
| Hildebrand et al30 (2006) | 56 | 17 | 21.2 | 28 | 34 |
| Aloia et al31 (2006) | 30 | 37 | 31.3 | 57% 3-year survival | NR |
| Sorensen et al32 (2007) | 102 | NR | NR | 32 | 7 |
| Lee et al33 (2008) | 37 | 29.7 | 48.2 | 40 | NR |
| Gleisner et al34 (2008) | 66 | NR | NR | 38.1 | NR |
| Park et al35 (2008) | 30 | 23.3 | 49 | 36 | NR |
| Hur et al36 (2009) | 25 | 2.8 | 42 | 60% 3-year survival | NR |
| Knudsen et al37 (2009) | 36 | NR | NR | 39 | 11 |
| Solbiati et al38 (2012) | 99 | 11.9 | 72 | 53.2 | 1.3 |
| Stintzing et al39 (2013) | 30 | 65% (1 year) → local control rate | NR | 52.2 | No major side effects |
| Babawale et al40 (2015) | 49 | 4.8 % after 1 month | 60 | 28.5 | NR |
| Lee et al41 (2015) | 51 | NR | 45 | 62.4% 3 year survival | NR |
NR, not reported.
Microwave ablation
In recent years, MWA has been introduced as a new ablative therapy option for the treatment of colorectal liver metastases. Although the first use of microwave coagulation was reported in the 1970s, MWA as an ablation technique has primarily been established during the past 10 years.38
MWA uses electromagnetic waves with frequencies from 900 to 2450 MHz, which can be used to agitate water molecules that produce friction and heat. This results in coagulation necrosis of the surrounding cells. After the procedure, tissue temperatures of 63–67 °C are measured in the ablated tissue. MWA creates a broad field of power density, allowing ablation zones of up to 2 cm. Owing to the convection profile, MWA is suitable for treatment of larger metastases with tumour diameter ≤6 cm, such as liver metastases of colorectal carcinoma.39
Percutaneous MWA is usually performed under ultrasound or CT guidance.40
In comparison to other ablative techniques such as RFA, MWA produces consistently higher intertumoural temperatures, larger tumour ablation volumes, faster ablation times and less procedural pain.39 Moreover, the heat sink effect of MWA is minimal and usually does not create ablation zone distortions in the vicinity of large blood vessels (Figure 1).39
Most studies reported MWA to be a relatively safe method with low complication rates. The most common complications of MWA are pain, fever, ascites, bile duct injury and pleural effusion (Table 4).41
Table 4.
Key studies employing microwave ablation
| Study (years) | Number of patients | Local reoccurrences (%) | Median follow-up (months) | Median survival (months) | Major complications (%) |
|---|---|---|---|---|---|
| Martin et al43 (2007) | 20 | 11.3 | 5 | NR | 4.0 |
| Iannitti et al44 (2007) | 33 | 2.7 | 19 | NR | 19.0 |
| Zhou et al45 (2009) | 35 | 11.3 | 5 | NR | NR |
| Groeschl et al46 (2014) | 198 | NR | 19 | 32.1 | NR |
NR, not reported.
Cryoablation
Cryoablation is the oldest of the ablative techniques used for treatment of malignant tumours.43 It was a fairly common technique in the 2000s but has lost popularity in the past few years. In cryoablation, liquid oxygen or nitrogen are used to freeze the tumour. Temperatures as low as −30 °C are achieved, creating an ice ball. Repeated cycles of freezing are used to ensure necrosis of the tumour.44
Although generally considered as safe, a unique complication that cryoablation can cause is cryoshock, in which the remnants of tumour cells are released into the system. This can cause a potentially life-threatening systemic inflammatory response with multiorgan failure.45
In Bhardwaj et al,47 viable cells were found in adjacent vascular structures after cryotherapy thereby leading to the development of further metastases and patient detriment. This does not occur in other ablation options such as MWA and RFA. Therefore, thermal ablation techniques should be rather used than cryotherapy, if a local ablative procedure is indicated.46
Combination therapy
A combination of locoregional therapies might offer a new tool for the treatment of liver metastases, especially in a neoadjuvant setting. However, there are few studies describing such an approach, and these are mostly based on individual therapies for certain patients. A systematic use of combined locoregional therapies could be an interesting field of further research.8
Clinical indications
The indication for the most interventional locoregional oncologic therapies (TACE, embolization with drug-eluting beads, TAE, SIRT) of liver metastases in patients with colorectal cancer is primarily palliative or sometimes symptomatic. In some patients, there is a shift of indication during the course of treatment from palliative to neoadjuvant. Palliative treatment is defined as therapy for asymptomatic patients intended mainly to prolong survival and to preserve and improve the quality of life without curing the disease. Symptomatic treatment is defined as a therapy intended to alleviate or decrease tumour-related symptoms (e.g. pain, bulk related symptoms). Neoadjuvant treatment is defined as a clinical situation in which the previous treatment with TAE, TACE or SIRT caused a significant decrease of the size and number of hepatic lesions so that a possible curative local treatment, such as hepatic resection or RFA with curative intent, could be performed. The criteria for RFA and MWA are most commonly defined as five or fewer metastases and a size of ≤3 cm in diameter. Patients who met such inclusion criteria for local ablation treatment before TACE, TAE or SIRT also underwent embolization before ablation to decrease the tumour activity and tumour vascularity and thus maximize the ablative effect of local thermal ablation methods on the tumour.11
DISCUSSION
Isolated and combined local and regional minimal invasive therapies were developed and improved since the 1990s and have proven to be useful in the treatment of colorectal liver metastases. Even if surgery and systemic chemotherapy are ineffective, they can lead to an increase in overall survival or, in the palliative setting, can result in an increased quality of life for the patient whilst having relatively few major complications and side effects.48 Most therapies have developed over the years and lead to an increase in overall survival. Considering RFA, for example, Solbiati et al38 documented an overall survival of 53.2 months, whereas Sorensen et al32 who recruited a similar number of patients documented an overall survival of 32 months. Differences, however, may not be as significant as the numbers suggest because Sorensen et al had 26% of patients with synchronous metastases, whereas Solbiati et al only included patients with metachronus malignancies, which have a better survival rate.31,38
The local recurrence of the listed studies also varies. Aloia et al31 reported a recurrence rate of 37%, whereas Hur et al36 only reported a recurrence rate of 2.8%. This may be due to the inclusion of patients eligible for resection but preferring RFA, which was also suggested by Hur et al.32,49
Complication in MWA seems to have been reduced over time. Although early studies of Zhang et al50 and Iannitti et al had complication rates of 19% and 16.1%, more recent studies such as Livraghi et al51 had complication rates of 2.9%. This may be due to increased experience of the doctors performing the treatment and more advanced MWA systems. This statement, however, is limited by the relatively small numbers of patients participating in the study of Iannitti et al.44
In SIRT, overall survival ranges between 8.3 and 12.6 months. Interestingly, the most recent study of Seidensticker et al,25 reported the lowest overall survival with 8.3 months, whereas Cosimelli et al,24 2 years earlier, recorded an overall survival of 12.6 months. This, however, can be explained by the fact that Seidensticker et al only included patients with extensive liver involvement (≥20%) and a palliative indication in his study, whereas Cosimelli et al also included patients with <20% liver involvement (40% of patients).24,25
Considering the TACE studies listed in Table 1, it is notable that conventional TACE has median survival rates ranging from 7.7 to 14 months, whereas DEBIRI TACE has median survival rates ranging from 22 to 28.6 months. This suggests that the newer DEBIRI TACE is more effective in treating colorectal liver metastases than conventional TACE. For further evaluation of the potential increase in overall survival, controlled trials should be performed.
CONCLUSION
Interventional oncologic therapies are a safe and effective method for treating patients with unresectable and chemotherapy-refractory colorectal liver metastases. Current data have demonstrated the great benefit of such procedures as described above. Nonetheless, this facet of radiological interventional oncology is still evolving, and there are great opportunities to combine percutaneous and transcatheter procedures with a view to provide a significant meaningful improvement in end points such as overall survival and quality of life parameters, both of which are of the utmost importance. This is a constantly evolving landscape with advances in the development of new therapeutic agents and improved technological developments and is an exciting area of research.
Contributor Information
Tatjana Gruber-Rouh, Email: tgruberrouh@googlemail.com.
Christian Marko, Email: s0761609@stud.uni-frankfurt.de.
Axel Thalhammer, Email: Axel.Thalhammer@kgu.de.
Nour-Eldin Nour-Eldin, Email: nour410@hotmail.com.
Marcel Langenbach, Email: marcel.langenbach@me.com.
Martin Beeres, Email: beeres@gmx.net.
Nagy N Naguib, Email: nagynnn@yahoo.com.
Stephan Zangos, Email: Zangos@em.uni-frankfurt.de.
Thomas J Vogl, Email: t.vogl@em.uni-frankfurt.de.
REFERENCES
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009; 22: 191–7. doi: 10.1055/s-0029-1242458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 2003; 8: 553–66. doi: 10.1634/theoncologist.8-6-553 [DOI] [PubMed] [Google Scholar]
- 3.Wang DS, Louie JD, Sze DY. Intra-arterial therapies for metastatic colorectal cancer. Semin Intervent Radiol 2013; 30: 12–20. doi: 10.1055/s-0033-1333649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasson AR, Sigurdson ER. Surgical treatment of liver metastases. Semin Oncol 2002; 29: 107–18. doi: 10.1053/sonc.2002.31676 [DOI] [PubMed] [Google Scholar]
- 5.Xing M, Kooby DA, El-Rayes BF, Kokabi N, Camacho JC, Kim HS. Locoregional therapies for metastatic colorectal carcinoma to the liver—an evidence-based review. J Surg Oncol 2014; 110: 182–96. doi: 10.1002/jso.23619 [DOI] [PubMed] [Google Scholar]
- 6.Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology 2013; 266: 407–30. doi: 10.1148/radiol.12112544 [DOI] [PubMed] [Google Scholar]
- 7.Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology 2009; 250: 281–9. doi: 10.1148/radiol.2501080295 [DOI] [PubMed] [Google Scholar]
- 8.Vogl TJ, Mack MG, Eichler K, Zangos S, Naguib NN, Gruber-Rouh T. Chemoperfusion and embolization in the treatment of liver metastases. [In German.] Rofo 2011; 183: 12–23. doi: 10.1055/s-0029-1245880 [DOI] [PubMed] [Google Scholar]
- 9.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983; 148: 397–401. doi: 10.1148/radiology.148.2.6306721 [DOI] [PubMed] [Google Scholar]
- 10.Albert M, Kiefer MV, Sun W, Haller D, Fraker DL, Tuite CM, et al. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2011; 117: 343–52. doi: 10.1002/cncr.25387 [DOI] [PubMed] [Google Scholar]
- 11.Vogl TJ, Jost A, Nour-Eldin NA, Mack MG, Zangos S, Naguib NNN. Repeated transarterial chemoembolisation using different chemotherapeutic drug combinations followed by MR-guided laser-induced thermotherapy in patients with liver metastases of colorectal carcinoma. Br J Cancer 2012; 106: 1274–9. doi: 10.1038/bjc.2012.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol 2007; 17: 1025–34. doi: 10.1007/s00330-006-0372-5 [DOI] [PubMed] [Google Scholar]
- 13.Bester L, Meteling B, Boshell D, Chua TC, Morris DL. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: a review of the literature. J Med Imaging Radiat Oncol 2014; 58: 341–52. doi: 10.1111/1754-9485.12163 [DOI] [PubMed] [Google Scholar]
- 14.Hong K, McBride JD, Georgiades CS, Reyes DK, Herman JM, Kamel IR, et al. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J Vasc Interv Radiol 2009; 20: 360–7. doi: 10.1016/j.jvir.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 15.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 2010; 17: 171–8. doi: 10.1245/s10434-009-0686-z [DOI] [PubMed] [Google Scholar]
- 16.Aliberti C, Fiorentini G, Muzzio PC, Pomerri F, Tilli M, Dallara S, et al. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res 2011; 31: 4581–7. [PubMed] [Google Scholar]
- 17.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res 2012; 32: 1387–95. [PubMed] [Google Scholar]
- 18.Hartnell GG, Gates J, Stuart K, Underhill J, Brophy DP. Hepatic chemoembolization: effect of intraarterial lidocaine on pain and postprocedure recovery. Cardiovasc Intervent Radiol 1999; 22: 293–7. doi: 10.1007/s002709900391 [DOI] [PubMed] [Google Scholar]
- 19.Romano M, Giojelli A, Tamburrini O, Salvatore M. Chemoembolization for hepatocellular carcinoma: effect of intraarterial lidocaine in peri- and post-procedural pain and hospitalization. [In Italian.] Radiol Med 2003; 105: 350–5. [PubMed] [Google Scholar]
- 20.Mitchell JW, O'Connell WG, Kisza P, Klyde DP, Gonzalez SF, Maldjian P, et al. Safety and feasibility of outpatient transcatheter hepatic arterial embolization for hepatocellular carcinoma. J Vasc Interv Radiol 2009; 20: 203–8. doi: 10.1016/j.jvir.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 21.Nasser F, Cavalcante RN, Galastri FL, de Rezende MB, Felga GG, Travassos FB, et al. Safety and feasibility of same-day discharge of patients with hepatocellular carcinoma treated with transarterial chemoembolization with drug-eluting beads in a liver transplantation program. J Vasc Interv Radiol 2014; 25: 1012–17. doi: 10.1016/j.jvir.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 22.Paul SB, Sharma H. Role of transcatheter intra-arterial therapies for hepatocellular carcinoma. J Clin Exp Hepatol 2014; 4(Suppl. 3): S112–21. doi: 10.1016/j.jceh.2014.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE, Jr, Loehr SP, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006; 65: 412–25. doi: 10.1016/j.ijrobp.2005.12.051 [DOI] [PubMed] [Google Scholar]
- 24.Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010; 103: 324–31. doi: 10.1038/sj.bjc.6605770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol 2012; 35: 1066–73. doi: 10.1007/s00270-011-0234-7 [DOI] [PubMed] [Google Scholar]
- 26.Hipps D, Ausania F, Manas DM, Rose JDG, French JJ. Selective interarterial radiation therapy (SIRT) in colorectal liver metastases: how do we monitor response? HPB Surg 2013; 2013: 570808. doi: 10.1155/2013/570808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan H, Hoffmann M. Selective internal radiotherapy (SIRT) of liver tumors. [In German.] Radiologe 2015; 55: 48–52. doi: 10.1007/s00117-014-2708-5 [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Baton O, Sideris L, Boige V, Malka D, Liberale G, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol 2005; 90: 36–42. doi: 10.1002/jso.20237 [DOI] [PubMed] [Google Scholar]
- 29.Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging 2005; 30: 419–26. doi: 10.1007/s00261-004-0256-6 [DOI] [PubMed] [Google Scholar]
- 30.Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch HP, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol 2006; 32: 430–4. doi: 10.1016/j.ejso.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 31.Aloia TA, Vauthey J, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastases: resection determines outcome. Arch Surg 2006; 141: 460–6; discussion 466–7. doi: 10.1001/archsurg.141.5.460 [DOI] [PubMed] [Google Scholar]
- 32.Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 2007; 48: 253–8. doi: 10.1080/02841850601161539 [DOI] [PubMed] [Google Scholar]
- 33.Lee W, Yun SH, Chun H, Lee WY, Kim SJ, Choi SH, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastases. J Clin Gastroenterol 2008; 42: 945–9. doi: 10.1097/MCG.0b013e318064e752 [DOI] [PubMed] [Google Scholar]
- 34.Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008; 143: 1204–12. doi: 10.1001/archsurg.143.12.1204 [DOI] [PubMed] [Google Scholar]
- 35.Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, Kim JC. Radiofrequency ablation for metachronous liver metastases from colorectal cancer after curative surgery. Ann Surg Oncol 2008; 15: 227–32. doi: 10.1245/s10434-007-9625-z [DOI] [PubMed] [Google Scholar]
- 36.Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009; 197: 728–36. doi: 10.1016/j.amjsurg.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 37.Knudsen AR, Kannerup A, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases downstaged by chemotherapy. Acta Radiol 2009; 50: 716–21. doi: 10.1080/02841850902991634 [DOI] [PubMed] [Google Scholar]
- 38.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012; 265: 958–68. doi: 10.1148/radiol.12111851 [DOI] [PubMed] [Google Scholar]
- 39.Stintzing S, Grothe A, Hendrich S, Hoffmann RT, Heinemann V, Rentsch M, et al. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncol 2013; 52: 971–7. doi: 10.3109/0284186X.2013.766362 [DOI] [PubMed] [Google Scholar]
- 40.Babawale SN, Jensen TM, Frøkjær JB. Long-term survival following radiofrequency ablation of colorectal liver metastases: a retrospective study. World J Gastrointest Surg 2015; 7: 33–8. doi: 10.4240/wjgs.v7.i3.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Heo JS, Cho YB, Yun SH, Kim HC, Lee WY, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastases: a propensity score analysis. World J Gastroenterol 2015; 21: 3300–7. doi: 10.3748/wjg.v21.i11.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol 2014; 4: S104–11. doi: 10.1016/j.jceh.2014.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin RC, Scoggins CR, McMasters KM. Microwave hepatic ablation: initial experience of safety and efficacy. J Surg Oncol 2007; 96: 481–6. doi: 10.1002/jso.20750 [DOI] [PubMed] [Google Scholar]
- 44.Iannitti DA, Martin RCG, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumor ablation with clustered microwave antennae: the US phase II trial. HPB (Oxford) 2007; 9: 120–4. doi: 10.1080/13651820701222677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009; 13: 318–24. doi: 10.1007/s11605-008-0710-9 [DOI] [PubMed] [Google Scholar]
- 46.Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 2014; 259: 1195–200. doi: 10.1097/SLA.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 47.Bhardwaj N, Strickland AD, Ahmad F, Dennison AR, Lloyd DM. Liver ablation techniques: a review. Surg Endosc 2010; 24: 254–65. doi: 10.1007/s00464-009-0590-4 [DOI] [PubMed] [Google Scholar]
- 48.Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol 2009; 21: 599–605. doi: 10.1097/MEG.0b013e328318ed04 [DOI] [PubMed] [Google Scholar]
- 49.Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16346 treated nodules in 13283 patients. Hepatol Res 2012; 42: 1058–64. doi: 10.1111/j.1872-034X.2012.01025.x [DOI] [PubMed] [Google Scholar]
- 50.Zhang XG, Zhang ZL, Hu SY, Wang SL. Ultrasound-guided ablative therapy for hepatic malignancies: a comparison of the therapeutic effects of microwave and radiofrequency ablation. Acta Chir Belg 2014; 114: 40–5 [PubMed] [Google Scholar]
- 51.Livraghi T, Meloni F, Sobiati L, Zanus G; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumours: results of a multicenter study. Cardiovasc Intervent Radiol 2012; 35: 868–74 [DOI] [PubMed] [Google Scholar]