Abstract
Objective:
To explore the feasibility of morphological and functional evaluation of the lateral pterygoid muscle (LPM) by diffusion tensor imaging (DTI) in vivo.
Methods:
30 healthy volunteers underwent DTI with the jaw in the rest position, opening and clenching. Diffusion parameters of the superior head of the LPM (SHLP) and the inferior head of the LPM (IHLP) at different jaw positions were calculated.
Results:
When the jaw was in the rest position, λ3 of the SHLP was significantly lower than that of the IHLP; fractional anisotropy (FA) value of the SHLP was significant higher than that of the IHLP. There was no significant difference in λ1, λ2 and apparent diffusion coefficient (ADC) value. During jaw opening, there was significant increase of all three eigenvalues and ADC value, and significant decrease of FA value both at the SHLP and IHLP. Clenching caused a significant increase in the ADC and all three eigenvalues, and caused a significant decrease of FA at the SHLP. However, at the IHLP, the variations of all diffusion parameters by clenching in the intercuspal position showed no significance when compared with those at rest.
Conclusion:
The morphological and functional changes of LPM fibres caused by jaw movements could be sensitively detected by DTI which may serve as a new and non-invasive method for simultaneously investigating the functional and morphological features of the LPM during jaw movement.
Advances in knowledge:
A new application of DTI is proposed for the morphological and functional evaluation of the LPMs. The results show that the significant change of three eigenvalues indicates the activity of the LPM in a specific jaw movement, a finding that shows the potential value of DTI serving as a new and non-invasive method for investigation of the LPM.
INTRODUCTION
The lateral pterygoid muscle (LPM), consisting of a superior head and inferior head, is one of the most important masticatory muscles due to its morphological and functional relationship with jaw movement. LPM dysfunction,1–4 including muscle hyperfunction, muscle hypofunction and low muscular co-ordination between the two heads of the muscle, is closely associated with temporomandibular joint disorders (TMJDs), thus it is important to evaluate in vivo the morphology and function of the LPM in clinic.
In previous studies about the LPM, the evaluation of its function during movement of the jaw has been limited to electromyography (EMG), in which single motor units were recorded from the superior head of the LPM (SHLP) and the inferior head of the LPM (IHLP) with the placement of stainless steel wire electrodes within the muscles while the electrodes were carried by a spinal needle directed below the zygomatic arch and retracted to leave the wires within the muscle. EMG study had been proved as a useful and accurate way to evaluate the function of the LPM in jaw movement.5–10 However, EMG could neither demonstrate the morphological characters of the muscles nor show the morphological change induced by muscle activity. In addition, EMG studies do not achieve a consensus result about the function of the IHLP in clenching. Murray et al5 showed that no electromyographic activities were recorded at the IHLP during clenching. On the contrary, Huo et al11 found that the IHLP was active during the intercuspal clenching, and the action potential of the IHLP was slightly lower than that of the SHLP.
MRI, with high spatial resolution, has been used in muscular anatomical imaging. In addition, functional MRI technique, such as diffusion tensor imaging (DTI), has been successfully used to non-invasively investigate the function of muscular tissues. Based on the restriction of water diffusion by cell membranes and other subcellular structures and via measuring the intensity of water diffusion in three orthogonal directions, including the primary (λ1), secondary (λ2) and tertiary (λ3) eigenvalues, DTI allows the mapping of the diffusion characteristic of water molecules of biological tissues in vivo. From the previous studies, DTI has been successfully used to non-invasively investigate the diffusive characteristics and microstructure changes of muscular tissues, such as the diffusive properties of the extraocular muscle,12 the effect of blood flow obstruction and reperfusion,13 skeleton muscle injury14,15 and changes after exercise.16,17 The results demonstrated that there was a geometric correlation between the eigenvalues and muscle fibre architecture.18,19 Therefore, DTI has a huge potential to be a non-invasive imaging tool for both functional and morphological evaluation of the LPM, which may subsequently be beneficial for patients with TMJD.
To the best of our knowledge, there is so far no study evaluating the influence of occlusal changes on the LPM using DTI. Therefore, we designed this prospective study to explore the feasibility of DTI in the functional evaluation of the LPM in vivo in the healthy volunteers.
METHODS AND MATERIALS
Subjects
This study was approved by the institutional review board of Tongji Medical College, Huazhong University of Science and Technology, and a written consent form was obtained from each participant. 30 healthy volunteers (22 males and 8 females; mean age, 24 ± 2.3 years; age range, 19–26 years) were enrolled. All volunteers were students at Tongji Medical College, Huazhong University of Science and Technology; all of whom gave informed consent for participation in the study before the examination. The inclusion criteria included: no missing tooth, no TMJDs, no jaw muscle dysfunction, no chew-side preference and with normal occlusion.
MRI
MRI of bilateral LPM was performed at a 3.0-T MR unit by using an eight-channel head coil for each subject. The imaging parameters of DTI were as follows: repetition time/echo time, 6000/65.6 ms; matrix, 128 × 128; thickness/gap, 2.0/0 mm; field of view, 22 cm; number of excitations, 2; and motion-probing gradients were applied for 25 different directions with b-values of 0 and 600 s mm−2. The total imaging time was 318 s. To specify the regions of interest (ROIs) for the selected muscle, a high-resolution three-dimensional (3D) brain volume imaging (3D-BRAVO, GE Medical Systems, Milwaukee, WI) was obtained with the following parameters: repetition time/echo time, 6.8/2.5 ms; matrix, 256 × 256; thickness/gap, 0.9/0 mm; number of excitations, 1; and field of view, 22 mm. The total imaging time was 138 s.
In this study, postural position was imposed as a supine position rather than upright position to adapt to the criteria of MR examination. The supine position was defined as the position in which the angle between the floor and Frankfort horizontal plane was 90°. According to Yotsuya et al,20 the anteroposterior postural change did not affect function of the LPM, therefore, it is possible to use supine position as postural position in this study.
Initially, the subject underwent both DTI and 3D BRAVO sequence after lying down on the examining table and keeping the jaw in the postural position for 15 min. Then, they underwent the second DTI and 3D BRAVO sequences with their mouth opened by holding a 20-ml syringe barrel covered with a sheet of gauze between the upper and lower incisors. Finally, the syringe barrel was removed; the subject had a rest for 15 min and was then instructed to imitate masticatory movement for 3 min; then, the third DTI was performed when the subject was instructed to bite slightly in the centric occlusion. The 3D BRAVO sequence was not performed in the centric occlusion so as to reduce the examination time. Owing to intolerability of some volunteers to the discomfort caused by the opening aid device, only 23 volunteers underwent the 3D BRAVO sequences with their mouth opened. The 15 min of jaw rest before the entire MR examination and the 15 min of jaw rest between the second and third DTI acquisitions was determined by the previous study performed by Chikui et al.21
Image analysis
The diffusion-related parameters including the apparent diffusion coefficient (ADC), fractional anisotropy (FA) and three eigenvalues (λ1, λ2, λ3) were calculated using the Diffusion Tensor Visualize Tool implemented in a dedicated off-line workstation (AW4.5; GE Medical Systems). The ROI was manually placed by tracing the contours of bilateral SHLP and IHLP on B0 images. In addition, the BRAVO images (anatomical images) were superimposed on the DTI images in order to confirm the level of ROIs. Fascia, blood vessels and fat were avoided from the ROIs to keep the measurements consistent and accurate.
The cross-sectional areas (CSAs) of the SHLP and IHLP when the jaw was in the postural position and open state were calculated at the centre of the muscles; meanwhile, the maximum length of the SHLP and IHLP was also measured.
All the measurements were repeated three times by two radiologists, and the mean value was present as the final result for each parameter.
Statistical analyses
Statistical analysis was performed by using SPSS® v. 19.0 for Windows® (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). The paired t-test was applied to evaluate the symmetry between the right and left sides of the LPM as well as the difference between the SHLP and IHLP when the jaw was in the posture position. The paired t-test was also used to evaluate whether occlusal changes had an effect on the DTI-derived parameters, the CSA and the length of the SHLP and IHLP. The statistical significance was set to p < 0.05 for all analyses.
RESULTS
DTI permits to directly observe the geometrical morphology of muscles via fibre tractography and to quantitatively assess the changes caused by occlusal variation (Figures 1–4).
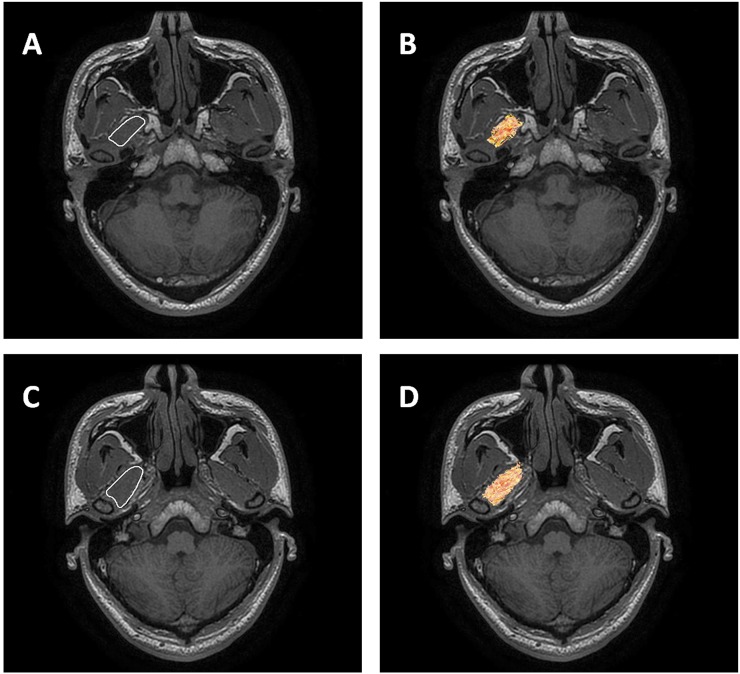
Figure 1.
The method for setting the region of interest at the superior head of the lateral pterygoid muscle (SHLP) (a) and the inferior head of the lateral pterygoid muscle (IHLP) (c). We traced the SHLP and IHLP on the b0 images using axial three-dimensional brain volume imaging (3D-BRAVO, GE Medical Systems, Milwaukee, WI) (a, c) images as anatomical images; the diffusion tensor tractography images (axial) were fused with 3D BRAVO images (axial) to provide detailed anatomy of SHLP (c) and IHLP (d).
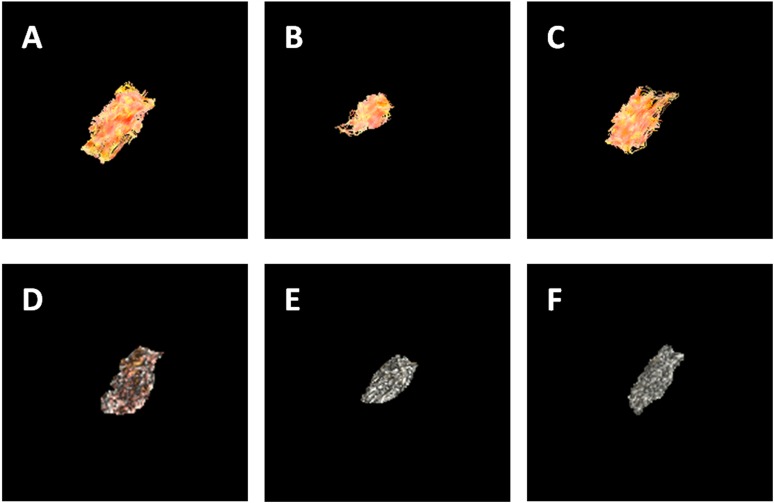
Figure 4.
The three-dimensional (3D) representations of axial views of diffusion tensor fibres and volume rendering (VR) images of the inferior head of lateral pterygoid muscle (IHLP) at different occlusal status (a–c) corresponding to diffusion tensor tractography images of rest, open and intercuspal position (ICP) clench; (d–f) corresponding to VR images of rest, open and ICP clench. During jaw opening, both diffusion-tensor imaging and 3D BRAVO detect a shortening of fibre length and an increase of cross-sectional area in comparison to the postural position, reflecting muscle contraction. Non-significant changes of the IHLP are observed during jaw clenching.
Figure 2.
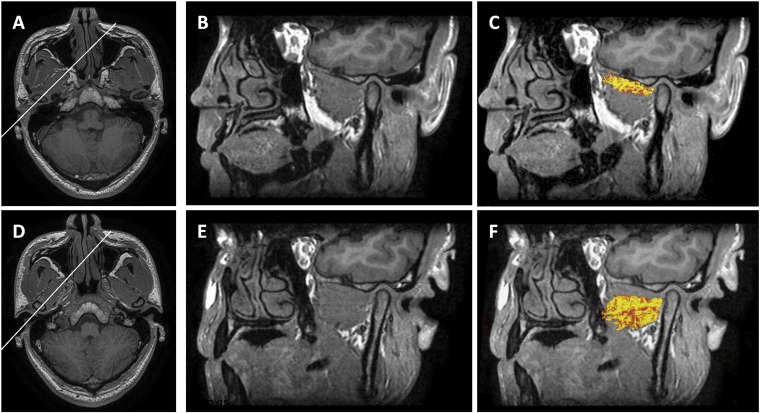
The oblique sagittal fused images of the superior head of the lateral pterygoid muscle (LPM) (c) and the inferior head of the LPM (f) using diffusion tensor tractography images and three-dimensional brain volume imaging (3D BRAVO, GE Medical Systems, Milwaukee, WI) images (b, e). The white lines in axial 3D BRAVO (a, superior head of LPM; d, inferior head of LPM) supplied as a reference image of the oblique sogittal images (b, c, e, f).
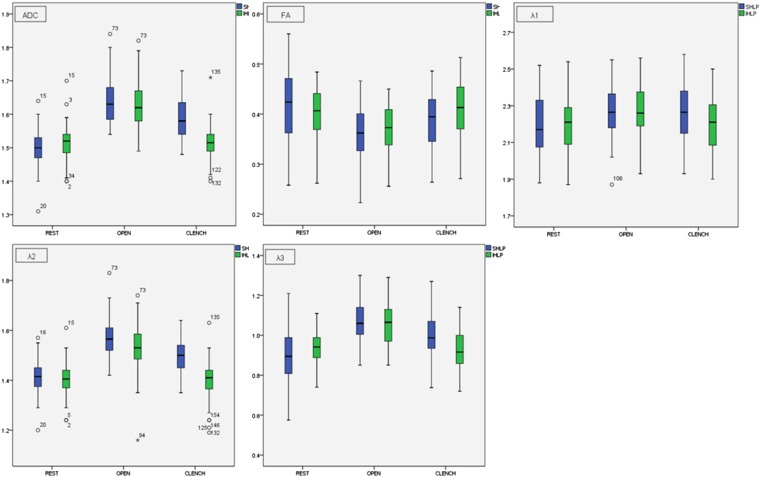
All the DTI-derived parameters (ADC, FA, λ1, λ2 and λ3), CSA and fibre length had no significant difference between the right and left sides of the SHLP and IHLP, respectively (Table 1). Therefore, the mean values from the bilateral SHLP and IHLP were used for analyses (Figure 5).
Table 1.
Symmetry between the right and left sides of the superior head of the lateral pterygoid muscle (SHLP) and the inferior head of the lateral pterygoid muscle (IHLP)
| Muscles | Side | Diffusion parameters |
Morphological parameters |
|||||
|---|---|---|---|---|---|---|---|---|
| ADC | FA | λ1 | λ2 | λ3 | CSA | Fibre length | ||
| SHLP | Left | 1.49 ± 0.06 | 0.41 ± 0.08 | 2.17 ± 0.15 | 1.41 ± 0.07 | 0.89 ± 0.14 | 79.47 ± 17.08 | 35.60 ± 2.61 |
| Right | 1.51 ± 0.05 | 0.42 ± 0.07 | 2.20 ± 0.15 | 1.42 ± 0.07 | 0.90 ± 0.12 | 75.33 ± 16.16 | 36.04 ± 2.41 | |
| p-value | 0.115 | 0.668 | 0.106 | 0.793 | 0.665 | 0.089 | 0.154 | |
| Mean value | 1.50 ± 0.05 | 0.42 ± 0.07 | 2.19 ± 0.15 | 1.41 ± 0.07 | 0.90 ± 0.13 | 77.40 ± 16.57 | 35.82 ± 2.49 | |
| IHLP | Left | 1.51 ± 0.06 | 0.40 ± 0.05 | 2.17 ± 0.14 | 1.39 ± 0.07 | 0.95 ± 0.07 | 228.92 ± 29.92 | 35.36 ± 2.20 |
| Right | 1.52 ± 0.06 | 0.41 ± 0.05 | 2.20 ± 0.15 | 1.41 ± 0.06 | 0.93 ± 0.08 | 232.52±38.42 | 35.47 ± 2.45 | |
| p-value | 0.379 | 0.177 | 0.186 | 0.189 | 0.357 | 0.282 | 0.694 | |
| Mean value | 1.51 ± 0.06 | 0.40 ± 0.05 | 2.19 ± 0.14 | 1.40 ± 0.07 | 0.94 ± 0.08 | 230.72 ± 34.10 | 35.41 ± 2.30 | |
λ1–λ3, three eigenvalues; ADC, apparent diffusion coefficient; CSA, cross-sectional areas; FA, fractional anisotropy.
Figure 5.
Changes in diffusion parameters [three eigenvalues (λ1–λ3), apparent diffusion coefficient (ADC) and fractional anisotropy (FA)] when the superior head of the lateral pterygoid muscle (LPM) and the inferior head of the LPM are at different occlusal status. Data are mean values (×10−3 mm2 s−1) of all the DTI-derived parameters.
When the jaw was in the postural position, λ3 of the SHLP was significantly lower than that of the IHLP (p = 0.002), and the FA value of the SHLP was significantly higher than that of the IHLP (p = 0.03). There were no significant differences in λ1, λ2 and the ADC value (Table 2).
Table 2.
Differences of diffusion tensor imaging-derived parameters of the superior head of the lateral pterygoid muscle (SHLP) and the inferior head of the lateral pterygoid muscle (IHLP) at rest
| Muscles | ADC | FA | λ1 | λ2 | λ3 |
|---|---|---|---|---|---|
| SHLP | 1.49 ± 0.05 | 0.42 ± 0.07 | 2.19 ± 0.15 | 1.41 ± 0.07 | 0.90 ± 0.13 |
| IHLP | 1.51 ± 0.06 | 0.40 ± 0.05 | 2.19 ± 0.14 | 1.40 ± 0.07 | 0.94 ± 0.08 |
| p-value | 0.102 | 0.030 | 1.000 | 0.237 | 0.002 |
λ1–λ3, three eigenvalues; ADC, apparent diffusion coefficient; FA, fractional anisotropy.
During jaw opening, all three eigenvalues and ADC values of the SHLP and IHLP were significantly higher than those at rest. The FA values of the SHLP and IHLP were significantly lower than those at rest (Table 3). Morphologically, the CSA of both heads were significantly larger than those at rest, whereas the muscle length got significantly shorter (Table 4, Figures 3 and 4).
Table 3.
Comparisons of diffusion tensor imaging-derived parameters of the superior head of the lateral pterygoid muscle (SHLP) and the inferior head of the lateral pterygoid muscle (IHLP) during occlusal changes
| Muscles | Parameters | Rest | Opening | ICP clench |
|---|---|---|---|---|
| SHLP | ADC | 1.50 ± 0.05 | 1.64 ± 0.07a | 1.59 ± 0.06b |
| FA | 0.42 ± 0.07 | 0.36 ± 0.05a | 0.39 ± 0.05b | |
| λ1 | 2.19 ± 0.15 | 2.27 ± 0.14a | 2.25 ± 0.16b | |
| λ2 | 1.41 ± 0.07 | 1.57 ± 0.08a | 1.50 ± 0.07b | |
| λ3 | 0.90 ± 0.13 | 1.07 ± 0.10a | 1.01 ± 0.10b | |
| IHLP | ADC | 1.51 ± 0.06 | 1.63 ± 0.07a | 1.51 ± 0.05 |
| FA | 0.40 ± 0.05 | 0.37 ± 0.05a | 0.41 ± 0.06 | |
| λ1 | 2.19 ± 0.14 | 2.27 ± 0.13a | 2.20 ± 0.14 | |
| λ2 | 1.40 ± 0.07 | 1.54 ± 0.10a | 1.40 ± 0.08 | |
| λ3 | 0.94 ± 0.08 | 1.05 ± 0.10a | 0.93 ± 0.09 |
λ1–λ3, three eigenvalues; ADC, apparent diffusion coefficient; FA, fractional anisotropy; ICP, intercuspal position.
p < 0.01 for the comparison between rest and opening.
p < 0.01 for the comparison between rest and ICP clench.
Table 4.
Comparisons of cross-sectional areas and muscle length of the superior head of the lateral pterygoid muscle (SHLP) and the inferior head of the lateral pterygoid muscle (IHLP) during occlusal changes
| Muscles | Cross-sectional areas |
Muscle length |
||
|---|---|---|---|---|
| Rest | Open | Rest | Open | |
| SHLP | 77.40 ± 16.57 | 110.93 ± 21.41a | 35.82 ± 2.49 | 24.77 ± 2.50a |
| IHLP | 230.72 ± 34.10 | 286.28 ± 43.82a | 35.41 ± 2.30 | 28.35 ± 2.44a |
p < 0.01 for the comparison between rest and opening.
Figure 3.
The three-dimensional (3D) representations of axial views of diffusion tensor fibres and volume rendering (VR) images of the superior head of the lateral pterygoid muscle at different occlusal status (a–c) corresponding to diffusion tensor tractography images of rest, open and clench; (d–f) corresponding to VR images of rest, open and clench. During jaw opening and clenching, both diffusion tensor imaging and 3D brain volume imaging (3D BRAVO, GE Medical Systems, Milwaukee, WI) detect a shortening of fibre length and an increase of cross-sectional area in comparison to the postural position, reflecting muscle contraction.
Clenching induced a significant increase in ADC and all three eigenvalues, and a significant decrease in FA value at the SHLP. However, at the IHLP, the variations of all the DTI parameters by clenching in the intercuspal position showed no significant difference as compared with those at rest (Table 3).
DISCUSSION
In the current study, we investigated the feasibility of DTI in evaluation of the function of the LPM in vivo. Our results demonstrate that the changes of geometrical morphology and diffusive properties caused by occlusal variation of the SHLP and IHLP could be sensitively detected by DTI. The variations of the diffusion parameters imply the presence of a functional difference between the SHLP and IHLP in specific jaw movements.
Unlike EMG, MRI could spontaneously achieve morphological visualization and functional evaluation of the LPM. 3D BRAVO with isotropic imaging and high spatial resolution enables reformations in arbitrary planes, optimizing visualization of the muscle and is helpful in quantitative assessment of the CSA and length in different status. Diffusion tensor tractography could directly depict the geometrical morphology of the SHLP and IHLP in different status via fibre tractography, which has a high concordance with the 3D BRAVO reformation images.
In the current study, we successfully calculate all the DTI-derived parameters (ADC, FA, λ1, λ2 and λ3) of the bilateral SHLP and IHLP, respectively. We demonstrated the symmetry between the left and the right sides of the two heads of the LPM and the difference of diffusive parameters between the SHLP and IHLP. Previous DTI studies18,19 have demonstrated that there was a geometric correlation between the eigenvalues and muscle fibre architecture. λ1, defined as the diffusivity parallel to the long axis of the muscle fibre, has been verified.19,22 λ2 was assumed to represent the diffusive transport along the sheets of individual muscle fibres within the endomysium. The SHLP and IHLP are two heads of the LPM, consisting of the same type of muscle fibre and making the water diffusion parallel to the long axis (λ1) and within the endomysium (λ2) non-significant when the jaw is at the postural position in this study. λ3 might be related to the physiological cross-sectional area (PCSA) through the radii of the muscle fibre.23 Theoretically, the PCSA is the sum of the CSAs of all the muscle fibres within the muscle. Our results demonstrated that the λ3 of the IHLP was statistically higher than that of the SHLP in the postural position, which may suggest that the IHLP has a bigger PCSA than the SHLP. This is consistent with the results from studies using cadavers,24 in which the IHLP (2.82 cm2) has a much bigger PCSA than the SHLP (0.95 cm2). The PCSA is the only architectural parameter that is directly proportional to the maximum tetanic tension generated by the muscles,23,25 making it an important architectural parameter in defining the function role of the muscles. In this study, we demonstrated that a higher λ3 was associated with a bigger PCSA, since the PCSA is directly proportional to the maximum tetanic tension; our result may indicate that the λ3 may be related to the ability to produce the maximum muscle force, making it a possible index in assessing the function of muscles. The ADC and FA were calculated from the three eigenvalues as follows:
According to these equations, the changes of ADC and FA values in this study were brought about by the three eigenvalues.
During the jaw-opening process, morphological changes were observed both in reformatted images of 3D BRAVO and the diffusion tensor tractography images. We observed a significantly increasing CSA and decreasing muscle length both in the SHLP and IHLP. This may demonstrate a reciprocal activity of both heads of the LPM in the generation and control of forces to the temporomandibular joint (TMJ) required in the jaw-opening process. This result is consistent with the result from EMG studies,8,10 which showed that both the SHLP and IHLP contracted to generate forces in fine controlling of the protrusive, opening and contralateral jaw movement tasks. Besides the changes of the CSA and the muscle length, a significant increase in the three eigenvalues of both the SHLP and IHLP was observed. These increased diffusion parameters at the SHLP and IHLP reflected the anatomical changes of muscle fibres caused by the contraction of the SHLP and IHLP. Physiologically, muscle contraction (a shortening of muscular fibres) will result in a decrease of muscle fibre length, an increase of muscle fibre diameter and a diastolic of the endomysium. These changes will significantly affect the water molecular diffusion parallel of the long axis of the muscle fibres and diffusion along the sheets of individual muscle fibres within the endomysium, as well as the diffusion within the cross-section of individual muscle fibres, which may cause an increase of λ2 and λ3 and a decrease of λ1, respectively. The changes of λ2 and λ3 were also proved by Schwenzer et al,26 who reported that increased fibre radius due to muscular shortening allows for facilitated diffusion of water in the radial directions, resulting in an increase of λ2 and λ3. According to our prediction, the muscle activity should cause a decrease of λ1; however, in the current study, we noticed a slight increase of λ1. We assume this contradicting change of λ1 may be a comprehensive impact of two factors. The presence of some subcellular structure, such as the sarcoplasmic reticulum, may serve as a strong barrier to radial diffusion instead of to axial diffusion. Therefore, the changes in λ1 due to muscle shortening are minor regarding diffusion hindrances.26 Meanwhile, the exercise of the muscular tissues was accompanied by heat production27 and the increasing of local blood perfusion.28 Both will promote the water diffusion in vivo. The slight increase of λ1 probably suggests that the promotion of water diffusion caused by heat production and the increase of local blood perfusion may exceed the hindrance caused by muscle shortening. Thus, we assume that the changes of the diffusion parameters (especially λ2 and λ3) reflect the morphological and functional changes of the LPM, and they could be used as an index for assessment.
According to Karibe et al,29 the occlusal force distribution on a dental arch had its own pattern and that the clenching strength had no effect on that pattern. Chikui et al21 showed that the variation of bite force had no influence on the diffusion parameters of the masseter muscle. In this study, there is no method for conducting the chewing and for monitoring the bite force during MRI examination; therefore, this study focused on the effect of the distribution of the bite force by static clenching in the intercuspal position on the diffusion parameters.
During the clenching process, morphological changes and significant variations of the three eigenvalues were observed in the SHLP but not in the IHLP. This finding indicates that SHLP contraction is closely associated with the jaw-clenching movement, whereas IHLP may not participate or just plays a less prominent role in this movement. Our results reveal the difference in morphology and diffusion parameters; this may reflect the functional difference of the SHLP and IHLP in the jaw-clenching movement. There was an argument about whether SHLP and IHLP played an important role in clenching.5,11,30 From the prospective of morphology and function, our findings are consistent with the EMG study of Murray et al,5 who believed that intercuspal clenching may activate the SHLP and other muscles, such as the medial pterygoid muscle, instead of the IHLP. Therefore, DTI has a great applied potential in the functional evaluation of muscles.
Recently, many investigations31–34 about the relationship between LPM attachment and internal derangement of the TMJ have been reported already. However, these studies focused on attachment of the LPM to the disc-condyle complex and found no consensus regarding the correlation between LPM attachment and TMJ pathology. In our study, DTI successfully demonstrated changes of the LPM during different jaw movement tasks. The morphological and functional evaluation method of the SHLP, as a whole muscle bundle, can offer a new idea for further research of TMJD's aetiopathogenesis.
This study has several limitations. First, due to the intolerability to maintain clenching, only a few volunteers underwent 3D BRAVO sequence during jaw clenching, thus the CSA and muscle length in clenching in the intercuspal position could not be used in statistication. Second, the sample size is relatively small, with a narrow range of subjects' age. Finally, the water diffusion in vivo is a comprehensive process influenced by many factors. Our result showed that the diffusion parameters are sensitive to changes by jaw movement, and further studies are required to specify the relationship between the changes of diffusion parameters and the physiology of muscle fibres, studies about DTI in detecting the function of LPM when it is under pathological conditions are also required.
CONCLUSION
DTI successfully demonstrated the morphological and functional changes of the LPM during different jaw movement tasks, it also proved synchronous action of the SHLP and IHLP in the jaw opening as well as the action of SHLP rather than IHLP in the jaw clenching. DTI may serve as a new and non-invasive method for simultaneously investigating the functional and morphological features of the LPM during jaw movement. Further studies could potentially use DTI to evaluate the LPM dysfunction in patients with TMJD.
FUNDING
This research was supported by the project from the National Scientific Foundation of China (No. 81401388) and the National Scientific Foundation of Hubei Province (No. 2014CFB292 and No. 2014CFB150).
Contributor Information
Simin Liu, Email: liusm117@163.com.
Min Wang, Email: wang951208@163.com.
Tao Ai, Email: aitao007@hotmail.com.
Qiuxia Wang, Email: 29654590@qq.com.
Renfa Wang, Email: wangrenfa2014@126.com.
Weiwei Chen, Email: chenweiwei101205@gmail.com.
Chu Pan, Email: panchu1008@hotmail.com.
Wenzhen Zhu, Email: zhuwenzhen@hotmail.com.
REFERENCES
- 1.Fujita S, Iizuka T, Dauber W. Variation of heads of lateral pterygoid muscle and morphology of articular disc of human temporomandibular joint—anatomical and histological analysis. J Oral Rehabil 2001; 28: 560–71. doi: 10.1046/j.1365-2842.2001.00691.x [DOI] [PubMed] [Google Scholar]
- 2.Hiraba K, Hibino K, Hiranuma K, Negoro T. EMG activities of two heads of the human lateral pterygoid muscle in relation to mandibular condyle movement and biting force. J Neurophysiol 2000; 83: 2120–37. [DOI] [PubMed] [Google Scholar]
- 3.Juniper RP. Temporomandibular joint dysfunction: a theory based upon electromyographic studies of the lateral pterygoid muscle. Br J Oral Maxillofac Surg 1984; 22: 1–8. doi: 10.1016/0266-4356(84)90001-9 [DOI] [PubMed] [Google Scholar]
- 4.ter Haar Romeny BM, van der Gon JJ, Gielen CC. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol 1984; 85: 631–50. [DOI] [PubMed] [Google Scholar]
- 5.Murray GM, Phanachet I, Uchida S, Whittle T. The role of the human lateral pterygoid muscle in the control of horizontal jaw movements. J Orofac Pain 2000; 15: 279–92; discussion 292–305. [PubMed] [Google Scholar]
- 6.Phanachet I, Whittle T, Wanigaratne K, Klineberg IJ, Sessle BJ, Murray GM. Functional heterogeneity in the superior head of the human lateral pterygoid. J Dent Res 2003; 82: 106–11. doi: 10.1177/154405910308200206 [DOI] [PubMed] [Google Scholar]
- 7.Bakke M, Werdelin LM, Dalager T, Fuglsang-Frederiksen A, Prytz S, Moller E. Reduced jaw opening from paradoxical activity of mandibular elevator muscles treated with botulinum toxin. Eur J Neurol 2003; 10: 695–9. doi: 10.1046/j.1468-1331.2003.00664.x [DOI] [PubMed] [Google Scholar]
- 8.Bhutada MK, Phanachet I, Whittle T, Wanigaratne K, Peck CC, Murray GM. Threshold properties of single motor units in superior head of human lateral pterygoid muscle. Arch Oral Biol 2007; 52: 552–61. doi: 10.1016/j.archoralbio.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Murray GM. The lateral pterygoid muscle: function and dysfunction. Semin Orthod 2012; 18: 44–50. doi: 10.1053/j.sodo.2011.10.001 [DOI] [Google Scholar]
- 10.Bhutada MK, Phanachet I, Whittle T, Peck CC, Murray GM. Activity of superior head of human lateral pterygoid increases with increases in contralateral and protrusive jaw displacement. Eur J Oral Sci 2007; 115: 257–64. doi: 10.1111/j.1600-0722.2007.00461.x [DOI] [PubMed] [Google Scholar]
- 11.Huo F, Geng-Sen Y, Yu-Jin L, Dong-Yun M, Xiao-Ru W. Comparison of the electromyographic changes between the normal lateral pterygoid muscle and the lateral pterygoid muscle spasm. Chin J Clin Rehabil 2004; 8: 662–3. [Google Scholar]
- 12.Seo HS, Kim SE, Rose J, Hadley JR, Parker DL, Jeong EK. Diffusion tensor imaging of extraocular muscle using two-dimensional single-shot interleaved multiple inner volume imaging diffusion-weighted EPI at 3 tesla. J Magn Reson Imaging 2013; 38: 1162–8. doi: 10.1002/jmri.24095 [DOI] [PubMed] [Google Scholar]
- 13.Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MF, Nicolay K, Strijkers GJ. DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med 2006; 56: 272–81. doi: 10.1002/mrm.20953 [DOI] [PubMed] [Google Scholar]
- 14.Zaraiskaya T, Kumbhare D, Noseworthy MD. Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging 2006; 24: 402–8. doi: 10.1002/jmri.20651 [DOI] [PubMed] [Google Scholar]
- 15.Holl N, Echaniz-Laguna A, Bierry G. Diffusion-weighted MRI of denervated muscle: a clinical and experimental study. Skeletal Radiol 2008; 37: 1111–17. doi: 10.1007/s00256-008-0552-2 [DOI] [PubMed] [Google Scholar]
- 16.Froeling M, Oudeman J, van den Berg S. Reproducibility of diffusion tensor imaging in human forearm muscles at 3.0 T in a clinical setting. Magn Reson Med 2010; 64: 1182–90. doi: 10.1002/mrm.22477 [DOI] [PubMed] [Google Scholar]
- 17.Deux JF, Malzy P, Paragios N. Assessment of calf muscle contraction by diffusion tensor imaging. Eur Radiol 2008; 18: 2303–10. doi: 10.1007/s00330-008-1012-z [DOI] [PubMed] [Google Scholar]
- 18.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000; 23: 1647–66. doi: [DOI] [PubMed] [Google Scholar]
- 19.Tseng WY, Wedeen VJ, Reese TG, Smith RN, Halpern EF. Diffusion tensor MRI of myocardial fibers and sheets: correspondence with visible cut-face texture. J Magn Reson Imaging 2003; 17: 31–42. doi: 10.1002/jmri.10223 [DOI] [PubMed] [Google Scholar]
- 20.Yotsuya M, Sato T, Kawamura S. Electromyographic response in inferior head of human LP muscle to anteroposterior postural changes during opening and closing of mouth. Bull Tokyo Dent Coll 2009; 50: 191–8. doi: 10.2209/tdcpublication.50.191 [DOI] [PubMed] [Google Scholar]
- 21.Chikui T, Shiraishi T, Ichihara T. Effect of clenching on T2 and diffusion parameters of the masseter muscle. Acta Radiol 2010; 51: 58–63. doi: 10.3109/02841850903280508 [DOI] [PubMed] [Google Scholar]
- 22.Damon BM, Ding Z, Anderson AW, Freyer AS, Gore JC. Validation of diffusion tensor MRI-based muscle fiber tracking. Magn Reson Med 2002; 48: 97–104. doi: 10.1002/mrm.10198 [DOI] [PubMed] [Google Scholar]
- 23.Galban CJ, Maderwald S, Uffmann K, de Greiff A, Ladd ME. Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf. Eur J Appl Physiol 2004; 93: 253–62. [DOI] [PubMed] [Google Scholar]
- 24.Van Eijden T, Koolstra J, Brugman P. Architecture of the human pterygoid muscles. J Dent Res 1995; 74: 1489–95. doi: 10.1177/00220345950740080901 [DOI] [PubMed] [Google Scholar]
- 25.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol 1984; 57: 1715–21. [DOI] [PubMed] [Google Scholar]
- 26.Schwenzer NF, Steidle G, Martirosian P. Diffusion tensor imaging of the human calf muscle: distinct changes in fractional anisotropy and mean diffusion due to passive muscle shortening and stretching. NMR Biomed 2009; 22: 1047–53. [DOI] [PubMed] [Google Scholar]
- 27.Fornasa F. Diffusion-weighted magnetic resonance imaging: what makes water run fast or slow? J Clin Imaging Sci 2011; 1: 27. doi: 10.4103/2156-7514.81294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isbell DC, Epstein FH, Zhong X. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 2007; 25: 1013–20. doi: 10.1002/jmri.20899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karibe H, Ogata K, Hasegawa Y, Ogihara K. Relation between clenching strength and occlusal force distribution in primary dentition. J Oral Rehabil 2003; 30: 307–11. doi: 10.1046/j.1365-2842.2003.01018.x [DOI] [PubMed] [Google Scholar]
- 30.Sasaki M, Sumi M, van Cauteren M, Obara M, Nakamura T. Intravoxel incoherent motion imaging of masticatory muscles: pilot study for the assessment of perfusion and diffusion during clenching. AJR Am J Roentgenol 2013; 201: 1101–7. doi: 10.2214/AJR.12.9729 [DOI] [PubMed] [Google Scholar]
- 31.Finden SG, Enochs WS, Rao VM. Pathologic changes of the lateral pterygoid muscle in patients with derangement of the temporomandibular joint disk: objective measures at MR imaging. AJNR Am J Neuroradiol 2007; 28: 1537–9. doi: 10.3174/ajnr.A0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonopoulou M, Iatrou I, Paraschos A, Anagnostopoulou S. Variations of the attachment of the superior head of human lateral pterygoid muscle. J Craniomaxillofac Surg 2013; 41: e91–7. doi: 10.1016/j.jcms.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 33.Dergin G, Kilic C, Gozneli R, Yildirim D, Garip H, Moroglu S. Evaluating the correlation between the lateral pterygoid muscle attachment type and internal derangement of the temporomandibular joint with an emphasis on MR imaging findings. J Craniomaxillofac Surg 2012; 40: 459–63. doi: 10.1016/j.jcms.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 34.Imanimoghaddam M, Madani AS, Hashemi EM. The evaluation of lateral pterygoid muscle pathologic changes and insertion patterns in temporomandibular joints with or without disc displacement using magnetic resonance imaging. Int J Oral Maxillofac Surg 2013; 42: 1116–20. doi: 10.1016/j.ijom.2013.01.022 [DOI] [PubMed] [Google Scholar]